Abstract
Background
We sought to estimate the maximum tolerated or recommended phase 2 dose and describe the pharmacokinetics and toxicities of enzastaurin, an oral inhibitor of protein kinase Cβ, in children with recurrent central nervous system malignancies.
Methods
Enzastaurin was administered continuously once daily at 3 dose levels (260, 340, and 440 mg/m2) and twice daily at 440 mg/m2/day. Plasma pharmacokinetics were evaluated following a single dose and at steady state. Inhibition of protein kinase C and Akt cell signaling in peripheral blood mononuclear cells was evaluated. Akt pathway activity was measured in pretreatment tumor samples.
Results
Thirty-three patients enrolled; 1 was ineligible, and 3 were nonevaluable secondary to early progressive disease. There were no dose-limiting toxicities during the dose-finding phase. Two participants receiving 440 mg/m2 given twice daily experienced dose-limiting toxicities of grade 3 thrombocytopenia resulting in delayed start of course 2 and grade 3 alanine transaminase elevation that did not recover within 5 days. There were no grade 4 toxicities during treatment. The concentration of enzastaurin increased with increasing dose and with continuous dosing; however, there was not a significant difference at the 440 mg/m2 dosing level when enzastaurin was administered once daily versus twice daily. There were no objective responses; however, 11 participants had stable disease >3 cycles, 7 with glioma, 2 with ependymoma, and 2 with brainstem glioma.
Conclusion
Enzastaurin was well tolerated in children with recurrent CNS malignancies, with chromaturia, fatigue, anemia, thrombocytopenia, and nausea being the most common toxicities. The recommended phase 2 dose is 440 mg/m2/day administered once daily.
Keywords: brain tumor, enzastaurin, pediatric, pharmacokinetic, phase 1
Central nervous system (CNS) tumors are the most common solid malignancy of childhood. In spite of significant improvements in therapy for pediatric malignancies over the past decades, pediatric CNS malignancy remains a leading cause of death due to cancer.1 Furthermore, acute and long-term morbidities from current therapies are significant.2 Thus, novel therapeutic agents that target molecular abnormalities in these cancers are greatly needed.
Enzastaurin is a potent oral serine/threonine kinase inhibitor of protein kinase Cβ, (PKCβ) and the PI3K/Akt pathways.3,4 Activation of PKCβ has been implicated in cell survival, growth, and invasiveness in solid and lymphoid malignancies.4,5 PKCβ lies in the signal cascade of vascular endothelial growth factor (VEGF). By blocking these pathways, enzastaurin and its metabolites (LY326020 and LY485912) have been shown in a number of tumor models, including glioblastoma, to suppress tumor cell proliferation, induce tumor cell death, and inhibit tumor-induced angiogenesis.3,6–8 Enzastaurin has also been shown to enhance the antiangiogenic effects of radiation, making it an appealing agent for development in pediatric CNS malignancies.9–11
Enzastaurin has been studied in adult clinical trials following once- or twice-daily dosing schedules at doses ranging from 20 to 900 mg/day.4,12–31 Oral doses of 500–525 mg have attained plasma exposures that exceeded a target concentration (1400 nM) associated with biologic activity.30 More recently, twice-daily dosing has been shown to further increase enzastaurin exposure.20,23
Initial phase 1 and 2 trials of enzastaurin in participants demonstrated encouraging antitumor activity in adults with glioblastoma.20,21 However, a subsequent phase 3 study in adults with recurrent glioblastoma failed to meet the endpoint of superiority over lomustine (CCNU). Nevertheless, enzastaurin was reasonably well tolerated compared with lomustine and had similar efficacy.12 Further studies of enzastaurin in combination with radiation, cytotoxics, and anti-angiogenic agents are ongoing.
We report the results of a phase 1 study to evaluate once-daily dosing of enzastaurin in children with recurrent or refractory CNS tumors. The primary objectives were to estimate the maximum tolerated dose (MTD) and/or recommend a phase 2 dose of enzastaurin and to describe the toxicities and pharmacokinetics of the recommended dose of enzastaurin when administered twice daily. The secondary objectives were to characterize the toxicities, pharmacokinetics, antitumor activity, and impact of enzastaurin on protein kinase C and Akt cell signaling in peripheral blood mononuclear cells.
Participants and Methods
Eligibility
Children younger than age 22 years with histologically verified recurrent, progressive, or refractory primary CNS tumors (histology was not required for intrinsic brainstem or optic pathway tumors) and a Karnofsky (age >16 y) or Lansky (age ≤16 y) performance status of ≥60% were eligible for this study. Participants were required to have recovered from the acute toxic effects of prior therapy and could not to have received myelosuppressive chemotherapy within 3 weeks (6 weeks if prior nitrosurea), biologic antineoplastic agents within 7 days, hematopoietic growth factors within 7 days (14 day for long-acting formulations), craniospinal or radiation therapy (RT) to 50% or more of the pelvis within 6 months, other substantial bone marrow RT within 6 weeks, local small port RT within 2 weeks, or autologous bone marrow transplant (BMT) within 3 months. Patients receiving dexamethasone had to be on a stable or decreasing dose. Other eligibility criteria included adequate hematologic function (absolute neutrophil count ≥1000/mm3, platelets ≥100 000/mm3, hemoglobin ≥8 g/dL), age-appropriate renal function, and adequate hepatic function (albumin ≥2.5 g/dL, bilirubin ≤1.5× and alanine transaminase ≤5× institutional upper limit of normal for age): Because the potential to prolong the corrected QT interval (QTc) was noted in some preclinical toxicology studies, patients were required to have a normal QTc for age and no clinically significant arrhythmias on electrocardiogram prior to registration. Exclusion criteria included receipt of other anticancer or experimental therapy, enzyme-inducing anticonvulsants or potent inducers, inhibitors or substrates of CYP3A4 or substrates of CYP2C8 or CYP2C9, known sensitivity to enzastaurin or its components, and any clinically significant unrelated systemic illness that would compromise their ability to tolerate protocol therapy. Patients who were pregnant or lactating were also excluded. Children with a body surface area less than 0.5 m2 were excluded because of inability to deliver the prescribed dose based on the fixed increment of the formulation. The institutional review boards of each Pediatric Brain Tumor Consortium (PBTC) institution approved the protocol prior to initial participant enrollment, and continuing approval was maintained throughout the study. Patients or their legal guardians signed written informed consent, and assent was obtained as appropriate at the time of enrollment.
Drug Administration and Study Design
Enzastaurin was supplied as enzastaurin monohydrochloride tablets containing 125 mg of active drug by Eli Lilly. Enzastaurin was administered orally once daily within 30 minutes after the ingestion of food, at the same time each day in courses of 28 days duration. A dosing nomogram was used to minimize interparticipant dosing variability with tablets rounded to the nearest increment of a whole or half tablet. Tablets could be swallowed whole or crushed and administered with a small quantity of food.
The starting enzastaurin dose was 260 mg/m2/day, which was 80% of the adult recommended dose, with dose escalations to 340 and 440 mg/m2. Dose escalation decisions between participant cohorts were based on toxicity observations during the first course of enzastaurin therapy. The 440 mg/m2 dose level was expanded to further evaluate the toxicity and the pharmacokinetics of daily (7 participants) and twice-daily (220 mg/m2 BID) dosing (14 participants). Participants consenting to pharmacokinetic studies received a single dose of enzastaurin on day −2 followed by pharmacokinetic sampling through day 1, when continuous daily enzastaurin dosing was initiated. In the absence of progressive disease or dose-limiting toxicity (DLT), enzastaurin dosing was continued without interruption for up to 26 courses.
Monitoring
Pretreatment evaluations included history and physical examination, assessment of performance status, MRI for disease evaluation, and basic laboratory studies including a pregnancy test for females of childbearing age. Participants were monitored with history, physical examination, and laboratory studies weekly during the first course and then at the start of each subsequent course, with weekly complete blood count continued through course 2. An electrocardiogram was obtained pretherapy and at the end of course 1. Disease evaluations with MRI including perfusion, diffusion, and gradient echo pulse sequences were obtained at baseline, after courses 1, 3, and 5, and then at the end of every third course.
Trial Design
Eligible participants receiving at least one dose of therapy were evaluable for toxicity and efficacy. In the absence of toxicity, participants were required to complete the first course of therapy to be considered fully evaluable for estimating the MTD. The modified continual reassessment method was used to estimate the MTD.32 Since enzastaurin is an oral drug available in fixed dosage strengths, which result in variability in the dose deliverable compared with the target dose, the continual reassessment method allows for use of the actual delivered dose rather than the assigned dose level in modeling the dose-toxicity relationship.
Toxicities were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE version 3.0)33 DLTs were defined as any of the following drug related adverse events: any grade 4 neutropenia or thrombocytopenia; any grade 3 or 4 nonhematologic toxicity with the specific exclusion of grade 3 nausea or emesis of <4 days duration; grade 3 aspartate aminotransferase or grade 3 alanine transaminase elevation that returned to meet eligibility criteria within 5 days of drug interruption; grade 3 fever or infection ≤5 days duration; grade 3 hypophosphatemia, hypomagnesemia, or hypokalemia responsive to oral supplementation; asymptomatic elevations in amylase or lipase resolving to grade 1 within 5 days of drug interruption; any grade 2 nonhematologic toxicity that persisted for >5 days and was sufficiently medically significant or intolerable that it required treatment interruption; or any enzastaurin-related adverse event that resulted in delay of 8 days between courses or results in need for cessation of therapy for >5 days during the first course.
Standard 2-dimensional imaging criteria were used for response assessment. Response findings for stable disease or complete or partial response had to be maintained for at least 4 weeks and be accompanied by a stable or decreasing dexamethasone dose and a stable or improving neurological exam. Because enzastaurin is a potential cytostatic agent, participants could continue to receive enzastaurin despite a radiographic increase in tumor size (25%–49%) if the investigator and patient/family thought that the participant was benefiting from therapy.
Pharmacokinetics
Plasma samples for pharmacokinetic studies were obtained from consenting participants on days −2 and 28 of course 1. Starting on day −2, samples were obtained prior to and at 1, 2, 4, 6, 8, 24, 48, and 72 hours following a single enzastaurin dose. Samples were also obtained on day 28 of therapy, prior to and at 2, 4, 8, and 10–12 and 24 hours after the dose. In participants receiving twice-daily dosing, pharmacokinetic sampling was required, and the evening dose of enzastaurin was held on days −2 and 28. Blood samples (1 mL) were collected in EDTA, and the plasma was separated and stored at −20°C or lower. Plasma samples were analyzed at Advion BioServices Inc for enzastaurin (LY317615) and its active metabolite (LY326020) using a validated liquid chromatography/tandem mass spectrometry (LC/MS/MS).4 Concentration versus time data were analyzed for pharmacokinetic parameters by noncompartmental methods using WinNonlin® Enterprise Version 5.3.
After a single dose, the maximum observed drug concentration (Cmax), time of maximal observed concentration (tmax), area under the concentration versus time curve from zero to infinity (AUC0-∞), half-life associated with the terminal rate constant (λz) in noncompartmental analysis (t1/2), apparent volume of distribution during the terminal phase after extravascular administration (Vz/F), and apparent total body clearance of drug calculated after extravascular administration (CL/F) were estimated for enzastaurin. Cmax, tmax, AUC0–∞, t1/2, and metabolic ratio (MR; AUC0–∞, LY326020/AUC0–∞, enzastaurin) were estimated for LY326020. Cmax, tmax and AUC0–∞ were also estimated for the total analyte (enzastaurin + LY326020). Similar parameters were also assessed at steady state.
Pharmacodynamic Studies
Correlative molecular biology studies were conducted to investigate the relationship between enzastaurin exposure and in vivo inhibition of Akt signaling. Blood samples (2 mL) were collected from consenting participants in heparinized tubes before enzastaurin and at days 14 and 28 in course 1. Peripheral blood mononuclear cells (PBMCs) were isolated using standard methods. Protein was then extracted from PBMC by direct lysis in protein lysis buffer, and total protein levels were quantified using a colorimetric assay (Pierce). The expression of total and phosphorylated versions of 7 Akt-pathway proteins was assessed by a multiplexed, Luminex bead-based assay. All reagents were purchased as a kit from Life Technologies. Proteins assayed, along with their respective sites of phosphorylation, were as follows: IR[pYpY1162⁄1163], IGF-1R[pYpY1135⁄1136], IRS-1[pS312], AKT[pS473], PRAS40[pT246], P70S6K[pTpS421⁄424], and GSK-3β[pS9]. The assay was performed according to the manufacturer's instructions, and beads were counted using a BioPlex 200 flow system (Bio-Rad). Relative phosphorylation scores were generated as a ratio of the mean fluorescent intensity for each phosphorylated protein versus the mean fluorescent intensity of its total counterpart.
We also assessed prestudy tumor samples for evidence of activation of the Akt pathway. Formalin-fixed, paraffin-embedded tumor sections (5 µm) were dewaxed and rehydrated prior to undergoing antigen retrieval in a rice steam-cooker (30 min; Target Retrieval Solution, Dako,). Standard procedures were used to probe for pAkt and pS6 proteins (antibodies #3787 and #2211, Cell Signaling Technology). Primary antibodies were then detected (Vector Stain Elite kit, Vector Labs) and visualized by DAB staining (Dako), according to the manufacturers' instructions. Immunoreactivity in each section was scored quantitatively as a percentage of positive cells (0–100) and semiquantitatively as a measure of signal intensity (1–3, low to high).
Results
A total of 33 participants were enrolled in the study, and 29 were fully evaluable for toxicity. Three participants experienced disease progression prior to development of toxicity or completion of the first course and were therefore not fully evaluable. The treatment-related toxicities experienced by these participants while on therapy are, however, included in Table 2. One participant was determined to be ineligible due to initiation of therapy within insufficient time (<3 half-lives) after completion of bevacizumab therapy. This participant experienced rapid disease progression, developed an intratumoral hemorrhage on day 12 of enzastaurin therapy, and died the following day.
Table 2.
Adverse events attributed to enzastaurin during any course including the 30 days after discontinuation of treatment
| Grade 2 |
Grade 3/4 |
|||||
|---|---|---|---|---|---|---|
| Adverse Event | 260 mg/m2 (n = 3) | 340 mg/m2 (n = 3) | 440 mg/m2 (n = 13) | 440 mg/m2 bid (n = 13) | 440 mg/m2 (n = 12) | 440 mg/m2 bid (n = 12) |
| Anemia | 1 | 1* | ||||
| Leukopenia | 1 | |||||
| Lymphopenia | 1 | 2 | 2 | |||
| Thrombocytopenia | 1 | 2 | ||||
| Neutropenia | 1 | 1 | ||||
| Fatigue | 1 | 1 | ||||
| Hypotension | 1 | |||||
| Prolonged QTc | 1 | |||||
| Dyspnea | 1 | |||||
| Diarrhea | 1 | |||||
| Vomiting | 2 | |||||
| Hypoalbuminemia | 1 | 1 | ||||
| Pancreatitis | 1 | |||||
| ALT(SGPT) Elevation | 1 | |||||
| AST(SGOT) elevation | 1 | |||||
| Bilirubin increase | 1 | |||||
| GGT increase | 1 | |||||
| Proteinuria | 2 | |||||
Abbreviations: ALT (SGPT), alanine transaminase; AST (SGOT), aspartate aminotransferase; bid, twice a day; GGT, gamma-glutamyl transpeptidase.
Demographics and Baseline Characteristics
Patient characteristics for all enrolled participants are outlined in Table 1. Overall, the median age was 11.6 years (range, 3–21 years). Males and females were nearly equally represented (48.5% and 51.5%, respectively). Performance status (Lansky or Karnofsky) was 100% in 51.5% of participants (n = 17) and ≥80% in 87.9% of participants (n = 29). In this study, anaplastic ependymoma was the most common disease type (18.2%), followed by brain stem glioma (15.2%), and glioblastoma multiforme (15.2%).
Table 1.
Patient Characteristics (n = 33)
| Characteristic | No. of Patients |
|---|---|
| Total patients enrolled | 33 |
| Eligible | 32 |
| Evaluable | 29 |
| Inevaluable | 3 |
| Ineligible | 1 |
| Diagnosis | |
| Ependymoma, anaplastic | 6 |
| Embryonal | |
| Medulloblastoma | 1 |
| Atypical teratoid/rhabdoid tumor | 1 |
| Pineoblastoma | 1 |
| Primitive neuroectodermal tumor | 1 |
| Glial tumors | |
| Pilocytic astrocytoma | 2 |
| Anaplastic astrocytoma | 3 |
| Glioblastoma | 5 |
| Astrocytoma, NOS (no grade provided) | 4 |
| Glioma, NOS (no grade provided) | 3 |
| Brainstem glioma | 5 |
| Meningioma | 1 |
| Sex | |
| Male | 16 |
| Female | 17 |
| Age (years, at study entry) | |
| Median | 12 |
| Range | 3–21 |
| Race | |
| Black | 3 |
| White, non-Hispanic | 29 |
| Multiple race (White/Asian) | 1 |
Abbreviation: NOS, not otherwise specified.
Adverse events
Enzastaurin was generally well tolerated with mostly low-grade toxicities, the most common treatment-related toxicities being urine color change (34%), anemia (34%), fatigue (25%), thrombocytopenia (25%), diarrhea (22%), and nausea (25%). Treatment-related toxicities grade 2 and higher are presented in Table 2. There were no DLTs observed in any of the 10 evaluable participants enrolled in the dose-escalation phase or in the expanded cohort of 7 participants receiving once-daily dosing at the maximum dose level of 440 mg/m2/day. All toxicities observed in eligible participants with once-daily dosing were grade 2 or lower with the exception of one participant with grade 3 lymphopenia. With twice-daily dosing at 440 mg/m2/day, 2 of the 12 evaluable participants experienced DLTs, which were grade 3 thrombocytopenia (decreased in grade within 1 day but resulted in a delay in the start of the next course) and grade 3 ALT elevation (decreased in grade but did not return to baseline within the protocol-defined time period). Although still well tolerated, there appeared to be a slight increase in toxicities observed with the twice-daily dosing schedule. Five deaths (15.2% overall) occurred within 30 days of discontinuation of enzastaurin: 1 at 260 mg/m/day, 2 at 440 mg/m/day daily, and 2 at 440 mg/m/day twice-daily dose. All deaths were related to tumor progression.
Response
No objective antitumor responses were reported. However, 11 (34%) participants had stable disease (>3 cycles): 5 with astrocytoma, 2 with ependymoma, 2 with brainstem glioma, and 2 with glioma (not otherwise specified). Two participants (glioma and brainstem glioma) were on treatment for longer than 12 months. The median time on therapy was 1.9 months (range, 0.4–17.5 months).
Pharmacokinetics
Pharmacokinetic data are available from 16 participants, 10 of whom received twice-daily dosing (see Table 3). There was significant interpatient variability in all variables evaluated.
Table 3.
Pharmacokinetic parameters following enzastaurin administration
| Single Dose (day −2), Geometric mean (CV%) |
Steady State (day 28), mean (SD) |
||||||
|---|---|---|---|---|---|---|---|
| 260 mg/m2 daily (n = 1) | 340 mg/m2 daily (n = 1) | 440 mg/m2 daily (n = 4) | 440 mg/m2 bid (n = 10) | 340 mg/m2 daily (n = 1) | 440 mg/m2 daily (n = 4) | 440 mg/m2 bid (n = 7) | |
| Enzastaurin | |||||||
| Cmax (nmol/L) | 790 (NC) | 1418 (NC) | 1020 (60) | 1020 (74) | 1406 (NC) | 1920 (90) | 1610 (111) |
| Cavg, ss (nmol/L) | (NC) | (NC) | (NC) | (NC) | 680 (NC) | 1060 (142) | 11 150 (162)d |
| Tmax (h)a | 1.00 (NC) | 8.00 (NC) | 5.04 (2.00–6.00) | 4.00 (1.00–8.00) | 2.00 (NC) | 3.00 (2.00–8.00) | 2.05 (1.92–4.00) |
| t½ (h) | 13.7 (NC) | 8.68 (NC) | 15.3 (37) | 12.1 (43)c | (NC) | (NC) | (NC) |
| Cl/F (L/h) | 112 (NC) | 37.3 (NC) | 73.2 (59) | 32.2 (80)c | 52.0 (NC) | 51.3 (125) | 43.7 (90)d |
| Vz/F (L) | 2230 (NC) | 466 (NC) | 1620 (35) | 564 (132)c | (NC) | (NC) | (NC) |
| AUCb (nmol · h/L) | 5390 (NC) | 22 800 (NC) | 16 600 (88) | 16 100 (79)c | 16 300 (NC) | 25 400 (142) | 13 800 (162)d |
| LY326020 | |||||||
| Cmax (nmol/L) | 432 | 672 | 549 (23) | 334 (30) | 823 | 1210 (11) | 922 (54) |
| Cavg, ss (nmol/L) | (NC) | (NC) | (NC) | (NC) | 628 | 1000 (19) | 832 (60)d |
| Tmax (h) | 2.00 | 8.00 | 5.0 4 (4.00–6.00) | 6.00 (2.00–8.00) | 4.00 | 3.00 (2.00–8.00) | 2.00 (0.00–7.95) |
| t½ (h) | 22.2 | 24.8 | 41.5 (78) | 29.2 (32)c | (NC) | (NC) | (NC) |
| AUCb (nmol · h/L) | 12 400 | 26 900 | 29 600 (55) | 16 300 (36)c | 15 100 | 24 000 (19) | 9970 (60)d |
| Total Analyte | |||||||
| Cmax (nmol/l) | 1116 | 2090 | 1570 (41) | 1370 (55) | 2195 | 3210 (54) | 2590 (78) |
| Cavg, ss (nmol/l) | (NC) | (NC) | (NC) | (NC) | 1310 | 2220 (76) | 2110 (90) |
| Tmax (h) | 2.00 | 8.00 | 5.04 (2.00–6.00) | 6.00 (1.00–8.00) | 2.00 | 3.00 (2.00–8.00) | 2.00 (0.00–4.00) |
| AUCb (nmol · h/L) | 17 800 | 49 600 | 46 600 (66) | 33 400 (50)c | 31 400 | 53 300 (76) | 25 300 (90) |
aMedian (Min-Max).
bReported AUC represents AUC(0–∞) for single dose and AUCτ,ss at steady state (Tau = 12 for BID and 24 for QD).
cN = 9.
dN = 6.
AUC (area under the curve); Cavg, ss (average concentration at steady state); Cmax (maximum concentration); Cl/F (apparent clearance); %CV (coefficient of variability); NC (not calculated); SD (standard deviation); t½ (half-life); Tmax (time to Cmax); Vz/F (apparent volume of distribution).
There was accumulation of enzastaurin over time, with the Cmax approximately doubling from day −2 to day 28. The concentration at steady state of enzastaurin increased with increasing dose; however, there was no significant difference at the 440 mg/m2 dosing level when enzastaurin was administered once daily versus twice daily. The total analyte Cavg,ss for participants taking enzastaurin 440 mg/m2 as a single daily dose or twice-daily dose (220 mg/m2/dose) were 2220 and 2110 nmol/L, respectively. The half-life of enzastaurin ranged from 8.7 to 15.3 hours. There were insufficient participants in each dose group to assess dose proportionality for Cmax and AUC.
Correlative Biology
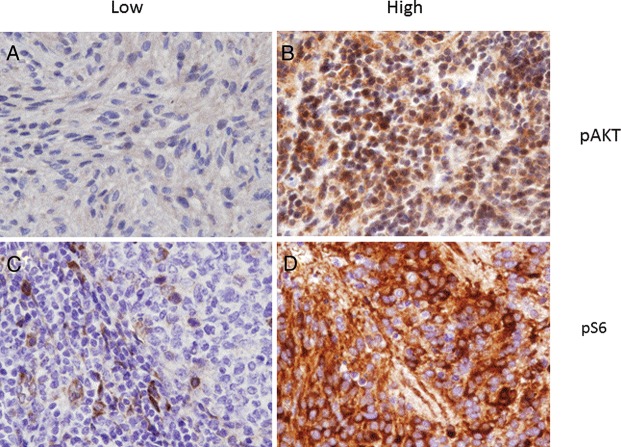
Sufficient archival tumor samples were available from 18 participants. Some degree of p-AKT expression was detected in 11 of 18 tumors, with 4 of the 18 samples having >50% of cells demonstrating at least moderately intense expression of the phosphorylated protein within the nucleus and cytoplasm (Figure 1). Immunohistochemical localization of p-S6 kinase was associated with the membrane in cells present in all 18 tumor samples, and 6 of 18 samples demonstrated that more than half of cells within the tumor had at least moderately intense staining. Thus, the Akt signaling pathway appears to be broadly expressed and is presumably active in several types of relapsed pediatric brain tumors.
Fig. 1.
Prominent expression of AKT signaling pathway components. Representative immunohistochemical localization of low and high expression of p-AKT within the nuclei of tumor cells from patients with meningioma (A) and medulloblastoma (B) respectively (magnification 100×). Also note, membranous immunolocalization of p-S6 kinase was present within the cells in all 18 tumor samples (representative samples from 2 patients with ependymoma are shown here, C and D). Variability of staining was noted within the tumor and demonstrated that more than half of cells within the tumor had at least moderate intensity of staining. Thus, the AKT signaling pathway appears to be broadly expressed and is presumably active in several types of relapsed pediatric brain tumors.
To address whether enzastaurin had any effect on AKT pathway activation in PBMCs, we evaluated phosphorylation of Akt, p70S6K, and GSK-3β in PBMCs collected from participants prior to enzastaurin and at 14 and 28 days of therapy by immunodetection. Out of 33 participants enrolled and treated, 18 had a baseline biology sample, 13 had both baseline and day 14 samples, 15 had both baseline and day 28 samples, and 10 had the biology sample at all 3 time points. Unfortunately, only 4 of these participants also had pharmacokinetics available for analysis, and it was not possible to show any statistically significant relationship between the pharmacokinetic parameters of AUC or Cmax of enzastaurin and the levels of protein phosphorylation. When the relative phosphorylation of day 14 and 28 samples was analyzed, there was also no significant difference in the phosphorylation level of any of the proteins from baseline.
Discussion
This is the first study that evaluated enzastaurin in children and established the recommended phase 2 dose for enzastaurin in children as 440 mg/m2/day given once daily. This is about 50% higher than the approximately 500 mg/day (∼280 mg/m2/day) dosing used in most adult studies. Similar to findings in adult studies, enzastaurin was very well tolerated as a single agent.4,12,14,17–21,25 Most toxicities were grade 1 and grade 2, with chromaturia and fatigue being the most commonly reported toxicities. Also similar to previous reports in adults, twice-daily dosing resulted in an increase of toxicity, with the only DLTs noted during the study occurring on the twice-daily dosing schedule.
Extended pharmacokinetic sampling was performed in consenting participants, and an additional cohort of patients were enrolled with required pharmacokinetic studies at the recommended phase 2 dose following daily and twice-daily dosing. The mean total analytes Cav,ss for participants taking 440 mg/m2/day as single or twice-daily doses were similar and reached target efficacious concentrations of 1400 nM. In contrast to other studies, the increase in toxicity noted with twice-daily dosing was not explained by an increase in average concentration at steady state or other pharmacokinetic parameters at the dose level studied.13,21,23 Pharmacokinetic parameters were otherwise similar to those described in adult studies. Since the twice-daily dosing was associated with increased toxicity without increased exposure, we recommend the once-daily dosing for the pediatric patient population.
Immunohistochemical analysis of protein phosphorylation of Akt and P6s confirms that there is evidence of Akt pathway activation in most of the tumor samples analyzed and strong immunolocalization in more than half of the samples. However, it is not known whether this presumed activity is promoting tumor growth and if it is a pathway whose activity is essential for tumor growth . Unfortunately, the sample numbers were so small that it was not possible to determine whether phosphorylation of these Akt pathway components in PBMCs would be useful for detecting biological activity of enzastaurin.
Although no objective responses were reported, 11 participants had stable disease, including a number of patients with gliomas (similar to prior experience in adults). Although enzastaurin demonstrated encouraging preliminary activity in adults with glioblastoma, results of the phase 3 study in recurrent glioblastoma were not impressive enough to continue development as a single agent in this patient population.12,20 However, preclinical studies have shown that enzastaurin enhances the activity of both cytotoxic and targeted agents in a variety of tumor types,34,35 and clinical trials evaluating its use in various combinations studies have been initiated.36–40
Preclinical studies of enzastaurin have also demonstrated that it enhances the effect of radiation in glioblastoma and other tumor models. Agents that sensitize tumors to RT are particularly appealing for the treatment of CNS malignancies because RT remains an important component of the treatment for most CNS malignancies. Early phase Clinical trials have been completed in adults with glioblastoma evaluating the use of enzastaurin in combination with RT.31,36,41
The results of our study, combined with available preclinical data and information from adult studies, suggest that future clinical trials of enzastaurin in children should combine it with other agents or radiotherapy. Its favorable toxicity profile, availability as an oral agent, and novel mechanism of action make it a promising agent for combination therapy with RT, antiangiogenic, or traditional chemotherapy.
Funding
This work was supported in part by NIH Grant K12 CA090433-06, NIH grant U01 CA81457 for the Pediatric Brain Tumor Consortium, NIH grant 5M01RR000188, NIH grant CA021765-34 for the Molecular Clinical Trials Core laboratory, and the American Lebanese Syrian Associated Charities, NIH Grant 5M01RR000188.
Acknowledgments
The authors and the PBTC acknowledge clinical research assistant support of Mr. Joyson Pekkattil.
Conflict of interest statement: Rodney Decker declares ownership of Eli Lilly stock. None of the other authors declare a conflict of interest.
References
- 1.Ries LAG, Smith MA, Gurney JG, et al. National Cancer Institute SP. Bethesda: NIH Pub; 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. No. 99-4649. [Google Scholar]
- 2.Turner CD, Rey-Casserly C, Liptak CC, et al. Late effects of therapy for pediatric brain tumor survivors. J Child Neurol. 2009;24(11):1455–1463. doi: 10.1177/0883073809341709. [DOI] [PubMed] [Google Scholar]
- 3.Graff JR, McNulty AM, Hanna KR, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65(16):7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 4.Carducci MA, Musib L, Kies MS, et al. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol. 2006;24(25):4092–4099. doi: 10.1200/JCO.2005.05.3447. [DOI] [PubMed] [Google Scholar]
- 5.Ma S, Rosen ST. Enzastaurin. Curr Opin Oncol. 2007;19(6):590–595. doi: 10.1097/CCO.0b013e3282f10a00. [DOI] [PubMed] [Google Scholar]
- 6.Keyes KA, Mann L, Sherman M, et al. LY317615 decreases plasma VEGF levels in human tumor xenograft-bearing mice. Cancer Chemother Pharmacol. 2004;53(2):133–140. doi: 10.1007/s00280-003-0713-x. [DOI] [PubMed] [Google Scholar]
- 7.Rizvi MA, Ghias K, Davies KM, et al. Enzastaurin (LY317615), a protein kinase Cbeta inhibitor, inhibits the AKT pathway and induces apoptosis in multiple myeloma cell lines. Mol Cancer Ther. 2006;5(7):1783–1789. doi: 10.1158/1535-7163.MCT-05-0465. [DOI] [PubMed] [Google Scholar]
- 8.Hanauske AR, Oberschmidt O, Hanauske-Abel H, et al. Antitumor activity of enzastaurin (LY317615.HCl) against human cancer cell lines and freshly explanted tumors investigated in in-vitro [corrected] soft-agar cloning experiments. Invest New Drugs. 2007;25(3):205–210. doi: 10.1007/s10637-007-9038-7. [DOI] [PubMed] [Google Scholar]
- 9.Willey CD, Xiao D, Tu T, et al. Enzastaurin (LY317615), a protein kinase C beta selective inhibitor, enhances antiangiogenic effect of radiation. Int J Radiat Oncol Biol Phys. 2010;77(5):1518–1526. doi: 10.1016/j.ijrobp.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudek AZ, Zwolak P, Jasinski P, et al. Protein kinase C-beta inhibitor enzastaurin (LY317615.HCI) enhances radiation control of murine breast cancer in an orthotopic model of bone metastasis. Invest New Drugs. 2008;26(1):13–24. doi: 10.1007/s10637-007-9079-y. [DOI] [PubMed] [Google Scholar]
- 11.Jasinski P, Terai K, Zwolak P, et al. Enzastaurin renders MCF-7 breast cancer cells sensitive to radiation through reversal of radiation-induced activation of protein kinase C. Eur J Cancer. 2008;44(9):1315–1322. doi: 10.1016/j.ejca.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanai C, Yamamoto N, Ohe Y, et al. A phase I study of enzastaurin combined with pemetrexed in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(7):1068–1074. doi: 10.1097/JTO.0b013e3181da3899. [DOI] [PubMed] [Google Scholar]
- 14.Robertson MJ, Kahl BS, Vose JM, et al. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2007;25(13):1741–1746. doi: 10.1200/JCO.2006.09.3146. [DOI] [PubMed] [Google Scholar]
- 15.Richards DA, Kuefler PR, Becerra C, et al. Gemcitabine plus enzastaurin or single-agent gemcitabine in locally advanced or metastatic pancreatic cancer: Results of a Phase II, randomized, noncomparative study. Invest New Drugs. 2011;29(1):144–153. doi: 10.1007/s10637-009-9307-8. [DOI] [PubMed] [Google Scholar]
- 16.Rademaker-Lakhai JM, Beerepoot LV, Mehra N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral protein kinase C beta-inhibitor enzastaurin in combination with gemcitabine and cisplatin in patients with advanced cancer. Clin Cancer Res. 2007;13(15 Pt 1):4474–4481. doi: 10.1158/1078-0432.CCR-06-2912. [DOI] [PubMed] [Google Scholar]
- 17.Mukohara T, Nagai S, Koshiji M, et al. Phase I dose escalation and pharmacokinetic study of oral enzastaurin (LY317615) in advanced solid tumors. Cancer Sci. 2010;101(10):2193–2199. doi: 10.1111/j.1349-7006.2010.01677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morschhauser F, Seymour JF, Kluin-Nelemans HC, et al. A phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Ann Oncol. 2008;19(2):247–253. doi: 10.1093/annonc/mdm463. [DOI] [PubMed] [Google Scholar]
- 19.Mina L, Krop I, Zon RT, et al. A phase II study of oral enzastaurin in patients with metastatic breast cancer previously treated with an anthracycline and a taxane containing regimen. Invest New Drugs. 2009;27(6):565–570. doi: 10.1007/s10637-009-9220-1. [DOI] [PubMed] [Google Scholar]
- 20.Kreisl TN, Kotliarova S, Butman JA, et al. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12(2):181–189. doi: 10.1093/neuonc/nop042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreisl TN, Kim L, Moore K, et al. A phase I trial of enzastaurin in patients with recurrent gliomas. Clin Cancer Res. 2009;15(10):3617–3623. doi: 10.1158/1078-0432.CCR-08-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocherginsky M, Cohen EE, Karrison T. Design of Phase II cancer trials for evaluation of cytostatic/cytotoxic agents. J Biopharm Stat. 2009;19(3):524–529. doi: 10.1080/10543400902802441. [DOI] [PubMed] [Google Scholar]
- 23.Hanauske AR, Lahn M, Musib LC, et al. Phase Ib safety and pharmacokinetic evaluation of daily and twice daily oral enzastaurin in combination with pemetrexed in advanced/metastatic cancer. Ann Oncol. 2009;20(9):1565–1575. doi: 10.1093/annonc/mdp049. [DOI] [PubMed] [Google Scholar]
- 24.Glimelius B, Spindler KL, Frodin JE, et al. Long-term follow-up of chemonaive patients with asymptomatic metastatic colorectal cancer treated with enzastaurin in a window of opportunity phase II study. Ann Oncol. 2010;21(5):1127–1128. doi: 10.1093/annonc/mdp526. [DOI] [PubMed] [Google Scholar]
- 25.Glimelius B, Lahn M, Gawande S, et al. A window of opportunity phase II study of enzastaurin in chemonaive patients with asymptomatic metastatic colorectal cancer. Ann Oncol. 2010;21(5):1020–1026. doi: 10.1093/annonc/mdp521. [DOI] [PubMed] [Google Scholar]
- 26.Dreicer R, Garcia J, Hussain M, et al. Oral enzastaurin in prostate cancer: A two-cohort phase II trial in patients with PSA progression in the non-metastatic castrate state and following docetaxel-based chemotherapy for castrate metastatic disease. Invest New Drugs. 2011;29(1):144–153. doi: 10.1007/s10637-010-9428-0. [DOI] [PubMed] [Google Scholar]
- 27.Clemons M, Joy AA, Abdulnabi R, et al. Phase II, double-blind, randomized trial of capecitabine plus enzastaurin versus capecitabine plus placebo in patients with metastatic or recurrent breast cancer after prior anthracycline and taxane therapy. Breast Cancer Res Treat. 2010;124(1):177–186. doi: 10.1007/s10549-010-1152-0. [DOI] [PubMed] [Google Scholar]
- 28.Chiappori A, Bepler G, Barlesi F, et al. Phase II, double-blinded, randomized study of enzastaurin plus pemetrexed as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(3):369–375. doi: 10.1097/JTO.0b013e3181cee24f. [DOI] [PubMed] [Google Scholar]
- 29.Casey EM, Harb W, Bradford D, et al. Randomized, double-blinded, multicenter, phase II study of pemetrexed, carboplatin, and bevacizumab with enzastaurin or placebo in chemonaïve patients with stage IIIB/IV non-small cell lung cance r: Hoosier Oncology Group LUN06-116. J Thorac Oncol. 2010;5(11):1815–1820. doi: 10.1097/JTO.0b013e3181ee820c. [DOI] [PubMed] [Google Scholar]
- 30.Camidge DR, Gail Eckhardt S, Gore L, et al. A phase I safety, tolerability, and pharmacokinetic study of enzastaurin combined with capecitabine in patients with advanced solid tumors. Anticancer Drugs. 2008;19(1):77–84. doi: 10.1097/CAD.0b013e3282f077b3. [DOI] [PubMed] [Google Scholar]
- 31.Butowski N, Chang SM, Lamborn KR, et al. Enzastaurin plus temozolomide with radiation therapy in glioblastoma multiforme: a phase I study. Neuro Oncol. 2010;12(6):608–613. doi: 10.1093/neuonc/nop070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onar A, Kocak M, Boyett JM. Continual reassessment method vs. traditional empirically based design: modifications motivated by Phase I trials in pediatric oncology by the Pediatric Brain Tumor Consortium. J Biopharm Stat. 2009;19(3):437–455. doi: 10.1080/10543400902800486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Cancer Institue N. Common Terminology for Adverse Events (CTCAE). Version 3.0. Vol NIH Pub. No 03-5410. Bethesda, MD: US Deparment of Health and Human Services, National Institutes of Health; 2009. [Google Scholar]
- 34.Jian W, Yamashita H, Levitt JM, et al. Enzastaurin shows preclinical antitumor activity against human transitional cell carcinoma and enhances the activity of gemcitabine. Mol Cancer Ther. 2009;8(7):1772–1778. doi: 10.1158/1535-7163.MCT-09-0141. [DOI] [PubMed] [Google Scholar]
- 35.Vogl UM, Berger W, Micksche M, et al. Synergistic effect of Sorafenib and Sunitinib with Enzastaurin, a selective protein kinase C inhibitor in renal cell carcinoma cell lines. Cancer Lett. 2009;277(2):218–226. doi: 10.1016/j.canlet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Wick W, Steinbach JP, Platten M, et al. Enzastaurin before and concomitant with radiation therapy, followed by enzastaurin maintenance therapy, in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation. Neuro Oncol. 2013;15(10):1405–1412. doi: 10.1093/neuonc/not100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vergote IB, Chekerov R, Amant F, et al. Randomized, phase II, placebo-controlled, double-blind study with and without enzastaurin in combination with paclitaxel and carboplatin as first-line treatment followed by maintenance treatment in advanced ovarian cancer. J Clin Oncol. 2013;31(25):3127–3132. doi: 10.1200/JCO.2012.44.9116. [DOI] [PubMed] [Google Scholar]
- 38.Gronberg BH, Ciuleanu T, Flotten O, et al. A placebo-controlled, randomized phase II study of maintenance enzastaurin following whole brain radiation therapy in the treatment of brain metastases from lung cancer. Lung Cancer. 2012;78(1):63–69. doi: 10.1016/j.lungcan.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Nwankwo N, Zhang Z, Wang T, et al. Phase I study of enzastaurin and bevacizumab in patients with advanced cancer: safety, efficacy and pharmacokinetics. Invest New Drugs. 2013;31(3):653–660. doi: 10.1007/s10637-012-9850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rampling R, Sanson M, Gorlia T, et al. A phase I study of LY317615 (enzastaurin) and temozolomide in patients with gliomas (EORTC trial 26054) Neuro Oncol. 2012;14(3):344–350. doi: 10.1093/neuonc/nor221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butowski N, Chang SM, Lamborn KR, et al. Phase II and pharmacogenomics study of enzastaurin plus temozolomide during and following radiation therapy in patients with newly diagnosed glioblastoma multiforme and gliosarcoma. Neuro-oncology. 2011;13(12):1331–1338. doi: 10.1093/neuonc/nor130. [DOI] [PMC free article] [PubMed] [Google Scholar]