Abstract
Invasion and proliferation in neoplasia require the cooperation of tumor cell and endothelial compartments. Glycogen synthase kinase-3 (GSK-3) is increasingly recognized as a major contributor to signaling pathways that modulate invasion and proliferation. Here we show that GSK-3 inhibitors of the indirubin family reduce invasion of glioma cells and glioma-initiating cell-enriched neurospheres both in vitro and in vivo, and we show that β-catenin signaling plays an important role in mediating these effects. Indirubins improved survival in glioma-bearing mice in which a substantial decrease in blood vessel density was seen in treated animals. In addition, indirubins blocked migration of endothelial cells, suggesting that anti-invasive glioma therapy with GSK-3 inhibitors in vivo not only inhibits invasion of tumor cells, but blocks migration of endothelial cells, which is also required for tumor angiogenesis. Overall, our findings suggest that indirubin inhibition of GSK-3 offers a novel treatment paradigm to target 2 of the most important interacting cellular compartments in heterotypic models of cancer.
Introduction
The formation of a cancerous mass requires contributions not only by neoplastic cells, but also by the stroma, which interact to cooperatively contribute to processes of tumorigenesis, such as proliferation and invasion/migration (1). Glioblastoma is a heterogeneous tumor composed primarily of tumor and endothelial cells, with some immune cells including microglia (2, 3). Glioblastoma cells extensively invade normal brain (4), which contributes to the continued poor prognosis for these tumors by preventing complete surgical resection. Invading tumor cells are resistant to conventional therapies (5) and invasiveness is enhanced by antiangiogenic approaches (6). Thus, an effective strategy to prevent invasion of glioma cells into surrounding normal brain is needed.
Recent studies have implicated glycogen synthase kinase-3 (GSK-3), a multifunctional serine-threonine protein kinase (7), in the regulation of cell motility in many cell types including astrocytes (8) and glioma cells in vitro (9). Two closely related isoforms, GSK-3α and GSK-3β, function in multiple proliferation- and migration-associated pathways including Wnt, Notch, growth factor, and G-protein–coupled receptor signaling. GSK-3 has many known substrates, including β-catenin, which is targeted for ubiquitylation and proteasomal degradation as a result of phosphorylation by GSK-3 (10). Targeting of GSK-3 for anti-invasive therapy has not yet been investigated in a relevant animal model of glioma. Here we show the beneficial effects of GSK-3 inhibitors of the indirubin family which act on both tumor cells blocking invasion and on endothelial cells blocking angiogenesis, providing a novel therapeutic paradigm for glioma treatment targeting tumor invasion and angiogenesis simultaneously.
Materials and Methods
Antibodies and reagents
Antibodies used were rabbit anti–GSK-3β (Cell Signaling Technology), mouse anti–phospho-GSK-3 (pY279/pY216) clone 5G-2F (Millipore), mouse anti–β-catenin (BD), and mouse anti-β-actin (Sigma-Aldrich). Peroxidase-conjugated secondary antibodies were from The Jackson Laboratories. Indirubins were from Calbiochem. Actinomycin D and LiCl were from Sigma-Aldrich.
Cell culture
Glioma cell lines were from American Type Culture Collection, primary human glioma GBM9 cells were derived from a human glioblastoma specimen and grown as tumor spheres (9). X12 glioma cells passaged as s.c. xenografts in nude mice were from Dr. C. David James (University of California, San Francisco, CA; ref. 10).
Transfection and cell-based assays
β-Catenin siRNA HP5 (Qiagen) was transfected as described (11). Spheroid and transwell assays were carried out as described (11). β-catenin reporter plasmid pSuper8XTOPflash or pSuper8XFOPflash (from Dr. Randall Moon, University of Washington, Seattle, WA; ref. 12) was used as described (11).
In vivo studies
For flank tumors, 1 × 106 Gli36 cells were injected into the rear flank of 5-week-old nude mice (National Cancer Institute). Twelve days later, vehicle [dimethyl sulfoxide (DMSO)/PBS] or 6-bromoindirubin acetoxime (BIA; 1 mg/kg in DMSO/PBS) was administered intraperitoneally every 2 days. Flank growth was monitored every 2 days with calipers to estimate tumor volume. Intracranial xenografts were done with X12, U87-ΔEGFR (a gift from Webster Cavenee, University of California, San Diego, CA), or GBM9-GFP cell lines. A total of 100,000 viable cells were stereotactically injected 2 mm right lateral, 1 mm frontal to the bregma (3 mm deep). On day 7, post-tumor injection animals began a dosing regimen of either vehicle (DMSO/PBS) or BIA (0.5 mg/kg or 1.0 mg/kg) every other day. For invasion studies, this dosing regimen was started on day 2 after injection.
Tissue preparation
Brains were harvested and placed in 4% paraformaldehyde for 24 hours, then in 30% sucrose for 48 hours. Tissue was embedded in Optimal Tissue Cutting Compound (OCT) and sectioned at 20 µm. Sections were then stained with hematoxylin and eosin and rat anti-mouse CD31 (Pharmingen). Sections were treated with 3% H2O2 in PBS for 10 minutes and washed in PBS. Slides were then blocked with the Elite Universal Vectastain ABC Blocking Kit (Vectorlabs) and treated with 10% normal goat serum and 0.5% Triton in PBS for 1 hour at room temperature, followed by Rat anti-CD31 (Pharmingen) diluted 1:20 in 0.5% Triton/PBS overnight at 4°C. The next day, staining was visualized using biotinylated goat anti-mouse IgG, diluted 1:200 in 0.5% Triton in PBS, followed by treatment with the Avidin–Biotin Blocking Kit (Vectorlabs). Stain was developed with DAB and costained with Eosin-Y, for 1 to 2 minutes.
Image processing
Ten images were selected and 10 areas measured from rim of tumor to furthest point migrated using ImageJ software.
Statistical analysis
Two sample t tests were used for all the continuous variables to compare experimental with control groups and pairwise comparisons were adjusted (Bonferroni's method). Statistically significant differences (adjusted P < 0.05) are marked with a single asterisk. Differences (adjusted P < 0.01) are marked with a double asterisk. For Fig. 4D an ANOVA model was fit using cell number as the outcome and the treatments as the independent variable. All treatments were compared with the control and P -values adjusted for multiple comparisons using Dunnet's method. For Kaplan–Meier curves (survival analysis, Fig. 3D), the log-rank method was used.
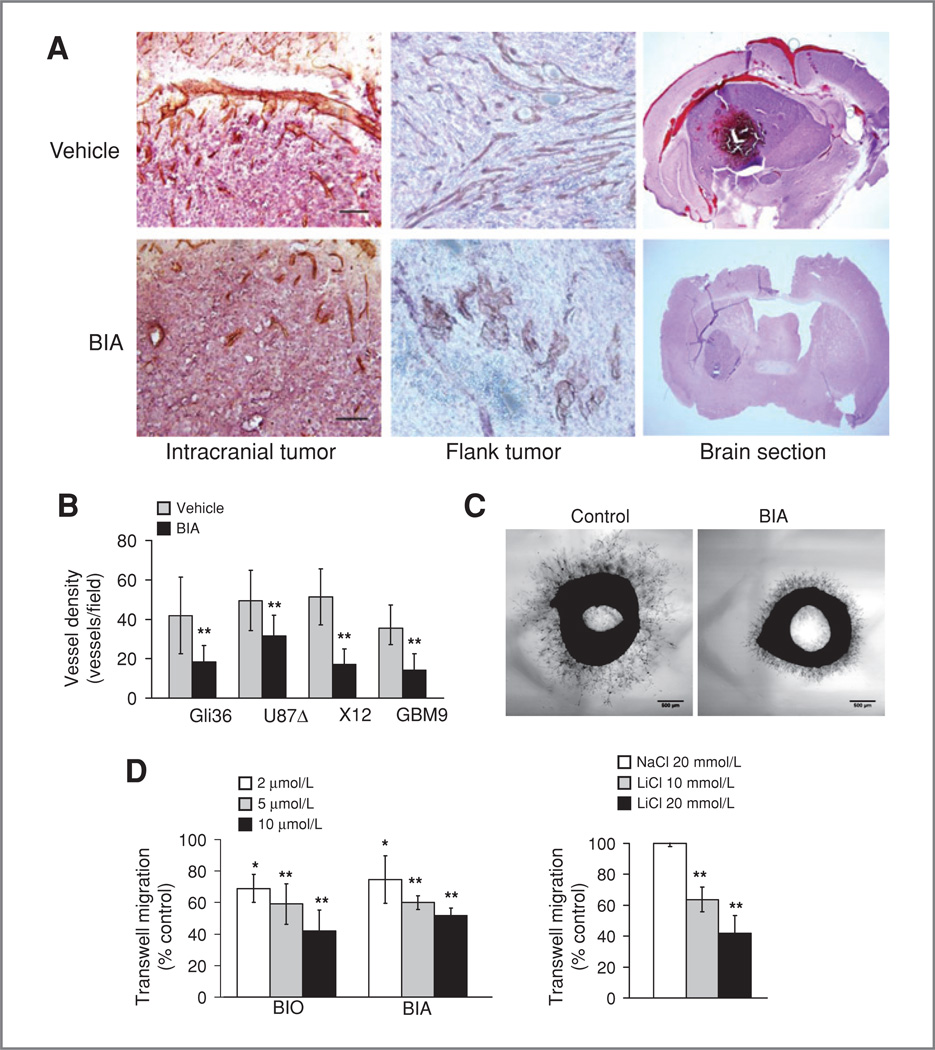
Figure 4.
Effects of BIA on angiogenesis. A, altered intratumoral blood vessel morphology in treated tumors (left), in flank tumors (middle), and in the hemorrhagic nature of tumor-bearing brains (right). B, reduced blood vessel density in 4 separate tumor models (Gli36 was quantitated from the flank, the rest were intracranial). C, indirubins reduce migration of rat endothelial cells in the aortic ring assay. D, left, indirubins reduce migration of human umbilical vein endothelial cells (HUVEC) in a transwell assay. Right, reduction of HUVEC cell motility by LiCl in the transwell assay.
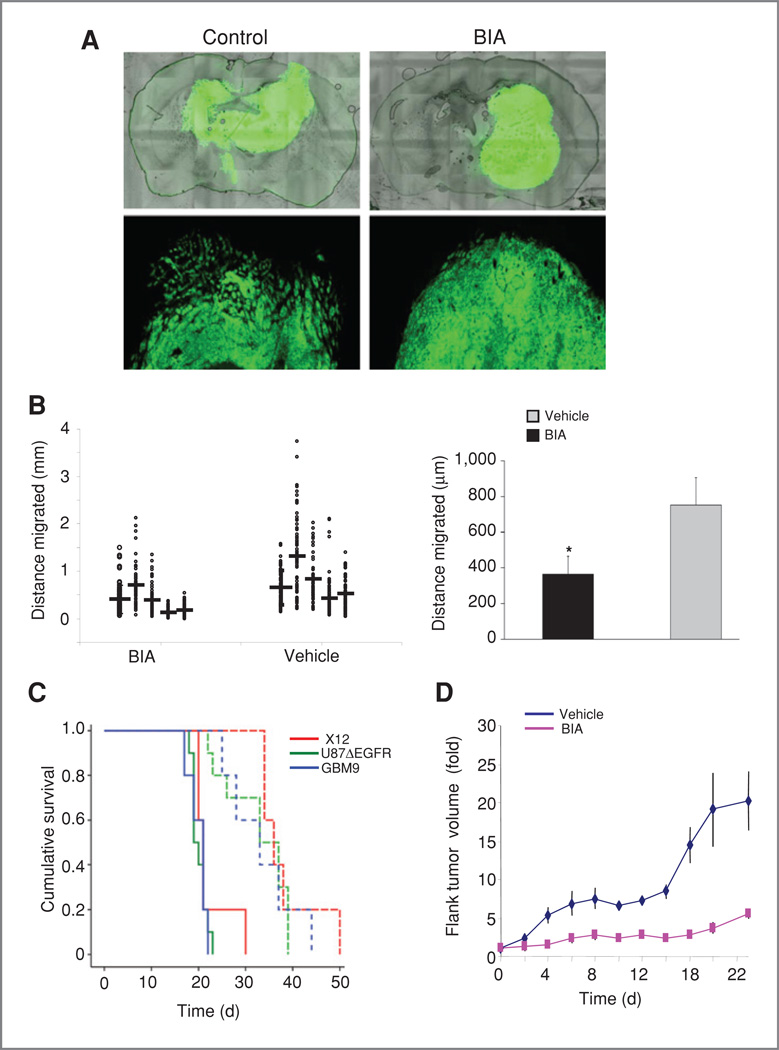
Figure 3.
BIA treatment blocks glioma growth and invasion in vivo. A, GBM9 GFP neurospheres were implanted intracranially and analyzed 14 days after implantation for invasiveness in BIA- or vehicle-treated mice. Photographs illustrate reduced invasion after BIA treatment at low (top) and high magnification (bottom). B, quantitation of invasiveness of intracranial GBM9 cells. Left, multiple tumors were examined and invasion measured in distance from the edge of the main tumor to the most distantly invaded cell around the circumference of the tumor. Each individual measure for each tumor is shown as a separate point. Mean migration for each tumor is shown by the horizontal bar. Right, combined migration data from the tumor measurements. C, Kaplan-Meier survival curves showing increased survival of intracranial tumor-bearing mice for 3 different glioma cell lines (vehicle-solid lines, BIA-dashed lines). D, measurement of tumor growth of U87ΔEGFR glioma cells implanted in the flanks of nude mice.
Results and Discussion
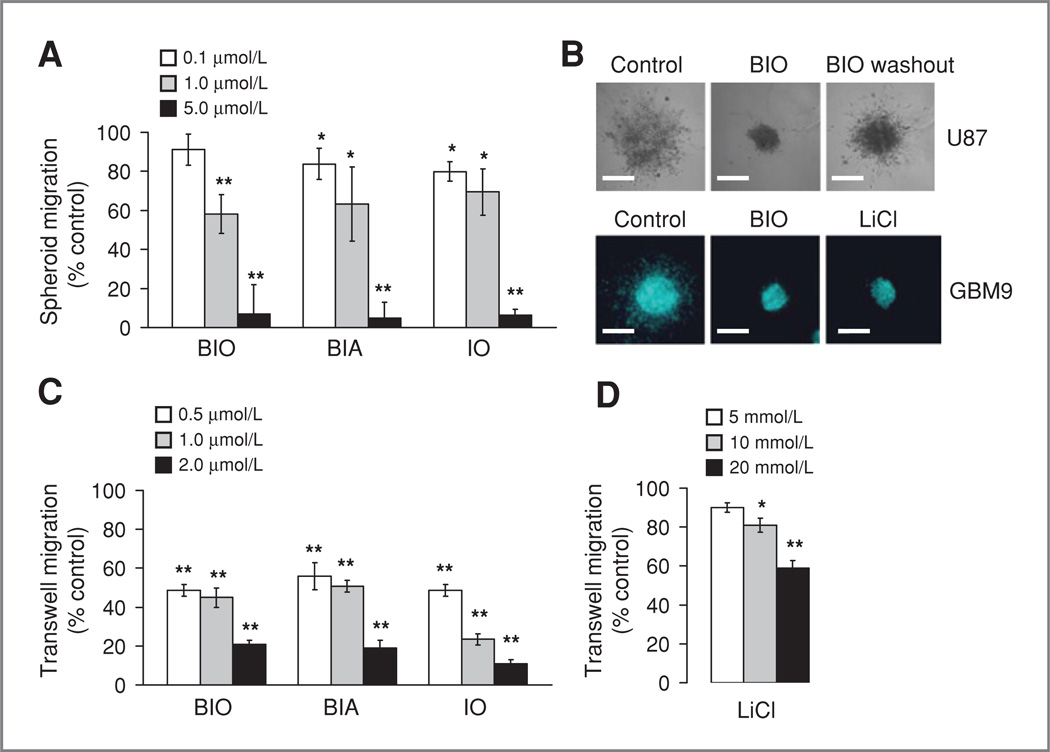
We found that indirubins, previously shown to be highly potent GSK-3 inhibitors with low nanomolar in vitro IC50 values (13, 14), blocked glioma migration at low concentrations compared with other compounds (data not shown). Indirubins were identified as the active component of the Chinese medicine Dang Gui Long Hui Wan, which has activity against leukemia (15). We examined 3 indirubin derivatives, 6-bromoindirubin-oxime (BIO), BIA, and indirubin 3′-oxime (10) in 3-dimensional (3-D) spheroid migration assays using U87 glioma cells. A similar dose-dependent blockade of migration was observed for each drug, with complete inhibition at 5 µmol/L (Fig. 1A; 1 µmol/L BIO reduced migration to 58.1% vs. vehicle control, P < 0.001; 5 µmol/L BIO-7.1% migration, P < 0.001: 1 µmol/L BIA–63.2%, P = 0.006, 5 µmol/L BIA-4.9%, P < 0.001; 1 µmol/L IO–69.5%, P = 0.09, 5 µmol/L IO-6.3%, P < 0.001). This is approximately 10-fold lower than the concentrations previously reported for other GSK-3 inhibitors and 4,000-fold lower than LiCl (11). When drug was washed from the spheres after 48 hours incubation, migration resumed (Fig. 1B), indicating a specific blockade of migration and not a general toxic effect. Migration of GBM9 glioblas-toma-derived neurosphere-initiating cells was also blocked by BIO (Fig. 1B), and experiments on U251 glioma cells in a transwell assay showed a dose-dependent reduction in migration (Fig. 1C; 0.5 µmol/L BIO reduced migration to 48.7% vs. vehicle control, P < 0.001, 2 µmol/L BIO–21.0%, P < 0.001; 0.5 µmol/L BIA–55.9%, P< 001,2 µmol/L BIA–19.1%, P< 0.001; 0.5 µmol/L IO–48.6%, P < 0.001; 2 µm IO–11%, P < 0.001). 1-Methyl-BIO (MeBIO), an indirubin derivative with negligible GSK-3 inhibitory activity (IC50 = 100 µmol/L), did not affect migration (data not shown). The effects of indirubins occur at lower concentrations than previously examined GSK-3 inhibitors and seem to be more pronounced, as seen in transwell assays (Fig. 1D; 5 mmol/L LiCl–90% migration, P = 0.216; 20 mmol/L LiCl–59%, P < 0.001).
Figure 1.
Indirubins potently block migration of glioma cells. A, treatment of U87 cells in the spheroid migration assay with BIO, BIA, or IO. Migration was measured after 48 hours with each drug at the concentrations shown. Migration is expressed as sphere perimeter related to DMSO-treated controls. B, spheroids pictured after treatment with BIO. Top: U87 spheroids after 48 hours in the spheroid assay. Control was treated with DMSO, BIO was used at 5 µmol/L, and migration 48 hours after washout is shown (right). The bottom panel shows a similar experiment done with a glioma neurosphere line (GBM9) in the presence of DMSO (control), BIO (5 µmol/L), or LiCl (20 mmol/L). Bar = 500 µm. C, effects of indirubins on U251 glioma cell migration in the transwell assay. Cells were treated with drugs at the concentrations indicated for 1 hour prior to transwell assays. Migration is shown relative to DMSO or NaCI-treated controls. D, cell viability was measured over a range of indirubin concentrations in U251 glioma cells. Cells were treated with drugs for 48 hours and viability measured with the Wst1 assay.
The effects of indirubins on cell migration were specific—only after 48 hours of treatment at high doses (10–50 µmol/L) with BIO, BIA, or IO was there a significant reduction in cell viability (Supplementary Fig. S1A). Cell proliferation with 5 µmol/L drug was not affected until 72 hours (Supplementary Fig. S1B). Treatment with MeBIO had no effect on cell viability in the concentration range tested. This shows that indirubins are relatively specific in their blockade of migration, which is observed very rapidly at low concentrations, with effects on cell viability observed only after prolonged exposure or at higher drug concentrations.
We used the TCF/LEF reporter plasmid TOPflash (12) as a reporter of β-catenin transcriptional coactivation in response to GSK-3 inhibition. This showed reporter activation after 24-hour treatment with BIO in 3 glioblastoma lines (Fig. 2A). GSK-3 inhibition with 20 mmol/L LiCl also led to an increase in TOP flash activity, but this was approximately 6-fold lower than with BIO (U87 cells–BIO RLU 6.0-fold higher than LiCl; U251 cells; GBM9 cells–7.9-fold. Adjusted P < 0.001 for each condition).
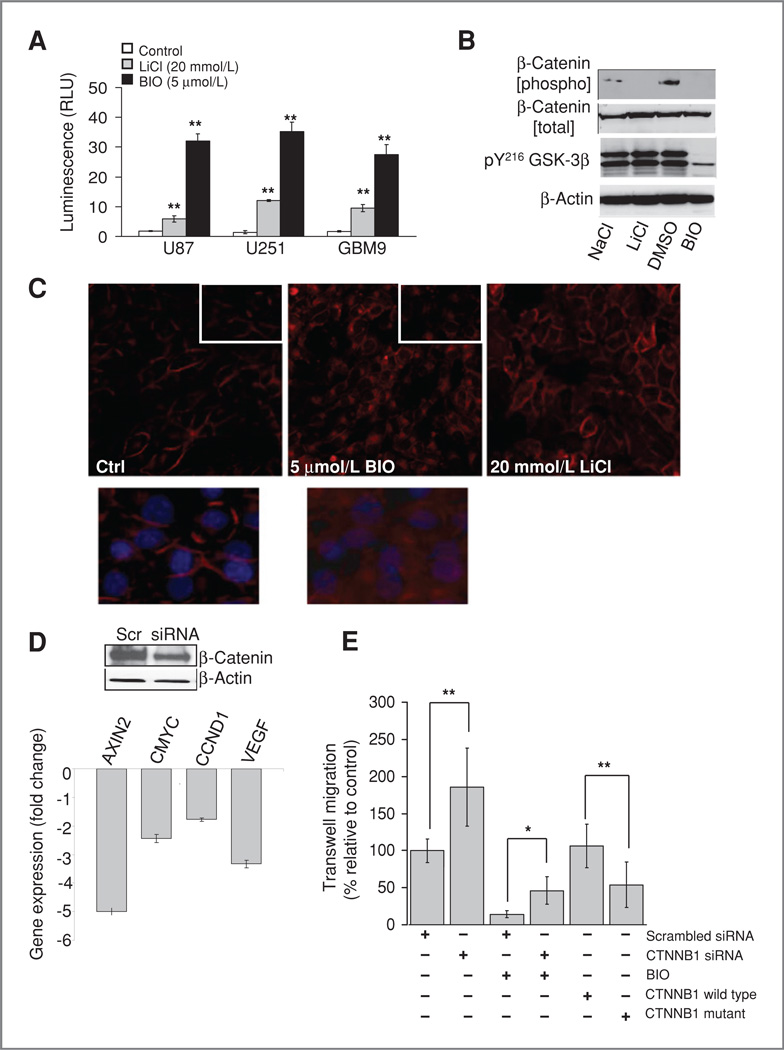
Figure 2.
β-Catenin contributes to the antimigratory effect of BIO. A, β-catenin transcriptional coactivation was measured by using the TOP-flash luciferase reporter vector transfected into glioma cell lines. Luminescence is expressed relative to controls after 24-hour drug treatment. B, phosphorylation of β-catenin (pS33,37, T41) and tyrosine phosphorylation of GSK-3 isoforms was measured by Western blotting 24 hours after treatment with 5 µmol/L BIO or 20 mmol/L LiCl. C, U251 cells were fixed and stained for β-catenin after 1 hour of drug treatment. Inset shows altered localization of β-catenin in BlO-treated cells at high magnification. D, Western blotting showed a reduction of β-catenin levels 48 hours after treatment with siRNA. Quantitative real-time-PCR showed a corresponding decrease in the levels of known β-catenin responsive genes. E, β-catenin levels affect glioma cell migration, β-catenin siRNA treatment leads to increased glioma invasion and partially rescues the effects of BIO in a transwell assay. Conversely overexpression of phospho-mutated β-catenin slows migration.
We observed a blockade of β-catenin phosphorylation after 24-hour treatment with either BIO (5 µmol/L) or LiCl (20 mmol/L). However, a reduction of the tyr216 phosphorylation of GSK-3β was only seen after BIO treatment, in contrast with LiCl (Fig. 2B). BIO also caused a rapid (1 hour) translocation of β-catenin (Fig. 2C), which was not seen with LiCl. These data suggest that BIO leads to more robust GSK-3 inactivation than LiCl.
β-catenin is not well studied in glioma: we therefore examined the effects of β-catenin knockdown on glioma cell migration. This led to almost a doubling of the migration of U251 in transwell assays compared with controls (P = 0.003; Fig. 2D), suggesting that GSK-3 activation promotes glioma migration through phosphorylation and downregulation of β-catenin. Knockdown of β-catenin led to a partial rescue of the antimigratory effects of BIO increasing migration 3-fold (P = 0.031). This suggests that GSK-3 inhibition and resulting β-catenin stabilization plays a role in the inhibition of migration by BIO. Conversely, glioma migration was reduced 50% by overexpression of mutated β-catenin, with its GSK-3 phosphorylation sites abolished (P = 0.002; Fig. 2D). Further support for a role for β-catenin in glioma migration was provided by studies which showed a large reduction in cell–cell adhesion after siRNA knockdown (Supplementary Fig. S2A). Finally, we analyzed β-catenin phosphorylation over a time course in a 3-D spheroid migration assay. This showed that over the course of the assay, β-catenin phosphorylation by GSK-3 increased (Supplementary Fig. S2B). Taken together, these data support a role for GSK-3 in glioma migration through regulation of β-catenin.
To determine whether indirubins could affect glioma invasion in vivo, we examined intracranial xenografts of an invasive human glioma–derived neurosphere line (GBM9) in athymic mice. Animals were sacrificed at day 14 after tumor implantation with or without BIA treatment. Reduced levels of invasion could be seen in treated animals in terms of diffuse migration. Strikingly, migration into the contralateral hemisphere was not observed in treated animals, and only single tumor foci were seen. A representative image of this effect is shown in Fig. 3A Quantitation of invasion across multiple sections of several animals revealed a significant reduction in migration of approximately 40% compared with controls (P < 0.001; Fig. 3B).
Most strikingly, increased animal survival was observed in intracranial models of 3 different glioma cell lines; U87ΔEGFR, X12, and GBM9 tumors (P = 0.002, 0.002, and <0.001, respectively, compared with untreated controls; Fig. 3C).
To further analyze the role of BIA in increased animal survival tumor volume was examined. This revealed a potential reduction in average tumor volume, although this did not reach significance (P = 0.156). Also, there was no significant reduction in mitotic index in treated tumors. However, examination of Gli36 glioma cells grown as flank tumors showed a significant 5-fold reduction in tumor size in BIA-treated animals compared with controls at day 22 after tumor implantation (P = 0.001; Fig. 3D). The occurrence of a clear anti-proliferative effect in flank tumors may reflect the increased availability of BIA to the tumor cells compared with those grown in the brain, and the potential direct effects on tumor cells merit further study. We noticed that untreated tumors were much darker and more hemorrhagic than those treated with BIA (Fig. 4A; Supplementary Fig. S3A). This strongly suggested alterations in blood flow after drug treatment. We therefore stained tumors using the endothelial marker CD31 to identify blood vessels. This revealed a significant alteration in blood vessel density and structure in treated tumors (Fig. 4A). Vessels were shorter both intratumorally and peritumorally; however, there was no significant change in their length in the contralateral hemisphere (intratumoral–69% vessel length compared with controls, P = 0.037; peritumoral–47%, P = 0.005; normal–85%, P = 0.33; Supplementary Fig. S4B and C). Also, CD31 showed diffuse staining in controls that was not observed in treated animals, likely as a result of increased vessel leakiness. A significant reduction in angiogenesis was observed in all tumor models tested (Gli36 flank–2.3-fold reduction, P = 0.004; U87ΔEGFR intracranial–1.6-fold, P = 0.007; X12 intra-cranial–3.0-fold, P < 0.001; GBM9 intracranial–2.5-fold, P < 0.001; Fig. 4B). We therefore investigated the effects of BIO in migration assays of endothelial cells in vitro. BIA (5 µmol/L) treatment led to a significant inhibition of cell migration in aortic ring assays (P = 0.001), and indirubins inhibited migration of endothelial cells in transwell assays (Fig. 4C and D; 10 µmol/L BIO–43.2% migration vs. control, P = 0.012; BIA-52.3%, P = 0.006; 20 mmol/L LiCl 40.8%, P = 0.003). Thus, BIA has the capacity to reduce angiogenesis through its effects on endothelial cell migration. This shows that indirubin-related compounds simultaneously block 2 major hallmarks of glioblastoma—angiogenesis and invasion—through effects on cell migration.
The invasive nature of gliomas is a major barrier to their effective clinical treatment. In fact, the recent FDA approval of bevacizumab has led to a clinical reduction of glioblastoma angiogenesis and observed tumor volumes, yet therapeutic failures are common potentially because of increased migration of treated tumor cells into the brain (6). Our data show that indirubins are far more potent in blocking migration than inhibitors previously examined (11) and that this inhibition functions to improve animal survival by acting on both the glioma and endothelial cell compartment.
A reversible inhibition of migration in spheroid assays was observed at 5 µmol/L indirubin, compared with our previous study (11) which showed 20 µmol/L for LiCl, AR-A014418 (50 µmol/L), and SB415286 (25 µmol/L). These values reflect the respective IC50 value of each compound for in vitro GSK-3 inhibition (indirubins–10–22 nmol/L; LiCl–2 mmol/L; AR-A014418–104 nmol/L; SB415286–78 nmol/L; ref. 13). Indirubins were more potent than LiCl in transwell assays and in β-catenin coactivation. The reasons for this may reflect the kinetics of drug import/export, stability, and their ability to inhibit GSK-3.
GSK-3 is known to play a role in migration of several cell types, by a range of mechanisms including Rac1 activation (16), microtubule activity (17), and focal adhesion dynamics (18). Here we show for the first time that β-catenin plays a role in mediating effects of GSK-3 on glioma cell motility. This conclusion is based on several lines of evidence: (i) a substantial increase in β-catenin promoter activity and cellular localization after BIO treatment; (ii) β-catenin phosphorylation is blocked by BIO treatment; (iii) siRNA mediated β-catenin knockdown increases migration and partially rescues the effects of BIA, whereas phospho-mutated β-catenin decreases glioma cell migration; (iv) β-catenin knockdown leads to a reduction in cell–cell adhesion; and (v) β-catenin phosphorylation increases during a glioma cell migration assay. Although well studied in other tumor types, relatively little is known of the role of β-catenin in glioma (19). Studies have shown that modulation of cell–cell contacts affects cell motility in glioma cells (20). It was also recently shown that the dual-specificity phosphatase DUSP26 regulates KIF3-dependent transport of β-catenin and N-cadherin complexes to the cell periphery promoting cell–cell adhesion (21). Our data suggest that altered β-catenin regulation is at least one of the mechanisms by which GSK-3 inhibition affects glioma cell motility, although, given the multifunctional nature of GSK-3, it is likely that other mechanisms also contribute.
The use of orthotopic xenograft models revealed that intraperitoneal administration of BIA leads to an increase in animal survival in 3 separate intracranial glioma models. Reduced invasion of glioma cells was observed in the glioma-initiating cell line GBM9. The observation of a striking reduction in hemorrhage in excised brains and tumor sections from treated animals prompted us to examine tumor-associated blood vessels which were significantly reduced in number after treatment with BIA. We then found that indirubins could inhibit endothelial migration, which suggest that treated animals have reduced vasculature and tumor invasion because of antimigratory effects of the drug on both tumor and endothelial cells. Interestingly, 2 recent studies suggested GSK-3 as a target in glioblastoma because of reduced proliferation observed with GSK-3 inhibitors (22, 23). However, angiogenesis was not examined in these studies. It is also a possibility that indirubins may have direct effects on tumor cell proliferation. We showed in vitro that this occurs at higher drug concentrations than are necessary for maximal blockade of migration and with prolonged incubation (Supplementary Fig. S1). On the basis of our observations, it is most likely that the increase in animal survival observed with BIA treatment is based on a combination of effects on cell motility, which limits tumor spread, and effects on tumor-associated blood vessels, which limits blood supply, and reduces vessel leakiness. Detailed studies of molecular alterations in treated tumors will be carried out to further address these questions. Thus, indirubins are candidates from which to build a combined antiangiogenic and anti-invasive approach for glioma treatment by acting on both tumor and endothelial compartments.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by the Esther L. Dardinger Endowment for Neurooncology and Neurosciences, a minority supplement (NIH CA69246-10A1 to S.P. Williams), the Jeffrey Thomas Hayden Foundation (J. Godlewski), and an American Brain Tumor Association postdoctoral grant (M.O. Nowicki).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumors. Nat Rev Neurosci. 2007;8:620–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 4.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 5.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 6.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 7.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–76. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 9.Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, et al. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68:9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- 10.Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowicki MO, Dmitrieva N, Stein AM, Cutter JL, Godlewski J, Saeki Y, et al. Lithium inhibits invasion of glioma cells; possible involvement of glycogen synthase kinase-3. Neuro Oncol. 2008;10:690–699. doi: 10.1215/15228517-2008-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 13.Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, et al. GSK-3-selective inhibitors derived from tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 16.Farooqui AA, Ong WY, Horrocks LA. Glycogen synthase kinase-3 acts upstream of ADP-ribosylation factor 6 and Rac1 to regulate epithelial cell migration. Pharmacol Rev. 2006;58:591–620. doi: 10.1016/j.yexcr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Owen R, Gordon-Weeks PR. Inhibition of glycogen synthase kinase 3β in sensory neurons in culture alters filopodia dynamics and microtubule distribution in growth cones. Mol Cell Neurosci. 2003;23:626–637. doi: 10.1016/s1044-7431(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 18.Cai X, Li M, Vrana J, Schaller MD. Glycogen synthase kinase 3-β and extracellular signal-regulated kinase-dependent phosphorylation of paxillin regulates cytoskeletal rearrangement. Mol Cell Biol. 2006;26:2857–2868. doi: 10.1128/MCB.26.7.2857-2868.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano H, Hara A, Takenaka K, Nakatani K, Shinoda J, Shimokawa K, et al. Differential expression of beta-catenin in human glioblastoma multiforme and normal brain tissue. Neurol Res. 2000;22:650–656. doi: 10.1080/01616412.2000.11740735. [DOI] [PubMed] [Google Scholar]
- 20.Perego C, Vanoni C, Massari S, Raimondi A, Pola S, Cattaneo MG, et al. Invasive behaviour of glioblastoma cell lines is associated with altered organisation of the cadherin-catenin adhesion system. J Cell Sci. 2002;115:3331–3340. doi: 10.1242/jcs.115.16.3331. [DOI] [PubMed] [Google Scholar]
- 21.Tanuma N, Nomura M, Ikeda M, Kasugai I, Tsubaki Y, Takagaki K, et al. Protein phosphatase Dusp26 associates with KIF3 motor and promotes N-cadherin-mediated cell–cell adhesion. Oncogene. 2009;28:752–761. doi: 10.1038/onc.2008.431. [DOI] [PubMed] [Google Scholar]
- 22.Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation. Cancer Res. 2008;68:6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyashita K, Kawakami K, Nakada M, Mai W, Shakoori A, Fujisawsa H, et al. Potential therapeutic effect of glycogen synthase kinase 3beta inhibition against human glioblastoma. Clin Cancer Res. 2009;15:887–897. doi: 10.1158/1078-0432.CCR-08-0760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.