Abstract
Background
This prospective and randomized study was designed to compare safety, potential complications, and patient and examiner satisfaction of 2 anesthetic combinations – etomidate-remifentanil and propofol-remifentanil – in elderly patients undergoing diagnostic gastroscopy.
Material/Methods
A group of 720 patients, aged 60–80 years, scheduled for diagnostic gastroscopy under sedation were prospectively randomized. After 0.4–0.6 μg kg−1 of remifentanil was infused, etomidate or propofol was administered. Patients in the etomidate group received doses of etomidate at 0.1–0.15 mg kg−1 followed by 4–6 mg. Patients in the propofol group received doses of propofol at 1–2 mg kg−1 followed by 20–40 mg. Physiological indexes were evaluated for the 715 of 720 patients that completed the treatment. The onset time, duration time, and discharge time were recorded. Physicians, anesthetists, and patients were surveyed to assess their satisfaction.
Results
Systolic pressure and diastolic pressure decreased significantly after the procedure in the propofol group (P<0.001). The average heart rate was significantly lower in the propofol group (P<0.05). No periods of desaturation (SpO2 <95%) were observed in either group. The onset time was earlier in the etomidate group (P=0.00). All adverse events, with the exception of myoclonus, were greater in the propofol group, and physician and patient satisfaction in both groups was similar.
Conclusions
Etomidate-remifentanil administration for sedation and analgesia during gastroscopy resulted in more stable hemodynamic responses and less adverse events in older patients.
MeSH Keywords: Etomidate, Gastroscopy, Propofol
Background
Gastroscopy is one of the most important diagnostic tools for upper gastrointestinal diseases. Routine gastroscopy can cause adverse reactions, including nausea, vomiting, throat bleeding, and anxiety [1]. Reduction of several physiological functions, respiratory symptoms, and a high incidence of cardiovascular diseases often occur, particularly in older patients. The administration of intravenous anesthesia during gastroscopy can prevent upper airway reflexes and improve the comfort of patients, which lead to an increase in the number of patients willing to undergo an endoscopy and accept a follow-up examination, thus improving the diagnosis rates of precancerous lesions of the upper gastrointestinal tract. However, older patients undergoing intravenous anesthesia for a gastroscopy are at the highest risk of hemodynamic instability, respiratory depression, and delays in recovery time associated with the use of narcotic analgesia [2]. Consequently, it is important for patients to have a safe and convenient anesthesia method when undergoing a gastroscopy.
The ideal agent for a gastroscopy should have a rapid onset, be effective throughout the procedure, and have a rapid recovery period with minimal adverse effects [3]. Propofol has been widely used as the anesthesia induction agent of choice for this procedure due to its enhanced depressant effects on the laryngeal reflexes compared with other induction agents [4]. Propofol has additional advantages such as effectiveness in a short period of time (approximately 30 s) and short action time (distribution half life of 2–4 s and elimination half life 30–60 s). Further, patients treated with propofol experience rapid and complete recovery. However, propofol may cause hemodynamic disturbances, including hypotension, respiratory depression, and loss of protective reflexes [5,6]. Moreover, the lack of analgesic effects restricts the use of propofol as a single drug and limits the implementation of a painless gastroscopy. A number of adjuncts, including midazolam and opioids, have been used to provide pain relief for patients [7]. However, the effective period of these drugs is longer than the period needed for a gastroscopic procedure, which may prolong discharge time and delay recovery. Consequently, a short-acting anesthetic agent seems to be urgently needed.
Remifentanil is a potent ester opioid because of its rapid onset of action (blood-brain equilibration time of 1 min), a higher clearance and shorter elimination half-life (<10 min), and minimal adverse effects on cardiovascular and respiratory parameters [8]. Previous studies have examined the etomidate-opioid combination as a sedative regimen for procedural sedoanalgesia [9–11]; however, few reports exist examining the application of an etomidate-remifentanil combination during gastroscopy. The aim of the present study was to compare etomidate-remifentanil and propofol-remifentanil on the basis of their effects on hemodynamic parameters, recovery, adverse effects, and physician-patient satisfaction in gastroscopy cases. This work provides a better implementation plan for the administration of anesthesia, ensuring a painless gastroscopy.
Material and Methods
Sample size analysis for detecting differences between groups was analyzed using a 2-group t-test with a 5% 2-sided significance level. The differences were assumed as a difference of 11.2 hemodynamic response with a 38.0 standard deviation [3]. The power analysis indicated that a study sample size of 360 subjects per group was sufficient to detect these differences between groups.
This study included 720 unmedicated ASA I–III patients (age 60–80 years) scheduled to undergo diagnostic gastroscopy at Daping Hospital. This study was approved by the Institutional Ethics Committee (Clinical trial registration no.: ChiCTR-TRC-12002340), with all patients providing written informed consent.
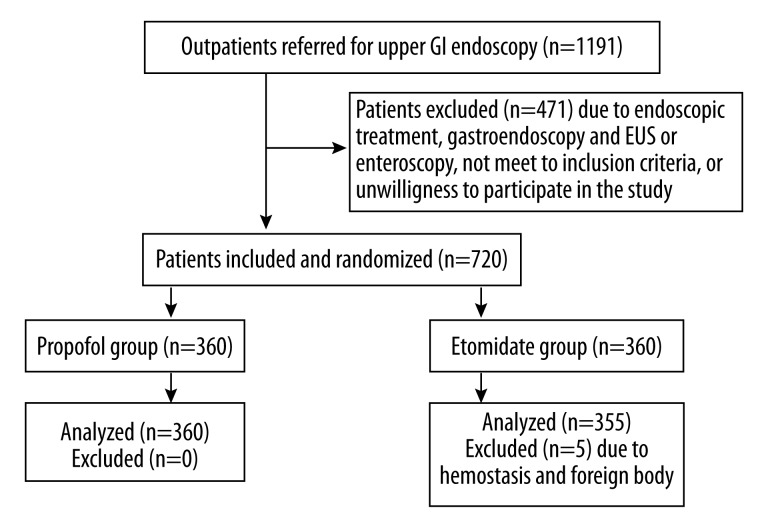
Our study population consisted of consecutive outpatients of either sex (n=1191) who visited our endoscopy centre between July 2012 and December 2012 after referral by general practitioners or gastroenterologists for upper gastrointestinal (GI) symptoms. These symptoms included upper abdominal pain, retrosternal pain, abdominal distension, dysphagia, loss of appetite, and upper GI bleeding. Inclusion criteria included patients aged 60–80 years who had ability to respond to a self-administered questionnaire. Exclusion criteria included cardiac, pulmonary, hepatic or nephritic disease, metabolic disease, electrolyte disturbance, blood pressure >180/110 mmHg, allergy to emulsion or opioid, second-degree atrioventricular block or complete left bundle branch block, and acute airway inflammation in the past 2 weeks. The 720 eligible patients were randomly assigned to propofol group (n=360) and etomidate group (n=360) using a computer-generated random allocation (Figure 1). Electrocardiograph (ECG), medical history, weight, height, and heart rate were recorded before the gastroscopy. Prior to gastroscopic examination, all patients underwent 12-lead electrocardiography, routine blood tests, and coagulation tests. All patients were premedicated with 30 ml 0.5% oral dimethicone powder (Honghe Pharmaceutical Co., Ltd., Zigong, China) 30 min before gastroscopy, and with 10 ml viscous oral lidocaine hydrochloride (Kangye Pharmaceutical Co., Ltd., Handan, China) 15 min before gastroscopy.
Figure 1.
Flow chart of patient assignment for endoscopy (GI, gastrointestinal).
Remifentanil (Yichang Humanwell, Hubei, China) was administered (0.4–0.6 μg/kg; intravenous) to all patients over a period of 60 s. Etomidate (Nhwa, Jiangsu, China) or propofol (AstraZeneca, Caponago, Italy) was administered after the remifentanil infusion began. Patients in the etomidate group (n=360) received etomidate (0.1–0.15 mg/kg) followed by a 4–6 mg additional dose intravenously by an independent anesthesiologist. Patients were maintained at a Ramsay Sedation Scale (RSS) score above 4 throughout the endoscopy; patients in the propofol group (n=360) received propofol (1–2 mg/kg) followed by a 20–40 mg additional dose by an independent anesthesiologist.
Venous access was performed with an indwelling needle and intravenous 0.9% normal saline infusion was initiated in all patients. During gastroscopy, 2 L min−1 oxygen was administered by nasal route. Hemodynamic parameters, including systolic pressure, diastolic pressure, heart rate, SpO2 and RSS (Ramsay sedation score), were measured and recorded before, during, and after the gastroscopy.
Total etomidate, propofol, and remifentanil dosage was completed throughout the gastroscopy, and recovery time was recorded. All occurrences of hypoxemia, apnea, myoclonus, decrease of SpO2 to less than 95%, and other adverse events were recorded. The satisfaction of the physician, anesthetist, and patient were evaluated and recorded using a 10-point scale (poor, 1–4; fair, 5–7; good, 8–10). Patients were surveyed after their full recovery to assess their satisfaction with the management of their sedation-analgesia.
Statistical analysis
Data with normal distribution are presented as mean ± standard deviation and compared by 1-way or repeated-measures analysis of variance (ANOVA). Data with abnormal distribution are presented as median (Q25, Q75) and compared using Wilcoxon signed-rank test. All qualitative data are expressed as n (%) and compared using the chi-square test or Fisher’s exact test. A P value of less than 0.05 was accepted as statistically significant.
Results
Baseline characteristics and endoscopic outcomes of patients
Of the 715 patients enrolled in the study, 403 were male, 312 were female, 360 patients were randomly assigned to the propofol group, and 355 patients received etomidate treatment. The characteristics of patients in both treatment groups are shown in Table 1. There was no statistical significance in age, sex, body weight, ASA physical status, or the underlying medical conditions of patients. Endoscopic diagnoses of patients treated with propofol-remifentanil or etomidate-remifentanil are shown in Table 2. There was no significant difference between the 2 groups except for benign disorders, chronic non-atrophic gastritis, and chronic atrophic gastritis (P<0.001, P=0.003, P=0.002, respectively).
Table 1.
Characteristics of patients undergoing gastroscopy with induction of anaesthesia using propofol-remifentanil or etomidate-remifentanil.
| Propofol group (n=360) | Etomidate group (n=355) | P-value | |
|---|---|---|---|
| Age (year) | 66.31±6.90 | 66.63±4.87 | 0.472 |
| Sex (M/F) | 203/157 | 200/155 | 1 |
| Body Mass Index | 21.86±3.40 | 21.58±3.45 | 0.288 |
| ASA physical status | 2.0 (2.0, 2.0) | 2.0 (2.0, 2.0) | 1 |
| Underlying medical conditions | |||
| Abnormal ECG | 182 (50.56%) | 165 (46.48%) | 0.295 |
| Hypertension | 64 (17.78%) | 64 (18.03%) | 1 |
| CVD | 24 (6.67%) | 21 (5.92%) | 0.759 |
| Diabetes | 6 (1.67%) | 11 (3.10%) | 0.229 |
| Respiratory disease | 18 (5.00%) | 14 (3.94%) | 0.588 |
| Allergy | 0 (0.00%) | 0 (0.00%) | NA |
ASA – American Society of Anesthesiologists; F – female; M – male; ECG – electrocardiograph; CVD – cardiovascular disease. NA – not applicable due to low event rate.
Table 2.
Endoscopic diagnoses of patients with successful anaesthesia using propofol-remifentanil or etomidate-remifentanil.
| Symptoms | Propofol group (n=360) | Etomidate group (n=355) | P-value |
|---|---|---|---|
| Benign disorders | 344 (95.56%) | 309 (87.04%) | <0.001 |
| Reflux esophagitis | 9 (2.50%) | 15 (4.23%) | 0.219 |
| Fungal esophagitis | 1 (0.28%) | 1 (0.28%) | 1 |
| Esophageal varices | 4 (1.11%) | 3 (0.85%) | 1 |
| Polyp | 39 (10.83%) | 40 (11.27%) | 0.905 |
| Gastrointestinal submucosal protrusive lesions | 14 (3.89%) | 13 (3.66%) | 1 |
| Ancylostomiasis | 3 (0.83%) | 3 (0.85%) | 1 |
| Hiatus hernia | 6 (1.67%) | 5 (1.41%) | 1 |
| Achalasia | 1 (0.28%) | 0 (0.00%) | 1 |
| Lemostenosis | 0 (0.00%) | 1 (0.28%) | 1 |
| Chronic nonatrophic gastritis | 216 (60.00%) | 173 (48.73%) | 0.003 |
| Chronic atrophic gastritis | 4 (1.11%) | 18 (5.07%) | 0.002 |
| Duodenitis | 24 (6.67%) | 14 (3.94%) | 0.133 |
| Peptic ulcer | 23 (6.39%) | 23 (6.48%) | 1 |
| Pre-or malignant disorders | 27 (7.50%) | 31 (8.73%) | 0.585 |
| Barrett’s esophagus | 4 (1.11%) | 9 (2.54%) | 0.173 |
| Esophageal cancer | 9 (2.50%) | 10 (2.82%) | 0.82 |
| Gastric cancer | 9 (2.50%) | 5 (1.41%) | 0.419 |
| Cardiac carcinoma | 2 (0.56%) | 3 (0.85%) | 0.684 |
| Low-grade intraepithelial neoplasia | 1 (0.28%) | 4 (1.13%) | 0.214 |
| High-grade intraepithelial neoplasia | 2 (0.56%) | 0 (0.00%) | 0.499 |
| Hp infection | 76 (21.11%) | 71 (20.00%) | 0.781 |
| Overall biopsy rate | 80 (22.22%) | 94 (26.48%) | 0.192 |
Cardiopulmonary responses of patients
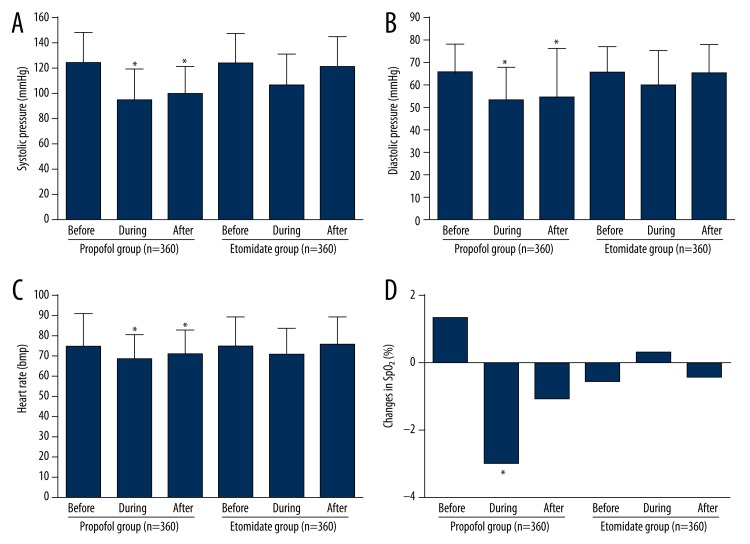
As presented in Figure 2, there was no significant difference in systolic pressure, diastolic pressure, heart rate, and SpO2 before the endoscopy between the 2 groups. However, systolic pressure, diastolic pressure, and heart rate significantly decreased during and after the endoscopy in the propofol-remifentanil group (P<0.05). Oxygen saturation levels in the propofol-remifentanil group were significantly reduced compared with the baseline values during the endoscopy (P<0.05). The decrease of these cardiopulmonary function parameters led to adverse effects in older patients. After the endoscopy, values increased to become not significantly different from baseline levels (P=0.282). In the etomidate-remifentanil group, no significant change was observed in hemodynamic indexes (systolic pressure, diastolic pressure, heart rate, and SpO2 reduction) during and after the endoscopy compared with the baseline values (Figure 2).
Figure 2.
Systolic pressure (A), diastolic pressure (B), heart rate (C), and changes in peripheral oxygen saturation (D) in patients with successful anesthesia using propofol or etomidate. * P<0.05.
Durations of endoscopy and satisfaction of physicians, anesthetists, and patients
There was no significant difference between groups for duration time, recovery time, and time to leave recovery room. However, values for onset time were significantly shorter (P=0) in the etomidate group (Table 3). There was no statistical significance in satisfaction of physicians, anesthetists, and patients (Table 4).
Table 3.
Mean drug doses, onset, duration, recovery and leave recovery room time values.
| Patients (n=60) | Propofol group (n=360) | Etomidate group (n=355) | P-value |
|---|---|---|---|
| Onset time (sec) | 85.14±24.90 | 78.16±21.60 | <0.001 |
| Duration time (sec) | 291.65±110.09 | 284.54±102.68 | 0.376 |
| Recovery time (sec) | 407.78±158.19 | 433.34±218.95 | 0.083 |
| Time to leave recovery room (sec) | 1064.85±170.07 | 1087.26±180.46 | 0.11 |
Data are expressed as mean ±SD.
Table 4.
Examiner and patient satisfaction survey.
| Propofol group (n=360) | Etomidate group (n=355) | |||||
|---|---|---|---|---|---|---|
| Good | Fair | Poor | Good | Fair | Poor | |
| Physician | 352 (97.78%) | 8 (2.22%) | 0 (0.00%) | 342 (96.34%) | 13 (3.66%) | 0 (0.00%) |
| Anesthetist | 339 (94.17%) | 21 (5.83%) | 0 (0.00%) | 329 (92.68%) | 24 (6.76%) | 2 (0.56%) |
| Patients | 360 (100.00%) | 0 (0.00%) | 0 (0.00%) | 355 (100.00%) | 0 (0.00%) | 0 (0.00%) |
Poor, 1–4; fair, 5–7; good, 8–10
Endoscopic morbidities and complications
The number of adverse events is depicted in Table 5. Patients in the propofol group had more adverse effects than in the etomidate group (P<0.05). There was significantly more hypoxemia, injection pain, and body quiver in the propofol group (P<0.005). There was significantly more myoclonus in the etomidate group (P<0.05).
Table 5.
Adverse events.
| Adverse events | Propofol group (n=360) | Etomidate group (n=355) | P-value |
|---|---|---|---|
| Yes | 224 (62.22%) | 166 (46.76%) | <0.001 |
| Upper airway obstruction | 2 (0.56%) | 1 (0.28%) | 1 |
| Hyoxemia | 77 (21.39%) | 45 (12.68%) | 0.002 |
| Apnoea | 0 (0.00%) | 0 (0.00%) | NA |
| Changes of heart rate and rhythm | 31 (8.61%) | 31 (8.73%) | 1 |
| Hypotension | 7 (1.94%) | 10 (2.82%) | 0.473 |
| Injection pain | 81 (22.50%) | 3 (0.85%) | <0.001 |
| Body quiver | 155 (43.06%) | 68 (69.15%) | <0.001 |
| Myoclonus | 3 (0.83%) | 16 (4.51%) | 0.002 |
| Nausea-vomiting | 1 (0.28%) | 0 (0.00%) | NA |
| Deliration/multilingual/hallucination | 0 (0.00%) | 0 (0.00%) | NA |
- Upper airway obstruction: point 0, no obstruction; point 1, mild snoring but normal aspiration; point 2, serious snoring or presence of depression sign on aspiration but with near normal ventilation; point 3, dependence on oropharyngeal airway or elevation of mandible.
- Hypoxaemia: point 1, SpO2 96–100%; point 2, 91–95%; point 3, 86–90%; point 4, <85% requiring assisted ventilation.
- Apnoea: point 0, apnoea ≤20 seconds; point 1, apnoea >20 seconds.
- Changes in heart rate and rhythm: point 0, heart rate ≥50 bpm or ≤120 bpm or absence of arrhythmia; point 1, heart rate <50 bpm or >120 bpm or presence of arrhythmia.
- Hypotension: point 0, systolic blood pressure ≥ 70% of the baseline or 80/50 mmHg; point 1, systolic blood pressure <70% of the baseline or 80/50 mmHg.
- Injection site pain: point 0, no response to the inquiry; point 1, mild response to the inquiry but without limb movement; point 2, moderate response to the inquiry but with obvious limb movement and spontaneous complaint of pain; point 3, severe response to the inquiry with attempt to withdraw the injected hand or presence of lacrimation. Injection site pain was continuously monitored until the patient became unconscious.
- Body movement: point 0, no body movement; point 1, digital movement not adversely affecting the examination; point 2, obvious limb or trunk movement adversely affecting the examination.
- Fasciculation: point 1, no visible muscle contraction; point 2, minimal extremity contraction; point 3, mild facial, trunk or limb muscle contraction; point 4, aggressive facial, trunk or limb muscle contraction even with limb twitch.
- Nausea and vomiting: point 1, no nausea or vomiting; point 2, mild nausea and upper abdominal discomfort without vomiting; point 3, obvious nausea and vomiting but without vomitus; point 4, serious vomiting of gastric fluid and other gastric content requiring medical intervention.
- Delirium/logorrhoea/delusion: point 0, absence of delirium/logorrhoea/delusion; point 1, presence of delirium/logorrhoea/delusion.
Discussion
The purpose of painless endoscopy is to improve patient adherence and help identify possible diseases, especially early-stage malignancies. There was no significant difference in diagnosis of cardiac carcinoma, oesophageal cancer, and gastric cancer between the 2 groups. Further, there was no obvious difference in diagnosis of precancerous lesions, including Barrett’s oesophagus, low-grade intraepithelial neoplasia, and high-grade intraepithelial neoplasia. In addition, no difference was observed in biopsy incidence. These results indicate that painless endoscopy could ensure accuracy of diagnoses and procedures by the physicians.
Taken together, our data suggest that etomidate-remifentanil is a superior anesthetic combination during gastroscopic procedures in older individuals when compared to propofol-remifentanil. One of the major advantages of etomidate is the lack of cardiovascular adverse effects. Clinical and experimental data reveal that etomidate is highly suitable for the induction of anesthesia, even in cardiac-compromised patients [12]. According our results, there was no significant difference in hemodynamics (such as blood pressure, heart rate, and SpO2) when etomidate bolus and remifentanil infusion were used for sedation during gastroscopy (Figure 1), indicating that etomidate did not affect the hemodynamic stability and suppress blood circulation. One of the most important etomidate-associated adverse effects is suppression of the adrenal cortex (data not shown) [13]. However, this adverse effect is manageable because the influence of etomidate on adrenocorticotropic hormone levels is temporary and reversible.
Propofol is widely considered the anaesthesia induction agent of choice for its enhanced depressant effects on the laryngeal reflexes compared with other induction agents [14]. In this study, systolic pressure, diastolic pressure, and heart rate decreased after induction of anaesthesia in the propofol-remifentanil group, suggesting it has inhibitory effects on cardiovascular and respiratory function. One reason for these symptoms may be the peripheral vasodilator and inhibitive effects on cardiomyocytes caused by propofol. In the study by Wihelm et al. [15], which compared the effects of remifentanil on the anesthetic induction characteristic of propofol and etomidate, mean arterial blood pressure and heart rate decreased significantly after anaesthesia induction with propofol. Therefore, etomidate seems to be the appropriate agent for providing hemodynamic stability and more is suitable for patients with cardiac problems.
Myoclonus is a prominent adverse effect experienced during the induction of anaesthesia with etomidate [16], and was the only adverse effect of significance observed in our study. The incidence of myoclonus has been reported to be as high as 50–80%, especially if etomidate is used without pre-medication [17]. However, the incidence of myoclonus after etomidate induction is reduced by remifentanil pre-treatment [10]. In this study, 4.51% of the patients treated with etomidate-remifentanil experienced myoclonus, but that number would be expected to be much higher if we had tested an etomidate alone group, further supporting the use of an etomidate-remifentanil combination. Due to short operation time of painless endoscopy and lower dosage of etomidate, myoclonus was only temporary and slight, and did not influence the physician’s decisions during the operation due to a shorter operation time and lower dosage of etomidate.
Propofol injection was associated with higher pain levels due to its strong vasodilatation effects [18]. Therefore, addition of lidocaine was used to alleviate injection pain, which consequently complicates and lengthens the procedure. In our study, incidence of pain at the injection site was lower in the etomidate group in older patients, a result consistent with a previous children’s study [19]. Injection of etomidate can help to reduce the incidence of hypoxemia and body movement, with no nausea and vomiting, which was more common in younger patients. Etomidate plays a neuroprotective role by reducing cerebral blood flow, intracranial pressure, and cerebral oxygen metabolism, which may explain its hypoxemia suppression effect. Remifentanil, an ultra–short-acting potent ester opioid, had a rapid onset of action in both group (78 s and 85 s) in this study (Table 3). However, there was no significant difference in duration time, recovery time, time to leave recovery room, or physician-patient satisfaction.
Conclusions
In conclusion, the present study compared the effects of anaesthesia induction by etomidate-remifentanil and propofol-remifentanil in patients undergoing gastroscopy. We found that etomidate-remifentanil infusions are well tolerated in older patients undergoing gastroscopy. Patients treated with etomidate-remifentanil had more stable blood pressure and heart rate. Further, etomidate-remifentanil had a more rapid onset of reaction and less adverse events. Additional studies will be required to identify optimal doses, as well as a safe and effective drug delivery system if etomidate is going to achieve wider use in patients undergoing gastroscopy.
Footnotes
Source of support: This study was supported by a grant from the National Natural Science Foundation of China (No.81171526) and Chongqing Natural Science Foundation (No. CSTC 2011 jjA10061)
References
- 1.Soweid AM, Yaghi SR, Jamali FR, et al. Posterior lingual lidocaine: A novel method to improve tolerance in upper gastrointestinal endoscopy. World J Gastroenterol. 2011;17:5191–96. doi: 10.3748/wjg.v17.i47.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amornyotin S. Sedation-related complications in gastrointestinal endoscopy. World J Gastrointest Endosc. 2013;5:527–33. doi: 10.4253/wjge.v5.i11.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucendo AJ, Arias Á, González-Castillo S, et al. Same-day bidirectional endoscopy with nonanesthesiologist administration of propofol: safety and cost-effectiveness compared with separated exams. Eur J Gastroenterol Hepatol. 2014;26:301–8. doi: 10.1097/MEG.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 4.Falk J, Zed PJ. Etomidate for procedural sedation in the emergency department. Ann Pharmacother. 2004;38:1272–77. doi: 10.1345/aph.1E008. [DOI] [PubMed] [Google Scholar]
- 5.Hsu WH, Wang SSW, Shih HY, et al. Low effect-site concentration of propofol target-controlled infusion reduces the risk of hypotension during endoscopy in a Taiwanese population. J Dig Dos. 2013;14:147–52. doi: 10.1111/1751-2980.12020. [DOI] [PubMed] [Google Scholar]
- 6.Turan A, Dalton JE, Kasuya Y, et al. Correlation between bispectral index, observational sedation scale, and lower esophageal sphincter pressure in volunteers using dexmedetomidine or propofol. Med Sci Monit. 2012;18(10):CR593–96. doi: 10.12659/MSM.883484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabbri LP, Nucera M, Marsili M, et al. Ketamine, propofol and low dose remifentanil versus propofol and remifentanil for ERCP outside the operating room: is ketamine not only a “rescue drug”? Med Sci Monit. 2012;18(9):CR575–80. doi: 10.12659/MSM.883354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beers R, Camporesi E. Remifentanil update: clinical science and utility. CNS Drugs. 2004;18:1085–104. doi: 10.2165/00023210-200418150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Akcaboy ZN, Akcaboy EY, Albayrak D, et al. Can remifentanil be a better choice than propofol for colonoscopy during monitored anesthesia care? Acta Anaesthesiol Scand. 2006;50:736–41. doi: 10.1111/j.1399-6576.2006.01047.x. [DOI] [PubMed] [Google Scholar]
- 10.Gasparovic S, Rustemovic N, Opacic M, et al. Clinical analysis of propofol deep sedation for 1,104 patients undergoing gastrointestinal endoscopic procedures: a three year prospective study. World J Gastroenterol. 2006;12:327–30. doi: 10.3748/wjg.v12.i2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt GS, Spencer MT, Hays DP. Etomidate and midazolam for procedural sedation: prospective, randomized trial. Am J Emerg Med. 2005;23:299–303. doi: 10.1016/j.ajem.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 12.Kelsaka E, Karakaya D, Sarihasan B, Baris S. Remifentanil pretreatment reduces myoclonus after etomidate. J Clin Anesth. 2006;18:83–86. doi: 10.1016/j.jclinane.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Yu JB, Dong SA, Gong LR, et al. Effect of electroacupuncture at Zusanli (ST36) and Sanyinjiao (SP6) acupoints on adrenocortical function in etomidate anesthesia patients. Med Sci Monit. 2015;21:406–12. doi: 10.12659/MSM.890111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uzun S, Gozacan A, Canbay O, Ozgen S. Remifentanil and etomidate for laryngeal mask airway insertion. J Int Med Res. 2007;35:878–85. doi: 10.1177/147323000703500616. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm W, Biedler A, Huppert A, et al. Comparison of the effects of remifentanil or fentanyl on anaesthetic induction characteristics of propofol, thiopental or etomidate. Eur J Anaesthesiol. 2002;19:350–56. doi: 10.1017/s026502150200056x. [DOI] [PubMed] [Google Scholar]
- 16.Isitemiz I, Uzman S, Toptaş M, et al. Prevention of etomidate-induced myoclonus: which is superior: Fentanyl, midazolam, or a combination? A Retrospective comparative study. Med Sci Monit. 2015;21:262–67. doi: 10.12659/MSM.889833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uzun Ş, Gözaçan A, Canbay Ö, et al. Remifentanil and etomidate for laryngeal mask airway insertion. J Int Med Res. 2007;35:878–85. doi: 10.1177/147323000703500616. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Zhu J, Xu L, et al. Efficacy and safety of flurbiprofen axetil in the prevention of pain on propofol injection: a systematic review and meta-analysis. Med Sci Monit. 2015;21:995–1002. doi: 10.12659/MSM.890102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyman Y, Von Hofsten K, Palm C, et al. Etomidate-Lipuro is associated with considerably less injection pain in children compared with propofol with added lidocaine. Br J Anaesth. 2006;97:536–39. doi: 10.1093/bja/ael187. [DOI] [PubMed] [Google Scholar]