Abstract
Background
Chronic rhinosinusitis is an important public health problem with substantial impact on patient quality of life and health care costs. We hypothesized that genetic variation may be one factor that affects this disease.
Objective
To identify genetic variation underlying susceptibility to chronic rhinosinusitis using a genome-wide approach.
Methods
We studied a religious isolate that practices a communal lifestyle and shares common environmental exposures. Using physical examination, medical interviews, and a review of medical records, we identified 8 individuals with chronic rhinosinusitis out of 291 screened. These 8 individuals were related to each other in a single 60 member, 9 generation pedigree. A genome-wide screen for loci influencing susceptibility to chronic rhinosinusitis using 1123 genome-wide markers was conducted.
Results
The largest linkage peak (P = 0.0023; 127.15 cM, equivalent to LOD=2.01) was on chromosome 7q31.1-7q32.1, 7q31 (127.15 cM; 1-LOD support region: 115cM to 135cM) and included the CFTR locus. Genotyping of 38 mutations in the CFTR gene did not reveal variation accounting for this linkage signal.
Conclusion
Understanding the genes involved in chronic rhinosinusitis may lead to improvements in its diagnosis and treatment. Our results represent the first genome-wide screen for chronic rhinosinusitis and suggest that a locus on 7q31.1-7q32.1 influences disease susceptibility. This may be the CFTR gene or another nearby locus.
Keywords: Chronic rhinosinusitis, Genetics, Linkage, Mapping, Polymorphism, Cystic fibrosis
INTRODUCTION
Chronic rhinosinusitis (CRS) is an important public health problem in the United States, with a substantial impact on the health care system and patient quality of life. A wide variety of factors are thought to contribute to CRS1. Prior work suggests that genetic factors may affect the development and presentation of CRS and related phenotypes2-4. CRS is associated with several well-characterized inherited disorders including Primary Ciliary Dyskinesia (PCD). Therefore, we employed a genetic perspective to gain information on this disease process.
Here, we report the first genome-wide screen for loci underlying susceptibility to CRS in the Hutterites, a founder population of European ancestry that lives on communal farms across the western United States and Canada. This population has several important advantages for genetic mapping studies including reduced genetic heterogeneity, a communal lifestyle which reduces confounding from environmental variables; and extensive experience with complex trait mapping in this population including successful identification of genes for related phenotypes such as asthma5-7.
METHODS
Sample Composition
In March, 2001, we conducted a population-based study of 291 Hutterites who were the subject of prior studies on the genetics of asthma8,9. Inclusion criteria for the CRS study consisted of the following: age over 13 years, ability to comply with the questionnaire, and presence in the colony (communal farm) on the day of the study visit. Informed consent was obtained and this study was approved by our Institutional Review Board.
Assessment of CRS
We developed a survey containing closed-and open-ended questions regarding sinonasal and medical history. These questionnaires were administered through interviews during the field trips and elicited information about the morbidities for sinonasal disease and nasal symptoms (congestion, postnasal drip, facial pressure, facial pain, headache, and nasal obstruction). The presence of atopy, a factor thought to influence CRS, was determined by skin prick testing (SPT) with 14 common allergens performed during prior visits to the colonies9. Subjects with symptoms consistent with CRS underwent anterior rhinoscopy and were consented for review of their medical records to search for history, physical findings, and imaging consistent with CRS. Nasal endoscopy was not possible due to logistical considerations.
To minimize the heterogeneity, CRS was defined as presence of CRS symptoms, a physician's diagnosis of CRS, and significant mucosal thickening on CT scan. This definition meets consensus clinical criteria. We excluded subjects with acquired causes (facial trauma), and subjects with confounding illness causing their disease (Cystic Fibrosis (CF), PCD, Young's syndrome, immunodeficiency, autoimmune disease, allergic fungal sinusitis or a history of sinonasal malignancy).
Genotyping
DNA was extracted from whole blood by standard methods. Genotyping of 658 autosomal microsatellite markers (screening sets 9 and 51) was performed by the Mammalian Genotyping Service (MGS) in Marshfield, Wisconsin (http://research.marshfieldclinic.org/genetics/Genotyping_Service/mgsver2.htm). An additional 226 microsatellites and 239 SNPs or insertion/deletions were genotyped in our laboratory in these individuals in selected regions according to standard methods. The 1123 microsatellite and SNP markers have an average spacing of 2.6 cM. The deCODE genetic map was used for reference in this analysis (www.decode.com)10.
In follow-up studies of the Cystic fibrosis transmembrane conductance regulator (CFTR) gene, 38 polymorphisms were genotyped using a version of the Linear Array (Roche Molecular Systems, Alameda, CA). The M1101K variant was genotyped using fluorescence polarization-single base extension.
Linkage Analysis
A multipoint non-parametric linkage (NPL) analysis was performed using Simwalk 2.8311, which uses a Markov Chain Monte Carlo approach to sample possible allele descent trees according to their likelihood. Three identity by descent (IBD) statistics that measure the degree of allele sharing among affected individuals were calculated. Stat E, equivalent to the Kong and Cox NPLall statistic as calculated in Genehunter-plus12, measures whether founder-alleles are overly represented in the affecteds, and is intended to detect linkage to traits under an additive model. Stat A and B calculate the number of founder-alleles contributing alleles to the affecteds, and the largest number of affecteds inheriting an allele from one founder-allele, respectively. The former performs better under recessive models, while the latter performs better under dominant models. P values were determined by comparison to a null distribution of these statistics when only the pedigree structure and the affection status of each person is available.
RESULTS
Phenotyping and Demographics
291 subjects participated in our study (117 males and 174 females; mean age ±SE, 35.2 ±0.97 years (range 14-78)). Based on interviews, we performed anterior rhinoscopy on 138 of these individuals and then requested medical records from 26 subjects. Of those, 8 subjects met criteria for CRS. This prevalence reflects our criteria which required imaging and is lower that those obtained in studies using self-report with its attendant inaccuracy. We note, however, that because our linkage analyses consider only affected individuals, underdiagnosing the phenotype will not comprise our results, although it does reduce our power. The remaining subjects had a variety of diagnoses (e.g., allergic rhinitis). Among the 8 subjects with CRS, there were 3 females and 5 males, ranging in age from 21 to 50 years (mean ±SE, 33 years±3.5 years). Four subjects were atopic (SPT positive ≥1 allergen) and the mean total IgE for the complete 8 subjects was 191.8 (range 8.8 – 953.5) (clinical details in Table 1). Three subjects were asthmatic and one demonstrated bronchial hyperreactivity by methacholine challenge.
Table 1.
Demographics/Clinical Characteristics of Subjects with CRS.
| Subject | Age | Sex | Asthma Status | Total IgE | SPT | Medical History | Medications |
|---|---|---|---|---|---|---|---|
| 1 | 50 | F | Normal | 31.0 | Mugwort, Cockroach | History of sinus disease, treated in the past with intranasal steroids, antibiotics, mucolytics | Premarin |
| 2 | 27 | M | Normal | 953.5 | Dust mite, cat, dog, alternaria, cockroach, rye grass, ragweed, mugwort | History of sinus disease, treated in the past with antibiotics, systemic steroids, antihistamine; prior immunotherapy | Claritin |
| 3 | 39 | M | Asthma | 31.9 | Negative | History of sinus disease, treated in the past with intranasal steroids, nasal saline; endoscopic sinus surgery recommended | “Antibiotic |
| 4 | 23 | M | Asthma | 34.4 | Negative | History of sinus disease, treated in the past with antibiotics, intranasal steroids; underwent endoscopic sinus surgery | “Antibiotic” |
| 5 | 32 | M | Normal | 21.8 | Cockroach, ragweed | History of sinus disease, gastroesophageal reflux; treated with antihistamines, intranasal steroids; underwent endoscopic sinus surgery | Aciphex |
| 6 | 21 | F | BHR | 74.0 | Cockroach | History of sinus disease, treated in the past with intranasal steroids; underwent endoscopic sinus surgery | |
| 7 | 42 | M | Normal | 8.8 | Negative | History of sinus disease, treated in the past with intranasal steroids, mucolytics, hypertonic saline; underwent endoscopic sinus surgery | |
| 8 | 33 | F | Asthma | 378.8 | Ragweed | History of sinus disease, deep venous thrombosis, diabetes | Coumadin, Aspirin |
BHR: bronchial hyperreactivity. SPT: Skin prick test.
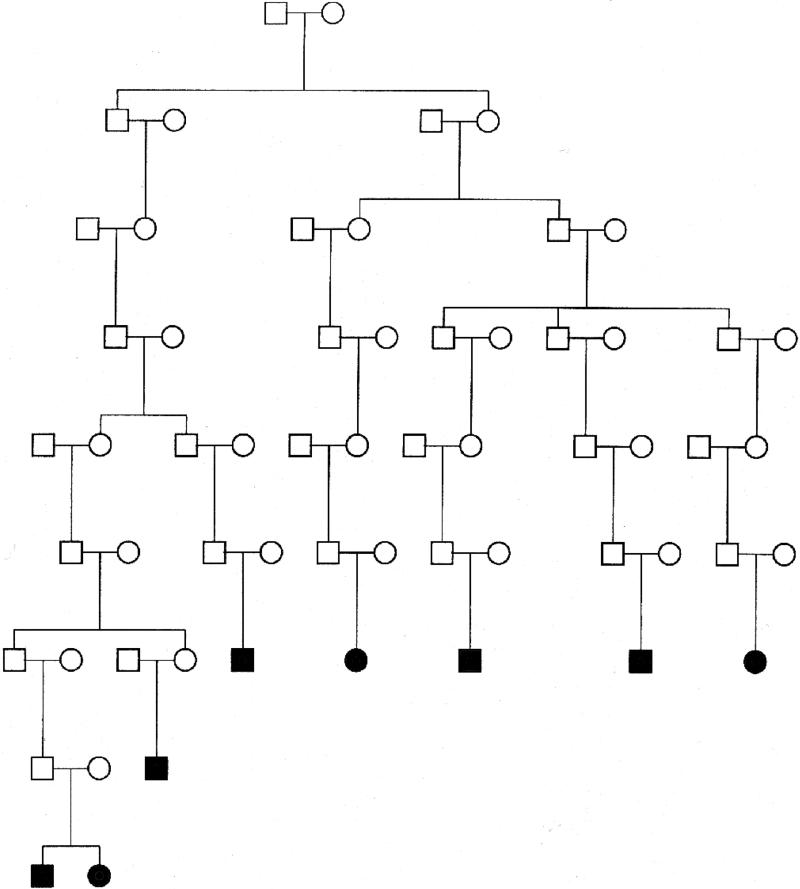
We then constructed a 60-member pedigree using PedHunter13, which was the smallest pedigree that included the 8 affected individuals. Because of the limitations of the mapping program Simwalk2, we broke all of the inbreeding loops in the pedigree, and conducted our mapping studies in this simplified, extended pedigree (Figure 1). Breaking inbreeding loops can result in loss of power and leads to an underestimation of the evidence for linkage14.
Figure 1.
Pedigree of subjects with CRS and their ancestors.
Linkage Analysis
NPL analysis is a powerful tool for genetic mapping15, since it is independent of specific models for the inheritance of the phenotype. It is based only on the probabilities that affected individuals share marker alleles identical by descent (IBD), i.e., inherited from a common ancestor, at the marker loci. If a marker is linked to a disease locus, one expects to see a clustering among the affected individuals of a few marker alleles descended from the pedigree founders.
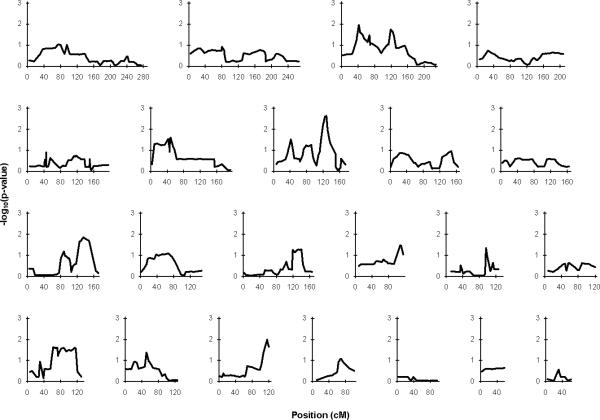
The P-values for the NPLall statistic across the 22 autosomes are plotted in Figure 2. The NPLpair results were virtually indistinguishable from the NPLall results and therefore are not shown. Nine of the twenty-two autosomes yielded regions with nominally significant evidence for linkage to CRS at a threshold of P<0.05 (Figure 2), with only the two largest linkage peaks (chromosome 7 and 18) showing significance at P<0.01. After these two, the linkage peaks in decreasing order of significance are found on chromosomes, 3, 10, 16, 6, 13, 17, and 14.
Figure 2.
P-values for Statistic E (NPLall) on a –log10 scale for each of the 22 autosomes.
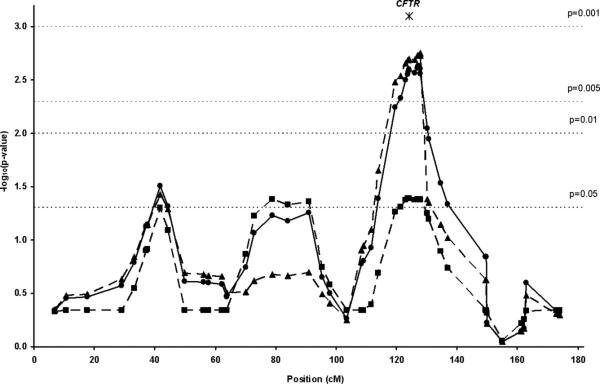
The largest linkage peak was observed on chromosome 7q31.1-7q32.1 with marker ttta001 (from the Marshfield screening set) at 127.15 cM from p-ter (P=0.0023) (Figure 3), nearly an order of magnitude lower than the next largest linkage signal on chromosome 18 (D18S1009, 116.2 cM, P= 0.010). This suggests that the most important locus in the Hutterites is on 7q31.1-7q32.1, and we focused our subsequent attention on this region. It should be noted that estimates of the empirical P-value using Simwalk2 were found to be conservative of the true P-value when compared with exact results for large simulated pedigrees where the exact results were known, and for pedigrees small enough so that exact P-values could be calculated11. Therefore, we are likely to be underestimating the true significance of our findings in these analyses.
Figure 3.
Linkage for susceptibility to CRS for chromosome 7. Statistic A (triangles) is the number of different founder-alleles contributing alleles to the affected individuals (best used to detect linkage to a recessive trait). Statistic B (squares) is the maximum number of alleles among the affected individuals descended from any one founder-allele (best used to detect linkage to a dominant trait). Statistic E (circles) is the extent of allele sharing among all affected individuals, equivalent to the NPLall statistic as implemented in GeneHunter15 (intended to detect linkage to traits in an additive model). The reduction in significance with Statistic B compared to the other statistics suggests that the 7q locus does not act in a dominant manner in the Hutterites. Position in cM according to the Decode genetic map is shown on the X-axis and –log10 (P- value) of the NPLall statistic on the Y-axis. Note: the position of the CFTR gene is 124.5 cM.
To define a critical region for further investigation, we set a P<0.01 significance threshold boundary of 118-131cM (Figure 4), corresponding to a 20Mb physical distance mapping to the ~108-128Mb position of chromosome 7 (NCBI Build 36.2). This region contains 96 genes (64 known genes, 32 hypothetical genes), and includes the CFTR gene.
CFTR as a Candidate Locus
Because this gene has been previously implicated in CRS in patients without CF and because CRS is a common finding in patients with CF, we conducted further studies in this gene. Neither of the only two known CF mutations present in this population (ΔF508 and M1101K)16,17 were present in the 8 affected individuals. One individual with CRS was heterozygous for both the PolyT variant (7/9) and the M470V variant (A/G), but homozygous for the normal allele at all other mutated sites on the array (Table 2). The remaining 7 sinusitis subjects were homozygotes for the wild type allele for all variants on the array.
Table 2.
CFTR genotypes.
| Subject | PolyT | M470V | ||
|---|---|---|---|---|
| 1 | 7/9 | A/G | ||
| 2 | 7/7 | A/A | ||
| 3 | 7/7 | A/A | ||
| 4 | 7/7 | A/A | ||
| 5 | 7/7 | A/A | ||
| 6 | 7/7 | A/A | ||
| 7 | 7/7 | A/A | ||
| 8 | 7/7 | A/A | ||
| Allele Frequency | 7 | 9 | A | G |
| 8 Sinusitis Subjects | 0.938 | 0.0625 | 0.938 | 0.0625 |
| Hutterite Population | 0.903 | 0.0970 | 0.662 | 0.338 |
DISCUSSION
CRS is a complex disorder with both environmental and genetic influences playing a role in disease development and expression. Several studies have reported associations between polymorphisms and CRS or related phenotypes, including in candidate genes in the HLA region18-20, the IL1A gene21, the LTC4S and PAI1 genes22, the TGFB1 gene23, and the IL1RA gene24. Functional effects of any of these findings are unclear.
We utilized a well characterized population with advantages for mapping genes that influence susceptibility to CRS. While several genomic regions showed nominally significant evidence for linkage, the strongest evidence for linkage to CRS occurs at 7q31.1-7q32.1 suggesting that a locus in this region influences disease susceptibility. In prior studies, nearly all of the common alleles that are present in the outbred European population are also present in the Hutterites at the same relative frequencies and show similar associations with common diseases, emphasizing the potential to generalize from these data.
In our study the largest linkage signal was near the CFTR gene. This ion channel could affect the development of CRS is through regulation of the inflammatory cascade25. Hence, the CFTR gene is an excellent positional and functional candidate locus. Prior work has demonstrated that the proportion of CRS patients with a CFTR mutation was higher compared to controls (7% vs. 2%, P=0.04)26. Furthermore, homozygotes for M470V (GG) were over-represented in the remaining sinusitis patients. Similar results have been reported in some27 but not all other studies28. Though these studies demonstrate an association with CRS, none of them identified conclusively the causal variant. Interestingly, a recent report described abnormal ion transport in CF heterozygotes in nasal epithelium; 3 of these patients had CRS, indicating that functional variation in this locus may be associated with clinical effects29.
Since there were only two known CFTR mutations in this founder population, and both of those were excluded as contributing to CRS in the Hutterites, we genotyped our sample for additional polymorphisms in the CFTR gene. One individual with CRS was a heterozygote for both the PolyT variant (7/9) which influences splicing and mRNA levels30 and the M470V variant (A/G) that has been associated with CRS26, but the remaining CRS subjects were homozygotes for the wild type allele for these and all other polymorphisms tested. Interestingly, the allele frequencies for both the PolyT variant and the M470V SNP differed from the population as a whole (Table 2), raising the possibility that haplotype differences reflecting other, unknown variation in this gene may underlie our linkage peak. The wide spectrum of severity in CF and the presence of a large number of mutations have led to a broad effort to understand the correlation between genotype and phenotype, especially in regards to diseases that extend beyond the classic definitions of CF. Our data is consistent with a concept of CRS as one of these manifestations, though extensive analysis of the largeCFTR locus, which harbors a large number of rare variants, would be necessary to confirm this speculation.
In summary, our genome-wide screen for CRS susceptibility identified a locus on 7q31.1-7q32.1. Although we cannot yet exclude unidentified variation in the CFTR gene as risk alleles for CRS, there are 96 known or hypothetical genes in the linked region that are potential candidates for involvement in this common condition.
Capsule Summary.
We utilized a population with advantages for gene mapping to perform the first genome wide search for loci that influence susceptibility to chronic rhinosinusitis. Our data suggest that a locus on 7q31.1-7q32.1 influences disease susceptibility.
ACKNOWLEDGEMENTS
CF genotyping arrays were a gift from Roche Molecular Sciences (Alameda, CA). Technical assistance was provided by Rebecca Anderson, Natasha Phillips, Robert Stanaker, Megan Burkhardt, Harvey Dytch, Erika Sorensen, Marcy DeTineo, Jamie Phillips, Justin Nguyen, and Darrel Waggonner, MD. Nancy J. Cox, PhD, provided helpful discussion. We thank Rodney Parry, MD, (University of South Dakota) for assistance with field trips and the Hutterites for their participation. JMP is supported by an American Geriatrics Society/Dennis W. Jahnigen Scholars Award.
Supported by the following NIH Grants: T32 DC 00058-04 (JMP), T32 HL 007605 (MGH), HL56399 (CO), HL66533 (CO), M01 RR00055 (The University of Chicago General Clinical Research Center), and HV-48141 (Mammalian Genotyping Service).
Abbreviations
- CRS
Chronic rhinosinusitis
- PCD
Primary ciliary dyskinesia
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- SPT
Skin prick testing
- MGS
Mammalian Genotyping Service
- NPL
Non-parametric linkage
- IBD
Identity by descent
- SE
standard error
REFERENCES
- 1.Hamilos DL. Chronic sinusitis. J Allergy Clin Immunol. 2000;106:213–227. doi: 10.1067/mai.2000.109269. [DOI] [PubMed] [Google Scholar]
- 2.Cutting GR. Genetics of Rhinosinusitis. In: Kennedy D, J Z, editors. Diseases of the Sinuses: Diagnosis and Management. BC Decker; Ontario: 2001. [Google Scholar]
- 3.Settipane GA. Epidemiology of nasal polyps. Allergy Asthma Proc. 1996;17:231–236. doi: 10.2500/108854196778662246. [DOI] [PubMed] [Google Scholar]
- 4.Cohen NA, Widelitz JS, Chiu AG, Palmer JN, Kennedy DW. Familial aggregation of sinonasal polyps correlates with severity of disease. Otolaryngol Head Neck Surg. 2006;134:601–604. doi: 10.1016/j.otohns.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Abney M, Ober C, McPeek MS. Quantitative-trait homozygosity and association mapping and empirical genomewide significance in large, complex pedigrees: fasting serum-insulin level in the Hutterites. Am J Hum Genet. 2002;70:920–934. doi: 10.1086/339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ober C, Pan L, Phillips N, Parry R, Kurina LM. Sex-specific genetic architecture of asthma-associated quantitative trait loci in a founder population. Curr Allergy Asthma Rep. 2006;6:241–246. doi: 10.1007/s11882-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 7.Ober C, Tan Z, Sun Y, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ober C, Tsalenko A, Willadsen S, et al. Genome-wide screen for atopy susceptibility alleles in the Hutterites. Clin Exp Allergy. 1999;29(Suppl 4):11–15. [PubMed] [Google Scholar]
- 9.Ober C, Tsalenko A, Parry R, Cox NJ. A second-generation genomewide screen for asthma-susceptibility alleles in a founder population. Am J Hum Genet. 2000;67:1154–1162. doi: 10.1016/s0002-9297(07)62946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong A, Gudbjartsson DF, Sainz J, et al. A high-resolution recombination map of the human genome. Nat Genet. 2002;31:241–247. doi: 10.1038/ng917. [DOI] [PubMed] [Google Scholar]
- 11.Sobel E, Lange K. Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker-sharing statistics. Am J Hum Genet. 1996;58:1323–1337. [PMC free article] [PubMed] [Google Scholar]
- 12.Kong A, Cox NJ. Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet. 1997;61:1179–1188. doi: 10.1086/301592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwala R, Biesecker LG, Hopkins KA, Francomano CA, Schaffer AA. Software for constructing and verifying pedigrees within large genealogies and an application to the Old Order Amish of Lancaster County. Genome Res. 1998;8:211–221. doi: 10.1101/gr.8.3.211. [DOI] [PubMed] [Google Scholar]
- 14.Dyer TD, Blangero J, Williams JT, Goring HH, Mahaney MC. The effect of pedigree complexity on quantitative trait linkage analysis. Genet Epidemiol. 2001;21(Suppl 1):S236–243. doi: 10.1002/gepi.2001.21.s1.s236. [DOI] [PubMed] [Google Scholar]
- 15.Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES. Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet. 1996;58:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- 16.Gallego Romero I, Ober C. CFTR mutations and reproductive outcomes in a population isolate. Hum Genet. 2008;122:583–588. doi: 10.1007/s00439-007-0432-1. [DOI] [PubMed] [Google Scholar]
- 17.Zielenski J, Fujiwara TM, Markiewicz D, et al. Identification of the M1101K mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and complete detection of cystic fibrosis mutations in the Hutterite population. Am J Hum Genet. 1993;52:609–615. [PMC free article] [PubMed] [Google Scholar]
- 18.Takeuchi K, Majima Y, Shimizu T, Ukai K, Sakakura Y. Analysis of HLA antigens in Japanese patients with chronic sinusitis. Laryngoscope. 1999;109:275–278. doi: 10.1097/00005537-199902000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi K, Majima Y, Sakakura Y. Tumor necrosis factor gene polymorphism in chronic sinusitis. Laryngoscope. 2000;110:1711–1714. doi: 10.1097/00005537-200010000-00027. [DOI] [PubMed] [Google Scholar]
- 20.Schubert MS, Hutcheson PS, Graff RJ, Santiago L, Slavin RG. HLA-DQB1 *03 in allergic fungal sinusitis and other chronic hypertrophic rhinosinusitis disorders. J Allergy Clin Immunol. 2004;114:1376–1383. doi: 10.1016/j.jaci.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Karjalainen J, Joki-Erkkila VP, Hulkkonen J, et al. The IL1A genotype is associated with nasal polyposis in asthmatic adults. Allergy. 2003;58:393–396. doi: 10.1034/j.1398-9995.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- 22.de Alarcon A, Steinke JW, Caughey R, et al. Expression of leukotriene C4 synthase and plasminogen activator inhibitor 1 gene promoter polymorphisms in sinusitis. Am J Rhinol. 2006;20:545–549. doi: 10.2500/ajr.2006.20.2934. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Park HS, Holloway JW, Shin HD, Park CS. Association between a TGFbeta1 promoter polymorphism and rhinosinusitis in aspirin-intolerant asthmatic patients. Respir Med. 2007;101:490–495. doi: 10.1016/j.rmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Cheng YK, Lin CD, Chang WC, et al. Increased prevalence of interleukin-1 receptor antagonist gene polymorphism in patients with chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2006;132:285–290. doi: 10.1001/archotol.132.3.285. [DOI] [PubMed] [Google Scholar]
- 25.Koehler DR, Downey GP, Sweezey NB, Tanswell AK, Hu J. Lung inflammation as a therapeutic target in cystic fibrosis. Am J Respir Cell Mol Biol. 2004;31:377–381. doi: 10.1165/rcmb.2004-0124TR. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Moylan B, Leopold DA, et al. Mutation in the gene responsible for cystic fibrosis and predisposition to chronic rhinosinusitis in the general population. Jama. 2000;284:1814–1819. doi: 10.1001/jama.284.14.1814. [DOI] [PubMed] [Google Scholar]
- 27.Coste A, Girodon E, Louis S, et al. Atypical sinusitis in adults must lead to looking for cystic fibrosis and primary ciliary dyskinesia. Laryngoscope. 2004;114:839–843. doi: 10.1097/00005537-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Hytonen M, Patjas M, Vento SI, et al. Cystic fibrosis gene mutations deltaF508 and 394delTT in patients with chronic sinusitis in Finland. Acta Otolaryngol. 2001;121:945–947. doi: 10.1080/000164801317166835. [DOI] [PubMed] [Google Scholar]
- 29.Sermet-Gaudelus I, Dechaux M, Vallee B, et al. Chloride transport in nasal ciliated cells of cystic fibrosis heterozygotes. Am J Respir Crit Care Med. 2005;171:1026–1031. doi: 10.1164/rccm.200406-740OC. [DOI] [PubMed] [Google Scholar]
- 30.Chillon M, Casals T, Mercier B, et al. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995;332:1475–1480. doi: 10.1056/NEJM199506013322204. [DOI] [PubMed] [Google Scholar]