Summary
Background
Nocardiosis primarily occurs in the setting of immunocompromising conditions. However, it may also occur in immunocompetent patients. We described computed tomography features of pulmonary nocardiosis and compared immunocompetent and immunocompromised patients.
Material/Methods
CT images of 25 patients (Mean age of 39.5 years; 76% male) with pulmonary nocardiosis proved by bronchoalveolar lavage or biopsy were reviewed by two experienced pulmonary radiologists and detailed findings were reported on. Fourteen patients (56%) were immunocompetent, while 44% had an underlying immunocompromising condition, including chronic granulomatous disease (CGD) (n=4), diabetes mellitus (DM) (n=2), malignancy (n=2), HIV (n=1), concomitant CGD and DM (n=1), and steroid therapy for nephrotic syndrome (n=1).
Results
Most patients had bilateral involvement with no zonal predominance. Multiple pulmonary nodules (96%) were the most common CT findings, followed by consolidation (76%) and cavity (52%). Other findings included bronchiectasis (48%), pleural thickening (40%), ground glass opacity (32%), mass-like consolidation (20%), intrathoracic lymphadenopathy (16%), pleural effusion (12%), reticular infiltration (4%), and pericardial effusion (4%). There was no statistically significant difference in the CT findings of immunocompromised and immunocompetent groups.
Conclusions
Pulmonary nocardiosis presents mainly as multiple pulmonary nodules, consolidations, and cavity in both immunocompromised and immunocompetent patients. However, these features are more suggestive of nocardiosis in the setting of an underling immunocompromised condition.
MeSH Keywords: Immunocompetence; Immunocompromised Host; Lung Diseases, Fungal; Multidetector Computed Tomography; Nocardia Infections
Background
Nocardiosis is a rare infection which primarily occurs in the setting of immunocompromising conditions such as human immunodeficiency virus (HIV) infection, transplantation, malignancy, and steroid or immunosuppressive therapy [1–6]. However, up to one third of the patients may be immunocompetent [7].
The lungs are the most common site of nocardiosis [1,7]. Clinical and radiological manifestations of pulmonary nocardiosis are nonspecific and definite microbiological data are lacking for most nocardia species. Therefore, this disease may be underestimated.
Chest radiograph is the first imaging study and shows nonspecific findings such as pulmonary nodules, consolidation, cavity, pulmonary infiltrations and pleural effusion [5,6,8–10]. Although, computed tomography (CT) shows more detailed features [1–4,11,12], no specific pattern has been yet reported on and also there is no study comparing CT presentations of pulmonary nocardiosis between immunocompromised and immunocompetent patients. Achieving a dominant CT presentation pattern for pulmonary nocardiosis could be helpful to prompt the diagnosis, decrease the morbidity and mortality, especially in immunocompromised patients, as well as to obviate the need for biopsy.
Material and Methods
Twenty-five consecutive patients (mean age 39.5±17 years; 76% male) with proven pulmonary nocardiosis (by CT-guided lung biopsy [n=8] or bronchoalveolar lavage [n=17]) who were admitted to a tertiary center for lung diseases from March 2001 to March 2011 were included in the study. Patients with co-infections (including two cases of tuberculosis) were excluded. None of the patients had pneumonia or primary or metastatic lung cancer.
Fourteen patients (56%) were immunocompetent and 11 cases (44%) had underlying immunosuppressive conditions, including chronic granulomatous disease (CGD) (n=4), diabetes mellitus (DM) (n=2), malignancy (n=2), HIV (n=1), concomitant CGD and DM (n=1), and steroid therapy for nephrotic syndrome (n=1). There were no significant differences between immunocompromised and immunocompetent patients with regard to age (mean age: 42.5 vs. 35.5 years, respectively; p>0.05) and sex (90% vs. 70% males, respectively; p>0.05).
Chest CT was performed using a 16-slice multi-detector CT scanner (SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany). Image acquisition was spaced from the lung apex to the base (1.5–3-mm collimation at 10-mm intervals) with scan parameters of 120 kVp, 200 mA and scan duration of 3 seconds. All chest CT images were reviewed by two expert pulmonary radiologists with 17 and 10 years of experience with almost perfect interobserver agreement (kappa=.81). The radiologists were blinded to the medical history and clinical status of the patients.
The comparison between subgroups was performed by SPSS version 17.0 (SPSS, Inc., Chicago, IL) using Chi2 and Fisher’s exact test. A p-value of less than 0.05 was considered significant.
Results
All CT findings of pulmonary nocardiosis and comparison of immunocompromised and immunocompetent patients are summarized in Table 1. There was no statistically significant difference in the CT findings of immunocompromised and immunocompetent groups. Although the cavity/cavitary consolidation in the immunocompromised patients (72.7%) was more common than in immunocompetent patients (35.7%), that difference was not statistically significant.
Table 1.
Frequency (%) of CT findings in patints with pulmonary nocardiosis.
| Nodules | Consolidation | Cavity | Bronchiectasis | Pleural thickening | Pleural effusion | Ground glass opacity | Lmphadenopathy | Reticular pattern | Pericardial effusion | p value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Male (n=19) | 94.7 | 79 | 52.6 | 52.6 | 31.6 | 10.5 | 36.8 | 13.8 | 5.3 | 0.0 | >0.05* |
| Female (n=6) | 100 | 66.7 | 50 | 33.3 | 66.7 | 16.7 | 16.7 | 16.7 | 0.0 | 16.7 | ||
| Age group | 20–40 (n=4) | 100 | 88.2 | 65 | 29.4 | 35.3 | 5.9 | 53.3 | 11.8 | 0.0 | 0.0 | >0.05** |
| 41–60 (n=17) | 75 | 50 | 25 | 100 | 25 | 25 | 25 | 25 | 25 | 0.0 | ||
| ≥61 (n=4) | 100 | 50 | 25 | 75 | 75 | 25 | 25 | 25 | 0.0 | 25 | ||
| Immunity status | Immunocompetent (n=14) | 93 | 91 | 72.7 | 57.1 | 35.7 | 14.2 | 45.5 | 7 | 7 | 7 | >0.05 |
| Immunocompromised (n=11) | 100 | 64.3 | 35.7 | 34.6 | 45.5 | 0 | 27.3 | 27 | 0 | 0 | ||
| Total (n=25) | 96 | 76 | 52 | 48 | 40 | 12 | 32 | 16 | 4 | 4 |
There was no cavitary or tree-in-bud pulmonary nodules in the male patients, while both type of nodules were seen in 33% of the females (p=0.003);
The mean age of patients with bronchiectasis was significantly higher than that of patients without bronchiectasis (46.5±11.8 vs. 33±13 year; p=0.04).
In overall, the most common CT finding was pulmonary nodules (96%) (Figures 1 and 2). All pulmonary nodules were multiple (ranging from 3 nodules to bilateral diffuse nodules). The size of fifty-two percent of nodules was <3 mm and 48% were of ≥3 mm in size. Cavitary nodules were seen in two patients and tree-in-bud pattern was seen in another two cases. The distribution of pulmonary nodules was as follows: bilateral diffuse nodules in 40%, single lobe in 32% (most common in the left lower lobe, 16%), and bi-lobe involvement in 24% (most common in bilateral upper lobes, 16%). All cavitary and tree-in-bud nodules were seen in female patients, while none of male patients had cavitary or tree-in-bud nodules (p=0.003).
Figure 1.
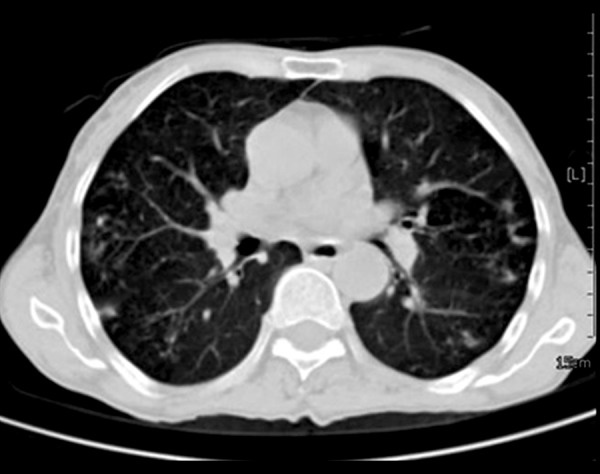
Bilateral pulmonary nodules of a different size in a 60-year-old diabetic man with pulmonary nocardiosis.
Figure 2.
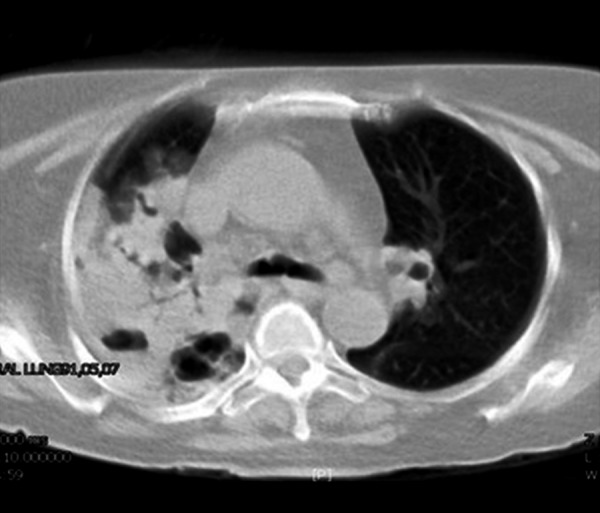
Cavitary consolidation in a 51-year-old HIV-positive woman with pulmonary nocardiosis.
The second most common CT finding was lung consolidation (76%, including 52% of cavitary consolidation) (Figures 2 and 3). More than half (58%) of consolidations were bilateral and multifocal. Single lobe consolidation was seen in five cases, all in the right upper lobe. Thirteen out of 19 lung consolidations were cavitary consolidation, and five cases were mass-like consolidation.
Figure 3.
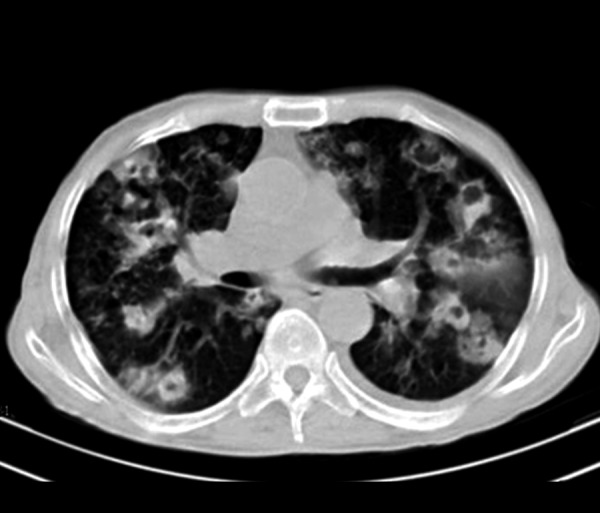
Bilateral cavitary consolidation and cavitary nodules in a 59-year-old man who presented with cough, fever and weight loss. Bronchoalveolar lavage revealed nocardiosis. Mild pleural effusion is also evident on the left side.
Bronchiectasis was the third CT finding with a frequency of 40%. Seventy-five percent of patients with bronchiectasis had single lobe involvement with a relatively similar distribution in bilateral upper and lower lobes. The mean age of the patients with bronchiectasis was significantly higher than that of patients without bronchiectasis (46.5±11.8 vs. 33±13 years; p=0.04).
The ground glass opacities were seen in about one third (32%) of the patients and showed nonspecific distribution.
Pleural thickening (40%) was seen predominantly as bilateral multifocal irregular thickening (Figure 4). All three pleural effusions (12%) were unilateral.
Figure 4.
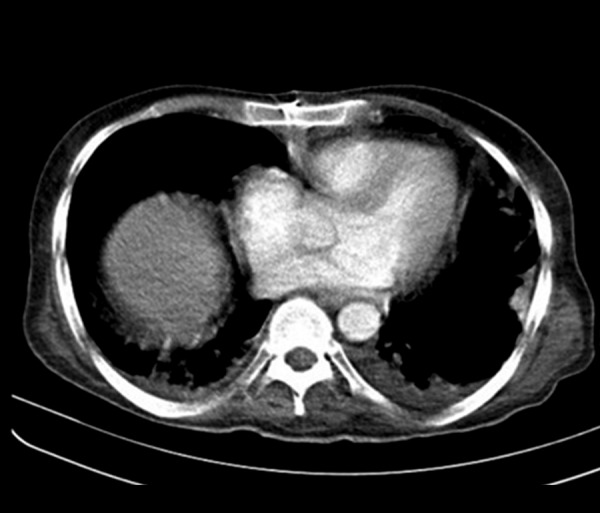
Bilateral irregular pleural thickenig in a 39-year-old man with chronic granulomatous disease. Pulmonary nocardiosis was diagnosed by CT-guided biopsy.
Discussion
Pulmonary nocardiosis most often presents as an opportunistic infection in patients with underlying immunodeficiency/immunosuppressive conditions [1–6]. Previous studies showed that up to one third of these patients can be healthy immunocompetent individuals [7]. That rate was higher in our series and more than half (56%) of cases was immunocompetent patients. Although the presence of an immunocompromising condition in patients with lung consolidations or nodules on CT may suggest an opportunistic organism, a significant portion of patients with nocardiosis may not have such a condition. Therefore, finding a specific radiologic presentation may be helpful for diagnosing pulmonary nocardiosis.
To the best of our knowledge, there is no previous comprehensive study comparing CT presentations of pulmonary nocardiosis between immunocompromised and immunocompetent patients. Only Blackmon et al. [1] in their review of CT features of pulmonary nocardiosis found discrete nodules to be more often associated with immunosuppression. In that study, we did not find any significant difference between immunocompromised and immunocompetent patients. The main CT pattern of pulmonary nocardiosis in both immunocompromised and immunocompetent patients in our study was a combination of pulmonary nodules, consolidation and cavity. That was relatively similar to the results of the previous studies; for example, in the study by Blackman et al., consolidation was the most common (64.2%) presentation of nocardiosis, followed by pulmonary nodules (57%) and cavity (40%) [1]. Consolidation, pulmonary nodules and masses were also common CT features of nocardiosis in the study by Kanne et al. Cavitation was also seen in some cases [12]. In another study, on 24 patients with nocardiosis, the most common CT findings were single or multiple pulmonary nodules and masses (83%). Other findings were cavity (33%), consolidation (33%) and pleural thickening (29%) [3]. In overall, based on our study and previous studies [2,3,5,6,11,12], in CT images which show lung consolidations, nodules, masses (or mass-like consolidation), and cavity, pulmonary nocardiosis can be suggested, especially in the setting of immunocompromising conditions.
The incidence of some less common findings in the present study was different from the one in the previous studies; pleural effusion was seen in 8% of our patients, while its frequency reached 80% in the study by Yoon et al. [11] and 75% in the study by Raby et al. [6]. Cavity (12.5%) and pulmonary nodules (37.5%) in the study by Raby et al. [6] were considerably less frequent than in our study (52% and 96%, respectively). The differences between our findings and the two mentioned studies may be due to the limited number of patients in those studies; there were only five patients in Yoon et al. study [11], and eight patients in Raby et al. study [6].
Bronchiectasis and ground glass opacities were the relatively common findings in the present study, whereas they were not reported or reported with considerably lower frequencies in other studies [1,3,6,11]. Interstitial involvement in Blackman et al. study (38%) [1] was significantly higher than in our study.
Although, based on our and other studies, consolidation, nodules, masses, and cavity are the main CT presentations of pulmonary nocardiosis, these findings may also be seen in the other conditions, such as tuberculosis, invasive aspergillosis, other pneumonias, and lung cancer [13–20]. For example, in the studies by Cha et al. [13], Yoem et al. [14], Chung et al. [15], and Kim et al. [16], pulmonary nodules, consolidation, and cavity were the most common features of tuberculosis. Not only are the radiological findings of pulmonary nocardiosis and other conditions (especially tuberculosis or invasive pulmonary aspergillosis) similar, these diseases occur mostly in the setting of underlying immunocompromising conditions. This is a diagnostic challenge. With respect to lower prevalence of nocardiosis in comparison to other mentioned diseases, it will be very difficult to suggest nocardiosis at the top of the list of differential diagnoses. Therefore, the mentioned findings are not specific and do not obviate the need for additional diagnostic tests, especially in immunodeficient patients.
Conclusions
Pulmonary nocardiosis has similar CT presentations in both immunocompromised and immunocompetent patients, which mainly consist of co-existing multiple pulmonary nodules, consolidations, and cavity. However, the findings are more suggestive of nocardiosis in the setting of an underling immunocompromised condition.
Footnotes
Conflict of interest
None.
References
- 1.Blackmon KN, Ravenel JG, Gomez JM, et al. Pulmonary nocardiosis: computed tomography features at diagnosis. J Thorac Imaging. 2011;26(3):224–29. doi: 10.1097/RTI.0b013e3181f45dd5. [DOI] [PubMed] [Google Scholar]
- 2.Kanne JP, Yandow DR, Mohammed TL, et al. CT findings of pulmonary nocardiosis. Am J Roentgenol. 2011;197(2):266–72. doi: 10.2214/AJR.10.6208. [DOI] [PubMed] [Google Scholar]
- 3.Buckley JA, Padhani AR, Kuhlman JE. CT features of pulmonary nocardiosis. J Comput Assist Tomogr. 1995;19(5):726–32. doi: 10.1097/00004728-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Oszoyoglu AA, Kirsch J, Mohammed TL. Pulmonary nocardiosis after lung transplantation: CT findings in 7 patients and review of the literature. J Thorac Imaging. 2007;22(2):143–48. doi: 10.1097/01.rti.0000213583.21849.5c. [DOI] [PubMed] [Google Scholar]
- 5.Hui CH, Au VW, Rowland K, et al. Pulmonary nocardiosis re-visited: experience of 35 patients at diagnosis. Respir Med. 2003;97(6):709–17. doi: 10.1053/rmed.2003.1505. [DOI] [PubMed] [Google Scholar]
- 6.Raby N, Forbes G, Williams R. Nocardia infection in patients with liver transplants or chronic liver disease: radiologic findings. Radiology. 1990;174(3 Pt 1):713–16. doi: 10.1148/radiology.174.3.2406779. [DOI] [PubMed] [Google Scholar]
- 7.Lederman ER, Crum NF. A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine. 2004;83:300–13. doi: 10.1097/01.md.0000141100.30871.39. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JH, Koh WJ, Suh GY, et al. Pulmonary nocardiosis with multiple cavitary nodules in a HIV-negative immunocompromised patient. Intern Med. 2004;43(9):852–54. doi: 10.2169/internalmedicine.43.852. [DOI] [PubMed] [Google Scholar]
- 9.Conant EF, Wechsler RJ. Actinomycosis and nocardiosis of the lung. J Thorac Imaging. 1992;7(4):75–84. doi: 10.1097/00005382-199209000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Kramer MR, Uttamchandani RB. The radiographic appearance of pulmonary nocardiosis associated with AIDS. Chest. 1990;98(2):382–85. doi: 10.1378/chest.98.2.382. [DOI] [PubMed] [Google Scholar]
- 11.Yoon HK, Im JG, Ahn JM, et al. Pulmonary nocardiosis: CT findings. J Comput Assist Tomogr. 1995;19(1):52–55. doi: 10.1097/00004728-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Tsujimoto N, Kikuchi K, Takata S, et al. High-resolution CT findings of patients with pulmonary nocardiosis. J Thorac Dis. 2012;4(6):577–82. doi: 10.3978/j.issn.2072-1439.2012.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha J, Lee HY, Lee KS, et al. Radiological findings of extensively drug-resistant pulmonary tuberculosis in non-AIDS adults: comparisons with findings of multidrug-resistant and drug-sensitive tuberculosis. Korean J Radiol. 2009;10(3):207–16. doi: 10.3348/kjr.2009.10.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoem JA, Jong YJ, Jeon D, et al. Imaging findins of primary multidrug-resistive tuberculosis: acomparison with finding of drug-sensitive tuberculosis. J Comput Assist Tomogr. 2009;33(6):956–60. doi: 10.1097/RCT.0b013e31819877ab. [DOI] [PubMed] [Google Scholar]
- 15.Chung MJ, Lee KS, Koh WJ, et al. Drug-sensitive tuberculosis, multidrug-resistant tuberculosis, and nontuberculous mycobacterial pulmonary disease in nonAIDS adults: comparisons of thin-section CT findings. Eur Radiol. 2006;16:1934–41. doi: 10.1007/s00330-006-0174-9. [DOI] [PubMed] [Google Scholar]
- 16.Kim HC, Goo JM, Lee HG, et al. Multidrug-Resistant Tuberculosis Versus Drug-Sensitive Tuberculosis in Human Immunodeficiency Virus-Negative Patients Computed Tomography Features. J Comput Assist Tomogr. 2004;28:366–71. doi: 10.1097/00004728-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Mehrad B, Paciocco G, Martinez FJ, et al. Spectrum of Aspergillus Infection in Lung Transplant Recipients: Case Series and Review of the Literature. Chest. 2001;119:169–75. doi: 10.1378/chest.119.1.169. [DOI] [PubMed] [Google Scholar]
- 18.Kenney HH, Agrons GA, Shin JS. Best Cases from the AFIP Invasive Pulmonary Aspergillosis: Radiologic and Pathologic Findings. Radiographics. 2002;22:1507–10. doi: 10.1148/rg.226025101. [DOI] [PubMed] [Google Scholar]
- 19.Bruno C, Minniti S, Vassanelli A, et al. Comparison of CT features of Aspergillus and bacterial pneumonia in severely neutropenic patients. J Thorac Imaging. 2007;22(2):160–65. doi: 10.1097/RTI.0b013e31805f6a42. [DOI] [PubMed] [Google Scholar]
- 20.Qin J, Meng X, Fang Y, et al. Computed Tomography and Clinical Features of Invasive Pulmonary Aspergillosis in Liver Transplant Recipients. J Thorac Imaging. 2012;27(2):107–12. doi: 10.1097/RTI.0b013e31820bb462. [DOI] [PubMed] [Google Scholar]


