Abstract
In Arabidopsis, much of what we know about the phytohormone salicylic acid (SA) and its role in plant defense comes from experiments using young plants. We are interested in understanding why young plants are susceptible to virulent strains of Pseudomonas syringae, while mature plants exhibit a robust defense response known as age-related resistance (ARR). SA-mediated signaling is important for defense in young plants, however, ARR occurs independently of the defense regulators NPR1 and WHY1. Furthermore, intercellular SA accumulation is an important component of ARR, and intercellular washing fluids from ARR-competent plants exhibit antibacterial activity, suggesting that SA acts as an antimicrobial agent in the intercellular space. Young plants accumulate both intracellular and intercellular SA during PAMP- and effector-triggered immunity, however, virulent P. syringae promotes susceptibility by suppressing SA accumulation using the phytotoxin coronatine. Here we outline the hypothesis that mature, ARR-competent Arabidopsis alleviates coronatine-mediated suppression of SA accumulation. We also explore the role of SA in other mature-plant processes such as flowering and senescence, and discuss their potential impact on ARR.
Keywords: Arabidopsis thaliana, Pseudomonas syringae pv. tomato, age-related resistance, salicylic acid, antimicrobial, flowering, senescence, intercellular space
INTRODUCTION
The phenolic phytohormone salicylic acid (SA) contributes to a number of developmental and physiological responses in plants. SA is predominately known for its role in initiating defense responses against pathogens such as Pseudomonas syringae (reviewed in Vlot et al., 2009; An and Mou, 2011), a hemibiotrophic bacterial pathogen. Seminal research established SA as an essential player in plant defense. Wild-type plants respond to microbial attack by accumulating high levels of SA, which induces expression of PATHOGENESIS-RELATED (PR) proteins, ultimately allowing the plant to respond in a resistant manner (Malamy et al., 1990; Métraux et al., 1990). Importantly, plants with reduced SA levels due to ectopic expression of a bacterial SA-hydroxylase gene (NahG) are unable to activate defense responses and are highly susceptible to pathogen attack (Gaffney et al., 1993; Delaney et al., 1994). The level of pathogen-induced SA accumulation is correlated with the magnitude of pathogen resistance, where high levels of SA are associated with resistance and low levels of SA are associated with susceptibility. Thus, SA is a focal point in the tug-of-war between plants and pathogens, with each side attempting to regulate SA levels for its own benefit. Not surprisingly, plant and pathogen genotypes play a large role in dictating the outcome of this tug-of-war, however, an often-overlooked aspect in this struggle is the developmental stage of the plant. In this perspective, we outline the profound impact that developmental age has on SA-mediated plant-pathogen interactions in Arabidopsis.
GENERAL PLANT DEFENSE RESPONSES
Plant defense is comprised of several overlapping layers that include PAMP-triggered immunity (PTI), effector-triggered susceptibility (ETS), and effector-triggered immunity (ETI; reviewed in Jones and Dangl, 2006). Basal defenses such as PTI are induced upon the recognition of common microbial epitopes or PAMPs (pathogen-associated molecular patterns) such as flagellin or chitin by cognate pattern-recognition receptors. The PTI response includes accumulation of SA (reviewed in Boller and Felix, 2009; Meng and Zhang, 2013). SA is synthesized through two distinct metabolic routes. It can be generated from L-phenylalanine via the PAL (PHENYLALANINE AMMONIA LYASE) pathway or from chorismate via ICS1/SID2 (ISOCHORISMATE SYNTHASE1/SALICYLIC ACID INDUCTION DEFICIENT2) the latter of which is responsible for the bulk of chloroplast-derived SA produced during pathogen infection in Arabidopsis (reviewed in Vlot et al., 2009; Dempsey et al., 2011). Arabidopsis sid2 mutants produce little SA and are defective in basal/PTI responses (Nawrath and Métraux, 1999; Wildermuth et al., 2001). To overcome PTI, adapted pathogens employ virulence effector proteins that translocate into plant cells via the type 3 secretion system (T3SS), as well as small diffusible phytotoxins such as coronatine. Once inside the cell, some effector proteins and phytotoxins target host proteins to interfere with PTI, resulting in host susceptibility or enhanced pathogenicity. The mechanisms by which effectors and phytotoxins suppress defense vary, however many suppress plant defenses such as SA accumulation and PR gene expression (Xin and He, 2013). To overcome the suppression of plant defense by effector proteins, plants employ ETI. To initiate ETI, an effector protein is first recognized by a highly specific Resistance (R) receptor protein, either directly or indirectly. Recognition of an effector or “avirulence” protein by its cognate R receptor initiates a signaling cascade that results in SA accumulation, PR gene expression, and a form of programmed cell death known as the hypersensitive response (Jones and Dangl, 2006). This form of resistance is highly specific and affords a high degree of resistance. Both ETI and PTI also initiate systemic acquired resistance (SAR), a defense response in which an initial local infection induces long-distance signaling to protect distant uninfected leaves against future pathogen attack (reviewed in Champigny and Cameron, 2009; Shah and Zeier, 2013). Much like PTI and ETI, plants defective in SA accumulation are defective in SAR. Although SA itself is not the long-distance SAR signal (Rasmussen et al., 1991; Vernooij et al., 1994), the SA conjugate methyl salicylate (MeSA) participates in SAR (Park et al., 2007; Vlot et al., 2008; Liu et al., 2011).
MECHANISM OF SA SIGNAL TRANSDUCTION
Salicylic acid accumulation initiates a complex signaling cascade that includes hallmark PR gene expression. Early genetic screens for mutants defective in SA signaling discovered NPR1 (NON-EXPRESSOR OF PR1), a transcriptional co-activator important for plant defense (Cao et al., 1997). Our current understanding of SA signaling places NPR1 in a central role as the master-regulator of SA-induced signal transduction (reviewed in Vlot et al., 2009; An and Mou, 2011; Yan and Dong, 2014). In brief, SA accumulation leads to a change in cellular redox status that facilitates the monomerization of a cytosolic oligomer pool of NPR1, which translocate to the nucleus and interact with TGA transcription factors to regulate gene expression (Mou et al., 2003). Although NPR1 plays a central role in signaling, its inability to reliably bind SA in conventional ligand-binding assays suggests that it is not the SA receptor. A search for the SA receptor demonstrated that NPR1 homologs NPR3 and NPR4 bind SA and regulate NPR1 protein stability to mediate SA-signaling (Fu et al., 2012). Based on their findings, the authors depict a model wherein SA levels affect the ability of NPR3 or NPR4 to target NPR1 for ubiquitinylation and degradation via the proteasome. At the lowest and highest levels of SA, the NPR1 homologs direct NPR1 degradation, preventing SA signaling. At intermediate SA levels, NPR1 is no longer targeted for degradation and can participate in SA signaling (reviewed in Yan and Dong, 2014). This regulatory module ensures that SA induces defense gene expression only when necessary and prevents constitutive SA-mediated immune signaling, which is generally detrimental to growth and development (reviewed in Durrant and Dong, 2004; Rivas-San Vicente and Plasencia, 2011).
MATURITY AND DEFENSE—UNCONVENTIONAL DISEASE RESISTANCE
Much of what we know about SA signaling and its impact on induced resistance comes from experiments using young plants. In the P. syringae–Arabidopsis pathosystem, young plants inoculated with virulent P. syringae pv. tomato (Pst) support high levels of in planta bacterial growth and are susceptible to disease, while mature plants support low levels of in planta bacterial growth and are resistant (Kus et al., 2002). This phenomenon, known as age-related resistance (ARR), is a highly robust form of developmentally regulated resistance. The focus of this perspective is ARR in Arabidopsis, however, developmentally regulated disease resistance has been observed in a variety of other plants (reviewed in Whalen, 2005; Develey-Rivière and Galiana, 2007). Much like defense in young plants, the ability to accumulate SA in response to pathogen infection is required for ARR in Arabidopsis. Plants defective in SA biosynthesis or accumulation (sid2, eds1, eds5/sid1, NahG) are ARR-defective such that mature plants remain susceptible to Pst at later stages of development (Kus et al., 2002; Carviel et al., 2009, 2014). Unlike defense in young plants, NPR1 is not required for ARR (Kus et al., 2002; Cameron and Zaton, 2004), suggesting that although SA accumulation is critical, NPR1-dependent SA signaling is dispensable during ARR. This led us to speculate that ARR may employ NPR1-independent SA signaling. Our knowledge of NPR1-independent SA signaling is less extensive in comparison to NPR1-dependent responses, however, the ssDNA-binding transcription factor WHIRLY1 (WHY1) is among a small number of genes thought to be involved in NPR1-independent SA signaling and defense (reviewed in Desveaux et al., 2005; An and Mou, 2011). WHY1 is required for SA and pathogen-induced PR1 expression irrespective of NPR1. Moreover, ssDNA-binding activity of WHY1 is induced by SA treatment in both wild-type and npr1-1 plants, suggesting that WHY1 functions to induce PR expression independent of NPR1 (Desveaux et al., 2004). To investigate the requirement of NPR1-independent SA signaling for ARR, we compared the ARR phenotypes of two independent why1 T-DNA insertion mutants (why1-1, why1-2) to wild-type Col-0 and the SA-deficient sid2-1 mutant. Plants were inoculated with 106 colony-forming units per ml (cfu ml–1) of virulent Pst (DC3000) at 4 and 7 weeks post-germination (wpg) followed by determination of in planta bacterial density 3 days later (Figure 1). For both wild-type Col-0 and the why1 mutants, young plants supported high in planta bacterial densities (2–5 × 106 cfu per leaf disk [cfu ld–1]), whereas mature plants displayed reduced bacterial densities (3–6 × 104 cfu ld–1) consistent with a strong ARR response. In comparison, the SA-deficient sid2-1 mutant displayed a characteristic ARR-defective phenotype, with high in planta bacterial densities (>1 × 107 cfu ld–1) at 4 and 7 wpg. These data suggest that WHY1 function is not required for ARR. Given that WHY1 and NPR1 are not required for ARR competence, we suggest that SA signaling through these proteins is not an important component of ARR. Indeed, we previously demonstrated that ARR-competent plants express less PR1 in response to virulent Pst compared to young plants (Kus et al., 2002; Rusterucci et al., 2005), indicating that ARR represents an unconventional SA-dependent defense response that occurs in older plants. Although it is possible that SA plays an NPR1- and WHY1-independent signaling role that is not associated with PR1 expression, we propose that SA may play a different role altogether during ARR.
FIGURE 1.
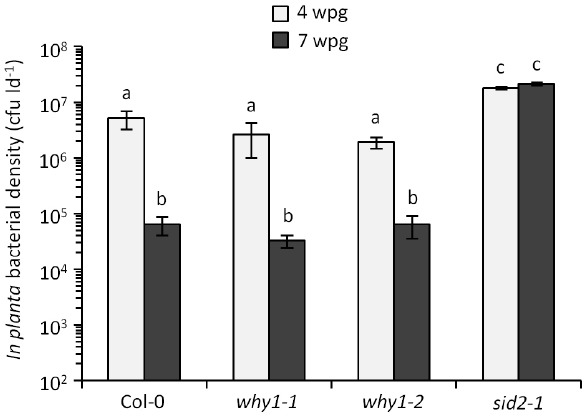
WHIRLY1 is not required for ARR. Plants were inoculated with 106 cfu ml–1 of virulent Pst DC3000 at 4 and 7 weeks post-germination (wpg). In planta bacterial density [colony-forming units per leaf disk (cfu ld–1)] was determined 3 days later and is presented as the mean ± standard deviation of three sample replicates. Different letters indicate statistically significant differences between means (ANOVA, Tukey’s HSD, P < 0.05). This experiment was performed three times with similar results. Plant growth, inoculations, and quantification of bacterial levels were performed as described previously (Carviel et al., 2014). The T-DNA mutants why1-1 (SALK_023713C) and why1-2 (SALK_147680C) were obtained from TAIR and have been characterized previously (Isemer et al., 2012).
A POTENTIAL NON-SIGNALING ROLE FOR SA IN PLANT DEFENSE RESPONSES
An alternative, non-signaling role for SA during ARR was explored by Cameron and Zaton (2004), who hypothesized that SA may act as an antimicrobial agent in the intercellular space (apoplast) during ARR. This hypothesis arose from the observation that intercellular washing fluids (IWFs) collected from mature (ARR-competent) plants inoculated with Pst possessed antimicrobial activity that was not present in corresponding IWFs from young (ARR-incompetent) plants (Kus et al., 2002). Moreover, antimicrobial activity was absent in IWFs from mature NahG plants, suggesting that SA accumulation is required for the antimicrobial activity observed in mature wild-type plants. Further investigation revealed that SA accumulated in IWFs from mature plants but not young plants following inoculation with Pst (Cameron and Zaton, 2004). Infiltration of exogenous SA into the intercellular space rescued the ARR-defect in sid2-1 but not NahG. Conversely, addition of the SA-degrading salicylate hydroxylase enzyme to the intercellular space impaired the ARR response of wild-type plants. Together these data suggest that SA accumulation in the intercellular space is a key aspect of the ARR response. The antimicrobial effect of SA on Pst in vitro (Cameron and Zaton, 2004) suggests that SA itself could be acting as an antimicrobial agent in planta during ARR. Moreover, SA and structurally related compounds possess antimicrobial activity against a variety of other phytopathogens in vitro (Prithiviraj et al., 1997; Georgiou et al., 2000; Amborabé et al., 2002; El-Mougy, 2002; Martín et al., 2010).
Mature plants accumulate high levels of intercellular SA in response to virulent Pst, while young plants accumulate relatively little (Cameron and Zaton, 2004; Carviel et al., 2014). We therefore propose that pathogen-mediated suppression of intercellular SA accumulation contributes to disease susceptibility in young plants, and that mature plants are able to overcome this virulence strategy. In young plants the P. syringae phytotoxin coronatine has been shown to suppress SA accumulation at the whole-leaf level (deTorresZabala et al., 2009; Zheng et al., 2012) as well as in the intercellular space (Carviel et al., 2014). Young plants inoculated with a coronatine-deficient Pst mutant accumulated higher levels of intracellular and intercellular SA, and supported lower bacterial levels compared to plants inoculated with wild-type Pst (Carviel et al., 2014). This suggests that intercellular SA accumulation is a component of the basal defense response that is suppressed by Pst. A specific signaling pathway for coronatine-mediated suppression of SA accumulation in young plants has recently been uncovered (Zheng et al., 2012), and we hypothesize that ARR involves the activity of one or more developmentally regulated gene products that alleviate coronatine-mediated suppression of defense (Wilson et al., 2014). Similar to mature plants responding to virulent Pst, young plants responding to avirulent Pst also accumulated high levels of SA in IWFs (Carviel et al., 2014). Thus, intercellular SA accumulation may also contribute to ETI.
SA-ASSOCIATED MATURE-PLANT PROCESSES AND ARR COMPETENCE
Our ARR research has revealed novel aspects of SA-mediated defense in both young and mature plants. However, the fundamental question, “how do mature plants become competent for ARR?,” remains to be answered. In Arabidopsis, several mature-plant developmental processes have been associated with SA accumulation (reviewed in Rivas-San Vicente and Plasencia, 2011). We speculate that these SA-dependent processes may contribute to ARR competence. Below, we briefly describe two major developmental processes, the transition to flowering and leaf senescence, and our efforts to understand their contribution to SA accumulation and ARR.
IMPACT OF LEAF SENESCENCE AND SA CATABOLISM ON ARR
Leaf senescence is an actively regulated developmental process that coordinates the reallocation of metabolic resources from leaves to reproductive tissues in older plants (reviewed in Lim et al., 2007). As a mature-plant process, leaf senescence could contribute to ARR competence. In a recent study, Zhang et al. (2013) identified the Arabidopsis S3H (SA-3-HYDROXYLASE) protein, which is responsible for the catabolism of SA to 2,3-dihydroxybenzoic acid (DHBA) and 2,5-DHBA. Arabidopsis s3h mutants accumulated high levels of SA and underwent leaf senescence early, whereas transgenic Arabidopsis overexpressing S3H had low levels of SA, high levels of 2,3-DHBA sugar conjugates, and were delayed in senescence (Zhang et al., 2013). This study demonstrates the strong positive correlation between SA levels and the induction of leaf senescence. The authors also determined that 2,3-DHBA and its xyloside conjugate 2,3-DHB3X accumulated with age (Zhang et al., 2013). In a previous study, 2,3-DHBA was identified as an EDS1-dependent metabolite that accumulated in response to P. syringae infection and with age (Bartsch et al., 2010). Although 2,3-DHBA does not possess a strong capacity to induce PR1 gene expression, the authors propose that it may contribute to EDS1-dependent defense. We agree with the authors’ idea that 2,3-DHBA, an isochorismate-derived metabolite that accumulates with age and is dependent on EDS1, may contribute to ARR. Their finding that 2,3-DHBA was a poor inducer of PR1 expression is in agreement with our observations that ARR-competent plants do not express PR1 to high levels and that ARR doesn’t require NPR1 or WHY1. Whether 2,3-DHBA plays a role in ARR could be addressed by quantifying 2,3-DHBA and 2,3-DHB3X in IWFs collected from young and mature plants inoculated with Pst, and by determining if DHBA contributes to the antimicrobial activity of IWFs from ARR-competent plants. However, ARR competence is not associated with early-stage senescence marker gene expression (SAG-13) or senescence-induced leaf tip chlorosis (Kus et al., 2002), suggesting that senescence is not a developmental cue for ARR competence. Rather, aspects of leaf aging such as an increase in SA catabolism and DHBA accumulation may contribute to ARR competence in Arabidopsis independent of leaf senescence.
THE TRANSITION TO FLOWERING IS ASSOCIATED WITH ARR
The transition from vegetative to reproductive growth is a highly regulated process that relies on multiple endogenous and environmental cues (reviewed in Amasino, 2010). Interestingly, SA appears to act as a positive regulator of flowering in Arabidopsis, as SA-deficient mutants flower later than wild-type plants (Martínez et al., 2004). Detailed genetic analyses indicated that the promotion of flowering by SA appears to proceed through several independent mechanisms, involving components of the autonomous and photoperiod flowering pathways (Martínez et al., 2004). In both short- and long-day-grown Arabidopsis the floral transition occurs at approximately the same time as the onset of ARR (Rusterucci et al., 2005). This led us to speculate that the transition to flowering could be a developmental cue for ARR competence. However, further investigation effectively separated the transition to flowering from ARR competence (Wilson et al., 2013). Early-flowering mutants and wild-type plants forced to flower early by transient exposure to long days did not exhibit early ARR, nor did late-flowering mutants display delayed ARR. Together these data suggest that the transition to flowering is neither sufficient nor required for the onset of ARR competence.
Unexpectedly, our analysis of flowering-time mutants revealed that early-flowering svp-31 was ARR-incompetent. SVP (SHORT VEGETATIVE PHASE) is a MADS-domain transcription factor that acts as a negative regulator of the floral transition (Hartmann et al., 2000). A genome-wide ChIP-chip study (Tao et al., 2012) identified many SVP target genes including three NAC transcription factors that have been shown to mediate the suppression of SA accumulation by coronatine (Zheng et al., 2012). Our current efforts are focused on elucidating the role of SVP in ARR and determining whether SVP suppresses NAC gene expression to prevent coronatine-mediated suppression of SA accumulation in mature plants.
CONCLUSION—DEVELOPMENTAL DIFFERENCES IN SA-MEDIATED DEFENSE
It is clear that SA plays a central role in immune responses to Pst in both young and mature Arabidopsis. Moreover, Arabidopsis ARR is also effective against the biotrophic pathogen Hyaloperonospora arabidopsidis (Hpa; Rusterucci et al., 2005; Carviel et al., 2009). Since several Hpa effectors have been shown to suppress SA-mediated immunity in young plants, (Anderson et al., 2012; Caillaud et al., 2013; Asai et al., 2014) we speculate that suppression of SA-mediated defense by Hpa is also alleviated in mature ARR-competent plants. Our current model of ARR and the role that SA plays in mature versus young plants is illustrated in Figure 2. At earlier developmental stages, plants support high levels of bacterial growth and are susceptible to Pst. The phytotoxin coronatine contributes to the suppression of SA accumulation in young plants to prevent SA-mediated immune signaling, thus promoting disease susceptibility. At later stages of development, plants gain competence for ARR and are resistant to Pst infection. This is associated with the accumulation of high levels of SA, which may act as an antimicrobial agent in the intercellular space. The transition to flowering overlaps with the onset of ARR, however, it is not the developmental cue for ARR competence. Interestingly, our recent studies with SVP, a negative regulator of the transition to flowering, suggest that this transcription factor may contribute to ARR by limiting coronatine-mediated suppression of SA accumulation. Further, we hypothesize that the SA-catabolite 2,3-DHBA, acts as an antimicrobial agent in the intercellular space similar to SA. Future research is required to address the key questions posed by our model and clarify the role of SA during plant-pathogen interactions in mature versus young Arabidopsis.
FIGURE 2.
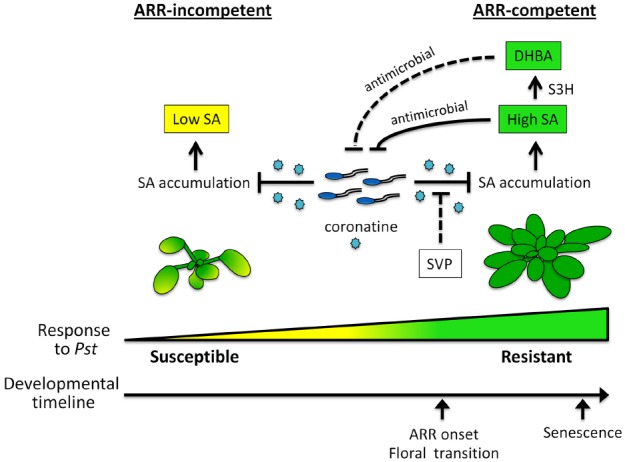
Salicylic acid-mediated disease resistance in young and mature Arabidopsis. The model illustrates key aspects of the Arabidopsis age-related resistance (ARR) response to Pseudomonas syringae pv. tomato (Pst) with a focus on salicylic acid (SA) accumulation in young and mature plants. In young plants, coronatine produced by Pst suppresses the accumulation of SA to dampen defense, resulting in susceptibility to disease. At later stages of development, plants acquire ARR competence and become resistant to Pst. Mature plants infected with virulent Pst accumulate high levels of SA despite the presence of coronatine. Our accumulated evidence supports the idea that intercellular SA acts as an antimicrobial agent to limit Pst growth. The onset of ARR competence coincides with the transition to flowering whereas leaf senescence occurs well after. We hypothesize that the floral repressor SHORT VEGETATIVE PHASE (SVP) contributes to ARR by alleviating coronatine-mediated suppression of SA. SA-3-HYDROXYLASE (S3H) converts SA to 2,3-dihydroxybenzoic acid (DHBA), which accumulates with age and contributes to leaf senescence. We hypothesize that DHBA contributes to ARR as an antimicrobial agent in the intercellular space. Dashed bar—hypothesized relationship, solid bar—relationship supported by evidence.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGMENTS
This research was funded by a Natural Sciences and Engineering Research Council of Canada (NSERC) grant to Robin K. Cameron, an NSERC graduate scholarship to Daniel C. Wilson, and an Ontario Graduate Scholarship to Philip Carella.
REFERENCES
- Amasino R. (2010). Seasonal and developmental timing of flowering. Plant J. 61, 1001–1013. 10.1111/j.1365-313X.2010.04148.x [DOI] [PubMed] [Google Scholar]
- Amborabé B., Fleurat-Lessard P., Chollet J., Roblin G. (2002). Antifungal effects of salicylic acid and other benzoic acid derivatives towards Eutypa lata: structure–activity relationship. Plant Physiol. Biochem. 40, 1051–1060 10.1016/S0981-9428(02)01470-5 [DOI] [Google Scholar]
- An C., Mou Z. (2011). Salicylic acid and its function in plant immunity. J. Integer. Plant Biol. 53, 412–428. 10.1111/j.1744-7909.2011.01043.x [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Casady M. S., Fee R. A., Vaughan M. M., Deb D., Fedkenheuer K., et al. (2012). Homologous RXLR effectors from Hyaloperonospora arabidopsidis and Phytophthora sojae suppress immunity in distantly related plants. Plant J. 72, 882–893. 10.1111/j.1365-313X.2012.05079.x [DOI] [PubMed] [Google Scholar]
- Asai S., Rallapalli G., Piquerez S. J. M., Caillaud M.-C., Furzer O. J., Ishaque N., et al. (2014). Expression profiling during Arabidopsis/downy mildew interaction reveals a highly-expressed effector that attenuates responses to salicylic acid. PLoS Pathog. 10:e1004443. 10.1371/journal.ppat.1004443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch M., Bednarek P., Vivancos P. D., Schneider B., von Roepenack-Lahaye E., Foyer C. H., et al. (2010). Accumulation of isochorismate-derived 2,3-dihydroxybenzoic 3-O-β-D-xyloside in Arabidopsis resistance to pathogens and ageing of leaves. J. Biol. Chem. 285, 25654–25665. 10.1074/jbc.M109.092569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Felix G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by patter-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. 10.1146/annurev.arplant.57.032905.105346 [DOI] [PubMed] [Google Scholar]
- Caillaud M.-C., Asai S., Rallapalli G., Piquerez S., Fabro G., Jones D. G. J. (2013). A downy mildew effector attenuates salicylic acid-triggered immunity in Arabidopsis by interacting with the host mediator complex. PLoS Biol. 11:e1001732. 10.1371/journal.pbio.1001732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. K., Zaton K. (2004). Intercellular salicylic acid accumulation is important for age-related resistance in Arabidopsis to Pseudomonas syringae. Physiol. Mol. Plant Pathol. 66, 197–209 10.1016/j.pmpp.2005.02.002 [DOI] [Google Scholar]
- Cao H., Glazebrook J., Clarke J. D., Volko S., Dong X. (1997). The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88, 57–63 10.1016/S0092-8674(00)81858-9 [DOI] [PubMed] [Google Scholar]
- Carviel J. L., Al-Daoud F., Neumann M., Mohammad A., Provart N. J., Moeder W., et al. (2009). Forward and reverse genetics to identify genes involved in the age-related resistance response in Arabidopsis thaliana. Mol. Plant Pathol. 10, 621–634. 10.1111/J.1364-3703.2009.00557.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carviel J. L., Wilson D. C., Isaacs M., Carella P., Catana V., Golding B., et al. (2014). Investigation of intercellular salicylic acid accumulation during compatible and incompatible Arabidopsis-Pseudomonas syringae interactions using a fast neutron-generated mutant allele of EDS5 identified by genetic mapping and whole-genome sequencing. PLoS ONE 9:e88608. 10.1371/journal.pone.0088608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champigny M. J., Cameron R. K. (2009). “Action at a distance: long-distance signals in induced resistance,” in Plant Innate Immunity, Vol. 51, ed. Van Loon L. C. (London: Academic Press; ), 123–171. [Google Scholar]
- de Torres Zabala M., Bennett M. H., Truman W. H., Grant M. R. (2009). Antagonism between salicylic acid and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J. 59, 375–386. 10.1111/j.1365-313X.2009.03875.x [DOI] [PubMed] [Google Scholar]
- Delaney T. P., Uknes S., Vernooij B., Friedrich L., Weymann K., Negrotto D., et al. (1994). Central role of salicylic acid in plant disease resistance. Science 266, 1247–1250. 10.1126/science.266.5188.1247 [DOI] [PubMed] [Google Scholar]
- Dempsey D. A., Vlot A. C., Wildermuth M. C., Klessig D. F. (2011). Salicylic acid biosynthesis and metabolism. Arabidopsis Book 9, 1–24. 10.1199/tab.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desveaux D., Subramaniam R., Despres C., Mess J. N., Levesque C., Fobert P. R., et al. (2004). A “Whirly” transcription factor is required for salicylic acid-dependent disease resistance in Arabidopsis. Dev. Cell 6, 229–240 10.1016/S1534-5807(04)00028-0 [DOI] [PubMed] [Google Scholar]
- Desveaux D., Marechal A., Brisson N. (2005). Whirly transcription factors: defense gene regulation and beyond. Trends Plant Sci. 10, 95–102. 10.1016/j.tplants.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Develey-Rivière M., Galiana E. (2007). Resistance to pathogens and host developmental stage: a multifaceted relationship within the plant kingdom. New Phytol. 175, 405–416. 10.1111/j.1469-8137.2007.02130.x [DOI] [PubMed] [Google Scholar]
- Durrant W. E., Dong X. (2004). Systemic acquired resistance. Annu. Rev. Phytopathol. 42, 185–209. 10.1146/annurev.phyto.42.040803.140421 [DOI] [PubMed] [Google Scholar]
- El-Mougy N. S. (2002). In vitro studies on antimicrobial activity of salicylic acid and acetylsalicylic acid as pesticidal alternatives against some soilborne plant pathogens. Egypt. J. Phytopathol. 30, 41–55. [Google Scholar]
- Fu Z. Q., Yan S. P., Saleh A., Wang W., Ruble J., Oka N., et al. (2012). NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228–232. 10.1038/nature11162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T., Friedrich L., Vernooij B., Negrotto D., Nye G., Uknes S., et al. (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754–756. 10.1126/science.261.5122.754 [DOI] [PubMed] [Google Scholar]
- Georgiou C. D., Tairis N., Sotiropoulou A. (2000). Hydroxyl radical scavengers inhibit lateral-type sclerotial differentiation and growth in phytopathogenic fungi. Mycologia 95, 825–834 10.2307/3761577 [DOI] [Google Scholar]
- Hartmann U., Höhmann S., Nettesheim K., Wisman E., Saedler H., Huijser P. (2000). Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J. 21, 351–360. 10.1046/j.1365-313x.2000.00682.x [DOI] [PubMed] [Google Scholar]
- Isemer R., Krause K., Grabe N., Kitahata N., Asami T., Krupinska K. (2012). Plastid located WHIRLY1 enhances the responsiveness of Arabidopsis seedlings toward abscisic acid. Front. Plant Sci. 3:283. 10.3389/fpls.2012.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. D. J., Dangl J. F. (2006). The plant immune system. Nature 444, 323–329. 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- Kus J. V., Zaton K., Sarkar R., Cameron R. K. (2002). Age-related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae. Plant Cell 14, 479–490. 10.1105/tpc.010481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P. K., Kim H. J., Nam H. G. (2007). Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136. 10.1146/annurev.arplant.57.032905.105316 [DOI] [PubMed] [Google Scholar]
- Liu P. P., von Dahl C. C., Klessig D. F. (2011). The extent to which methyl salicylate is required for signaling systemic acquired resistance is dependent on exposure to light after infection. Plant Physiol. 157, 2216–2226. 10.1104/pp.111.187773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J., Carr J. P., Klessig D. F., Raskin I. (1990). Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250, 1002–1004. 10.1126/science.250.4983.1002 [DOI] [PubMed] [Google Scholar]
- Martín J. A., Solla A., Witzell J., Gil L., García-Vallejo M. C. (2010). Antifungal effect and reduction of Ulmus minor symptoms to Ophiostoma novo-ulmi by carvacrol and salicylic acid. Eur. J. Plant Pathol. 127, 21–32 10.1007/s10658-009-9567-3 [DOI] [Google Scholar]
- Martínez C., Pons E., Prats G., León J. (2004). Salicylic acid regulates flowering time and links defense responses and reproductive development. Plant J. 37, 209–217. 10.1046/j.1365-313X.2003.01954.x [DOI] [PubMed] [Google Scholar]
- Meng X., Zhang S. (2013). MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 51, 245–266. 10.1146/annurev-phyto-082712-102314 [DOI] [PubMed] [Google Scholar]
- Métraux J.-P., Signer H., Ryals J., Ward E., Wyss-Benz M., Gandin J., et al. (1990). Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250, 1004–1006. 10.1126/science.250.4983.1004 [DOI] [PubMed] [Google Scholar]
- Mou Z., Fan W., Dong X. (2003). Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113, 935–944 10.1016/S0092-8674(03)00429-X [DOI] [PubMed] [Google Scholar]
- Nawrath C., Métraux J.-P. (1999). Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 8, 1393–1404 10.1105/tpc.11.8.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-W., Kaimoyo E., Kumar D., Mosher S., Klessig D. F. (2007). Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 113–116. 10.1126/science.1147113 [DOI] [PubMed] [Google Scholar]
- Prithiviraj B., Manickam M., Singh U. P., Ray A. B. (1997). Antifungal activity of anacardic acid, a naturally occurring derivative of salicylic acid. Can. J. Bot. 75, 207–211 10.1139/b97-021 [DOI] [Google Scholar]
- Rasmussen J. B., Hammerschmidt R., Zook M. N. (1991). Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv. syringae. Plant Physiol. 97, 1342–1347 10.1104/pp.97.4.1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-San Vicente M., Plasencia J. (2011). Salicylic acid beyond defence: its role in plant growth and development. J. Exp. Bot. 62, 3321–3338. 10.1093/jxb/err031 [DOI] [PubMed] [Google Scholar]
- Rusterucci C., Zhao Z., Haines K., Mellersh D., Neumann M., Cameron R. K. (2005). Age-related resistance to Pseudomonas syringae pv. tomato is associated with the transition to flowering in Arabidopsis and is effective against Peronospora parasitica. Physiol. Mol. Plant Pathol. 66, 222–231 10.1016/j.pmpp.2005.08.004 [DOI] [Google Scholar]
- Shah J., Zeier J. (2013). Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 4:30. 10.3389/fpls.2013.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z., Shen L., Liu C., Liu L., Yan Y., Yu H. (2012). Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis. Plant J. 70, 549–561. 10.1111/j.1365-313X.2012.04919.x [DOI] [PubMed] [Google Scholar]
- Vernooij B., Friedrich L., Morse A., Reist R., Kolditz-Jawhar R., Ward E., et al. (1994). Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell 6, 959–965. 10.1105/tpc.6.7.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot A. C., Liu P. P., Cameron R. K., Park S. W., Yang Y., Kumar D., et al. (2008). Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J. 56, 445–456. 10.1111/j.1365-313X.2008.03618.x [DOI] [PubMed] [Google Scholar]
- Vlot A. C., Dempsey D. A., Klessig D. F. (2009). Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 47, 177–206. 10.1146/annurev.phyto.050908.135202 [DOI] [PubMed] [Google Scholar]
- Whalen M. C. (2005). Host defence in a developmental context. Mol. Plant Pathol. 6, 347–360. 10.1111/j.1364-3703.2005.00286.x [DOI] [PubMed] [Google Scholar]
- Wildermuth M. C., Dewdney J., Wu G., Ausubel F. M. (2001). Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565. 10.1038/35107108 [DOI] [PubMed] [Google Scholar]
- Wilson D. C., Carella P., Cameron R. K. (2014). Intercellular salicylic acid accumulation during compatible and incompatible Arabidopsis-Pseudomonas syringae interactions. Plant Signal. Behav. 9:e29362. 10.4161/psb.29362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. C., Carella P., Isaacs M., Cameron R. K. (2013). The floral transition is not the developmental switch that confers competence for the Arabidopsis age-related resistance response to Pseudomonas syringae pv. tomato. Plant Mol. Biol. 83, 235–246. 10.1007/s11103-013-0083-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X.-F., He S.-Y. (2013). Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu. Rev. Phytopathol. 51, 473–498. 10.1146/annurev-phyto-082712-102321 [DOI] [PubMed] [Google Scholar]
- Yan S., Dong X. (2014). Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol. 20, 64–68. 10.1016/j.pbi.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Halitschke R., Yin C., Liu C.-J., Gan S.-S. (2013). Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc. Natl. Acad. Sci. U.S.A. 110, 14807–14813. 10.1073/pnas.1302702110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. Y., Spivey N. W., Zeng W., Liu P. P., Fu Z. Q., Klessig D. F., et al. (2012). Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11, 587–596. 10.1016/j.chom.2012.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]


