Abstract
Background
Serious adverse events that occur in hospitals rank as a leading cause of preventable death in the United States. Many states operate reporting systems to monitor and publicly report serious adverse events, a subset that falls under Medicare’s Hospital-Acquired Conditions (HACs).
Purpose(s)
Identify and describe state efforts, and the supporting role of federal initiatives, to track and report HACs and other serious adverse events.
Data Sources
Document review of state and federal reports, databases, and policies for HACs and other serious adverse events; conduct semi-structured telephone interviews with state health department officials and directors of patient safety organizations.
Results
Thirty-two states and the District of Columbia (D.C.) track at least one Medicare HAC. Five states collect nearly all ten Medicare HACs (9–10). Eighteen states and D.C. track events through both a state-based reporting system and the Centers for Disease Control National Healthcare Safety Network (NHSN) for health-care associated infections (HAI). For serious adverse events, most states either partially or fully adopted the National Quality Forum’s Serious Reportable Events. For HAIs, thirty states and D.C. mandate reporting through NHSN. States interviewed reported that Medicare’s choice of HACs for nonpayment had at least a partial influence on which serious adverse events required reporting.
Conclusions
Many states use the collected data on HACs and other events for quality improvement initiatives and to provide greater transparency through public reporting. More work and research is needed to develop a national reporting system template that has standard definitions, methodology, and reporting.
Keywords: Medicare, Medicaid, qualitative research, quality of care, patient safety (measurement), state health policies
Introduction
The Institute of Medicine’s (IOM’s) landmark publication in 1999, To Err Is Human, called for a nationwide public mandatory reporting system to identify and learn from medical errors and other adverse events (Kohn, Corrigan, & Donaldson, 2000; IOM, 1999). Under the reporting system, state governments would be required to collect standardized information about adverse medical events that result in death and serious harm. Subsequently, the National Quality Forum (NQF) released Serious Reportable Events in Healthcare in 2002 (NQF, 2002). This groundbreaking document reflected consensus on a list of 28 serious, preventable, adverse events that could form the basis for a national reporting system and lead to substantial improvements in patient safety.
Since that time, state activity has focused on the development and improvement of reporting systems that can help improve quality and outcomes by identifying system weaknesses, complement other state functions, and help safeguard the health care consumer (Rosenthal & Takach, 2007). Numerous adverse event reporting systems are in operation, and there is growing evidence that these efforts have been bringing positive change to the quality of care delivered (Leape & Berwick, 2005). Federal legislation in recent years has prompted both federal and state-level payment reforms in Medicare and Medicaid, as both health insurance programs for the nation’s elderly, disabled, and poor now prohibit payments to hospitals, and other facility types in some states, for hospital-acquired conditions (HAC) and other serious adverse events.
The Centers for Medicare and Medicaid Services (CMS) contracted with RTI International to conduct a three-year evaluation of the Hospital Acquired Condition—Present on Admission (HAC-POA) payment policy.1 The evaluation sought to answer a broad set of research questions, one of which was to identify what states are doing to track and report HACs as defined by both CMS for Medicare payment purposes and states for payment or reporting purposes. The purpose of our study was three-fold: (1) to document recent federal initiatives that support states in their efforts to improve health care quality and patient safety through better reporting of HACs and other serious adverse events; (2) to compile an inventory of state-based reporting systems for HACs; and (3) to conduct an in-depth review of a select number of states that track a majority of Medicare HACs that publicly report and use the collected data for statewide quality improvement initiatives. In particular, we sought to investigate the rigors of data utilization, data validity, quality improvement initiatives, and public reporting among our selected states. The four selected states (California, Connecticut, Nevada, Pennsylvania) met the following criteria: (1) data is collected on 8 to 10 categories of Medicare HACs; (2) data collection process is systematic and uniform; (3) at least 3 different U.S. Census regions (i.e., Northeast, Mid-Atlantic, and West) are represented; and (4) both small and large states, in terms of population size, are represented. For example, California and Pennsylvania are ranked in the top 10 most populous states, and Nevada and Connecticut are ranked in the bottom 20 (United States Census, 2010).
Data and Methods
Our study data collection approach entailed the following: (1) document review of federal reports, databases, and policies for Medicare HACs and other serious adverse events; (2) document review of state reports, databases, or policies that track or report HACs; and (3) semi-structured telephone interviews with state officials from four selected states with active and robust reporting systems that track nearly all Medicare HACs.
We developed an inventory matrix beginning in late 2009 that captured state reporting system activity. During a three-year period, we continuously updated this information as reporting activities changed or underwent updates. Our information was derived from several sources, including recent Health and Human Services’ (HHS) Office of Inspector General (OIG) reports describing state adverse event reporting systems, and the National Academy of State Health Policy (NASHP) patient safety toolbox (OIG, 2008; NASHP, 2010). Recent Government Accountability Office (GAO) reports on healthcare-associated infection (HAI) reporting systems and the role of the Patient Safety Act also informed our document review activities (GAO, 2010). Furthermore, we substantiated information collected from these research efforts by reviewing state health department or hospital association Web sites that provide information on the reporting systems or served as the portal for public reporting of HAC data.
We collected state reports, typically in the form of an annual patient safety or adverse event report, from state health department or other state government Web sites. We reviewed at least 25 state reports to determine their serious reportable event list (e.g., National Quality Forum [NQF] list or state defined), their mechanism for collecting the data, and whether the data were reported on individual facilities or in aggregate for all facilities.
Finally, we conducted telephone interviews using semi-structured interview guides with state officials, or their state health department designees, who direct state-based reporting systems and related patient safety or quality activities. Our interview guide contained discussion items that focused on uses of the data, validity of the data, degree of engagement or collaboration that the state office had in fostering patient safety or quality improvements, and the extent of publicly reported information the state captured and analyzed.
Results
Federal Policies and Initiatives in the New Millennium
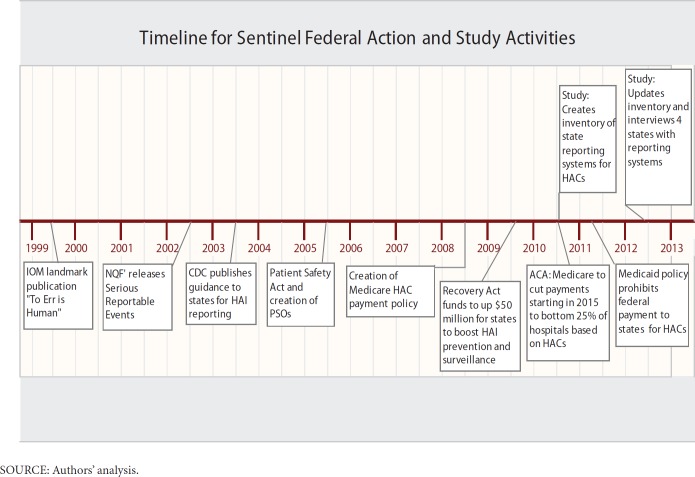
Several states operated mandatory reporting systems prior to the 1999 IOM report. Exhibit 1 shows a timeline of sentinel federal actions to address adverse events beginning with the landmark 1999 IOM Report, and RTI’s study activities that occurred over a three year period from 2011 to 2013. However, these reporting systems were used primarily to hold providers accountable for their errors and often involved public disclosure. Confidential, voluntary systems for reporting of medical errors were less common. The IOM report noted that health care providers are often reluctant to report or publicly disclose their medical errors and to participate in related learning efforts out of fear of incurring legal liability or professional sanctions. To address these concerns, the IOM recommended the expanded use of voluntary medical error reporting systems that allow confidential reporting. Partially because of the IOM report, Congress responded with subsequent legislation to encourage and fund voluntary reporting systems and other patient safety initiatives. In 2003, the Centers for Disease Control and Prevention’s (CDC) Healthcare Infection Control Practices Advisory Committee (HICPAC) published guidelines for states to implement healthcare–associated infection (HAI) public reporting, including CDC’s National Healthcare Safety Network (NHSN). States responded with a grassroots movement toward public reporting of HAI rates by facility with many states opting to use NHSN as the system to track nosocomial infections.
Exhibit 1. Timeline for Sentinel Federal Action and Study Activities.
The focus on patient safety improvement also led state legislators to impose disclosure requirements of adverse events to patients. There is a dynamic tension between the movement for greater transparency about adverse events and the need to keep information about reported adverse events confidential to encourage reporting (Mello, Kelly, & Brennan, 2005). Some state legislatures have attempted to encourage physicians and health care facilities to disclose medical errors by enacting “apology laws.” Physician groups, in particular, have raised serious concerns about disclosure of medical errors. Thus, state legislators have taken steps to protect those who provide information about adverse events from suffering legal consequences. Many states have provided protections making patient safety data contained in reporting systems confidential and protected from subpoena and discovery in lawsuits (Hanscom et al., 2003). States have also passed laws to protect patient safety whistle-blowers from retaliation.
Some argue that as the public’s awareness of medical errors deepens, plaintiffs’ attorneys will grow more empowered and aggressive, which will in turn increase the pressure of the current tort (medical malpractice) crisis and the defensiveness of the medical profession (Mello et al., 2005). This conflict between tort liability and patient safety laws was raised at the federal level in the early 2000s, which subsequently led to the creation of the Patient Safety and Quality Improvement Act of 2005 (the Patient Safety Act). The legislation directed HHS to create a list of public or private organizations known as patient safety organizations (PSOs), and it prohibits unauthorized disclosure of certain types of data regarding patient safety events that providers send to the PSOs (Government Accountability Office [GAO], 2010). PSOs certify that they will analyze data regarding patient safety events, provide feedback to providers, and develop and disseminate information on ways providers can improve patient safety. To support PSOs and providers in their efforts to develop and adopt improvements in patient safety, the Agency for Healthcare Research and Quality (AHRQ) has created a network of patient safety databases (NPSDs). These databases collect and aggregate nonidentifiable data on patient safety events voluntarily submitted by the PSOs and providers. Patient safety data are aggregated and analyzed nationally.
More recent federal initiatives have sought to strengthen the capabilities and accountability of states to monitor adverse events occurring in health care settings. The American Recovery and Reinvestment Act of 2009 (the Recovery Act) authorized $50 million to support states in the prevention and reduction of healthcare-associated infections (HAIs). CDC is the federal agency responsible for distributing the Recovery Act funds to state health departments through cooperative agreements that support programs to boost surveillance and prevention of HAIs, encourage collaboration, train the workforce in HAI prevention, and measure outcomes. These efforts are consistent with recommendations outlined in the National Action Plan to Prevent Healthcare-Associated Infections: Roadmap to Elimination (Office of the Assistant Secretary for Health, 2012). As part of the effort, all 50 states received Preventive Health and Health Services Block Grant funds from CDC to reduce HAIs. CDC also provides training support and technical assistance to states that monitor HAI occurrences using the NHSN, which has become the primary means of states’ data collection from health care facilities through the Recovery Act agreements. NHSN is a voluntary, secure, Internet-based surveillance system operated by CDC that is open to all types of health care facilities in the United States. CDC currently supports more than 4,400 health care facilities that are using NHSN, and as of June 2012, 27 states and the District of Columbia require—or will require—hospitals to report HAIs using NHSN.
Medicare and Medicaid Payment Reforms
In addition to federal initiatives aimed at improving the surveillance and reporting of healthcare-acquired conditions, Congress and the U.S. Department of Health and Human Services (HHS) directed the Centers for Medicare & Medicaid Services (CMS) to undertake a series of payment reforms to adjust payments made to hospitals and other health care facilities for HACs and other provider preventable conditions. The Deficit Reduction Act of 2005 (the Act) modified payment for hospitalizations of Medicare fee-for-service beneficiaries if a complicating condition that could have reasonably been prevented occurred during the hospitalization by creating the HAC-POA payment policy.2
Through collaboration with other HHS agencies, CMS selected 10 HAC categories, shown in Exhibit 1, that identify conditions considered to be preventable under accepted evidence-based guidelines and targeted these for application of the case-based HAC payment policy. Section 2702 of the Patient Protection and Affordable Care Act of 2010 (hereafter referred to as the Affordable Care Act) directs CMS to transition the HAC-POA program from being a case-based to rate-based payment adjustment (CMS, 2012).3 Beginning in 2015, hospitals scoring in the top quartile for the rate of HACs as compared to the national average will have their Medicare payments reduced by 1 percent for all DRGs. In calculating the rates, the Secretary will establish and apply an appropriate risk adjustment methodology (Patient Protection and Affordable Care Act, 2010).
In addition to these Medicare payment changes, Section 2702 also directs the Secretary to issue Medicaid regulations effective as of July 1, 2011, prohibiting federal payments to states for any amounts expended for providing medical assistance for health care–acquired conditions (HCACs) and other provider-preventable conditions (PPC) that can be identified by states, but must be approved by CMS. As shown in Exhibit 2, the HCACs include the 10 categories of Medicare HACs as well as the three surgical event errors4 that are a part of Medicare’s National Coverage Determinations (NCD) exclusions. Such regulations must ensure that the prohibition of payment for HCACs and other PPCs does not result in a loss of access to care or services for Medicaid beneficiaries. In a preamble to the final rule, CMS stated that compliance action would not be undertaken against states until July 1, 2012. The Center for Medicaid and State Operations (CMSO) issued a survey to states in 2011 to obtain information on current state Medicaid practices for prohibiting payments for HCACs. CMS found that 21 states had HCAC-related nonpayment policies prior to the new regulation, most of which identify at least the Medicare HACs for nonpayment in hospitals. Half exceeded the Medicare policies in terms of the conditions, systems used to indicate the conditions, or the settings to which the nonpayment policies apply.
Exhibit 2. Healthcare-Acquired Conditions and Other Provider Preventable Conditions.
| Category 1: Healthcare-acquired conditions (also Medicare’s list of hospital-acquired conditions) | Foreign object retained after surgery |
| Air embolism | |
| Blood incompatibility | |
| Stage III and IV pressure ulcers | |
| Falls and trauma; including fractures, dislocations, intracranial injuries, crushing injuries, and burns | |
| Catheter-associated urinary tract infections (CAUTI) | |
| Vascular catheter-associated infections | |
| Manifestations of poor glycemic control | |
| Surgical site infections following certain orthopedic procedures | |
| Deep vein thrombosis/pulmonary embolism | |
| Category 2: Other provider preventable conditions | Wrong surgical or other invasive procedure performed on a patient |
| Surgical or other invasive procedure performed on the wrong body part | |
| Surgical or other invasive procedure performed on the wrong patient | |
| Other conditions identified in the State’s plan and according to requirements of the final regulation | |
State Adverse Event and Medical Error Reporting Systems
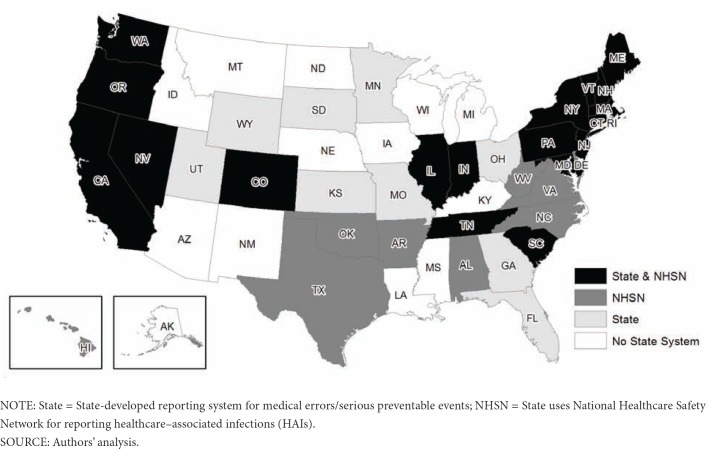
As of June 2012, 27 states and the District of Columbia (DC) enacted legislation to authorize and establish reporting systems for healthcare-associated adverse events or medical errors. Our selection of states was consistent with the criteria also used by the National Academy of State Health Policy (NASHP) patient safety toolbox and the Office of Inspector General (OIG) Report on State Adverse Event Reporting Systems. Twenty of these states implemented an adverse event reporting system within the last ten years; New Hampshire being the most recent in 2010. States vary widely regarding which adverse events are reported through these state-based reporting systems. Many states require the reporting of the NQF list of serious reportable events (SREs), whereas others have defined their own list of events, including only a portion of the NQF events, and still others include the AHRQ patient safety indicators or the more prevalent HAIs as reportable events. Some states have both a state-based reporting system for medical errors and track HAIs separately through NHSN, which is increasingly more common as states go “live” with their collection of at least one HAI using NHSN. The map in Exhibit 3 illustrates the different scenarios states use to report HAIs either through a state-based reporting system for medical errors and adverse events, through NHSN, or through both.
Exhibit 3. Reporting System Type by State.
As the map illustrates, currently 18 states (California, Colorado, Connecticut, Illinois, Indiana, Maine, Maryland, Massachusetts, Nevada, New Hampshire, New Jersey, New York, Oregon, Pennsylvania, South Carolina, Tennessee, Vermont, and Washington) and the District of Columbia both maintain a state-based reporting system for health care-acquired conditions or other adverse events and track, or will soon track, HAIs through NHSN. The 9 states that track HAIs through NHSN, but do not have a mandated state-based reporting system for health care-acquired conditions or adverse events are Alabama, Arkansas, Delaware, Hawaii, North Carolina, Oklahoma, Texas, Virginia, and West Virginia. Currently, 8 states (Florida, Georgia, Kansas, Minnesota, Ohio, Rhode Island, Utah, and Wyoming) maintain a state-based reporting system and do not participate in NHSN. The remaining 15 states (Alaska, Arizona, Idaho, Iowa, Kansas, Kentucky, Louisiana, Michigan, Mississippi, Montana, Nebraska, New Mexico, North Dakota, South Dakota, and Wisconsin) neither track HAIs through NHSN nor maintain a mandated state-based reporting system.
National Healthcare Safety Network and Healthcare-Associated Infections
Of the 30 states and District of Columbia that have mandated HAI reporting, 27 states and the District of Columbia now use the NHSN. Five states mandated NHSN use during 2011: Arkansas, Hawaii, Indiana, Maine, and North Carolina. NHSN may be used to monitor health care–associated events, including facility-acquired infections, health care personnel influenza vaccination, and reactions associated with transfusions of blood or blood products. Device-associated infections are measured for bloodstream infections, urinary tract infections, and pneumonia. Surgical site infections are measured according to selected procedures. The NHSN captures central line–associated bloodstream infections (CLABSI), which is a more narrow condition than the HAC-defined vascular catheter-associated infection. Within the NHSN application, facilities can compare themselves with risk-adjusted, national aggregate data for local quality improvement purposes. Facilities can also use the system to develop surveillance and analytic methods that allow timely recognition of patient safety problems for prompt intervention. Alaska, Arizona, New Mexico and Ohio, states without mandatory or voluntary reporting, were considering whether to mandate HAI reporting during the time of our analysis. The state of New Mexico enacted the Hospital Infection Act in 2009, which formalized its HAI Advisory Committee and its role while keeping HAI data submission voluntary. The Committee is facilitated by the New Mexico Department of Health and is currently working toward its goals to develop a public reporting system and prevent HAIs.
As previously noted, New Hampshire recently enacted mandatory reporting legislation of the 29 NQF serious reportable events. This results in 15 states and the District of Columbia that track all HACs that are part of the NQF’s list of 29 SREs. These HACs are (1) foreign object retained after surgery, (2) air embolism, (3) blood incompatibility, (4) Stage III and IV pressure ulcers, (5) falls and trauma, and (6) manifestations of poor glycemic control.
In addition, three of the Medicare HAC categories are HAIs, which include catheter-associated urinary tract infections (CAUTI), vascular catheter-associated infections or central line associated blood infections (CLABSI), and certain surgical site infections (SSIs) after certain orthopedic and cardiac surgeries. Three states (Nevada, New York, and Pennsylvania) historically tracked selected HAIs through their state’s adverse event report systems, but transitioned to now track HAIs through the NHSN. Connecticut continues to track nosocomial or healthcare-associated infections that result in death or serious injury through its state-based adverse event reporting system, and also mandates reporting CAUTI, CLABSI, and SSI through the NHSN. CLABSI, a subset of vascular catheter–associated infections, continues to be the most common mandatory reported HAI through NHSN (27 states and D.C.). Peripheral line infections, another subset of vascular catheter–associated infections, are not reportable to NHSN. Reporting of surgical site infections via NHSN is or will be mandated by 20 states, whereas only 6 states require reporting of CAUTI via NHSN. Many more states plan to begin using NHSN to track at least one HAI as part of their HAI Recovery Act State Plan. CDC reviewed these plans to help understand how state activities can contribute to the HHS HAI goals, identify gaps, and determine means of additional support. The Office of Healthcare Quality (OHQ) has since offered project funding to address some of these gaps.
States not listed do not track any of the Medicare HACs through a state-authorized reporting system or NHSN. It is possible that these states submit reports through PSOs and are not listed here. Such reports would not necessarily, or likely, be reported statewide, given that individual health care facilities have agreements with a state-designated PSO to voluntarily and confidentially report medical errors.
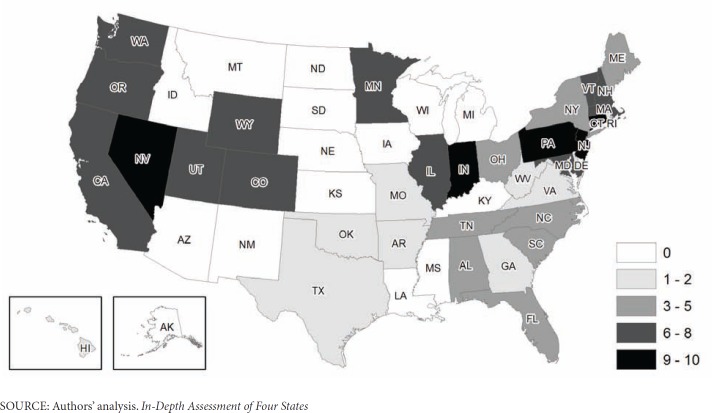
As Exhibit 4 shows, five states (Connecticut, Indiana, New Jersey, Nevada, and Pennsylvania) collect 9 to 10 categories of Medicare HACs. Twelve states plus the District of Columbia collect 6 to 8 HACs: California, Colorado, Illinois, Maryland, Massachusetts, Minnesota, New Hampshire, Oregon, Utah, Vermont, Washington, and Wyoming. Another eight states (Alabama, Florida, Maine, New York, North Carolina, Ohio, South Carolina and Tennessee) collect between 3 and 5 HACs. Ten states (Arkansas, Delaware, Georgia, Hawaii, Missouri, Oklahoma, Rhode Island, Texas, Virginia, and West Virginia) collect either 1 or 2 HACs.
Exhibit 4. Number of Medicare-Listed Hospital-Acquired Conditions Reported by States.
All four states that were part of the in-depth review, as shown in Exhibit 5, recently expanded their list of reportable conditions. It appears that CMS’ HAC payment policy did have an effect on state decisions to do so, as we confirmed that states do take into consideration what conditions are of national importance in terms of Medicare and Medicaid payment policy and quality reporting. Another finding is that there is increasing importance placed on data accuracy and validity, as evidenced by clinicians’ reviews of incident reports or collected data on HACs. Connecticut conducts a thorough review of each reported incident and clinical teams comprised of various medical professionals review all event reports for validation and intervene with facilities when needed. In Nevada, health facility surveyors cross-reference incident reports completed during inspections with facility self-reports documented in the state’s patient safety registry. Follow-up is conducted with the facility when there are discrepancies.
Exhibit 5. In-Depth Review of Four States’ Reporting Systems.
| State | Medicare HACs tracked | Mandated HAIs reported to NHSN | Data Validity | Public Reporting | Quality Improvement and Patient Safety | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| California |
|
|
|
|
|
||||||
| Connecticut |
|
|
|
|
|
||||||
| Nevada |
|
|
|
|
|
||||||
| Pennsylvania |
|
|
|
|
|
||||||
SOURCE: Authors’ analysis. Limitations
In terms of public reporting, all four states are expanding or developing systems and Web portals to collect and share data. This is consistent with the larger national movement to have state and federal-level HAC data be more transparent to providers, insurers, patients, and consumers through different levels of reporting. Some states have developed specific state-based quality improvement teams (preventionists) to collaborate with facilities to improve care processes and outcomes for specific HACs. This is particularly true for HAIs. There is some movement, particularly in California, to link central line insertion practice (CLIP) process data with outcome data (CLABSI) to analyze the connection between process improvement and outcome improvement. Among the four states we interviewed, the utility of a national data base that includes aggregated adverse event rates was recognized. A national adverse event data base could be used for inter- and intra-state comparisons among healthcare organizations if both adverse event definitions and data collection methods were standardized. States with reporting systems appear to be committed to improving patient safety within their health care facilities and provide patients and consumers with more information on the quality of care being provided.
The information in this report reflects our findings from the aforementioned document review activities and semi-structured interviews with state officials. We verified that our updates on state-level information already collected from NASHP and OIG were still current and that they reflect state mandates still in place for medical error reporting. However, states’ efforts to collect data and report on medical errors, particularly on HACs from the Medicare list, constitute a fluid and evolving activity in that greater federal involvement is having an impact on HAC reporting at the state level. We cannot guarantee that all findings reflect the most recent and ongoing changes to state tracking of HACs. Furthermore, our findings assume that states are using the reported information in the manner described by their state reporting system documentation or annual state reports. We did not independently verify the validity of their description of reporting activities beyond the four states that received a more in-depth review. In addition, available project resources limited us to select only four states to review more in-depth through telephone interviews and close examination of their reporting system characteristics.
Discussion
The IOM’s recommendation for a nationwide mandatory public reporting system, whereby states would be required to collect standardized information about adverse medical events, has not been fully realized. Since that time, however, the federal government, many states, and patient safety organizations have played a significant role in improving patient safety in our nation’s hospitals (Wachter, 2010). According to the author, many states have stepped up their oversight of hospitals and providers to promote safety, particularly in regard to error reporting and greater transparency. State requirements that hospitals report serious adverse events appear to be prompting institutions to initiate more rapid and thorough analyses of such events—a positive development (Rosenthal & Takach, 2007). The emergence of the NQF’s “never events” list coupled with CDC’s NHSN for HAIs has served as the basis for more effective adverse event reporting systems. Originally envisioned as a set of events that might form the basis for a national state-based reporting system, the NQF’s SREs continue to fill that purpose as states and many patient safety organizations have put them into practice. Six of the ten Medicare HACs were adopted from the SREs. Additionally, SREs have been used or adapted by national entities with the goal of illuminating such events to facilitate learning and improvement (NQF, 2011). In our four-state, in-depth assessment, we found that effective analysis is a critical component of an adverse event reporting system. These states were in the early stages of using the reported data to make systematic improvements in the delivery of healthcare, not merely improving individual provider performance. In this age of patient safety enlightenment, we are evolving from a culture of “blame and shame” to “blame and train” (Clarke, 2006).
Federal policies and initiatives serve as catalysts for increasing public reporting and heightened surveillance of serious adverse events, including HACs. The HAI Recovery Act initiative, implemented by CDC, prompted a large public health expansion of state-level surveillance and reporting of HAIs. The Patient Protection and Affordable Care Act of 2010 transitions the HAC-POA program from a case-based to rate-based payment policy by providing an annual Medicare payment adjustment to qualifying hospitals as an incentive for reducing HACs beginning in 2015. Section 2702 of the legislation also prohibits federal payments to states’ Medicaid programs for the Medicare list of HACs, along with other provider preventable conditions the states might include. Furthermore, CMS’ Hospital Compare Web site now provides facility-level results on rates for eight of the ten HACs for most hospitals throughout the country paid on a prospective basis by Medicare. Similarly, private Medicare Advantage Organizations are now mandated to report HACs received on Medicare claims. This development enables consumers to obtain information regarding what their local hospitals are doing to prevent these types of events and how they compare with other hospitals.
Voluntary reporting initiatives occurring at both the national and state level for specific HACs also play a prominent role in promoting greater safety in our nation’s hospitals. One such initiative, funded by the Agency for Healthcare Research and Quality and run by the Comprehensive Unit-Based Safety Program (CUSP), is a partnership between the American Hospital Association (AHA), Johns Hopkins University, and the Michigan Health & Hospital Association’s Keystone Center for Patient Safety & Quality. Known as “On the Cusp: Stop BSI” and “On the Cusp: Stop CAUTI,” the goal of these programs is to eliminate up to 60,000 bloodstream infections in the critically ill and reduce CAUTI rates in hospitals by 25 percent. A key focus of these programs is the expansion of state-level capacity to implement quality and patient safety improvement projects, whereby states can be instrumental in coordinating activities among state hospital associations, quality improvement oorganizations, and patient safety organizations.
Our study provides insight into some of the challenges of developing a nationwide reporting system that would be inclusive of all payers. Currently, states select the adverse medical events to report, define the reporting structure and process, and utilize the findings in different ways (e.g., publicly report facility-level vs. aggregated data, sponsor quality improvement initiatives, impose financial penalties and other corrective actions). Federal initiatives have bolstered HAC reporting activities at the state level, yet there is still wide variability and lack of standardization across state reporting systems. These differences make the data unsuitable to identify national incidence and trends for HACs. Underreporting of HAC data also makes it problematic to make significant inferences or to track improvement over time. The evidence to show the effects of public reporting of HACs, other related adverse events, and the outcomes of improving patient safety continue to evolve. Fung, Lim, Mattke, Damberg, and Shekelle (2008) synthesized the peer-reviewed literature on using publicly reported data to improve quality. They concluded that the evidence suggests that publicly releasing performance data stimulates quality improvement activity at the hospital level. The usefulness of public reporting on effectiveness and safety remains uncertain as few studies have assessed these end points. Lamb, Smith, Weeks, and Queram (2013) conducted one of the first studies on the impact of public reporting on providers and provider groups. Their results showed that physician groups reported that publicly reported performance data motivated them to address some quality measures, but not all.
The level of reporting varies across states, but two of the states in our in-depth review were transitioning from aggregate to facility-level reporting of HAC data during 2012 (Nevada and Connecticut). It will be interesting to see how this evolves as health care consumers and patients become more knowledgeable about what is available and how the information affects them. It appears that the Medicare HAC payment policy has had some effect on state decisions to increase transparency and public reporting of HACs, as states examine what conditions are of national importance in terms of Medicare and Medicaid payment policy and quality reporting. Increasing importance is placed on data accuracy and transparency with HACs and other serious events reported at the state level. Consequently, there is a movement for states to expand or develop information technology systems and share data that can be available to providers, insurers, and ultimately consumers and the public. As more states initiate public reporting through NHSN or state reporting systems, research should focus on studies that examine the empirical causal pathways through which public reporting influences quality of care (Fung et al., 2008).
Disclaimer
The authors have been requested to report any funding sources and other affiliations that may represent a conflict of interest. The authors report there are no conflicts of interest sources. The statements contained in this manuscript are solely those of the authors and do not necessarily reflect the views or policies of neither the Centers for Medicare & Medicaid Services nor the Department of Health and Human Services. The authors assume responsibility for the accuracy and completeness of the information contained in this manuscript.
Footnotes
The Deficit Reduction Act of 2005 modified payment for acute-care hospitalizations of Medicare fee-for-service beneficiaries if a complicating condition occurred during the hospitalization that could have reasonably been prevented. In response to the legislation, CMS developed the Hospital-Acquired Condition-Present on Admission (HAC-POA) payment policy, whereby inpatient prospective payment system cases can no longer be assigned to higher-paying Medicare Severity–Diagnosis Related Groups (MS-DRGs) on the basis of reasonably preventable selected complicating conditions that are acquired during the hospital stay. CMS identified 10 categories of HACs as being preventable under accepted guideline-consistent care and targeted these for application of the HAC payment policy. Effective for discharges occurring on or after October 1, 2008, Medicare no longer assigns an inpatient hospital discharge to a higher paying MS-DRG if a selected condition is not POA, and that condition is the only reason why the discharge would be assigned to the higher-paying MS-DRG.
These conditions must meet the following three criteria: (1) they are high cost, high volume, or both; (2)they are assigned to a higher-paying Medicare Severity-Diagnosis Related Group (MS-DRG) when present as a secondary diagnosis; and (3) they could reasonably have been prevented through the application of evidence-based guidelines.
The HAC policy under the 2005 Deficit Reduction Act is “case based,” meaning that CMS is required to identify specific discharges meeting the definitions of a HAC; potential reductions in the payment may apply only to the specific discharge when a HAC occurs. Section 1886(p) of the Social Security Act, as added by section 3008(a) of The Affordable Care Act, requires CMS to make risk-adjusted “rate-based” payment adjustments to IPPS hospitals with relatively poor performance on HACs identified and any other condition acquired during a stay in a hospital determined appropriate by the Secretary, effective for discharges from applicable hospitals during FY 2015. This additional rate-based HAC adjustment will reduce the DRG-based payment amount by one percent for all Medicare discharges in applicable hospitals, those with rates in the top quartile of the distribution of hospital HAC rates.
(1) A different procedure altogether; (2) The correct procedure, but on the wrong body part; or (3) The correct procedure, but on the wrong patient. Publication 100-03 Medicare National Coverage Determinations (June 12, 2009). CMS Manual System.Transmital 101, retrieved from http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R101NCD.pdf
References
- Centers for Medicare and Medicaid Services (CMS) Report to Congress: Assessing the Feasibility of Extending the Hospital Acquired Conditions (HAC) IPPS Payment Policy to Non-IPPS Settings. U.S. Department of Health and Human Services. 2012 Retrieved from http://innovation.cms.gov/Files/x/HospAcquiredConditionsRTC.pdf.
- Clarke SP. Organizational Climate and Culture Factors. Annual Review of Nursing Research. 2006;24:255–272. [PubMed] [Google Scholar]
- Fung CH, Lim Y, Mattke S, Damber C, Shekelle P. Systematic Review: The Evidence that Publishing Patient Care Performance Data Improves Quality of Care. Annals of Internal Medicine. 2008;148:111–123. doi: 10.7326/0003-4819-148-2-200801150-00006. [DOI] [PubMed] [Google Scholar]
- GAO (Government Accountability Office) Patient Safety Act: HHS Is in the Process of Implementing the Act So Its Effectiveness Cannot Yet Be Evaluated (GAO Pub.No.GAO-10-281) Washington, DC: U.S. Government Accountability Office; 2010. Retrieved from http://www.gao.gov/new.items/d10281.pdf. [Google Scholar]
- Hanscom R, Mello M, Powers R, Sato L, Schaefer M, Studdert D. Legal Liability and Protection of Patient Safety Data. Commissioned Paper for the Institute of Medicine Committee on Patient Safety Data Standards 2003.
- Institute of Medicine (IOM) To err is human: Building a safer health care system. Washington, DC: National Academy Press; 1999. p. 2000. [Google Scholar]
- Kohn L, Corrigan J, Donaldson M, editors. To Err is Human: Building A Safer Health System. Washington DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- Lamb GC, Smith M, Weeks W, Queram C. Publicly Reported Quality-of-Care Measures Influenced Wisconsin Physician Groups to Improve Performance. Health Affairs. 2013;32(3):536–543. doi: 10.1377/hlthaff.2012.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leape LL, Berwick D. Five Years After To Err is Human: What Have We Learned? Journal of the American Medical Association. 2005;293:2384–2390. doi: 10.1001/jama.293.19.2384. [DOI] [PubMed] [Google Scholar]
- Mello MM, Kelly C, Brennan T.2005Fostering Rational Regulation of Patient Safety Journal of Health Politics, Policy and Law, 303375–426. 10.1215/03616878-30-3-375 [DOI] [PubMed] [Google Scholar]
- NASHP (National Academy of State Health Policy) Patient Safety Toolbox. 2010 Retrieved from http://www.nashp.org/pst-welcome.
- NQF. Serious Reportable Events in Healthcare: A Consensus Report. Washington, DC: National Quality Forum; 2002. [Google Scholar]
- NQF (National Quality Forum) Serious Reportable Events in Healthcare–2011 Update: A Consensus Report. Washington, DC: National Quality Forum; 2011. Retrieved from http://www.qualityforum.org/Publications/2011/12/Serious_Reportable_Events_in_Healthcare_2011.aspx. [Google Scholar]
- Office of the Assistant Secretary for Health. National Action Plan to Prevent Healthcare-Associated Infections: Roadmap to Elimination. 2012 Retrieved from http://www.hhs.gov/ash/initiatives/hai/infection.html.
- OIG (Office of Inspector General) Adverse Events in Hospitals: State Reporting Systems. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- Patient Protection and Affordable Care Act (Affordable Care Act) Washington, DC: U.S. Government Printing Office; 2010. P.L. 111–148 Retrieved from http://frwebgate.access.gpo.gov/cgibin/getdoc.cgi?dbname=111_cong_bills&docid=f:h3590enr.txt.pdf. [Google Scholar]
- Rates for hospital-acquired conditions released on hospital compare amid controversy. The Joint Commission Benchmark. 2011;13(6):1–4. [Google Scholar]
- Rosenthal J, Takach M. 2007 Guide to State Adverse Event Reporting Systems. Portland: National Academy for State Health Policy; 2007. [Google Scholar]
- United States Census. 2010 census data. 2010 Retrieved from http://2010.census.gov/2010census/data/
- Wachter RM. Patient Safety at Ten: Unmistakable Progress, Troubling Gaps. Health Affairs. 2010;29(1):165–173. doi: 10.1377/hlthaff.2009.0785. [DOI] [PubMed] [Google Scholar]