Abstract
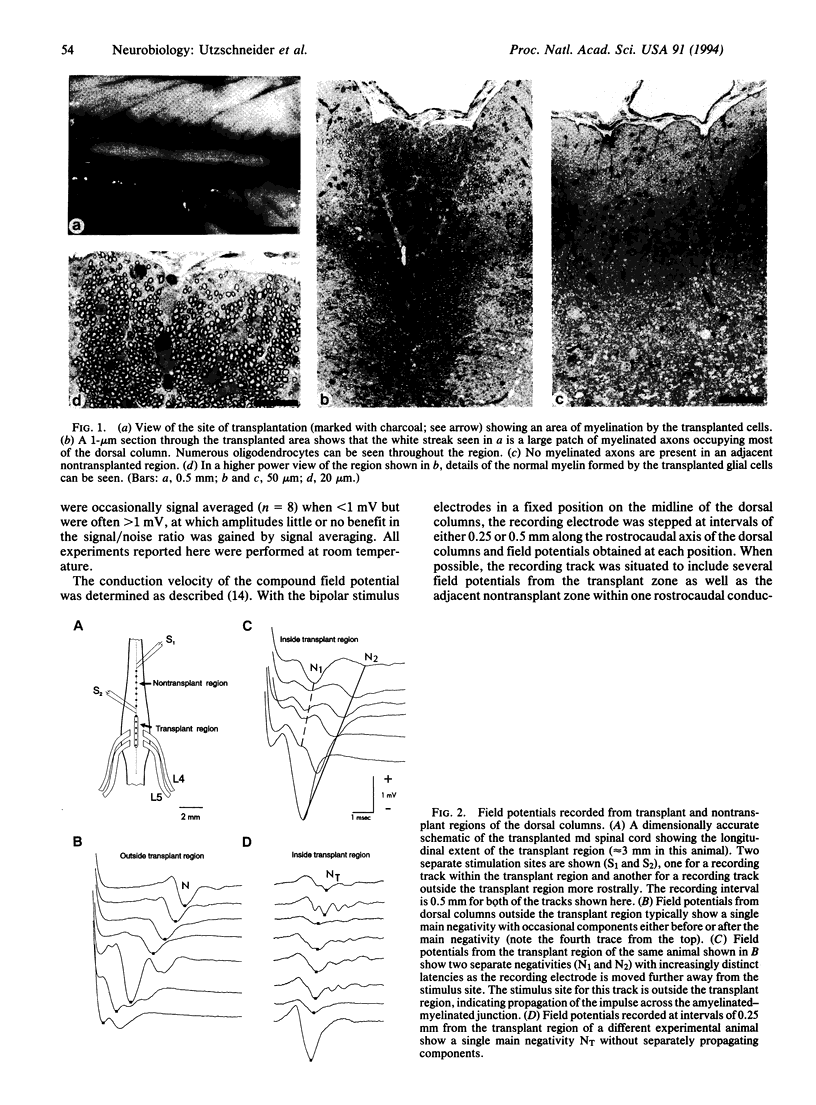
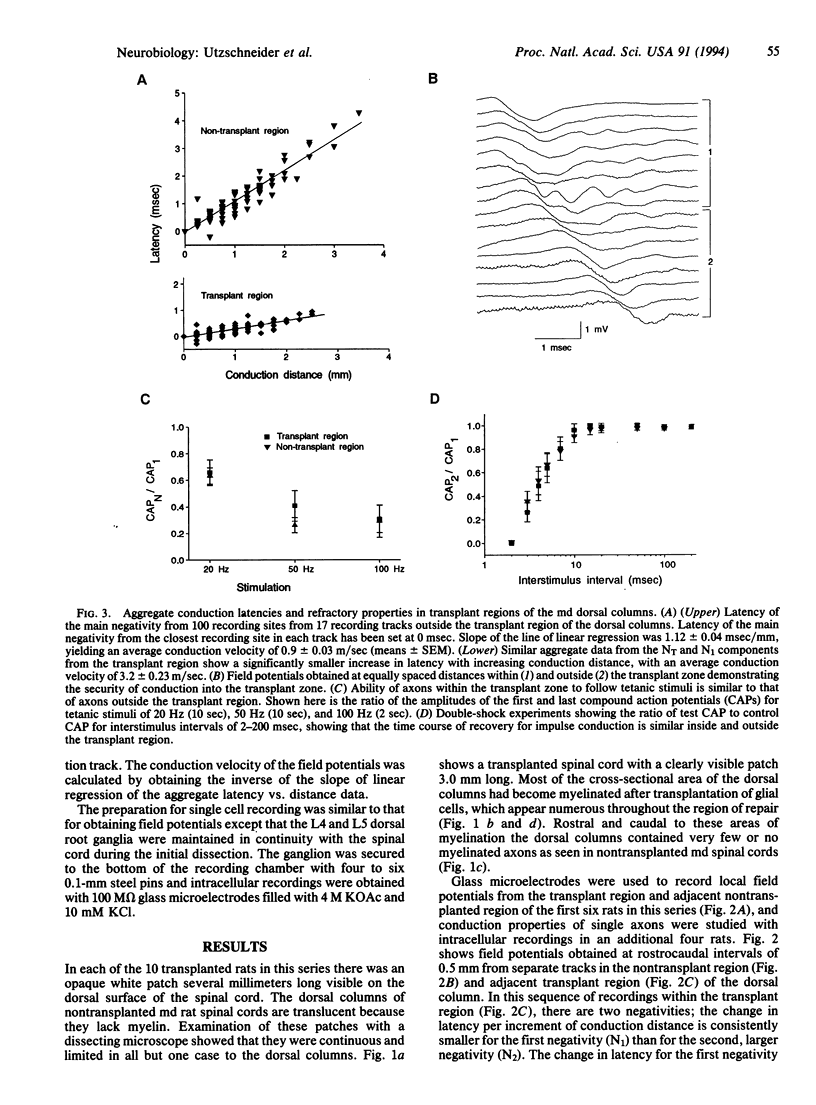
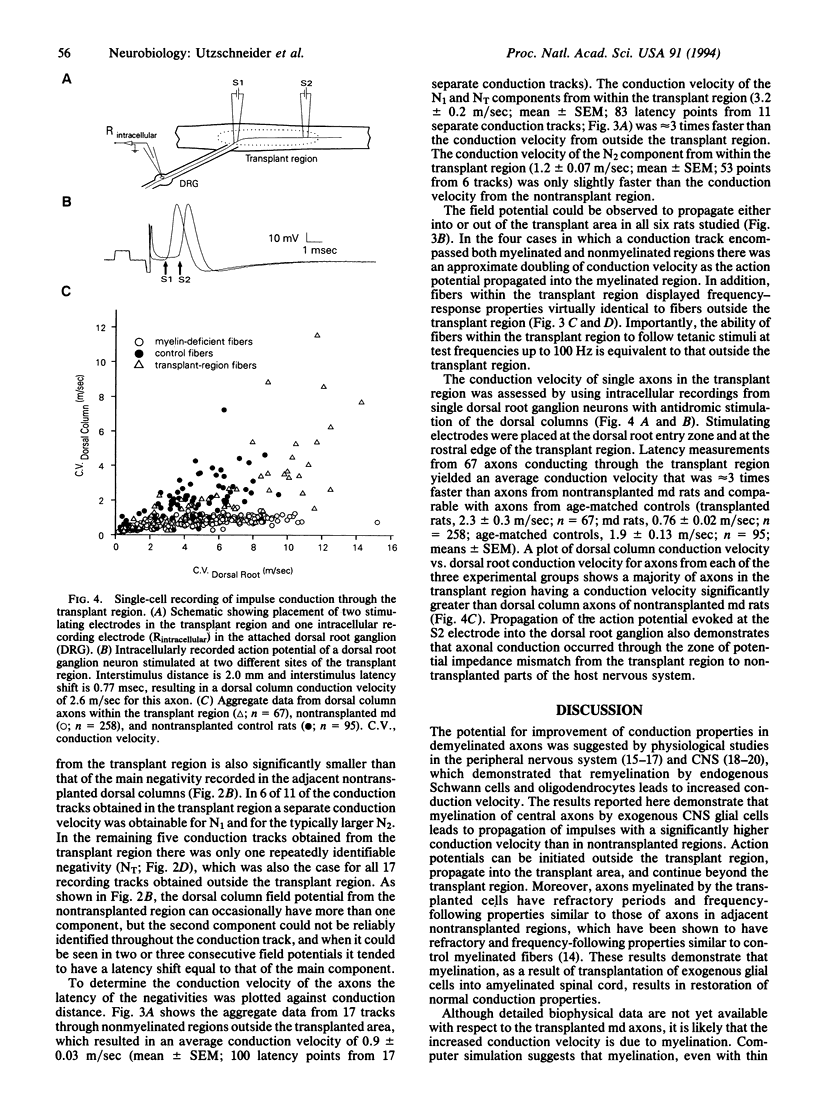
A central issue in transplantation research is to determine how and when transplantation of neural tissue can influence the development and function of the mammalian central nervous system. Of particular interest is whether electrophysiological function in the traumatized or diseased mammalian central nervous system can be improved by the replacement of cellular elements that are missing or damaged. Although it is known that transplantation of neural tissue can lead to functional improvement in models of neurological disease characterized by neuronal loss, less is known about results of transplantation in disorders of myelin. We report here that transplantation of glial cells into the dorsal columns of neonatal myelin-deficient rat spinal cords leads to myelination and a 3-fold increase in conduction velocity. We also show that impulses can propagate into and out of the transplant region and that axons myelinated by transplanted cells do not have impaired frequency-response properties. These results demonstrate that myelination following central nervous system glial cell transplantation enhances action potential conduction in myelin-deficient axons, with conduction velocity approaching normal values.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black J. A., Felts P., Smith K. J., Kocsis J. D., Waxman S. G. Distribution of sodium channels in chronically demyelinated spinal cord axons: immuno-ultrastructural localization and electrophysiological observations. Brain Res. 1991 Mar 22;544(1):59–70. doi: 10.1016/0006-8993(91)90885-y. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F. Remyelination of CNS axons by Schwann cells transplanted from the sciatic nerve. Nature. 1977 Mar 3;266(5597):68–69. doi: 10.1038/266068a0. [DOI] [PubMed] [Google Scholar]
- Blight A. R., Young W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci. 1989 Jun;91(1-2):15–34. doi: 10.1016/0022-510x(89)90073-7. [DOI] [PubMed] [Google Scholar]
- Brill M. H., Waxman S. G., Moore J. W., Joyner R. W. Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. J Neurol Neurosurg Psychiatry. 1977 Aug;40(8):769–774. doi: 10.1136/jnnp.40.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I. D., Hammang J. P., Gilmore S. A. Schwann cell myelination of the myelin deficient rat spinal cord following X-irradiation. Glia. 1988;1(3):233–239. doi: 10.1002/glia.440010309. [DOI] [PubMed] [Google Scholar]
- Duncan I. D., Hammang J. P., Jackson K. F., Wood P. M., Bunge R. P., Langford L. Transplantation of oligodendrocytes and Schwann cells into the spinal cord of the myelin-deficient rat. J Neurocytol. 1988 Jun;17(3):351–360. doi: 10.1007/BF01187857. [DOI] [PubMed] [Google Scholar]
- Duncan I. D., Paino C., Archer D. R., Wood P. M. Functional capacities of transplanted cell-sorted adult oligodendrocytes. Dev Neurosci. 1992;14(2):114–122. doi: 10.1159/000111655. [DOI] [PubMed] [Google Scholar]
- Felts P. A., Smith K. J. Conduction properties of central nerve fibers remyelinated by Schwann cells. Brain Res. 1992 Mar 6;574(1-2):178–192. doi: 10.1016/0006-8993(92)90815-q. [DOI] [PubMed] [Google Scholar]
- Funch P. G., Faber D. S. Measurement of myelin sheath resistances: implications for axonal conduction and pathophysiology. Science. 1984 Aug 3;225(4661):538–540. doi: 10.1126/science.6204382. [DOI] [PubMed] [Google Scholar]
- Groves A. K., Barnett S. C., Franklin R. J., Crang A. J., Mayer M., Blakemore W. F., Noble M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993 Apr 1;362(6419):453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- Koles Z. J., Rasminsky M. A computer simulation of conduction in demyelinated nerve fibres. J Physiol. 1972 Dec;227(2):351–364. doi: 10.1113/jphysiol.1972.sp010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachapelle F., Lapie P., Gumpel M. Oligodendrocytes from jimpy and normal mature tissue can be 'activated' when transplanted in a newborn environment. Dev Neurosci. 1992;14(2):105–113. doi: 10.1159/000111654. [DOI] [PubMed] [Google Scholar]
- Moore J. W., Joyner R. W., Brill M. H., Waxman S. D., Najar-Joa M. Simulations of conduction in uniform myelinated fibers. Relative sensitivity to changes in nodal and internodal parameters. Biophys J. 1978 Feb;21(2):147–160. doi: 10.1016/S0006-3495(78)85515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender M. P. Recovery from acute experimental allergic encephalomyelitis in the Lewis rat. Early restoration of nerve conduction and repair by Schwann cells and oligodendrocytes. Brain. 1989 Apr;112(Pt 2):393–416. doi: 10.1093/brain/112.2.393. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rang H. P., Pellegrino R. Sodium and potassium channels in demyelinated and remyelinated mammalian nerve. Nature. 1981 Nov 19;294(5838):257–259. doi: 10.1038/294257a0. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. Density of sodium channels in mammalian myelinated nerve fibers and nature of the axonal membrane under the myelin sheath. Proc Natl Acad Sci U S A. 1977 Jan;74(1):211–215. doi: 10.1073/pnas.74.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J., Hasegawa M., Shirasaki N., Rosen C. L., Liu Z. Myelin formation following transplantation of normal fetal glia into myelin-deficient rat spinal cord. J Neurocytol. 1990 Oct;19(5):718–730. doi: 10.1007/BF01188040. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Bostock H., Sheratt M. The pathophysiology of demyelination and its implications for the symptomatic treatment of multiple sclerosis. Neurology. 1978 Sep;28(9 Pt 2):21–26. doi: 10.1212/wnl.28.9_part_2.21. [DOI] [PubMed] [Google Scholar]
- Shrager P. Ionic channels and signal conduction in single remyelinating frog nerve fibres. J Physiol. 1988 Oct;404:695–712. doi: 10.1113/jphysiol.1988.sp017314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Blakemore W. F., McDonald W. I. The restoration of conduction by central remyelination. Brain. 1981 Jun;104(2):383–404. doi: 10.1093/brain/104.2.383. [DOI] [PubMed] [Google Scholar]
- Smith K. J., Bostock H., Hall S. M. Saltatory conduction precedes remyelination in axons demyelinated with lysophosphatidyl choline. J Neurol Sci. 1982 Apr;54(1):13–31. doi: 10.1016/0022-510x(82)90215-5. [DOI] [PubMed] [Google Scholar]
- Targ E. F., Kocsis J. D. 4-Aminopyridine leads to restoration of conduction in demyelinated rat sciatic nerve. Brain Res. 1985 Mar 4;328(2):358–361. doi: 10.1016/0006-8993(85)91049-2. [DOI] [PubMed] [Google Scholar]
- Utzschneider D., Black J. A., Kocsis J. D. Conduction properties of spinal cord axons in the myelin-deficient rat mutant. Neuroscience. 1992 Jul;49(1):221–228. doi: 10.1016/0306-4522(92)90090-o. [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Brill M. H. Conduction through demyelinated plaques in multiple sclerosis: computer simulations of facilitation by short internodes. J Neurol Neurosurg Psychiatry. 1978 May;41(5):408–416. doi: 10.1136/jnnp.41.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G., Swadlow H. A. The conduction properties of axons in central white matter. Prog Neurobiol. 1977;8(4):297–324. doi: 10.1016/0301-0082(77)90009-0. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Waxman S. G., Stohlman S. A., Kwan A. Remyelination following viral-induced demyelination: ferric ion-ferrocyanide staining of nodes of Ranvier within the CNS. Ann Neurol. 1980 Dec;8(6):580–583. doi: 10.1002/ana.410080606. [DOI] [PubMed] [Google Scholar]




