Abstract
Current osteoinductive protein therapy utilizes bolus administration of large doses of bone morphogenetic proteins (BMPs), which is costly, and may not replicate normal bone healing. The limited in vivo biologic activity of BMPs requires the investigation of growth factors that may enhance this activity. In this study, we utilized the C3H10T1/2 murine mesenchymal stem cell line to test the hypotheses that osteoactivin (OA) has comparable osteoinductive effects to bone morphogenetic protein-2 (BMP-2), and that sustained administration of either growth factor would result in increased osteoblastic differentiation as compared to bolus administration. Sustained release biodegradable hydrogels were designed, and C3H10T1/2 cells were grown on hydrogels loaded with BMP-2 or OA. Controls were grown on unloaded hydrogels, and positive controls were exposed to bolus growth factor administration. Cells were harvested at several time points to assess osteoblastic differentiation. Alkaline phosphatase (ALP) staining and activity, and gene expression of ALP and osteocalcin were assessed. Treatment with OA or BMP-2 resulted in comparable effects on osteoblastic marker expression. However, cells grown on hydrogels demonstrated osteoblastic differentiation that was not as robust as cells treated with bolus administration. This study shows that OA has comparable effects to BMP-2 on osteoblastic differentiation using both bolus administration and continuous release, and that bolus administration of OA has a more profound effect than administration using hydrogels for sustained release. This study will lead to a better understanding of appropriate delivery methods of osteogenic growth factors like OA for repair of fractures and segmental bone defects.
Osseous defect reconstruction is a complex surgical challenge in patients suffering from malignancies, trauma, and congenital skeletal deformities. It is estimated that in the United States over 30,000 patients per year may require craniofacial reconstructive surgery (Garcia-Godoy and Murray, 2006). Also, 15.3 million fractures are sustained in this country yearly, with 5–10% resulting in delayed or impaired healing (American Academy of Orthopaedic Surgeons, 2008). Bone grafting is frequently required for treatment of these clinical problems.
Bone grafting, one of the oldest reconstructive methods, is associated with a significant failure rate due to graft resorption, as well as potential donor site morbidity, and at times insufficient donor bone quantities. Homologous and heterologous bone grafts are infrequently used because they carry the added risks of disease transmission and host immune system activation (Toriumi et al., 1991). Bridging metal and resorbable reconstruction plates and trays, with and without bone grafts, as well as polymers such as polymethylmethacrylate, have been used for bony defect repair since the 1980s. Complications recognized with these reconstructive methods include stress shielding, implant infection and exposure, hardware failure, and limited esthetic and functional restoration (Arden et al., 1999; Blackwell and Lacombe, 1999; Boyd et al., 1995; Disher et al., 1993). Distraction osteogenesis has been used for bone lengthening but is associated with lengthy distraction and consolidation processes, and is often complicated by hardware failure, scarring, nonunion, malocclusion, relapse and the need for multiple surgical procedures. For these reasons, craniofacial tissue engineering is an active field of study encompassing the disciplines of cell and molecular biology, polymer chemistry, molecular genetics, materials science, robotics and mechanical engineering (Mao et al., 2006).
The discovery of the osteo-inductive properties of demineralized bone (DB) eventually led to the purification of the bone morphogenetic proteins (BMPs) (Urist et al., 1983). The BMPs (except for bone morphogenetic protein-1) are members of the transforming growth factor-beta (TGF-β) superfamily of polypeptide growth factors. Approximately 40 bone morphogenetic protein (BMP) isoforms have been identified, and they differ in their effects, which may be mitogenic, chemotactic, morphogenic, or apoptotic depending on the cell type to which the growth factor is exposed and the growth factor concentration (Reddi, 2000; Spector et al., 2001). It is recognized that mixtures of BMPs derived from DB are up to a thousand times more potent for bone induction than any specific recombinant BMP (DeGroot, 1998). This is indicative of the fact that the activity of native BMPs is a combination of the synergistic activities of several growth factors (Hing, 2004).
Currently bone morphogenetic protein-2 and -7 (BMP-2 and -7) are the only biologic modifiers that have received United States Food and Drug Administration approval for limited orthopedic clinical applications. The BMP low biologic activity is demonstrated by the fact that commercially available BMP-2- and -7-containing products deliver protein doses of tens of milligrams, whereas naturally occurring BMPs exist in concentrations on the order of several micrograms per kilogram of bone (Urist et al., 1983; Aono et al., 1995). BMP therapeutic doses in preclinical and clinical trials varied by as much as 100-fold, demonstrating less than reproducible effects on bone repair (Salgado et al., 2004). Species-related differences in osteoblastic responses to BMP signaling have also been demonstrated, indicating that human osteoblasts, in comparison to murine osteoblasts, require dexamethasone in addition to BMP-2, -4 and -7 in order to upregulate alkaline phosphatase (ALP) activity, a marker of osteoblastic differentiation and function (Diefenderfer et al., 2003). For these reasons the investigation of complimentary osteoinductive factors is necessary.
The BMP-2 osteoinductive signal is transduced after binding of the BMP receptors and results in the induction of expression of osteoblastic markers such as ALP, type I collagen, vascular endothelial growth factor (VEGF), osteocalcin (OCN), osteopontin, and osteoactivin (OA). The initial identification of OA emerged from studies using an animal model of osteopetrosis, in which it is overexpressed (Safadi et al., 2001; Owen et al., 2003; Selim 2009). Other groups have identified the same protein in different species and have designated different names such as glycoprotein nmb in melanoma cell lines (Weterman et al., 1995; Okamoto et al., 2005; Kuan et al., 2006; Tse et al., 2006; Pollack et al., 2007) and melanocytes (Anderson et al., 2002, 2006), dendritic cell heparin sulfate proteoglycan integrin dependent ligand in dendritic and T-cells (Shikano et al., 2001; Chung et al., 2007), and human hematopoietic growth factor inducible neurokinin in tumor cells (Bandari et al., 2003). In osteoblasts, OA exists as a 65-kDa transmembrane protein and a 115-kDa secreted glycoprotein (Safadi et al., 2001). The osteoinductive effects of BMP-2 are dependent on OA (Abdelmagid et al., 2007). OA has the ability to regulate cell proliferation, adhesion, differentiation and synthesis of ECM proteins in various cell types (Adema et al., 1994; Jager et al., 2000; Anderson et al., 2001, 2002, 2006; Safadi et al., 2001; Bandari et al., 2003; Onaga et al., 2003; Ott et al., 2003; Safadi et al., 2003; Yasumoto et al., 2004; Boissy et al., 2005; Ogawa et al., 2005; Okamoto et al., 2005; Brunberg et al., 2006; Hoashi et al., 2006; Abe et al., 2007; Helip-Wooley et al., 2007; Nakamura et al., 2007; Ripoll et al., 2007). OA messenger ribonucleic acid (mRNA) and protein are expressed by human and rodent osteoblasts (Safadi et al., 2001; Owen et al., 2003). OA down-regulation decreases osteoblast differentiation and function (Abdelmagid et al., 2007). These properties suggest that OA may be a novel osteoinductive agent that is less costly than BMPs, and that OA may be able to augment the effects of BMPs.
In this report, we hypothesized that OA has similar osteoinductive effects in murine mesenchymal cells to BMP-2, and that continuous release of BMP and OA using biodegradable gelatin hydrogels would result in increased osteoblastic differentiation of mesenchymal cells as compared to bolus administration.
Materials and Methods
Development of controlled release hydrogels for delivery of BMP and OA
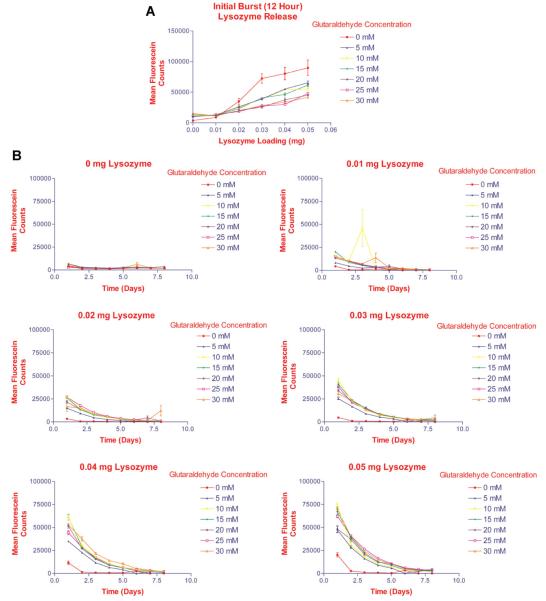
Controlled-release hydrogels for delivery of the growth factors were developed (Raiche and Puleo, 2001, 2004). In order to determine optimal glutaraldehyde concentration ranges for crosslinking of the gelatin-based hydrogels and loading doses of protein, gelatin (type 2A, 300 bloom from porcine skin, Sigma Chemical Company, St. Louis, MO) was dissolved, 5 g/100 ml, in PBS containing various concentrations of fluorescein-labeled lysozyme (0.01, 0.02, 0.03, 0.04, or 0.05 mg/ml). Lysozyme was chosen to initially define the release kinetics from the hydrogels because it has an isoelectric point and molecular weight (MW) similar to that of BMP-2 (Raiche and Puleo, 2001). Lysozyme was labeled as previously described (Goding, 1986). The gelatin solutions were placed in 24-well plates (0.5 ml per well) and dried under laminar airflow for 8 h. Controls consisted of gelatin solution without lysozyme. The gelatin hydrogels were covered with glutaraldehyde solution at various concentrations (0, 5, 10, 15, 20, 25, and 30 mM), and allowed to crosslink at 4°C for 24 h. Each experimental group contained three replicates. The gelatin hydrogels were treated with 100 mM glycine in PBS at 4°C for 1 h to remove unbound or partially reacted glutaraldehyde and poorly bound protein (Raiche and Puleo, 2004). After drying, the plates were sterilized under ultraviolet light for 5 h. To determine release kinetics from the hydrogels, PBS was added to each well. The plates were placed in a 5% CO2 incubator at 37°C. After 12 h and then daily, the supernatants were aspirated completely, and replaced with new PBS for 10 days to maintain sink conditions. Samples were analyzed by measuring fluorescence at an excitation wavelength (λ) = 495 nm and λemission=515 nm. Lysozyme release from the constructs was averaged and plotted against time (Fig. 1).
Fig. 1.
Determination of optimal glutaraldehyde concentration ranges required for crosslinking of the gelatin-based hydrogels. Gelatin was dissolved in PBS containing various concentrations of fluorescein-labeled lysozyme. The gelatin solutions were placed in 24-well plates and dried under laminar airflow for 8 h. Controls consisted of gelatin solution without lysozyme. The gelatin sheets were covered with glutaraldehyde solution at various concentrations, and allowed to crosslink at 4°C for 24 h. PBS was added to each well and the plates were placed in a 5% CO2 incubator at 37°C. After 12 h and then daily, the supernatants were aspirated completely, and replaced for 10 days to maintain sink conditions. Samples were analyzed by measuring fluorescence at an excitation wavelength (λ) = 495 nm and λemission = 515 nm. A: Initial burst release of lysozyme from hydrogels during first 12 h. Lysozyme release from the hydrogels correlated with the loading doses, and varied inversely to the degree of crosslinking of the hydrogels (glutaraldehyde concentration). B:Lysozyme release from hydrogels over 8 days suggested that crosslinking with 30 mM glutaraldehyde resulted in the most delayed release of lysozyme.
In order to determine whether the glutaraldehyde used for crosslinking of the hydrogels was toxic to cells, cells were plated onto unloaded hydrogels crosslinked with various concentrations of glutaraldehyde. Cell viability was determined using inverted microscopy (data not shown). Glutaraldehyde concentration did not appear to affect cellular proliferation (Fig. 2).
Fig. 2.
Cell viability in response to glutaraldehyde used for crosslinking of the hydrogels. Cells were plated onto unloaded (no protein added) hydrogels crosslinked with various concentrations of glutaraldehyde. Glutaraldehyde concentration did not appear to affect cellular viability/proliferation using bright field inverted microscopy. 10× magnification.
Based on data from the lysozyme release assays (Fig. 1), gelatin constructs were then designed to study the release kinetics of BMP and OA. These hydrogels were loaded with 0.1 μg/ml recombinant human bone morphogenetic protein-2 (rhBMP-2), the recombinant human BMP-2/7 heterodimer (rhBMP-2/7) or recombinant mouse osteoactivin (rmOA). Growth factors were obtained from R&D Systems (Minneapolis, MN). Controls consisted of unloaded hydrogels. The hydrogels were crosslinked with glutaraldehyde solution at 2 or 5 mM at 4°C for 24 h. Each experimental group contained three replicates. The aldehyde groups were blocked with glycine as described above,and afterdrying, the plates weresterilized.
To determine release kinetics from the hydrogels, PBS was added to each well. The plates were placed in a 5% CO2 incubator at 37°C. After 12 h and then daily, the supernatants were sampled, aspirated completely, and replaced for 21 days. The release kinetics of rhBMP-2 from the hydrogels was defined using ELISAs (R&D Systems). Release of rhBMP-2/7 could not be confirmed with ELISAs, and therefore this growth factor was not used for subsequent experiments. Because there was no commercially available ELISA for OA at the time of this experiment, release of OA from the hydrogels was demonstrated with Western blot analysis as previously described (Abdelmagid et al., 2007).
Treatment of mesenchymal cells with controlled release growth factors
Gelatin hydrogels were constructed in 24-well tissue culture plates as described above by loading with either 0.75 μg/well rhBMP-2 or 100 ng/well rmOA, and crosslinked with different concentrations of glutaraldehyde (2 or 5 mM). Other hydrogels were constructed, loaded with rhBMP-2 at several loading doses (1, 2, or 4 μg/well) or with rmOA at several loading doses (1, 10, 50, or 100 ng/well), and crosslinked with 20 mM glutaraldehyde. C3H10T1/2 cells (passage 8) were plated on to the hydrogels at a density of 2.5 × 104 cells per well and grown in Dulbecco's modified Eagle's medium with 100 U/ml penicillin/streptomycin and 10% fetal bovine serum at 37°C in a 5% CO2 atmosphere. Every 3 days the medium was sampled, aspirated completely, and replaced. On day 21 cells were harvested for ALP staining and activity. Medium samples were analyzed by ELISA to confirm release of BMP. Western blot analysis was used to confirm release of OA. Positive controls consisted of cells grown on unloaded hydrogels with media containing 0.75 μg/ml rhBMP-2 OR 100 ng/ml rmOA. Negative controls consisted of cells grown on unloaded hydrogels with media not containing growth factors. Media were also changed every 3 days for the positive and negative controls.
Titration of BMP and OA bolus doses
In order to determine dose ranges of BMP and OA that would yield optimal osteoblastic differentiation, C3H10T1/2 cells (passage 8) were treated with various bolus doses of rhBMP-2 (1, 2, or 4 μg/ml) or rmOA (1, 10, 50, or 100 ng/ml). C3H10T1/2 cells were plated in 24-well plates at a density of 2.5 × 104 cells per well in Dulbecco's modified Eagle's medium with 100 U/ml penicillin/streptomycin and 10% fetal bovine serum at 37°C in a 5% CO2 atmosphere. After 24 h, cells were treated with growth factor-containing medium, and the medium was changed every 3 days, refreshing the growth factor dose. Positive controls consisted of ROS 17/2 osteosarcoma cells grown in medium without growth factors. Cells were harvested after 7, 14, and 21 days, stained for ALP, and assayed for matrix mineralization using von Kossa staining (day 21).
Comparative osteoblastic differentiation effects of BMP-2 and OA using bolus growth factor administration
The comparative effects of BMP-2 and OA on mesenchymal cell osteoblastic differentiation were assessed using the ALP activity assay, and measurement of ALP and OCN messenger ribonucleic acid (mRNA) expression. Concentrations of the growth factors used were based on the titration assays.
Alkaline phosphatase (ALP) activity assays
C3H10T1/2 cells (passage 8) were grown in supplemented medium containing 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate with 100 ng/ml rhBMP-2 or 100 ng/ml rmOA. Positive controls consisted of ROS 17/2 osteosarcoma cells grown in supplemented medium without growth factors. Negative controls consisted of C3H10T1/2 cells grown in supplemented medium without the addition of growth factors. After 7, 14, and 21 days cells were washed with HBSS and lysed in 500 μl of 0.1% Triton X-100 for 30 min at 37°C. A 20 μl sample of the cell lysates was mixed with 100 μl of working solution containing 2.0 mM MgCl2, 0.1 M carbonate buffer, pH 9.8, and a p-nitrophenylphosphate (pNPP) substrate (final concentration 6.7 mM, LabAssay™ ALP, Wako Chemicals, Richmond, Virginia). After a 15-min incubation at 37°C, plates were read at 405λ in a microplate reader. ALP activity per minute was calculated by comparison to a p-nitrophenol standard curve and normalized to protein content. Total protein content was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Cells were grown in differentiation medium as described for ALP activity assays. Again, positive controls consisted of ROS cells, while negative controls consisted of C3H10T1/2 cells grown in medium without growth factors. After 7, 14, and 21 days, cells were harvested then homogenized in Trizol (Invitrogen, Carlsbad, CA). Cellular homogenates were separated into organic and aqueous layers by chloroform extraction, and ribonucleic acid (RNA) was recovered from the aqueous phase by isopropyl alcohol precipitation. Pellets were washed with 70% ethanol and RNA concentrations were measured by optical densitometry at 260 and 280 nm. RNA integrity was evaluated by agarose gel electrophoresis.
One microgram of total RNA from each sample was reverse transcribed into complementary deoxyribonucleic acid (cDNA) with a SuperScript First Strand kit (Invitrogen) according to the manufacturer's recommendations. Reverse transcriptase polymerase chain reaction was performed in triplicate on an ABI 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) using the SYBR green PCR Master mix from the same company. The sequences of primers for ALP (AKP-2), osteocalcin (OC) and β-actin genes are given in Table 1. Fifteen microliters of 2.0×SYBR green Master mix was added to each well of an optical 96-well plate, with 2 μl (10 mM) of both forward and reverse primers, 5 μl of cDNA sample and deionized distilled water to complete a 25 μl total reaction volume. The cycling program was as follows: 50°C for 2 min, 95°C for 10 min; 40 cycles of 95°C for 15 sec followed by 60°C for 1 min. Samples were normalized to β-actin expression.
TABLE 1.
Forward (F) and reverse (R) primers for target genes
| Gene name | Amplicon length (bp) | 5′–3′ primer sequence |
|---|---|---|
| AKP-2 (NM007431) | 164 | F5′-TGCCTACTTGTGTGGCGTGAA-3′ |
| OC (NM007541) | 178 | R5′-TCACCCGAGTGGTAGTCACAATG-3′ F5′-AGCAGCTTGGCCCAGACCTA-3′ |
| β-Actin (NM007393) | 131 | R5′-TAGCGCCGGAGTCTGTTCACTAC-3′ F5′-TGACAGGATGCAGAAGGAGA-3′ |
| R5′-GCTGGAAGGTGGACAGTGAG-3′ |
Statistical analysis
Data analysis was performed with SPSS statistical software (SPSS, Incorporated, Chicago, IL). One-way analyses of variance were used to identify differences in mean values for ALP activity (expressed as nM p-nitrophenol/minute/μg protein), and ALP and OCN mRNA (expressed as relative mRNA expression) between experimental groups. A P < 0.05 was considered statistically significant for all comparisons. The Bonferroni post hoc multiple analysis test was used to identify significant pairwise differences. The importance of significant differences (effect size) was estimated using a Hayes ω2 statistic. The effect size indicates the percentage of the total variances that is explained by the independent variable. An ω2 of 0.01 through 0.05 indicates a small effect, 0.06–0.13 indicates a medium effect, and 0.14 or greater indicates a large effect.
Results
We first tested the hypothesis that mesenchymal cells treated with sustained release of the osteoinductive growth factors, BMP-2 and OA, would demonstrate increased osteoblastic differentiation as compared to cells treated with bolus administration of the growth factors. To test this hypothesis, we developed biodegradable crosslinked gelatin hydrogels for growth factor delivery.
Release of fluorescein-labeled lysozyme from the gelatin hydrogels
Protein release from the hydrogels were first confirmed using lysozyme, which is less costly than the growth factors, but has an isoelectric point and MW similar to that of BMP-2 (Raiche and Puleo, 2001). Lysozyme was released from the hydrogels in a dose dependent-manner, with the rate of release being inversely proportional to the degree of crosslinking (i.e., the glutaraldehyde concentration used to crosslink the hydrogels). Initial burst release is depicted in Figure 1A. Release over 8 days is shown in Figure 1B. Lysozyme release from the hydrogels correlated with the loading doses, and varied inversely to the degree of crosslinking of the hydrogels (glutaraldehyde concentration).
Effects of continuous growth factor release on mesenchymal cell differentiation
After demonstrating that lysozyme could be released from the hydrogels in a dose-dependent manner predictable on differential crosslinking of the gelatin hydrogels, growth factor release from the hydrogels and release-dependent effects on cellular differentiation were examined.
Effects of differential crosslinking of the gelatin hydrogels
First we examined whether glutaraldehyde had toxic effects on the C3H10T1/2 cells. Cells grown on the unloaded hydrogels demonstrated normal cellular proliferation at all of the concentrations of glutaraldehyde tested (Fig. 2). However, in the presence of mesenchymal cells, growth factor-loaded hydrogels crosslinked with low concentrations of glutaraldehyde (2–10 mM) did not maintain their integrity for more than 1 week. Thus for experiments assessing the effects of growth factor dose titration using the hydrogels, 20 mM glutaraldehyde was used for crosslinking (see below).
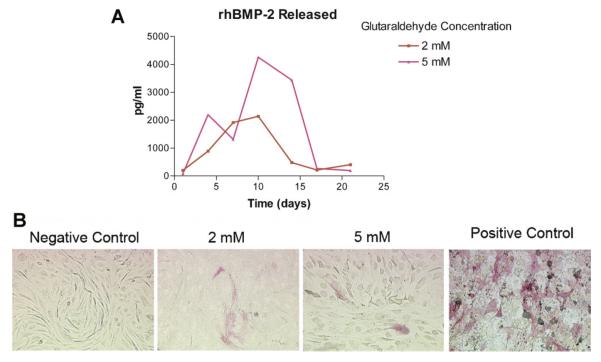
Next, we assessed the effects of sustained growth factor release from the hydrogels on cellular differentiation. The effects of differential crosslinking of the hydrogels in the presence of mesenchymal cells were assessed using ALP staining as a marker of osteoblastic differentiation (Risteli and Risteli, 1993; Abdelmagid et al., 2007). The release of rhBMP-2 from the gelatin constructs as measured by ELISA is shown in Figure 3A. Few differentiated cells were noted on the rhBMP-2-loaded hydrogels as compared to the positive control, which consisted of cells treated with growth factor-containing medium on unloaded hydrogels (Fig. 3B). ALP activity was too low to be measured in any of the cells grown on the hydrogels.
Fig. 3.
Effects of differential crosslinking of the hydrogels on BMP-2 release in the presence of mesenchymal cells and release-dependent effects on mesenchymal cell differentiation. Gelatin hydrogels were constructed in 24-well tissue culture plates, loaded with 0.75 μg/well rhBMP-2, and crosslinked with different concentrations of glutaraldehyde. A: BMP-2 release from hydrogels. rhBMP-2 release from differentially crosslinked hydrogels was assessed by ELISA. rhBMP-2 maximum release was achieved between days 10 and 14 in culture from hydrogels crosslinked with 5 mM glutaraldehyde. B: Photomicrographs of ALP staining of C3H10T1/2 cells grown on rhBMP-2-loaded hydrogels (molarities refer to glutaraldehyde concentration used for crosslinking) at 14 days in culture. Positive controls were cells grown on unloaded hydrogels with medium containing rhBMP-2. Negative controls were cells grown on unloaded hydrogels with medium containing no growth factor. rhBMP-2 release from the hydrogels had very little effect on cell differentiation as determined by ALP staining. 20× magnification.
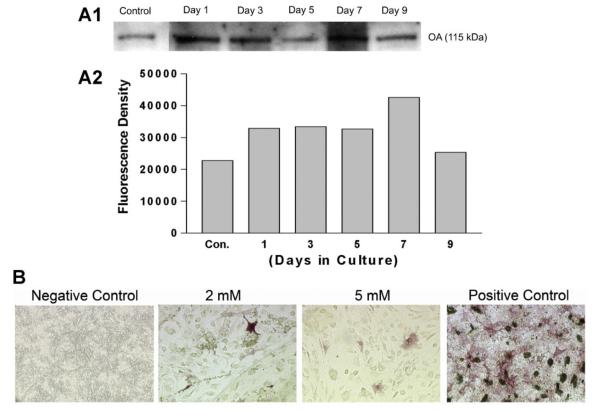
To assess rmOA release from the hydrogels, medium was collected from cells plated over hydrogels at different time points (0, 1, 3, 5, 7, and 9 days), then subjected to Western blot analysis and probed with anti-OA antibody. The control sample contained some OA secreted by C3H10T1/2 cells. The amount of OA released into medium increased daily to reach a maximum at 7 days. These data suggest that OA was released/eluted out of the gelatin constructs in a time dependent manner. The Western blot analysis also demonstrated that the secreted/released OA was the fully glycosylated form without degradation (Fig. 4A). As with the cells grown on the rhBMP-2-loaded constructs, there were few differentiated cells on the rmOA-loaded constructs as demonstrated by ALP staining (Fig. 4B). The degree of crosslinking of the hydrogels did not seem to correlate with the numbers of differentiated cells grown on either rhBMP-2- or rmOA-loaded hydrogels (data not shown).
Fig. 4.
Effects of differential crosslinking of the hydrogels on OA release in the presence of mesenchymal cells, and release-dependent effects on mesenchymal cell differentiation. Gelatin hydrogels were constructed in 24-well tissue culture plates, loaded with 100 ng/well rmOA, and crosslinked with different concentrations of glutaraldehyde. A: OA release from hydrogels. Western blot analysis of media from C3H10T1/2 cells grown on rmOA-loaded hydrogels. Graph represents densitometric analysis of Western blot. rmOA release was time-dependent. B: ALP staining of C3H10T1/2 cells grown on rmOA-loaded hydrogels (molarities refer to glutaraldehyde concentration used to crosslink gelatin) at 14 days. rmOA release from the hydrogels had very little effect on cell differentiation as determined by ALP staining. 20× magnification.
Effects of growth factor dose titration
Next, the effects of differential loading of the hydrogels in the presence of mesenchymal cells were assessed. Hydrogels crosslinked with 20 mM glutaraldehyde maintained their integrity for longer periods than those crosslinked with lesser concentrations of glutaraldehyde. Nevertheless, very few differentiated cells were identified on the hydrogels despite differential loading with various concentrations of both growth factors (Fig. 5A). More differentiated cells were noted on the rmOA-loaded hydrogels, and there seemed to be a dose–response relationship (Fig. 5A). Again, there were insufficient numbers of differentiated cells on the hydrogels to measure ALP activity. Nevertheless, matrix mineralization was noted with von Kossa assays, demonstrating osteoblastic differentiation (Fig. 5B).
Fig. 5.
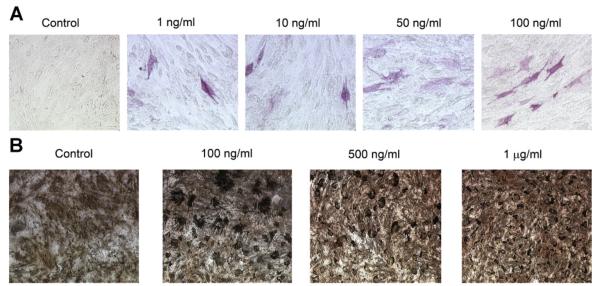
Effects of differential loading of the hydrogels on mesenchymal cell differentiation. Hydrogels were constructed, loaded with rhBMP-2 at several loading doses (1, 2, or 4 μg/well) or with rmOA at several loading doses (1, 10, 50, or 100 ng/well), and crosslinked with 20 mM glutaraldehyde. A: Effects of differential loading of hydrogels with OA on ALP staining of C3H10T1/2 cells at 21 days. Concentrations represent loading concentrations of rmOA in gelatin solution. 20× magnification. B: Von Kossa staining of C3H10T1/2 cells grown on hydrogels differentially loaded with rmOA, and crosslinked with 20 mM glutaraldehyde. Concentrations represent loading concentrations of rmOA in gelatin solution. 20× magnification.
Titration of BMP and OA bolus doses
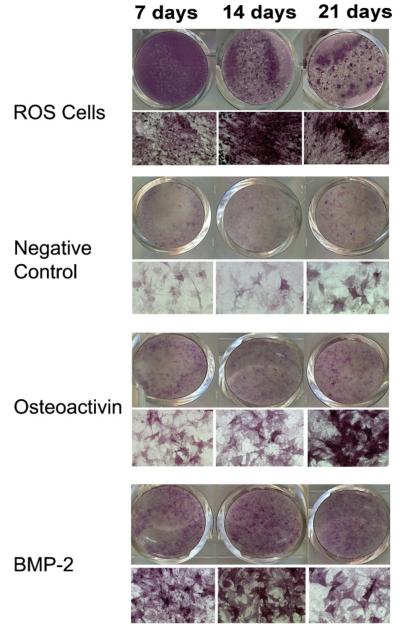
In this study we titrated concentrations of BMP-2 and OA administered to cells in bolus to give a maximum response of osteoblastic differentiation as determined by ALP staining in order to compare the osteoblastic effects of BMP-2 and OA. For C3H10T1/2 cells treated with bolus growth factors, little to no ALP staining was evident at 7 days. ALP staining was most intense for cells at 14 days (Fig. 6B). Cells treated with exogenous rhBMP-2 at 1 μg/ml had the most intense ALP staining as compared to cells treated with 2 or 4 μg/ml (data not shown). Cells treated with rmOA demonstrated a dose–response relationship with the numbers of stained cells correlating with the dose of rmOA up to 100 ng/ml (data not shown). Exogenously applied rmOA at 500 ng/ml was toxic to cells. Extensive, diffuse matrix mineralization as demonstrated by von Kossa staining was also demonstrated in all experimental groups. However, cells treated with growth factors stimulated mineralizing bone-forming nodules (data not shown).
Fig. 6.
Concentrations of BMP-2 and OA administered to cells in bolus were titrated to give a maximum response of osteoblastic differentiation as determined by ALP staining in order to compare the osteoblastic effects of BMP-2 and OA. ALP staining of ROS 17/2 osteosarcoma cells grown in medium without growth factors demonstrated an increase in ALP staining with time in cultures. ALP staining of C3H10T1/2 cells at 7, 14, and 21 days: Cells shown in the OA group were grown in medium containing 100 ng/ml rmOA. Cells shown in the BMP-2 group were grown in medium containing 1 μg/ml rhBMP-2. In both conditions, there was an increase in ALP activity in response to rmOA and rhBMP-2, and this response was time-dependent.
Comparative osteoblastic differentiation effects of BMP-2 and OA
Based on the results of the bolus titration study, the osteoinductive effects of BMP-2 and OA were compared by measuring ALP activity, and ALP and OCN mRNA expression in mesenchymal cells (Risteli and Risteli, 1993; Abdelmagid et al., 2007).
ALP activity
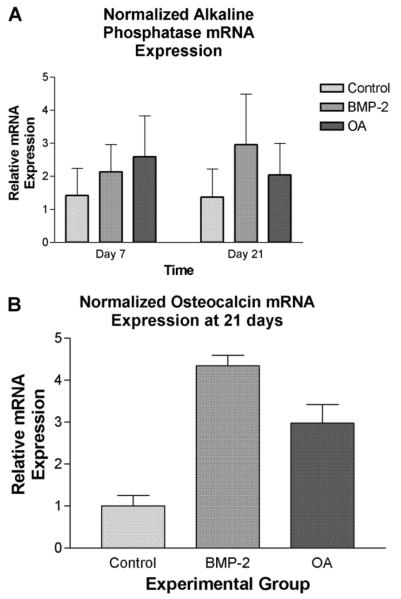
C3H10T1/2 cells treated with rhBMP-2 (100 ng/ml) had significantly increased ALP activity as compared to negative controls on days 7 and 14, but not on day 21 (P < 0.0001, ω2 = 0.70). ALP activity between cells treated with rmOA (100 ng/ml) and rhBMP-2 (100 ng/ml) was not statistically significant. All groups demonstrated decreased ALP activity by day 21, which is consistent with known osteoblastic differentiation patterns (Fig. 7) (Risteli and Risteli, 1993). ROS osteosarcoma cells (used as a positive control) expressed significantly more ALP activity at all time points measured as compared to C3H10T1/2 mesenchymal cells treated with either rhBMP-2 or rmOA, and in comparison to negative controls (P = 0.001, ω2 = 0.79, data not shown).
Fig. 7.
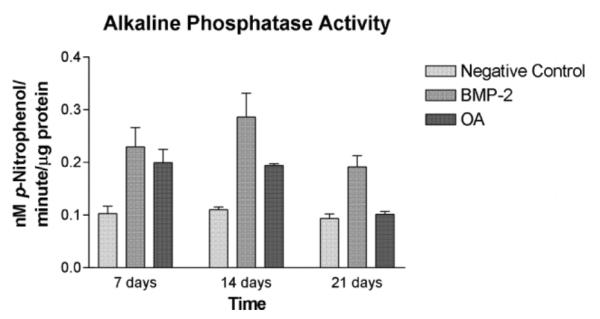
C3H10T1/2 cells were grown in medium containing 100 ng/ml rhBMP-2 or 100 ng/ml rmOA. Negative controls consisted of C3H10T1/2 cells grown in medium without growth factors. After 7, 14, and 21 days cells were washed and lysed. Cell lysates were tested for ALP activity. Error bars indicate standard deviations.
ALP mRNA expression
ALP mRNA expression as determined by qRT-PCR was greater in cells treated with rhBMP-2 (100 ng/ml) or with rmOA (100 ng/ml) as compared to the untreated control at 7 days (P = 0.0011, ω2 = 0.85, Fig. 8A). By 21 days, ALP mRNA expression in the rmOA-and rhBMP-2-treated groups both exceeded that in the control group, and there were higher levels of ALP mRNA in the rhBMP-2-treated cells than in the rmOA-treated cells (P < 0.0001, ω2 = 0.97).
Fig. 8.
C3H10T1/2 cells were grown in medium containing 100 ng/ml rhBMP-2 or 100 ng/ml rmOA. Negative controls consisted of C3H10T1/2 cells grown in medium without growth factors. After 7, 14, and 21 days, cells were harvested then homogenized for qRT-PCR analysis for ALP and OCN mRNA. A: ALP mRNA expression in experimental groups at 7 and 21 days. Error bars indicate standard deviations. B: OCN mRNA expression in experimental groups at 21 days. Error bars indicate standard deviations.
OCN mRNA expression
While the expression of OCN mRNA in the experimental groups was measured at days 7 and 14, it was insignificant as would be expected because OCN is expressed during the matrix mineralization phase of osteoblastic differentiation (data not shown). At 21 days, expression of OCN mRNA in rhBMP-2-treated cells was significantly greater than its expression in the negative control, but was not significantly different from cells treated with rmOA (P = 0.0011, ω2 = 0.85, Fig. 8B).
Discussion
Several growth factors have been implicated in bone healing include TGF-β1–3, insulin-like growth factor, VEGF, fibroblast growth factor, interleukins, prostaglandins, parathyroid hormone, estrogen, connective tissue growth factor, platelet-derived growth factor and OA (Blom et al., 1994; Dennison et al., 1994; Safadi et al., 2001, 2003; Peng et al., 2002; Owen et al., 2003; Song et al., 2007). However, the BMPs are thought to be the most potent inducers of osteoblastic differentiation in mesenchymal progenitor cells (Haidar et al., 2009a). OA acts downstream of BMP-2, and our results indicate that OA may have similar osteoinductive effects to BMP-2.
Commercially available BMP-2- and -7-containing products deliver BMP doses of tens of milligrams per application, whereas naturally occurring BMPs comprise 0.1% of total bone weight, indicating that they exist in concentrations on the order of several micrograms per kilogram of bone (Urist et al., 1983; Aono et al., 1995). BMP therapeutic doses in preclinical and clinical trials varied by as much as one hundred-fold (Salgado et al., 2004). The relative inefficiency of in vivo BMP applications is thought to be due to the solubility of BMPs, and their rapid clearance and degradation. Moreover, the supraphysiologic dosages of BMPs used in current osteoinductive therapies are thought to contribute to the marked local inflammation associated with these therapies, as well as local bone resorption thought to be due to osteoclast activation secondary to induction of BMP inhibitors (Haidar et al., 2009a). These limitations of current osteoinductive protein therapy provide a rationale for the study of other osteoinductive growth factors, such as OA, that may be used alone or co-administered with BMPs to enhance the biologic activity of BMPs.
Immune stimulation has also been demonstrated with the in vivo use of BMPs, presumably due to the bovine collagen carriers approved for BMP-2 and -7 applications (Haidar et al., 2009b). BMP gene therapy is in its infancy as temporal and spatial control of growth factor expression is not currently possible with existing gene therapy methods. Consequently the development of delivery systems that retain the growth factor(s) at the defect site for duration of time pertinent to repair is an ongoing pursuit.
BMP sustained delivery is thought to more closely recapitulate natural healing, and has been shown to result in improved bone regeneration as compared to bolus delivery. Studies have demonstrated that sustained BMP delivery results in the formation of coarse, trabecular bone that is ideal for load-bearing, while bolus delivery results in the formation of thin, lace-like bone (Gazit et al., 1999; Lieberman et al., 1999). Woo et al. compared calvarial defect healing in rabbits using bolus and sustained delivery of identical doses of BMP-2 with a biodegradable carrier. In the animals implanted with the sustained release carrier, 75–79% of the defect area was repaired with new bone, as compared to 45% defect healing with immediate-release implant use (Woo et al., 2001).
A number of BMP carriers and delivery systems have been studied including natural polymers (collagen, fibrin glue, alginate, chitosan, hyaluronic acid, and gelatin), inorganic materials (calcium phosphate cements, bioactive glasses, hydroxyapatite (HA), tricalcium phosphates, metals, ceramics, and calcium sulfate), synthetic polymers (polymers and co-polymers of α-hydroxy esters) and composite materials (collagen-HA and titanium-l-polylactic acid) (Haidar et al., 2009b). The characteristics of the ideal growth factor carrier as described by Geiger et al. (2003) are listed in Table 2. While no ideal growth factor carrier currently exists, several release-controlled delivery systems have been studied including crosslinked collagen and gelatin, nanoparticles consisting of BMP-containing liposomes coated with natural occurring polymers, and injectable self-assembling peptide-amphiphile hydrogels (Haidar et al., 2009a). Release-controlled systems can be either diffusion-controlled, chemical and/or enzymatic reaction-controlled, or a combination of both (Haidar et al., 2009a). In this study, we used a crosslinked gelatin construct with both diffusion-controlled and degradation-controlled properties because of its relatively low cost and ease of production.
TABLE 2.
Characteristics of the ideal growth factor carrier adapted from Geiger et al. (2003)
| Biocompatibility, low immunogenicity and antigenicity |
| Biodegradability with biocompatible components, in predictable manner in concert with bone growth |
| Adequate porosity for cellular invasion and vascularization |
| Adequate compressive and tensile strength |
| Enhancement of cellular attachment, but without inducing fibrous tissue growth at the bone/carrier interface |
| Amenability to sterilization without loss of properties |
| Affinity to growth factor and host bone |
| Enhancement of osteogenic activity with a restrictive release of growth factor at an effective dose during a period coincident with the accumulation and proliferation of target cells |
| Adaptability to irregular wound site, malleability |
| Availability to surgeon on short notice |
Like Raiche and Puleo, we were able to demonstrate slow release of fluorescein-labeled lysozyme (Fig. 1) (Raiche and Puleo, 2001), rhBMP-2 (Fig. 3A, Raiche and Puleo, 2004), and rmOA (Fig. 4A) from our gelatin-based hydrogels, but not release of the rhBMP-2/7 heterodimer. While it is possible that rhBMP-2/7 crosslinking with the gelatin matrix of the hydrogels may have prevented its release, it is more likely that the BMP-2 and BMP-7 ELISA assays (data not shown) used to detect the released rhBMP-2/7 recognized hidden epitopes in the heterodimer that are not hidden in the homodimers. It may also be that lysozyme was more readily released from the constructs than the growth factors because of its enzymatic activity and ability to degrade the crosslinked gelatin constructs.
Unlike Raiche and Puleo (2004), we were not able to demonstrate significant osteoblastic differentiation of the C3H10T1/2 mesenchymal cells grown on the slow-release gelatin hydrogels. This decreased osteoblastic differentiation in the progenitor cells grown on slow release hydrogels most likely reflected insufficient exposure of the mesenchymal cells to growth factor due to the sink conditions used to maintain release from the hydrogels. This was especially true of the hydrogels crosslinked with 2 or 5 mM glutaraldehyde (Figs. 3B and 4B), as they did not maintain their integrity as long as the hydrogels crosslinked with 20 mM glutaraldehyde (Fig. 5A). Our growth factor-loaded hydrogels crosslinked with glutaraldehyde concentrations between 2 and 10 mM did not maintain their integrity in the presence of mesenchymal cells for more than 1 week. The effect of growth factor wash-out on exposure of cells to the growth factors and resultant osteoblastic differentiation is evident when comparing the experimental groups in Figure 4B to the 100 ng/ml group in Figure 5A, where the gels were loaded with the same amount of rmOA, but differentially crosslinked. The glutaraldehyde concentrations used to crosslink our gelatin hydrogels (2–20 mM) were similar to that used by Raiche and Puleo (2004) (4 mM), as were our rhBMP-2 loading doses (0.75–2 μg in our studies; 0.938–3.75 μg). Interestingly, more differentiated cells were noted on the rmOA-loaded hydrogels than on the rhBMP-2-loaded hydrogels despite the smaller loading doses of rmOA, again indicating that OA has comparable osteoinductive effects to BMP-2. The control samples contained some OA secreted by C3H10T1/2 cells presumably due to the constitutive excretion of BMP-2 by this cell line (Phimphilai et al., 2006).
In animal studies release-controlled delivery systems, including gelatin hydrogels, are advantageous because of their ability to retain growth factors at the application site (Yamamoto et al., 2003; Haidar et al., 2009a). However, the effective rhBMP-2 loading doses demonstrated using controlled-release gelatin hydrogels is relatively high (0.938–5 μg) (Yamamoto et al., 2003; Raiche and Puleo, 2004) as compared to bolus dosing. These studies indicate that the use of simple biological polymers such as crosslinked gelatin hydrogels may not effectively deliver adequate doses of bone growth factors to achieve bone healing in a cost-effective manner. In addition, cellular responses to slow-release growth factor administration have to be better understood in order to withdraw growth factors in a timely fashion so as to prevent the induction of growth factor inhibitors (Leclerc et al., 2004; Rifas 2007; Haidar et al., 2009a). The use of other controlled-release growth factor delivery methods, such as inducible viral vectors or the combination of bolus administration with subsequent sustained release of growth factors may prove to be more efficacious for osseous tissue engineering and deserve further study.
Our bolus dosing growth factor titration experiments demonstrated that the lower dose of rhBMP-2 (1 μg/ml) resulted in the best ALP staining at 21 days (data not shown). This is in accordance with previous studies indicating that BMP-2 is able to induce osteoblastic differentiation in C3H10T1/2 cells and C2C12 murine myoblasts at bolus doses between 100 and 1000 ng/ml (Wang et al., 1993; Katagiri et al., 1994; Lee et al., 1999; Ikeda et al., 2000). rmOA dosing up to100 ng/ml resulted in a dose-dependent increase in ALP staining at 14 and 21 days, while doses equal to or greater than 500 ng/ml were toxic to cells. Little to no ALP staining was evident in the C3H10T1/2 mesenchymal cells at 7 days, whether or not they were treated with growth factors. However, the ROS 17/2 osteosarcoma cells demonstrated strong ALP staining even at 7 days. Matrix mineralization as demonstrated by von Kossa staining supported the ALP staining assays.
Though increased ALP staining in the growth factor-treated progenitor cells was not evident at 1 week, increased ALP activity could be demonstrated in the cells treated with BMP-2 and OA as compared to the negative controls at 7 days (Fig. 7). While the ALP activity in the OA-treated cells was similar to that of the BMP-2-treated cells, ALP activity in the BMP-2 treated cells was statistically greater than that in the negative control. This apparent difference in osteoinductive capacity between OA and BMP-2 can be explained by the difference in dosing of the growth factors in this experiment (100 ng/ml for both cytokines, which is 7.81 pM for rhBMP-2 and 1.25 pM for rmOA). Despite this difference in dosing, expression of ALP mRNA between cells treated with BMP-2 and OA was equivalent at day 7, and greater in the BMP-2-treated cells only at day 21, indicating that OA even at a sixfold lower dose had comparable effects on ALP expression as BMP-2. This was also true of osteocalcin mRNA expression (Fig. 8B). These results support our hypothesis that OA has comparable osteoinductive effects to BMP-2 in mesenchymal cells.
Acknowledgments
This study was supported by an American Academy of Facial Plastic and Reconstructive Surgery Leslie Bernstein grant and a Temple University Junior Faculty grant to Dr. Arosarena.
Contract grant sponsor: American Academy of Facial Plastic Reconstructive Surgery Foundation.
Literature Cited
- Abdelmagid SM, Barbe MF, Arango-Hisijara I, Owen TA, Popoff SN, Safadi FF. Osteoactivin acts as a downstream mediator of BMP-2 effects on osteoblast function. J Cell Physiol. 2007;210:26–37. doi: 10.1002/jcp.20841. [DOI] [PubMed] [Google Scholar]
- Abe H, Uto H, Takami Y, Hasuike S, Kodama M, Nagata K, Moriuchi A, Numata M, Ido A, Tsubouchi H. Transgenic expression of osteoactivin in the liver attenuates hepatic fibrosis in rats. Biochem Biophys Res Commun. 2007;356:610–615. doi: 10.1016/j.bbrc.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Adema GJ, de Boer AJ, Vogel AM, Loenen WA, Figdor CG. Molecular characterization of the melanocyte lineage-specific antigen gp100. J Biol Chem. 1994;269:20126–20133. [PubMed] [Google Scholar]
- American Academy of Orthopaedic Surgeons Musculoskeletal injuries. The burden of musculoskeletal diseases in the United States: Prevalence, societal and economic cost. 2008:123–162. http://boneandjointburden.org/
- Anderson MG, Smith RS, Savinova OV, Hawes NL, Chang B, Zabaleta A, Wilpan R, Heckenlively JR, Davisson M, John SW. Genetic modification of glaucoma associated phenotypes between AKXD-28/Ty and DBA/2J mice. BMC Genet. 2001;2:1. doi: 10.1186/1471-2156-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Libby RT, Mao M, Cosma IM, Wilson LA, Smith RS, John SW. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006;4:20. doi: 10.1186/1741-7007-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun. 1995;210:670–677. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- Arden RL, Rachel JD, Marks SC, Dang K. Volume-length impact of lateral jaw resections on complication rates. Arch Otolaryngol Head Neck Surg. 1999;125:68–72. doi: 10.1001/archotol.125.1.68. [DOI] [PubMed] [Google Scholar]
- Bandari PS, Qian J, Yehia G, Joshi DD, Maloof PB, Potian J, Oh HS, Gascon P, Harrison JS, Rameshwar P. Hematopoietic growth factor inducible neurokinin-1 type: A transmembrane protein that is similar to neurokinin 1 interacts with substance P. Regul Pept. 2003;111:169–178. doi: 10.1016/s0167-0115(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Blackwell KE, Lacombe V. The bridging lateral mandibular reconstruction plate revisited. Arch Otolaryngol Head Neck Surg. 1999;125:988–993. doi: 10.1001/archotol.125.9.988. [DOI] [PubMed] [Google Scholar]
- Blom S, Holmstrup P, Dabelsteen E. A comparison of the effect of epidermal growth factor, platelet-derived growth factor, and fibroblast growth factor on rat periodontal ligament fibroblast-like dells' DNA synthesis and morphology. J Periodontol. 1994;65:373–378. doi: 10.1902/jop.1994.65.5.373. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Richmond B, Huizing M, Helip-Wooley A, Zhao Y, Koshoffer A, Gahl WA. Melanocyte-specific proteins are aberrantly trafficked in melanocytes of Hermansky-Pudlak syndrome-type 3. Am J Pathol. 2005;166:231–240. doi: 10.1016/S0002-9440(10)62247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JB, Mulholland RS, Davidson J, Gullane PJ, Rotstein LE, Brown DH, Freeman JE, Irish JC. The free flap and plate in oromandibular reconstruction: Long-term review and indications. Plast Reconstr Surg. 1995;95:1018–1028. doi: 10.1097/00006534-199505000-00010. [DOI] [PubMed] [Google Scholar]
- Brunberg E, Andersson L, Cothran G, Sandberg K, Mikko S, Lindgren G. A missense mutation in PMEL17 is associated with the Silver coat color in the horse. BMC Genet. 2006;7:46. doi: 10.1186/1471-2156-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Sato K, Dougherty II, Cruz PD, Jr., Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007;109:4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroot J. Carriers that concentrate native bone morphogenetic protein in vivo. Tissue Eng. 1998;4:337–341. doi: 10.1089/ten.1998.4.337. [DOI] [PubMed] [Google Scholar]
- Dennison DK, Vallone DR, Pinero GJ, Rittman B, Caffesse RG. Differential effect of TGF-β1 and PDGF on proliferation of periodontal ligament cells and gingival fibroblasts. J Periodontol. 1994;65:641–648. doi: 10.1902/jop.1994.65.7.641. [DOI] [PubMed] [Google Scholar]
- Diefenderfer DL, Osyczka AM, Garino JP, Leboy PS. Regulation of BMP-induced transcription in cultured human bone marrow stromal cells. J Bone Jt Surg Am. 2003;85-A:19–28. doi: 10.2106/00004623-200300003-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disher MJ, Esclamado RM, Sullivan MJ. Indications for the AO plate with a myocutaneous flap instead of revascularized tissue transfer for mandibular reconstruction. Laryngoscope. 1993;103:1264–1268. doi: 10.1288/00005537-199311000-00009. [DOI] [PubMed] [Google Scholar]
- Garcia-Godoy F, Murray PE. Status and potential commercial impact of stem cell-based treatments on dental and craniofacial regeneration. Stem Cells Dev. 2006;15:881–887. doi: 10.1089/scd.2006.15.881. [DOI] [PubMed] [Google Scholar]
- Gazit D, Turgeman G, Kelley P, Wang E, Jalenak M, Zilberman Y, Moutsatsos I. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: A novel cell-mediated gene therapy. J Gene Med. 1999;1:121–133. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<121::AID-JGM26>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Goding JW. Monoclonal antibodies: Principles and practice. Academic Press; Orlando: 1986. Immunofluorescence; pp. 241–280. [Google Scholar]
- Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A. Current challenges in BMP delivery. Biotechnol Lett. 2009a;31:1817–1824. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B. Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009b;31:1825–1835. doi: 10.1007/s10529-009-0100-8. [DOI] [PubMed] [Google Scholar]
- Helip-Wooley A, Westbroek W, Dorward HM, Koshoffer A, Huizing M, Boissy RE, Gahl WA. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5. J Invest Dermatol. 2007;127:1471–1478. doi: 10.1038/sj.jid.5700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hing KA. Bone repair in the twenty-first century: Biology, chemistry, or engineering? Philos Trans R Soc Lond A. 2004;362:2821–2850. doi: 10.1098/rsta.2004.1466. [DOI] [PubMed] [Google Scholar]
- Hoashi T, Muller J, Vieira WD, Rouzaud F, Kikuchi K, Tamaki K, Hearing VJ. The repeat domain of the melanosomal matrix protein PMEL17/GP100 is required for the formation of organellar fibers. J Biol Chem. 2006;281:21198–21208. doi: 10.1074/jbc.M601643200. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Hachisu R, Yamaguchi A, Gao YH, Okano T. Radiation retards muscle differentiation but does not affect osteoblastic differentiation induced by bone morphogenetic protein-2 in C2C12 myoblasts. Int J Radiat Biol. 2000;76:403–411. doi: 10.1080/095530000138745. [DOI] [PubMed] [Google Scholar]
- Jager E, Maeurer M, Hohn H, Karbach J, Jäger D, Zidianakis Z, Bakhshandeh-Bath A, Orth J, Neukirch C, Necker A, Reichert TE, Knuth A. Clonal expansion of Melan A-specific cytotoxic T lymphocytes in a melanoma patient responding to continued immunization with melanoma-associated peptides. Int J Cancer. 2000;86:538–547. doi: 10.1002/(sici)1097-0215(20000515)86:4<538::aid-ijc16>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan CT, Wakiya K, Dowell JM, Herndon JE, II, Reardon DA, Graner MW, Riggins GJ, Wikstrand CJ, Bigner DD. Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme. Clin Cancer Res. 2006;12:1970–1982. doi: 10.1158/1078-0432.CCR-05-2797. [DOI] [PubMed] [Google Scholar]
- Leclerc N, Luppen CA, Ho VV, Nagpal S, Hacia JG, Smith E, Frenkel B. Gene expression profiling of glucocorticoid-inhibited osteoblasts. J Mol Endocrinol. 2004;33:175–193. doi: 10.1677/jme.0.0330175. [DOI] [PubMed] [Google Scholar]
- Lee M, Javed A, Kim H, Shin HI, Gutierrez S, Choi JY, Rosen V, Stein JL, van Wijnen AJ, Stein GS, Lian JB, Ryoo HM. Transient upregulation of CBFA1 in response to bone morphogenetic protein-2 and transforming growth factor β1 in C2C12 myogenic cell coincides with suppression of the myogenic phenotype but is not sufficient for osteoblast differentiation. J Cell Biochem. 1999;73:114–125. [PubMed] [Google Scholar]
- Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, Kabo JM, Finerman GA, Berk AJ, Witte ON. The effect of regional gene therapy with bone morphogenetic protein-2 producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Ishii A, Ohata C, Komurasaki T. Early induction of osteoactivin expression in rat renal tubular epithelial cells after unilateral ureteral obstruction. Exp Toxicol Pathol. 2007;59:53–59. doi: 10.1016/j.etp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Nikawa T, Furochi H, Kosyoji M, Hirasaka K, Suzue N, Sairyo K, Nakano S, Yamaoka T, Itakura M, Kishi K, Yasui N. Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am J Physiol Cell Physiol. 2005;289:C697–C707. doi: 10.1152/ajpcell.00565.2004. [DOI] [PubMed] [Google Scholar]
- Okamoto I, Pirker C, Bilban M, Berger W, Losert D, Marosi C, Haas OA, Wolff K, Pehamberger H. Seven novel and stable translocations associated with oncogenic gene expression in malignant melanoma. Neoplasia. 2005;7:303–311. doi: 10.1593/neo.04514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaga M, Ido A, Hasuike S, Uto H, Moriuchi A, Nagata K, Hori T, Hayash K, Tsubouchi H. Osteoactivin expressed during cirrhosis development in rats fed a choline-deficient, L-amino acid-defined diet, accelerates motility of hepatoma cells. J Hepatol. 2003;39:779–785. doi: 10.1016/s0168-8278(03)00361-1. [DOI] [PubMed] [Google Scholar]
- Ott C, Iwanciw D, Graness A, Giehl K, Goppelt-Struebe M. Modulation of the expression of connective tissue growth factor by alterations of the cytoskeleton. J Biol Chem. 2003;278:44305–44311. doi: 10.1074/jbc.M309140200. [DOI] [PubMed] [Google Scholar]
- Owen TA, Smock SL, Prakash S, Pinder L, Brees D, Krull D, Castleberry TA, Clancy YC, Marks SC, Jr., Safadi FF, Popoff SN. Identification and characterization of the genes encoding human and mouse osteoactivin. Crit Rev Eukaroyt Gene Expr. 2003;13:205–220. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.130. [DOI] [PubMed] [Google Scholar]
- Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–646. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack VA, Alvarez E, Tse KF, Torgov MY, Xie S, Shenoy SG, MacDougall JR, Arrol S, Zhong H, Gerwien RW, Hahne WF, Senter PD, Jeffers ME, Lichenstein HS, LaRochelle WJ. Treatment parameters modulating regression of human melanoma xenografts by an antibody-drug conjugate (CR011-vcMMAE) targeting GPNMB. Cancer Chemother Pharmacol. 2007;60:423–435. doi: 10.1007/s00280-007-0490-z. [DOI] [PubMed] [Google Scholar]
- Raiche AT, Puleo DA. Triphasic release model for multilayered gelatin coatings that can recreate growth factor profiles during wound healing. J Drug Target. 2001;9:449–460. doi: 10.3109/10611860108998779. [DOI] [PubMed] [Google Scholar]
- Raiche AT, Puleo DA. Cell responses to BMP-2 and IGF-I released with different time-dependent profiles. J Biomed Mater Res. 2004;69A:342–350. doi: 10.1002/jbm.a.30006. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Bone morphogenetic proteins and skeletal development: The kidney-bone connection. Pediatr Nephrol. 2000;14:598–601. doi: 10.1007/s004670000364. [DOI] [PubMed] [Google Scholar]
- Rifas L. The role of noggin in human mesenchymal stem cell differentiation. J Cell Biochem. 2007;100:824–834. doi: 10.1002/jcb.21132. [DOI] [PubMed] [Google Scholar]
- Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol. 2007;178:6557–6566. doi: 10.4049/jimmunol.178.10.6557. [DOI] [PubMed] [Google Scholar]
- Risteli L, Risteli J. Biochemical markers of bone metabolism. Ann Med. 1993;25:385–393. doi: 10.3109/07853899309147301. [DOI] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Rico MC, Owen TA, Popoff SN. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J Cell Biochem. 2001;84:12–26. doi: 10.1002/jcb.1259. [DOI] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, Marks SC, Jr., Owen TA, Popoff SN. Expression of connective tissue growth factor in bone: Its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: State of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- Selim AA. Osteoactivin bioinformatic analysis: Prediction of novel functions, structural features, and modes of action. Med Sci Monit. 2009;15:MT19–MT33. [PubMed] [Google Scholar]
- Shikano S, Bonkobara M, Zukas PK, Ariizumi K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J Biol Chem. 2001;276:8125–8134. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- Song JJ, Aswad R, Kanaan RA, Rico MC, Owen TA, Barbe MF, Safadi FF, Popoff SN. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-β1 to induce mesenchymal cell condensation. J Cell Physiol. 2007;210:398–410. doi: 10.1002/jcp.20850. [DOI] [PubMed] [Google Scholar]
- Spector JA, Luchs JS, Mehrara BJ, Greenwald JA, Smith LP, Longaker MT. Expression of bone morphogenetic proteins during membranous bone healing. Plast Reconstr Surg. 2001;107:124–134. doi: 10.1097/00006534-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Toriumi DM, Kotler HS, Luxenberg DP, Holtrop ME, Wang EA. Mandibular reconstruction with a recombinant bone-inducing factor. Arch Otolaryngol Head Neck Surg. 1991;117:1101–1112. doi: 10.1001/archotol.1991.01870220049009. [DOI] [PubMed] [Google Scholar]
- Tse KF, Jeffers M, Pollack VA, McCabe DA, Shadish ML, Khramtsov NV, Hackett CS, Shenoy SG, Kuang B, Boldog FL, MacDougall JR, Rastelli L, Herrmann J, Gallo M, Gazit-Bornstein G, Senter PD, Meyer DL, Lichenstein HS, LaRochelle WJ. CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res. 2006;12:1373–1382. doi: 10.1158/1078-0432.CCR-05-2018. [DOI] [PubMed] [Google Scholar]
- Urist MR, DeLange RJ, Finerman GAM. Bone cell differentiation and growth factors. Science. 1983;220:680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- Weterman MA, Ajubi N, van Dinter IM, Degen WG, van Muijen GN, Ruitter DJ, Bloemers HP. Nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- Woo BH, Fink BF, Page R, Schrier JA, Jo YW, Jiang G, DeLuca M, Vasconez HC, DeLuca PP. Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm Res. 2001;18:1747–1753. doi: 10.1023/a:1013382832091. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Takahashi Y, Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375–4383. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- Yasumoto K, Watabe H, Valencia JC, Kushimoto T, Kobayashi T, Appella E, Hearing VJ. Epitope mapping of the melanosomal matrix protein gp100 (PMEL17): Rapid processing in the endoplasmic reticulum and glycosylation in the early Golgi compartment. J Biol Chem. 2004;279:28330–28338. doi: 10.1074/jbc.M401269200. [DOI] [PubMed] [Google Scholar]