Abstract
Adipose-derived stem cells (ADSCs) are a subset of mesenchymal stem cells (MSCs) that possess many of the same regenerative properties as other MSCs. However, the ubiquitous presence of ADSCs and their ease of access in human tissue have led to a burgeoning field of research. The plastic surgeon is uniquely positioned to harness this technology because of the relative frequency in which they perform procedures such as liposuction and autologous fat grafting. This review examines the current landscape of ADSC isolation and identification, summarizes the current applications of ADSCs in the field of plastic surgery, discusses the risks associated with their use, current barriers to universal clinical translatability, and surveys the latest research which may help to overcome these obstacles.
Keywords: stem cell, mesenchymal stem cell, adipose-derived stem cell, lipotransfer
Introduction
Methods of ADSC isolation & identification
-
Current applications
– Soft tissue
– Bone and cartilage
– Wound healing
– Peripheral nerve regeneration
Barriers to clinical entry
Future directions
Concluding remarks
Introduction
Recent advances in regenerative medicine, in particular the discovery of multipotent, easily accessible stem cells such as adipose-derived stem cells (ADSCs), have provided the opportunity of using autologous stem cell transplants as regenerative therapies. The field of plastic surgery, centred on the restoration and enhancement of the body, is logically positioned to utilize such new technologies focused on the repair and replacement of diseased cells and tissues [1]. The ability of stem cells to self-renew, to secrete trophic factors and to differentiate into different cell types has allowed for the development of more flexible therapies to redefine the classic ‘autologous tissue transplant’ and offer more customizable treatment options. ADSCs are being utilized for a variety of different applications in plastic surgery [2–11], and as our understanding of the basic science of stem cells continues to develop, the plastic surgeon should be prepared for the translational and clinical implications of this progress.
Adipose-derived stem cells are particularly useful as they can be easily harvested with minimal donor site morbidity and have a differentiation potential similar to other MSCs [12,13]. In addition, ADSCs have higher yields and greater proliferative rates in culture when compared to bone marrow stromal cells [14–16]. The discovery that ADSCs are not only precursors to adipocytes but also are multipotent progenitors to a variety of cells [17] including osteoblasts, chondrocytes, myocytes, epithelial cells and neuronal cells [18], creates the potential to treat a variety of tissue defects from a single, easily accessible autologous cell source.
Adult stem cell research has made significant strides as a therapeutic modality in recent years. However, there remain significant barriers to the safe and efficacious use of stem cell therapies. With regard to ADSCs, this includes better defining the source population of multipotent cells, optimizing the isolation of these cells in compliance with regulatory standards, and better understanding the behaviour of ADSCs in their transplanted niche. The purpose of this review is to (i) explore the utilization of ADSCs in plastic surgery, (ii) describe the current limitations of ADSC treatments with regard to developing translatable clinical therapies and (iii) describe certain techniques used in our laboratory that may help overcome these barriers. Understanding the current status of clinical ADSC treatments and defining the challenges ahead may bring us closer to achieving desired outcome while minimizing unwanted side effects with these therapies.
Methods of ADSC isolation & identification
The most commonly published method of ADSC isolation involves enzymatic digestion of lipoaspirate to release the stromal vascular fraction (SVF) of cells which include stromal & endothelial cells, pericytes, various white blood cells, red blood cells and stem/progenitor cells [19]. The enzyme preparations used to achieve this fraction include dispase, trypsin and more commonly collagenase. In our laboratory, we take freshly harvested lipoaspirate and wash it with sterile 1% PBS until golden in colour. The adipose tissue is then digested with 0.01% collagenase/PBS solution at a ratio of 1 ml of enzyme solution to 1 cm3 of adipose tissue. This mixture is incubated at 37°C with intermittent agitation until it becomes cloudy (usually 30 min.). The infranatant is then carefully aspirated, transferred to 50 ml conical tubes and centrifuged at 706 × g for 8 min. The supernatant is discarded and resulting pellet, the SVF, is resuspended in control media [DMEM supplemented with 10% foetal bovine serum (FBS), 500 IU penicillin and 500 μg streptomycin; Mediatech, Manassas, VA, USA]. The cells are then counted and plated in uncoated T75 flasks at a concentration of 1 × 106 cells. Consistently, 20 mg of lipoaspirate is ample tissue to harvest an adequate yield of SVF (>1 × 107 cells).
In 2006, the International Society for Cellular Therapy (ICTS) defined a set of minimal criteria for identifying cells as ADSCs. These include plastic adherence while maintained in standard culture conditions, expression of CD73, CD90 and CD105 while lacking the expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules [20]. In conjunction with the International Federation for Adipose Therapeutics and Science in 2013, the ICTS has denoted additional surface markers CD13, CD29 and CD44 as being constitutively expressed at >80% on the surface of ADSCs, while CD31, CD45 and CD235a are the primary negative markers that should be expressed on less than 2% of the cells [19]. Ultimately, the viability of the isolated cells should exceed 70% and the presence of at least two positive and two negative markers are necessary for foundational phenotyping. Finally, ADSCs must possess the ability to differentiate into osteoblasts, adipocytes and chondroblasts.
Identification of ADSCs in our laboratory is accomplished by labelling our plastic-adherent cells with a mesenchymal stem cell (MSC) phenotyping kit after the second passage (Miltenyi Biotec Inc, Auburn, CA, USA). Cells are analysed using a C6 Accuri Flow Cytometer (BD Biosciences, San Jose, CA, USA) which demonstrate positive staining for CD90 (81.3%), CD105 (86.6%) and CD73 (99.9%) and negative staining for CD14, CD20, CD34 and CD45 (1.97% – Fig. 1). To complete the identification of our ADSCs, we culture these cells in adipogenic, osteogenic, or chondrogenic conditions provided in commercially available kits (Cyagen Biosciences Inc., Sunnyvale, CA, USA). Cells subjected to adipogenic or osteogenic conditions reveal lipid droplets or calcium synthesis after staining with Oil Red O or Alizarin Red S, respectively, after fixation in 4% formalin. Cells subjected to chondrogenic conditions reveal proteoglycan synthesis upon staining with Alcian Blue after paraffin embedding (Fig. 2). The ease at which ADSCs can be isolated has led to rapid and widespread translational applications.
Fig. 1.
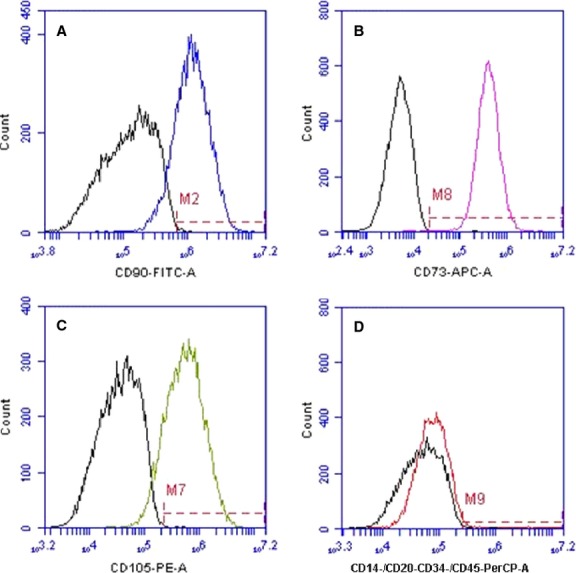
Flow cytometry analysis of isolated ADSCs after collagenase method. Cells stained (A) 81.3% positive for CD90, (B) 99.9% positive for CD73, (C) 86.6% positive for CD105 and (D) 1.97% positive for CD14, CD20, CD34 and CD45.
Fig. 2.
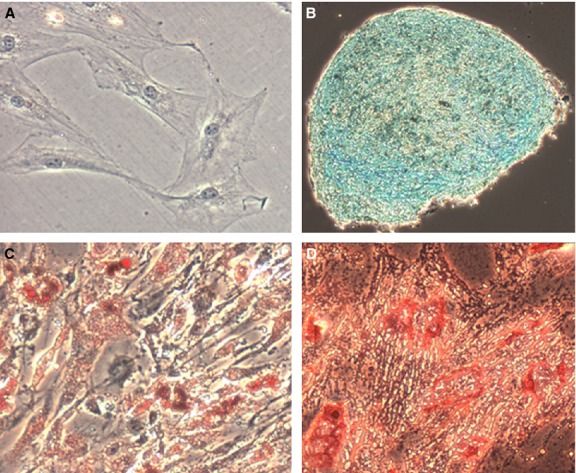
Undifferentated and differentiated ADSCs visualized using microscopy. Original magnification, 10×. (A) Control stain – uADSCs stained with Oil Red O (other controls not shown). (B) Staining with Alcian Blue revealing presence of chondroblasts. (C) Staining with Oil Red O revealing presence of adipocytes. (D) Staining with Alizarin Red S revealing presence of osteoblasts.
A number of groups have described the isolation of ADSCs using non-enzymatic methods. Studies show that ADSCs reside in the infranatant layer of the suction canister after liposuction and that these cells can be expanded ex vivo. And while these cells exhibit phenotypic and differentiation potential similar to ADSCs isolated via collagenase digestion, their presence is significantly lower with reported yields ranging from a 3- to 19-fold decrease in comparison [21–24]. Interestingly, it has been found that multiple variables, including medical comorbidities of the patient, location adipose tissue stores, and the method in which this tissue is harvested, all affect the properties of the ADSCs therein. For example, diabetic patient fat stores have been found to contain fewer ADSCs with a reduced phenotypic expression profile and ability to proliferate [3]. The anatomical location of adipose tissue harvest also appears to have an effect on the yield and characteristics of the isolated ADSCs [25,26]. More recently, Gnanasegaran et al. demonstrated that the gene expression levels and tendency towards specific germ layer differentiation is affected by whether the fat is harvested via liposuction versus lipectomy [27].
Clinical applications
Soft tissue
The regenerative potential of ADSCs has been demonstrated for several years with techniques such as fat grafting for replacement of soft tissue. The autologous tissue utilized in fat grafting contains a variety of cells, including ADSCs [28], which support tissue neo-vascularization [29] and regeneration through secretion of angiogenic growth factors [30]. Cell-assisted lipotransfer (CAL), a technique first described by Matsumoto et al. in 2006, combines aspirated fat with concentrated ADSCs to create stem cell-rich fat grafts [5]. This approach allows for marked improvements in the survival rate of transplanted fat with less fat resorption [31] as well as a decrease in adverse effects of lipoinjection such as fibrosis and cyst formation [5]. In breast tissue, fat grafting has also been associated with fat necrosis and calcifications which can complicate breast imaging and breast cancer surveillance [32,33]. Coincidentally, Yoshimura et al. used CAL in 2008 for cosmetic breast augmentation in 40 patients and reported favourable aesthetic outcome with no major complications [10]. Since then, CAL has been used in other studies for breast augmentation [2,34,35] as well as for facial lipoatrophy [11,36] and augmentation during face-lift and facial contouring surgeries, among other applications (Table 1) [37].
Table 1.
Clinical applications of ADSCs
| Application | Source articles |
|---|---|
| Soft tissue reconstruction | |
| Breast augmentation | Kamakura et al., 2011*; Tiryaki et al., 2011†; Wang et al., 2012*; Yoshimura et al., 2008* |
| Breast augmentation revision | Yoshimura et al., 2010* |
| Facial lipoatrophy | Castro-Govea et al., 2012*; Tiryaki et al., 2011†; Yoshimura et al., 2008* |
| Facial augmentation | Lee S. et al., 2012 |
| Lumpectomy reconstruction | Tiryaki et al., 2011† |
| Traumatic/iatrogenic soft tissue defects | Tiryaki et al., 2011† |
| Bony reconstruction | |
| Calvarial reconstruction | Lendeckel et al., 2004; Thesleff et al., 2011 |
| Maxilla reconstruction | Mesimaki et al., 2009 |
| Wound healing | |
| Radiation atrophy | Rigotti et al., 2007 |
| Ischaemic wounds | Lee H. et al., 2012 |
Cell-assisted lipotransfer (CAL).
Stem cell-enriched tissue injections.
ADSC, Adipose-derived stem cell.
Most recently, Kolle et al. demonstrated significant fat graft survival in human cases after ex vivo expansion of collagenase-processed ADSCs used for CAL when compared to the traditional method of fat grafting [38]. Alternative ADSC therapies have also been explored including techniques such as stem cell-enriched tissue injections that combine traditional fat grafts and subsequent ADSC injections [39,40]. These preliminary studies suggest that ADSCs might allow for improvements in the retention and volume-restoring capabilities of transplanted fat, though the applicability of these studies in the United States is limited because of the methods (enzymatic isolation) used.
Concern has been raised over the use of ADSCs in the form of CAL for breast augmentation after breast cancer therapy. For example, studies have demonstrated that MSCs potentiate the metastatic potency of breast cancer cells when the two are mixed and reimplanted [41–43]. While there is little evidence with regard to CAL therapy for breast augmentation post-mastectomy, the American Society of Plastic Surgeons has come to the conclusion that fat grafting does not increase the risk of breast cancer recurrence [44]. It appears that ADSCs may enhance the growth of active cells without affecting dormant cells, but because there is a need for more research in this setting, the current recommendations are such that CAL therapy should be delayed for reconstructive purposes in breast cancer treatment until there is no evidence of active disease for a period of 7 years [45].
Bone and cartilage
Mesenchymal stem cells were first identified in the bone marrow (BM-MSCs), but the costs of harvesting these cells seemed to rarely outweigh the benefits of their use. The discovery of MSCs in adipose tissue was met with great excitement. ADSCs harvest is safer, easier and yields as high as 500 times more cells than when harvesting stem cells from the same amount of BM tissue [46]. And while ADSCs are known to exhibit some differences in phenotypic, transcriptome and proteome expression when compared to BM-MSCs (e.g. CD34 expression), ADSCs have been found to have superior differentiation, proliferation and immunomodulatory effects [46].
Adipose-derived stem cells, by definition, are able to differentiate into osteoblasts and chondroblasts and have therefore been explored for bone and cartilage regeneration therapies. Current clinical stem cell therapies for bone regeneration have demonstrated promising results for craniofacial defects [4,8,47]. Studies have shown that ADSCs, either combined with autologous bone [4] or seeded alone in β-tricalcium phosphate (TCP) granules [8] are capable of forming new bone and repairing large calvarial defects in human cases. Stem cell treatments have also been used for repair of defects involving the maxilla and mandible. Multistep delayed procedures that combine ADSCs with growth factors [47] in muscle tissue, followed by transplanting the entire structure as a composite microvascular flap surrounding ectopic bone have yielded excellent functional and aesthetic results in maxilla repair [47]. Single-stage procedures involving ADSCs seeded on scaffolds of β-TCP and bone morphogenetic protein 2 have also been used to fill a mandibular defect [48]. Overall, these studies suggest that ADSCs are capable of ossifying bony defects and providing a non-invasive method of bony reconstruction without the associated donor site morbidity of traditional bone grafts [49].
Cartilage defects present a challenging reconstructive problem because of the tissue's limited intrinsic capacity for self-repair. To the best of the authors’ knowledge, there are no clinical trials utilizing ADSCs to treat cartilage defects, though several animal studies have yielded promising results. ADSCs cultured in a three-dimensional environment [50] and preconditioned with the appropriate growth factors, primarily those in the TGF-β superfamily [51], are capable of forming cartilage tissue in vivo [52]. In addition, undifferentiated ADSCs have fully repaired hyaline cartilage defects in patellofemoral joints [53] and ear auricle defects [54] in animals. The latter experiments suggest the intrinsic ability of ADSCs to adapt to their environment in vivo and create a promising direction for future clinical applications.
Wound healing
Adipose-derived stem cells are favourable candidates for wound therapies as they secrete numerous growth factors and cytokines critical in wound healing [55,56] and also increase macrophage recruitment, enhance granulation tissue, and improve vascularization (Table 2) [57,58]. Repeated transplants of purified autologous lipoaspirates into radiation-induced lesions in breast cancer patients have shown improvement of ultrastructural tissue characteristics with neovessel formation as well as significant clinical improvements [6]. ADSCs have also been used clinically to treat wounds complicated by ischaemia, such as in thromboangiitis obliterans and diabetes [3]. The angiogenic properties of ADSCs may contribute to the collateral vessel formation seen in these patients.
Table 2.
Selected ADSC secretomes and their functions
| Growth factor | Function | Source articles |
|---|---|---|
| Brain-derived growth factor (BDNF) | Nerve regeneration | Salgado et al., 2010; Lopatina et al. 2011; Reid et al., 2011; Sowa et al., 2012; Kingham et al., 2013 |
| Glial-derived growth factor (GDNF) | Nerve regeneration | Salgado et al., 2010; Reid et al., 2011; Lopatina et al. 2011; Kingham et al., 2013 |
| Hepatocyte growth factor (HGF) | Angiogenesis, wound healing, immunomodulation | Wang et al., 2006; Kapur et al., 2013 |
| Insulin-like growth factor-1 (IGF-1) | Wound healing, nerve regeneration, cardiac regeneration | Wang et al., 2006; Salgado et al., 2010; Kapur et al., 2013 |
| Nerve growth factor (NGF) | Nerve regeneration | Salgado et al., 2010; Reid et al., 2011; Lopatina et al. 2011; Sowa et al., 2012 |
| Vascular endothelial growth factor (VEGF) | Angiogenesis, wound healing, cardiac regeneration, immunomodulation | Wang et al., 2006; Sowa et al., 2012; Kingham et al., 2013; Kapur et al.,2013 |
| Transforming Growth Factor beta (TGF-β) | Angiogenesis, immunomodulation | Salgado et al., 2010; Lopatina et al. 2011; Kapur et al., 2013 |
| Basic Fibroblast Growth Factor (bFGF) | Angiogenesis | Salgado et al., 2010; Kapur et al., 2013 |
| Granulocyte colony-stimulating factor (G-CSF) | Angiogenesis, wound healing Wound healing | Kapur et al., 2013 |
| Interleukin 6 (IL-6) | Immunomodulation | Kapur et al., 2013 |
| Interleukin 8 (IL-8) | Wound healing | Kapur et al., 2013 |
ADSC, Adipose-derived stem cell.
In addition, ADSCs have also shown to be useful in treating pathological wound healing such as aberrant scar formation. Scars treated with ADSC injections in animal models exhibited subsequent reduction in surface area and improvements in colour and pliability when compared to controls [59]. Potential mechanisms of decreased scarring may involve targeting of the inflammatory processes associated with scar formation [60] as ADSCs have been shown to have anti-inflammatory and immunosuppressive effects [61,62].
Peripheral nerve regeneration
The use of autologous nerve grafts for the repair of peripheral nerve injuries (PNI) is limited by donor site morbidity and suboptimal functional recovery. As a result, alternative treatments have been investigated including several forms of regenerative and cellular therapies. The majority of research on PNI has focused on replacing host support cells, particularly the Schwann-cell (SC) population, as these cells are crucial in providing trophic, structural and directional support for regenerating axons [13]. In addition, in vivo studies have demonstrated that ADSCs can promote nerve regeneration by differentiating into neuron-like lineages [7].
Recent PNI research has shifted focus to the role of ADSCs providing support to host cells. In this setting, in vivo and in vitro studies that include the transplantation of undifferentiated ADSCs (uADSCs), and even ADSC-conditioned media, have demonstrated mechanisms of neurotrophic factor elaboration, including glial-derived growth factor, nerve growth factor, brain-derived growth factor, glial cell-derived neurotrophic factor, insulin-like growth factor, hepatocyte growth factor and VEGF as promoting significant nerve regeneration [63–67]. These findings support a role for ADSCs in providing a favourable microenvironment to support regenerating axons via paracrine mechanisms. In our laboratory, preliminary data support this paracrine role and indicates that undifferentiated ADSCs can be modulated towards neurotrophic secretome function when cultured in embryonic motor neuron-conditioned media. The current trends in PNI research with ADSCs and demonstration of nerve regeneration in many in vivo models indicate that clinical trials may be on the horizon.
Barriers to clinical entry
In Europe, ADSCs are considered Advanced Therapy Medicinal Products, as defined by the European Union (European Commission) 1394/2007 which contains rules for ‘authorization, supervision, and pharmacovigilance’ regarding the summary of product characteristics, labelling, and packaging of Advanced Therapy Medicinal Products that are prepared commercially and in academic institutions [68]. This regulation refers to the European good manufacturing process (eGMP) rules [69]. The process of converting protocols, including collagenase-processed ADSCs, into a process that is compliant with eGMP requires assays that have had careful consideration of all the risks and benefits for the patient end user. As a result, the general recommendation on the use of enzyme-processed CAL in the clinical setting is not prohibited as this technique has been demonstrated to provide satisfying results in terms of long-term outcome, most likely because of the dramatic release of angiogenic growth factors and the differentiation of ADSCs into adipocytes and vascular endothelial cells [5,10,11].
In the United States, the Food and Drug Administration (FDA) regulates Human Cells and Tissue-Based Products (HCT/P) intended for human transplant and maintains two levels of classifications: 361 and 351 products. HCT/P 361 encompasses ‘tissue’ (e.g. bone, ligaments, vein grafts, etc.) and their related procedures that take place in the same operative session, all of which fall under the jurisdiction of practice of medicine which is governed by state medical boards and professional societies; not the FDA. HCT/P 351, on the other hand, includes ‘drugs/biologics’ (e.g. cultured cells, lymphocyte immune therapy, cell therapy involving the transfer of genetic material, etc.) which is fully governed by FDA [70,71]. Regulation 21 CFR 1271 directly demonstrates the FDA's position on enzymatically isolated adipose stem cells derived from SVF for reconstructive purposes as beyond the scope of ‘minimal manipulation’ and therefore, a drug [72]. Thus, the practical implication is the need for any surgeon who wishes to use ADSCs isolated via collagenase to submit an Investigational New Drug application to the FDA and have an approved Institutional Review Board with the referring Institution.
Given the time, expense and complexity of the regulatory issues surrounding ADSCs intended for transplantation, it is evident that U.S. physicians are discouraged to perform any cell-supplemented lipotransfer techniques in the current commonly accepted practices. Furthermore, automated devices for separating adipose stem cells are regulated as class III medical devices by the FDA, and currently, none are approved for human use in the United States. Kolle et al. demonstrated that CAL, when supplemented with ADSCs expanded ex vivo after collagenase digestion, yields superior results when compared to lipotransfer alone [38]. The FDA restrictions that would preclude such a study to be conducted in the United States prompt an impetus to develop methods for CAL that results in ‘minimal manipulation’ of source adipose tissue.
Future directions
In 2006, Yoshimura et al. described a cell population in the liposuction aspirate fluid that exhibited similar phenotypic properties to ADSCs harvested in the traditional manner (collagenase) from processed lipoaspirate cells; however, the yield was reduced by a third when comparing to the two methods [23]. Since that time, additional studies have been published touting the benefits of non-enzymatic ADSC isolation. In 2010, Francis et al. described a method of ADSC ‘Rapid Isolation’ in ∼30 min. that excluded the use of collagenase, however, a significant disadvantage of this study was the low yield of ∼250,000 cells from a starting volume of ∼250 ml liposuction aspirate fluid [21]. Zeng et al. describe a ‘rapid and efficient’ form of non-enzymatic ADSC isolation in which adipose tissue is cut into tiny pieces and placed in culture flasks with 100% FBS in which the plastic-adherent cells were allowed to expand over a period of days [24]. One obvious downside to this method is the requirement to expand the cell population in calf serum. Most recently, Shah et al. describe a form of non-enzymatic ADSC isolation combining the cells of the liposuction aspirate fluid with the cells captured from the processed lipoaspirate tissue wash that is typically discarded prior to collagenase digestion [22]. They observed significant improvement in MSC-related phenotypic markers and similar adipogenic and osteogenic differentiation characteristics. While their isolation time was cut by one-third, they observed a 19-fold decrease in ADSC isolation when compared to the traditional method. In our laboratory, we have adopted a very similar protocol of non-enzymatic isolation that includes processing the processed lipoaspirate effluent. The primary difference in our protocol, however, is the method of plating cells. While Shah et al. plate the entire SVF pellets in T175 flasks, we resuspend our pellets in culture media and then plate the cells at specific concentrations. In one experiment for example, we plated the SVF pellet after collagenase digestion at a concentration of 5 × 105 in a T75 flask. Concurrently, we plated the SVF pellet obtained after non-enzymatic isolation at 2 × 106. After 6 days of culture, these two flasks appeared nearly identical in terms of confluence, correlating to a fourfold decrease in ADSC harvest when using the latter method. The two cell populations were then analysed under flow cytometry as previously described. There is little difference in the phenotypic expression between the two populations as demonstrated by >80% expression of CD90, CD73 and CD105 and <5% expression of CD14, CD20, CD 34 and CD45 (Fig. 3).
Fig. 3.
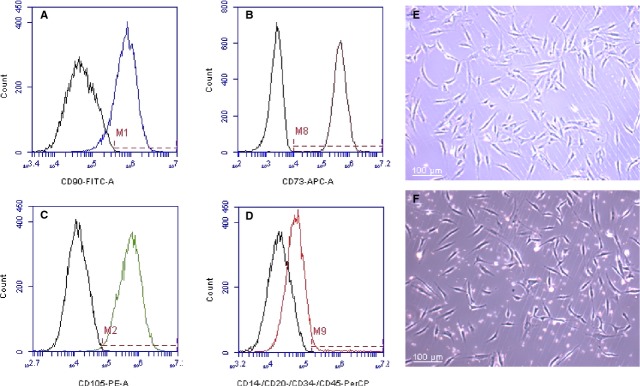
Flow cytometry analysis of isolated ADSCs after rapid isolation (no collagenase). Cells stained (A) 85.8% positive for CD90, (B) 99.9% positive for CD73, (C) 99.4% positive for CD105 and (D) 3.79% positive for CD14, CD20, CD34 and CD45. (E) Collagenase-isolated ADSCs after 6 days of primary culture seeded at 5 × 105 in T75 flask. (F) Rapid isolation ADSCs after 6 days primary culture seeded at 2 × 106 in T75 flask.
Most convincingly, Kolle et al. demonstrated a clear benefit to CAL over lipotransfer alone. They isolated and expanded ADSCs ex vivo from human cases followed by lipotransfer to the cases’ arms with or without ADSC supplementation. They demonstrated a 65% improvement in fat graft survival after 4 months in the experimental group [38]. The major drawback to their experimental model was that to achieve these results, the 34 ml of lipotransfer was supplemented with 6.5 × 108 ADSCs or 2000 times the physiological level [38]. The methods of ‘rapid isolation’, previously mentioned, demonstrate the ability to isolate ADSCs without the aid of enzymatic digestion, but at a cost of greatly reduced yields. There is significant doubt that ADSCs used at such low concentrations would serve for any clinical benefit. As previously discussed, ex vivo expansion of ADSCs is not practical for application in the United States or other principalities with strict regulations. Therein lies an impetus to discover innovative methods of ADSC isolation and characterization of the regenerative components of the SVF that might yield similar results to concentrated ADSCs alone.
There is promise in capitalizing on the plastic-adherent properties of ADSCs as a form of non-enzymatic isolation. The same group that first described the isolation of cells from the LAF, Doi et al., has demonstrated that an adherent column of rayon–polyethylene non-woven fabrics may also be used to isolate ADSCs, though at an inferior yield to the traditional method [73]. Further advancements in harnessing the plastic-adherent properties of these cells are clearly needed as Buschmann et al. demonstrated that 30–50% of ADSCs remain in suspension after 24 hrs of primary culture [74].
Concluding remarks
Many questions remain unanswered. A consensus on the phenotypic characterization of these ADSCs is still lacking as is a common method of isolation that will allow for direct translational applications worldwide. In addition, the long-term safety of CAL in areas of previous cancer remains unanswered. While we are still uncovering the exact mechanism of stem cell function, be it paracrine or differentiation induced, the discovery of the very abundant ADSC will allow for major advancements in regenerative medicine, particularly pertaining to therapeutics. To be truly translational, this research is challenged with producing minimally manipulated cells that can be used in the operating room either autogenously or in ‘off the shelf’ variations. In addition, it may well become clear that these therapies will need to be individually tailored if we discover that patients vary in their response to these cells or the trophic factors they produce. The ultimate answer will likely be achieved by the combined efforts of basic scientists, clinicians and biomedical engineers.
Acknowledgments
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR000153. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We want to thank Dr. Frank Zaldivar and the ICTS laboratory at UC Irvine for use of their Flow Cytometer. We also want to thank The Miami Project and California Stem Cells, Inc. for providing conditioned media.
Conflicts of interest
The authors declare no conflicts of interest with regard to this paper.
References
- 1.Walia B, Satija N, Tripathi RP, et al. Induced pluripotent stem cells: fundamentals and applications of the reprogramming process and its ramifications on regenerative medicine. Stem Cell Rev. 2012;8:100–15. doi: 10.1007/s12015-011-9279-x. [DOI] [PubMed] [Google Scholar]
- 2.Kamakura T, Ito K. Autologous cell-enriched fat grafting for breast augmentation. Aesthetic Plast Surg. 2011;35:1022–30. doi: 10.1007/s00266-011-9727-7. [DOI] [PubMed] [Google Scholar]
- 3.Lee HC, An SG, Lee HW, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012;76:1750–60. doi: 10.1253/circj.cj-11-1135. [DOI] [PubMed] [Google Scholar]
- 4.Lendeckel S, Jodicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Cranio-Maxillo-Facial Surg. 2004;32:370–3. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–82. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 6.Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–22. doi: 10.1097/01.prs.0000256047.47909.71. ;; discussion 23-4. [DOI] [PubMed] [Google Scholar]
- 7.Shen CC, Yang YC, Liu BS. Peripheral nerve repair of transplanted undifferentiated adipose tissue-derived stem cells in a biodegradable reinforced nerve conduit. J Biomed Mater Res, Part A. 2012;100:48–63. doi: 10.1002/jbm.a.33227. [DOI] [PubMed] [Google Scholar]
- 8.Thesleff T, Lehtimaki K, Niskakangas T, et al. Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery. 2011;68:1535–40. doi: 10.1227/NEU.0b013e31820ee24e. [DOI] [PubMed] [Google Scholar]
- 9.Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–70. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. ; discussion 6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178–85. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 12.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.di Summa PG, Kalbermatten DF, Pralong E, et al. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278–91. doi: 10.1016/j.neuroscience.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi A, Chuang CW, Ling QD, et al. Differentiation ability of adipose-derived stem cells separated from adipose tissue by a membrane filtration method. J Membr Sci. 2011;366:286–94. [Google Scholar]
- 15.Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–41. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 17.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 18.Brayfield C, Marra K, Rubin JP. Adipose stem cells for soft tissue regeneration. Handchir Mikrochir Plast Chir. 2010;42:124–8. doi: 10.1055/s-0030-1248269. [DOI] [PubMed] [Google Scholar]
- 19.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–8. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Francis MP, Sachs PC, Elmore LW, et al. Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis. 2010;6:11–4. doi: 10.4161/org.6.1.10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah FS, Wu X, Dietrich M, et al. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy. 2013;15:979–85. doi: 10.1016/j.jcyt.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 24.Zeng G, Lai K, Li J, et al. A rapid and efficient method for primary culture of human adipose-derived stem cells. Organogenesis. 2013;9:287–95. doi: 10.4161/org.27153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332:415–26. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schipper BM, Marra KG, Zhang W, et al. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–44. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gnanasegaran N, Govindasamy V, Musa S, et al. Different isolation methods alter the gene expression profiling of adipose derived stem cells. Int J Med Sci. 2014;11:391–403. doi: 10.7150/ijms.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown SA, Levi B, Lequeux C, et al. Basic science review on adipose tissue for clinicians. Plast Reconstr Surg. 2010;126:1936–46. doi: 10.1097/PRS.0b013e3181f44790. [DOI] [PubMed] [Google Scholar]
- 29.Sheng L, Yang M, Li H, et al. Transplantation of adipose stromal cells promotes neovascularization of random skin flaps. Tohoku J Exp Med. 2011;224:229–34. doi: 10.1620/tjem.224.229. [DOI] [PubMed] [Google Scholar]
- 30.Salgado AJ, Reis RL, Sousa NJ, et al. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–10. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 31.Koh KS, Oh TS, Kim H, et al. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg. 2012;69:331–7. doi: 10.1097/SAP.0b013e31826239f0. [DOI] [PubMed] [Google Scholar]
- 32.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119:775–85. doi: 10.1097/01.prs.0000252001.59162.c9. ; discussion 86-7. [DOI] [PubMed] [Google Scholar]
- 33.Zheng DN, Li QF, Lei H, et al. Autologous fat grafting to the breast for cosmetic enhancement: experience in 66 patients with long-term follow up. J Plast Reconstr Aesthet Surg. 2008;61:792–8. doi: 10.1016/j.bjps.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Lu Y, Luo X, et al. Cell-assisted lipotransfer for breast augmentation: a report of 18 patients. Zhonghua Zheng Xing Wai Ke Za Zhi. 2012;28:1–6. [PubMed] [Google Scholar]
- 35.Yoshimura K, Asano Y, Aoi N, et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16:169–75. doi: 10.1111/j.1524-4741.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 36.Castro-Govea Y, De La Garza-Pineda O, Lara-Arias J, et al. Cell-assisted lipotransfer for the treatment of parry-romberg syndrome. Arch Plast Surg. 2012;39:659–62. doi: 10.5999/aps.2012.39.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SK, Kim DW, Dhong ES, et al. Facial soft tissue augmentation using autologous fat mixed with stromal vascular fraction. Arch Plast Surg. 2012;39:534–9. doi: 10.5999/aps.2012.39.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolle SF, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382:1113–20. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhu M, Zhou Z, Chen Y, et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222–8. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 40.Tiryaki T, Findikli N, Tiryaki D. Staged stem cell-enriched tissue (SET) injections for soft tissue augmentation in hostile recipient areas: a preliminary report. Aesthetic Plast Surg. 2011;35:965–71. doi: 10.1007/s00266-011-9716-x. [DOI] [PubMed] [Google Scholar]
- 41.Eterno V, Zambelli A, Pavesi L, et al. Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget. 2014;5:613–33. doi: 10.18632/oncotarget.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 43.Rowan BG, Gimble JM, Sheng M, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS ONE. 2014;9:e89595. doi: 10.1371/journal.pone.0089595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Society of Plastic Surgeons. 2012. Post-Mastectomy Fat Graft/Fat Transfer ASPS Guiding Principles http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/2012Post-MastectomyFatGraft-FatTransferASPSGuidingPrinciples.pdf.
- 45.Zimmerlin L, Donnenberg AD, Rubin JP, et al. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strioga M, Viswanathan S, Darinskas A, et al. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 47.Mesimaki K, Lindroos B, Tornwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–9. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Sandor GK, Tuovinen VJ, Wolff J, et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J Oral Maxillofac Surg. 2013;71:938–50. doi: 10.1016/j.joms.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Sandor GK, Nish IA, Carmichael RP. Comparison of conventional surgery with motorized trephine in bone harvest from the anterior iliac crest. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:150–5. doi: 10.1067/moe.2003.42. [DOI] [PubMed] [Google Scholar]
- 50.Estes BT, Diekman BO, Gimble JM, et al. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294–311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hennig T, Lorenz H, Thiel A, et al. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–91. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 52.Lin Y, Luo E, Chen X, et al. Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J Cell Mol Med. 2005;9:929–39. doi: 10.1111/j.1582-4934.2005.tb00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang HN, Li L, Leng P, et al. Uninduced adipose-derived stem cells repair the defect of full-thickness hyaline cartilage. Chin J Traumatol. 2009;12:92–7. [PubMed] [Google Scholar]
- 54.Bahrani H, Razmkhah M, Ashraf MJ, et al. Differentiation of adipose-derived stem cells into ear auricle cartilage in rabbits. J Laryngol Otol. 2012;126:770–4. doi: 10.1017/S0022215112001065. [DOI] [PubMed] [Google Scholar]
- 55.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 56.Wang M, Crisostomo PR, Herring C, et al. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291:R880–4. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 57.Ebrahimian TG, Pouzoulet F, Squiban C, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009;29:503–10. doi: 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 58.Hong SJ, Jia SX, Xie P, et al. Topically delivered adipose derived stem cells show an activated-fibroblast phenotype and enhance granulation tissue formation in skin wounds. PLoS ONE. 2013;8:e55640. doi: 10.1371/journal.pone.0055640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yun IS, Jeon YR, Lee WJ, et al. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012;38:1678–88. doi: 10.1111/j.1524-4725.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 60.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–89. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 62.Pinheiro CH, de Queiroz JC, Guimaraes-Ferreira L, et al. Local injections of adipose-derived mesenchymal stem cells modulate inflammation and increase angiogenesis ameliorating the dystrophic phenotype in dystrophin-deficient skeletal muscle. Stem Cell Rev. 2012;8:363–74. doi: 10.1007/s12015-011-9304-0. [DOI] [PubMed] [Google Scholar]
- 63.Lopatina T, Kalinina N, Karagyaur M, et al. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE. 2011;6:e17899. doi: 10.1371/journal.pone.0017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lattanzi W, Geloso MC, Saulnier N, et al. Neurotrophic features of human adipose tissue-derived stromal cells: in vitro and in vivo studies. J Biomed Biotechnol. 2011;2011:468705. doi: 10.1155/2011/468705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reid AJ, Sun M, Wiberg M, et al. Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience. 2011;199:515–22. doi: 10.1016/j.neuroscience.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 66.Sowa Y, Imura T, Numajiri T, et al. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev. 2012;21:1852–62. doi: 10.1089/scd.2011.0403. [DOI] [PubMed] [Google Scholar]
- 67.Kingham PJ, Kolar MK, Novikova LN, et al. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014;23:741–54. doi: 10.1089/scd.2013.0396. [DOI] [PubMed] [Google Scholar]
- 68.Sensebe L, Bourin P, Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther. 2011;22:19–26. doi: 10.1089/hum.2010.197. [DOI] [PubMed] [Google Scholar]
- 69.European Commission. European Union. 2011. EudraLex, Volume 4. Good manufacturing practice (GMP) guidelines http://ec.europa.eu/health/documents/eudralex/vol-4/index_en.htm.
- 70.U.S. Food and Drug Administration. U.S. Food & Drug Administration. 2013. CFR - Code of Federal Regulations Title 21. Part 860: Medical device classification procedures http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=860.
- 71.U.S. Food and Drug Administration. U.S. Food & Drug Administration. 2013. CFR - Code of Federal Regulations Title 21. Part 814: Premarket approval of medical devices http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=814.
- 72.U.S. Food and Drug Administration. U.S. Food & Drug Administration. 2013. CFR - Code of Federal Regulations Title 21. Part 1271: Human cells, tissues and cellular and tissue-based products http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=1271.10.
- 73.Doi K, Kuno S, Kobayashi A, et al. Enrichment isolation of adipose-derived stem/stromal cells from the liquid portion of liposuction aspirates with the use of an adherent column. Cytotherapy. 2014;16:381–91. doi: 10.1016/j.jcyt.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Buschmann J, Gao S, Harter L, et al. Yield and proliferation rate of adipose-derived stromal cells as a function of age, body mass index and harvest site-increasing the yield by use of adherent and supernatant fractions? Cytotherapy. 2013;15:1098–105. doi: 10.1016/j.jcyt.2013.04.009. [DOI] [PubMed] [Google Scholar]


