Abstract
Background
The main goal of this study was to assess frequency, clinical correlates, and independent predictors of fatigue in a homogeneous cohort of well-defined glioblastoma patients at baseline prior to combined radio-chemotherapy.
Methods
We prospectively included 65 glioblastoma patients at postsurgical baseline and assessed fatigue, sleepiness, mean bedtimes, mood disturbances, and clinical characteristics such as clinical performance status, presenting symptomatology, details on neurosurgical procedure, and tumor location and diameter as well as pharmacological treatment including antiepileptic drugs, antidepressants, and use of corticosteroids. Data on fatigue and sleepiness were measured with the Fatigue Severity Scale and the Epworth Sleepiness Scale, respectively, and compared with 130 age- and sex-matched healthy controls.
Results
We observed a significant correlation between fatigue and sleepiness scores in both patients (r = 0.26; P = .04) and controls (r = 0.36; P < .001). Only fatigue appeared to be more common in glioblastoma patients than in healthy controls (48% vs 11%; P < .001) but not the frequency of sleepiness (22% vs 19%; P = .43). Female sex was associated with increased fatigue frequency among glioblastoma patients but not among control participants. Multiple linear regression analyses identified depression, left-sided tumor location, and female sex as strongest associates of baseline fatigue severity.
Conclusions
Our findings indicate that glioblastoma patients are frequently affected by fatigue at baseline, suggesting that factors other than those related to radio- or chemotherapy have significant impact, particularly depression and tumor localization.
Keywords: depression, fatigue, glioblastoma, sleepiness
Glioblastoma is the most common primary brain tumor in adults, with an estimated incidence of about 3 per 100 000 inhabitants per year in Europe and North America.1 The standard of care for newly diagnosed glioblastoma, subsequent to surgery, comprises radiotherapy with concomitant temozolomide followed by adjuvant temozolomide. In the study defining this treatment regimen, median survival was limited to 15 months,2 and median survival was reported to be only 12 months in a population-based analysis of more than 10 000 glioblastoma patients.3
Independent of any treatment, fatigue is a common symptom in cancer patients in general as well as in primary brain tumor patients, with an estimated prevalence of 50%–90% and 40%–70%, respectively.4,5 Cancer-related fatigue is defined by the National Comprehensive Cancer Network (NCCN) as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.6 The patients themselves indicate fatigue as one of the most distressing symptom related to cancer and its treatment.7 It is a strong predictor of decreased patient satisfaction and health-related quality of life (QoL) and may represent one of the key reasons for discontinuing treatment.8–10 Nevertheless, fatigue is believed to be underdiagnosed and underestimated in cancer patients despite its possible impact on treatment compliance.4,11 As a consequence, some groups have questioned whether the standard treatment for glioblastoma is justified in view of the limited benefit on survival and the severity of associated symptoms.12
Compared with other tumor types, namely breast and lung cancer, few studies have addressed the problem of fatigue in glioblastoma patients in depth, and several limitations have to be mentioned. First, many groups have included patients with all sorts of primary brain tumors despite large differences in underlying neurobiology, treatment procedures, and prognosis.5,13 Second, baseline data are often missing, particularly in studies using a cross-sectional design.5 As a consequence, fatigue was mainly assessed as a treatment complication, thereby failing to acknowledge the primary impact of the tumor itself and other treatment-independent factors.14 Third, the results of many older series cannot be directly compared with the current situation because of advances in radiation techniques. Finally, while many validated fatigue questionnaires are available,9,15 the large majority of neuro-oncological studies identified and quantified fatigue in a very rudimental way, using the Visual Analogue Scale (VAS) or one single fatigue item appearing in tools such as the European Organisation for Research and Treatment of Cancer (EORTC) QoL questionnaire, the M D Anderson Symptom Inventory–Brain Tumor Module, or the Symptom Distress Scale.9,12,16–18
Thus, in this prospective study, we aimed at examining frequency and predictors of fatigue severity in a homogeneous cohort of glioblastoma patients at baseline prior to combined radio-chemotherapy. For this goal, we used the Fatigue Severity Scale (FSS), which has been identified as the most widely adopted fatigue questionnaire in clinical practice and has been validated for a variety of neurological diseases.15,19,20 In addition, we explored the evolution of fatigue, sleepiness, and mood disorders during and after combined radio-chemotherapy.
Patients and Methods
This prospective, longitudinal study was conducted as a collaboration of the Departments of Neurology, Oncology, and Radiation Oncology of the University Hospital Zurich between October 2008 and October 2012. The study protocol was approved by the Ethics Committee of the Canton of Zurich, Switzerland, specialized subcommittee for Psychiatry, Neurology, Neurosurgery (Project E-43/2007), and informed consent was obtained from all patients prior to enrollment.
Participants and Controls
We prospectively included 65 patients with newly diagnosed and histologically proven glioblastoma, corresponding to an estimated 60%–65% of all eligible glioblastoma patients of the acquisition period. Patients aged ≥18 years undergoing postoperative standard radio-chemotherapy were eligible.2 They also had to be fluent in the German language. The participants were examined clinically and by questionnaires at 3 different time points: (i) 28 ± 7 days after the initial neurosurgical procedure (1 day prior to the first radiation), (ii) at the time of the last radiation, and (iii) prior to the initiation of adjuvant chemotherapy. Overall, the study period captured the first 10 weeks of postoperative standard treatment.
As control group, we included 130 healthy and age- and sex-matched individuals using a 1:2 case-control design. The controls were selected from a previously published cohort of 454 healthy subjects that we used in our original validation study of the German version of the FSS.19
Clinical Assessment and Questionnaires
Demographic variables included age, sex, and educational status (defined as highest degree attained). We ascertained several tumor characteristics including type of neurosurgical procedure (biopsy, partial, complete or unclear resection), tumor topography, and brain magnetic resonance imaging-based tumor diameter. To elucidate whether the presenting symptomatology affected fatigue severity, we divided the medical history into seizure, motor weakness, cognitive deficit, headache/nausea/vomiting, apathy/asthenia, visual deficit, and accidental finding. Seizure type was further classified as partial, generalized, complex-focal, and unclassified. To estimate the influence of pharmacological treatment, we included at each time point details on anticonvulsive drugs, antidepressants, anxiolytics, CNS stimulants, hypnotics, and whether or not participants received corticosteroids (including the dose of the steroids). Clinical performance status was assessed by the Karnofsky performance score (KPS), with 100% indicating perfect physical health and 0% death. As mentioned earlier, we measured fatigue by means of the FSS. This self-administered questionnaire comprises 9 items exploring fatigue severity in different situations during the previous week, and the final score ranges from 1 to 7 with the latter value indicating maximal fatigue. The presence of clinically significant fatigue was defined as an FSS score ≥4.0. The FSS has robust psychometric properties and has been validated for various neurological disorders but has not yet been validated for glioblastoma patients. We therefore performed a reliability statistic in our cohort, which revealed excellent internal consistency as reflected by a Cronbach's α of 0.94.21 We used the German version of the Epworth Sleepiness Scale (ESS) for assessment of sleepiness; a score of ≥10 indicates excessive daytime sleepiness (EDS).22 We also determined the prevalence of overlap between fatigue and EDS, when participants presented both a FSS score ≥4.0 and an ESS score ≥10. Sleep need was estimated using information on mean bedtimes. We arbitrarily defined mean bedtimes ≥10 hours as “long bedtimes”, probably indicating increased sleep need per 24 hours (ie, hypersomnia). Finally, for evaluation of anxiety and depression, we used the German version of the Hospital Anxiety and Depression Scale (HADS). It is a well-validated questionnaire, which is suitable for cancer populations because it contains only nonphysical symptoms for both anxiety and depression. Subjects indicate their agreement with each item on a scale ranging from 0 to 3. The questionnaire has 2 subscales for anxiety and depression, each consisting of 7 items. A score of >10 is considered to indicate overt anxiety or depression.23,24
Data Analysis and Statistics
We used SPSS (version 19.0) for statistical analysis. Group data are described by means, standard deviations (SD), and confidence intervals (95% CI). To compare the mean values of FSS and ESS scores between glioblastoma participants and controls, we used the Student' t test; the χ2 test was used to compare the frequency of fatigue and EDS between the 2 groups. Longitudinal differences of scores were assessed using the Student' paired t test. We applied Cronbach α statistics to calculate the internal consistency of the FSS in glioblastoma patients. To identify predictors of fatigue severity at baseline, we performed a multiple linear regression analysis with the FSS score as a dependent variable. Among the set of potential predictor variables (age, sex, education, KPS, ESS, bedtimes, anxiety and depression scores, tumor localization, and use of steroids or antidepressants), we evaluated each variable for an estimated effect of ≥0.2 or ≤−0.2 on the outcome score for fatigue severity and a P value <.05 in a univariate comparison. Those predictor variables fulfilling the 2 criteria were included in the multiple linear regression model. Significance was accepted at P <.05. 95% confidence intervals (CI) for mean differences between the groups were additionally presented when group comparisons revealed significant differences.
Results
Characterization of Glioblastoma Patients
We included 65 glioblastoma patients, of whom 44 (68%) were male. Mean age was 57.3 ± 10.1 years. The tumor was localized in the left brain hemisphere in 28 patients (43%), in the right brain hemisphere in 31 patients (48%), and bilateral in 6 patients (9%). A majority of tumors affected the frontotemporal lobes (57%) as compared with the parieto-occipital lobes (31%), basal ganglia (5%), or multiple sites (8%).
Comparison between Glioblastoma Patients and Controls
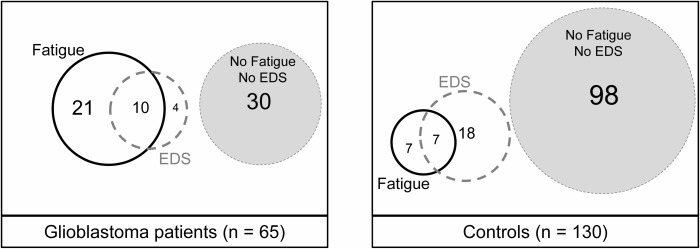
Glioblastoma patients had significantly higher FSS scores (3.9 ± 1.7 vs 2.8 ± 1.0; 95% CI 0,74–1.49; P < .001), fatigue frequency (48% vs 11%; P < .001), and longer bedtimes (8.8 ± 1.2 h vs 7.7 ± 0.9 h; 95% CI, 0.76–1.39; P < .001) than controls, whereas ESS scores and the prevalence of EDS were similar (Table 1). FSS and ESS scores were significantly correlated in both glioblastoma patients (r = 0.26; P = .04) and controls (r = 0.36; P < .001). In glioblastoma patients, overlap of both fatigue and EDS was observed in 15%, while “isolated fatigue” was much more common (32%) than “isolated EDS” (6%) (Fig. 1). Conversely, we observed more controls with isolated EDS (14%) than isolated fatigue (5%) (P < .001).
Table 1.
Comparison of frequency and severity of fatigue, sleepiness, and mean bedtimes between glioblastoma patients and controls. Values are mean ± standard deviation.
| Glioblastoma Patients (n = 65) | Controls (n = 130) | P Value | |
|---|---|---|---|
| Age (y) | 57.3 ± 10.1 | 57.4 ± 9.8 | .93 |
| Sex, male | 44 (68%) | 88 (68%) | .57 |
| FSS | 3.9 ± 1.7 | 2.8 ± 1.0 | <.001 |
| Fatigue (FSS >4.0) | 31 (48%) | 14 (11%) | <.001 |
| ESS | 5.9 ± 4.3 | 6.2 ± 3.6 | .67 |
| EDS (ESS > 10) | 14 (22%) | 25 (19%) | .43 |
| Mean bedtime (h) | 8.8 ± 1.2 | 7.7 ± 0.9 | <.001 |
| Long bedtime (>10 h) | 10 (16%) | 4 (3%) | .003 |
Abbreviations: EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; FSS, Fatigue Severity Scale; h, hours; y, years.
Fig. 1.
Frequency and overlap of fatigue and excessive daytime sleepiness in glioblastoma patients and controls. In glioblastoma patients, fatigue is often associated with excessive daytime sleepiness (EDS), but isolated EDS seldom occurs.
Comparison of Glioblastoma Patients With and Without Fatigue
Almost half of all glioblastoma patients suffered from fatigue prior to radiotherapy. When compared with those not having fatigue, glioblastoma patients with fatigue revealed a higher prevalence of EDS (32% vs 12%; P = .04), spent more time in bed (95% CI, 0.13–1.32; P = .02), and were more depressed (95% CI, 0.78–4.04; P =.005) (Table 2). In addition, sex distribution differed significantly: of the 21 female glioblastoma patients, 14 had fatigue (67%), while only 39% of all male patients had fatigue (P = .03). Of note, fatigue prevalence was similar in female and male controls (12% vs 10%; P = .49). Finally, glioblastoma patients affected by a tumor in the left brain hemisphere appeared to suffer more frequently from fatigue than those with right-sided tumors (Table 3). Patients with left-sided tumor localization were also more prone to anxiety and depression. On the other hand, we did not observe any group differences concerning educational status, presenting symptomatology, seizure type, use of antiepileptic drugs or corticosteroids, or extent of tumor resection.
Table 2.
Comparison of glioblastoma patients with and without fatigue at baseline. Data are described as mean ± standard deviation.
| Glioblastoma Patients | Glioblastoma Patients with Fatigue (n = 31) | Glioblastoma Patients without Fatigue (n = 34) | P Value |
|---|---|---|---|
| Age (y) | 57.4 ± 10.6 | 57.2 ± 9.7 | .94 |
| Male:female | 17 (55%):14 (45%) | 27 (79%):7 (21%) | .03 |
| KPS | 82.2 ± 15.1 | 85.5 ± 11.3 | .36 |
| Tumor diameter (cm) | 4.0 ± 1.5 | 4.0 ± 1.5 | .84 |
| Tumor localization | .03 | ||
| Left | 19 (61%) | 9 (26%) | |
| Right | 12 (39%) | 19 (56%) | |
| Bilateral | 0 | 6 (18%) | |
| Tumor topography | .59 | ||
| Frontotemporal | 20 | 17 | |
| Parieto-occipital | 7 | 13 | |
| Basal ganglia | 1 | 2 | |
| Frontotemporal + parieto-occipital | 3 | 1 | |
| Parieto-occipital + basal ganglia | 0 | 1 | |
| Presenting symptomatology | .18 | ||
| Seizure | 15 | 15 | |
| Motor weakness | 5 | 7 | |
| Cognitive deficit | 8 | 7 | |
| Headache, nausea, vomiting | 8 | 5 | |
| Apathy, asthenia | 0 | 2 | |
| Visual deficit | 0 | 3 | |
| Accidental finding | 0 | 1 | |
| Seizure type | .63 | ||
| No seizure | 6 | 12 | |
| Partial | 4 | 5 | |
| Generalized | 9 | 8 | |
| Complex-focal | 2 | 0 | |
| Unclassified | 1 | 1 | |
| Partial + generalized | 2 | 3 | |
| Complex-focal + generalized | 0 | 1 | |
| Antiepileptic drugs | 19 | 22 | .49 |
| Levetiracetam | 9 | 12 | |
| Phenytoin | 9 | 7 | |
| Clobazam | 5 | 6 | |
| Lamotrigin | 2 | 3 | |
| Clonazepam | 1 | 0 | |
| Topiramat | 1 | 0 | |
| Gabapentin | 0 | 2 | |
| Valproat | 1 | 1 | |
| Extent of resection | .74 | ||
| Biopsy | 2 | 4 | |
| Partial | 22 | 20 | |
| Complete | 6 | 8 | |
| Unclear | 1 | 2 | |
| ESS score | 7.1 ± 4.9 | 4.8 ± 3.4 | .03 |
| EDS (ESS score >10) | 10 (32%) | 4 (12%) | .04 |
| Mean bedtime (h) | 9.2 ± 1.2 | 8.5 ± 1.1 | .02 |
| Long bedtime (>10 h) | 6 (19%) | 4 (12%) | .25 |
| Anxiety score | 6.2 ± 4.0 | 5.3 ± 3.7 | .32 |
| Anxiety, prevalence | 7 (23%) | 4 (18%) | .20 |
| Depression score | 5.7 ± 3.5 | 3.3 ± 3.1 | .005 |
| Depression, prevalence | 4 (13%) | 2 (6%) | .29 |
| Corticosteroids [mg] | 2.7 ± 3.7 | 2.5 ± 2.7 | .79 |
| Activating antidepressants | 3 | 2 | .46 |
| Sedating antidepressants | 1 | 1 | .73 |
| Anxiolytics | 0 | 0 | |
| CNS stimulants | 0 | 0 | |
| Hypnotics | 1 | 2 | .54 |
EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; FSS, Fatigue Severity Scale; h, hours; KPS, Karnofsky performance status; y, years.
Table 3.
Comparison of fatigue, sleepiness, and mood disorders between patients with left-sided and right-sided glioblastoma at baseline prior to radio-chemotherapy. Data are described as mean ± standard deviation.
| Left-sided Tumor | Right-sided Tumor | P Value | |
|---|---|---|---|
| (n = 28) | (n = 31) | ||
| Age (y) | 55.8 ± 9.5 | 57.9 ± 10.5 | .43 |
| Male sex | 20 (71%) | 21 (68%) | .60 |
| Karnofsky performance status | 86 ± 14 | 83 ± 12 | .40 |
| Tumor diameter (cm) | 4.0 ± 1.7 | 4.0 ± 1.3 | .92 |
| FSS | 4.6 ± 1.3 | 3.5 ± 1.8 | .008 |
| Fatigue (FSS >4.0) | 19 (68%) | 12 (39%) | .004 |
| ESS | 5.8 ± 4.2 | 6.0 ± 4.7 | .83 |
| EDS (ESS >10) | 5 (18%) | 8 (26%) | .73 |
| Mean bedtime (h) | 9.1 ± 1.4 | 8.6 ± 1.0 | .13 |
| Long bedtime (>10 h) | 7 (25%) | 3 (11) | .18 |
| HADS anxiety score | 6.6 ± 3.8 | 5.1 ± 4.0 | .16 |
| Anxiety (prevalence) | 8 (30%) | 3 (10%) | .03 |
| HADS depression score | 5.3 ± 2.9 | 3.6 ± 3.8 | .05 |
| Depression, prevalence | 2 (9%) | 4 (13%) | .49 |
EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; FSS, Fatigue Severity Scale; h, hours; HADS, Hospital Anxiety and Depression Scale; y, years.
Predictors of Fatigue Severity in Glioblastoma Patients
Using a multiple linear regression model, we identified higher HADS depression score (estimated effect = 0.16 per 1-unit increase in depression score: P = .004), left-sided tumor location (estimated effect = −0.88; P = .002), and female sex (estimated effect = 0.89; P = .02) as significant associates of fatigue severity at baseline prior to radio-chemotherapy (Table 4). The adjusted r2 and the multiple r2 of our final model were 0.29 and 0.32, respectively.
Table 4.
Multiple linear regression model for coefficients of fatigue severity at baseline in glioblastoma patients
| Dependent Variable | Significant Coefficients* | Estimated Effect | Standard Error | t Value | P Value |
|---|---|---|---|---|---|
| FSS score (baseline) | HADS score for depression | 0.16 | 0.05 | 3.03 | .004 |
| Tumor localization (left) | 0.88 | 0.28 | 3.16 | .002 | |
| Female sex | 0.89 | 0.38 | 2.38 | .020 |
*Additional coefficients included in the model were age, education, Karnofsky performance status, Epworth Sleepiness Scale, bedtimes, Hospital Anxiety and Depression Scale score for anxiety, use of steroids, and use of antidepressants.
Abbreviations: FSS, Fatigue Severity Scale; HADS, Hospital Anxiety and Depression Scale.
Evolution of Fatigue, Excessive Daytime Sleepiness, and Mood Disorders
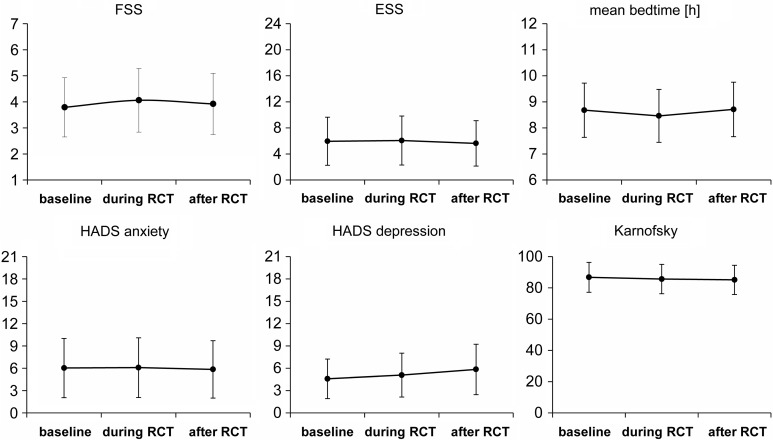
Unfortunately, the dropout rate was rather high: only 46 and 38 of the included 65 glioblastoma patients filled out all questionnaires during and after radio-chemotherapy, respectively. Mean values for FSS and ESS scores, mean bedtimes, mean values for HADS anxiety and depression scores, and KPS did not show significant changes at subsequent time points (Fig. 2).
Fig. 2.
Compared with postsurgical baseline, the scores of the Fatigue Severity Scale (FSS), Epworth Sleepiness Scale (ESS), mean bedtimes, HADS depression and anxiety, and Karnofsky performance status did not show any significant changes during and immediately after combined radio-chemotherapy (RCT). Error bars indicate standard deviations.
Discussion
Our prospective study demonstrated that fatigue is a prominent pretreatment symptom in patients with newly diagnosed and operated glioblastoma, reaching a prevalence of 48% compared with only 11% among healthy controls. Surprisingly, our data represent the first controlled assessment of fatigue frequency in a selected and homogeneous cohort of glioblastoma patients that was measured with a specific and validated fatigue questionnaire. Although direct comparison is obviously hampered by major methodological differences, our finding roughly matches the reported 40%–70% fatigue prevalence among patients with primary brain tumors.5,9,14 On the other hand, we have found higher fatigue prevalence in patients with other neurological disorders such as multiple sclerosis (69%), idiopathic Parkinson's disease (59%), episodic migraine (54%, unpublished data) or previous ischemic stroke (49%), always using the FSS.19,20
Fatigue in patients with primary brain tumors has repeatedly been reported in relation to radiotherapy.13,25,26 In contrast, our study challenges the view that fatigue in glioblastoma patients mainly represents a complication of radiotherapy and/or chemotherapy because the prevalence of fatigue was already high prior to radio-chemotherapy. Therefore, the contribution of toxicity from radio-chemotherapy to fatigue is probably only one factor among many. In addition, the longitudinal assessment of fatigue, sleepiness, and mood disturbances during and after radio-chemotherapy did not show significant changes, which might however reflect a bias due to the high dropout rate. It is conceivable, however, that the toxic effect of radio-chemotherapy on fatigue severity was obscured by the additional presence of many other fatigue-inducing factors in our cohort. Of related interest is our observation that pharmacological treatment, including antiepileptic drugs, antidepressants or corticosteroids, was not associated with fatigue. This is in contrast to a recent work by Struik et al, who reported an increase in fatigue severity among patients with low-grade glioma taking antiepileptic drugs and corticosteroids.27
While fatigue was more than 4 times more prevalent in glioblastoma patients than in controls, the frequency of excessive daytime sleepiness was similar (22% vs 19%). Fatigue and sleepiness are commonly regarded as 2 distinct symptoms, but they also present substantial overlap and presumably, at least to some extent, similar pathophysiology.28 Our finding is surprising because increased frequency of sleepiness is indeed common in many neurological disorders with prominent fatigue. For instance, we found excessive daytime sleepiness in 48% of patients with idiopathic Parkinson's disease and 38% of traumatic brain injury survivors, while other groups reported an even higher prevalence.20,29 Degenerative or trauma-induced disruption of arousal-promoting structures in the rostral brainstem and hypothalamus may cause both fatigue and sleepiness, and this assumption is increasingly supported by neuropathological evidence.30–32 On the other hand, fatigue is a complex symptom influenced by a large variety of factors, and the composition of these contributing factors most likely differs between neurological disorders commonly associated with high fatigue burden. Thus, it is tempting to speculate whether the selective increase of fatigue with normal prevalence of sleepiness may shed some light on the underlying etiology of glioblastoma-related fatigue. Of interest in this context, the discrepant prevalence of fatigue and sleepiness is reminiscent of patients with mood disorders, who often suffer from fatigue and insomnia, while sleepiness is not a consistent complaint.33 Along the same line, we could identify depression as an independent predictor of fatigue severity at baseline by using multiple regression analyses. To the best of our knowledge, this is the first study to highlight this important association between fatigue severity and depression in glioblastoma patients; similar correlations have been reported in breast cancer patients but never in patients with primary brain tumors.34,35
Anxiety and depression represent normal emotional reactions to the diagnosis of glioblastoma, and they are significant and independent contributors to fatigue severity. However, as emphasized by the overview of Litofsky and Resnick and further corroborated by our observations, other factors have to be considered.36 Of note, fatigue, anxiety and depression all appeared to be more common in glioblastoma patients with left-sided tumors compared with those having right-sided tumors; left-sided tumor location was consistently identified as an independent predictor of fatigue severity. Indeed, there is some evidence indicating that patients with left hemispheric lesions are prone to depressive reactions, whereas patients with right hemisphere lesions often show indifferent emotional reactions.37,38,39 Likewise, Klein et al found significant impairment of attentional and executive functioning in patients with left-sided high-grade glioma compared with right-sided.40 However, several studies failed to observe any association between depression and hemispheric laterality of gliomas.41 Data on depression in ischemic stroke provide a similarly inconsistent picture. Few studies suggested a higher prevalence of depression in left-sided ischemic stroke, but other studies could not confirm a significant impact of stroke location on depressive symptoms.42 Whether tumor laterality plays such a significant role in the severity of fatigue, as suggested by our study, remains to be confirmed by future works.
Similar to previous work, we found that female sex was associated with higher fatigue severity at baseline. Recently, Armstrong et al assessed fatigue in 201 patients with primary brain tumors and demonstrated that moderate-to-severe fatigue was more common in females, while low fatigue was more common in males.5
Several limitations of our study have to be acknowledged. First, the number of participants was relatively small. Second, dropout rates at subsequent time points were high and might have introduced significant bias. Our observations on evolution of fatigue, sleepiness, and mood disorders during and after radio-chemotherapy must therefore be considered with caution and require confirmation by larger studies with minimal dropout rates. However, elimination of a significant dropout will be challenging, as it is a well-known, notorious problem in longitudinal studies of primary brain tumor patients. Our dropout rate was similar, for instance, to that reported in a recent large randomized EORTC trial.43 Third, a certain selection bias is likely, as we included only 60%–65% of all eligible glioblastoma patients during the study period, which prevents direct generalizability of our findings. Finally, we did not measure the impact of fatigue and associated variables on health-related QoL.
In summary, roughly half of all glioblastoma patients are affected by fatigue at postsurgical baseline. Depression is among the strongest predictors of fatigue severity at baseline, which might also explain the unexpected absence of increased sleepiness in glioblastoma patients. Hence, treating physicians should be more vigilant for fatigue and depression in glioblastoma patients because they both represent frequent comorbidities with mutually negative repercussions and are known to negatively impact QoL, treatment compliance, and overall survival.12,16,18,44
Funding
There was no funding for this study.
Conflict of interest statement. None declared.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. Lyon: IARC; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DR, Ma DJ, Buckner JC, et al. Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer. 2012;118(22):5608–5613. doi: 10.1002/cncr.27590. [DOI] [PubMed] [Google Scholar]
- 4.Campos MP, Hassan BJ, Riechelmann R, et al. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22(6):1273–1279. doi: 10.1093/annonc/mdq458. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong TS, Cron SG, Bolanos EV, et al. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116(11):2707–2715. doi: 10.1002/cncr.25018. [DOI] [PubMed] [Google Scholar]
- 6.Berger AM, Abernethy AP, Atkinson A, et al. Cancer-related fatigue. J Natl Compr Canc Netw. 2010;8(8):904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- 7.Stone P, Richardson A, Ream E, et al. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol. 2000;11(8):971–975. doi: 10.1023/a:1008318932641. [DOI] [PubMed] [Google Scholar]
- 8.Lis CG, Rodeghier M, Grutsch JF, et al. Distribution and determinants of patient satisfaction in oncology with a focus on health related quality of life. BMC Health Serv Res. 2009;9:190. doi: 10.1186/1472-6963-9-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong TS, Gilbert MR. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro Oncol. 2012;4(suppl. 4):iv65–iv72. doi: 10.1093/neuonc/nos210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum. 1994;21(1):23–36. [PubMed] [Google Scholar]
- 11.Pachman DR, Barton DL, Swetz KM, et al. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687–3696. doi: 10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- 12.Taphoorn MJ, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: A randomized controlled trial. Lancet Oncol. 2005;6(12):937–944. doi: 10.1016/S1470-2045(05)70432-0. [DOI] [PubMed] [Google Scholar]
- 13.Faithfull S, Brada M. Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clin Oncol. 1998;10(4):250–254. doi: 10.1016/s0936-6555(98)80011-3. [DOI] [PubMed] [Google Scholar]
- 14.Lovely MP, Miaskowski C, Dodd M. Relationship between fatigue and quality of life in patients with glioblastoma multiformae. Oncol Nurs Forum. 1999;26(5):921–925. [PubMed] [Google Scholar]
- 15.Hjollund NH, Andersen JH, Bech P. Assessment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health Qual Life Outcomes. 2007;5:12. doi: 10.1186/1477-7525-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown PD, Ballman KV, Rummans TA, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76(3):283–291. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- 17.Flechl B, Ackerl M, Sax C, et al. Neurocognitive and sociodemographic functioning of glioblastoma long-term survivors. J Neurooncol. 2012;109(2):331–339. doi: 10.1007/s11060-012-0897-1. [DOI] [PubMed] [Google Scholar]
- 18.Flechl B, Ackerl M, Sax C, et al. The caregivers’ perspective on the end-of-life phase of glioblastoma patients. J Neurooncol. 2013;112(3):403–411. doi: 10.1007/s11060-013-1069-7. [DOI] [PubMed] [Google Scholar]
- 19.Valko PO, Bassetti CL, Bloch KE, et al. Validation of the fatigue severity scale in a Swiss cohort. Sleep. 2008;31(11):1601–1607. doi: 10.1093/sleep/31.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valko PO, Waldvogel D, Weller M, et al. Fatigue and excessive daytime sleepiness in idiopathic Parkinson's disease differently correlate with motor symptoms, depression and dopaminergic treatment. Eur J Neurol. 2010;17(12):1428–1436. doi: 10.1111/j.1468-1331.2010.03063.x. [DOI] [PubMed] [Google Scholar]
- 21.Cronbach LJ. Coefficient alpha and the internal structure of test. Psychometrika. 1951;16(3):97–102. [Google Scholar]
- 22.Bloch KE, Schoch OD, Zhang JN, et al. German version of the Epworth Sleepiness Scale. Respiration. 1999;66(5):440–447. doi: 10.1159/000029408. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale - a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41. doi: 10.1016/s0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 25.Drappatz J, Schiff D, Kesari S, et al. Medical management of brain tumor patients. Neurol Clin. 2007;25(4):1035–1071. doi: 10.1016/j.ncl.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Powell C, Guerrero D, Sardell S, et al. Somnolence syndrome in patients receiving radical radiotherapy for primary brain tumours: a prospective study. Radiother Oncol. 2011;100(1):131–136. doi: 10.1016/j.radonc.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Struik K, Klein M, Heimans JJ, et al. Fatigue in low-grade glioma. J Neurooncol. 2009;92(1):73–78. doi: 10.1007/s11060-008-9738-7. [DOI] [PubMed] [Google Scholar]
- 28.Hossain JL, Ahmad P, Reinish LW, et al. Subjective fatigue and subjective sleepiness: two independent consequences of sleep disorders? J Sleep Res. 2005;14(3):235–253. doi: 10.1111/j.1365-2869.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 29.Baumann CR, Werth E, Stocker R, et al. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130(7):1873–1883. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 30.Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130(6):1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 31.Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66(4):555–559. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrington ME. Neurobiological studies of fatigue. Prog Neurobiol. 2012;99(2):93–105. doi: 10.1016/j.pneurobio.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billiard M, Dolenc L, Aldaz C, et al. Hypersomnia associated with mood disorders: A new perspective. J Psychosom Res. 1994;38(suppl. 1):41–47. doi: 10.1016/0022-3999(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 34.Gaston-Johansson F, Fall-Dickson JM, Bakos AB, et al. Fatigue, pain, and depression in pre-autotransplant breast cancer patients. Cancer Pract. 1999;7(5):240–247. doi: 10.1046/j.1523-5394.1999.75008.x. [DOI] [PubMed] [Google Scholar]
- 35.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 36.Litofsky NS, Resnick AG. The relationships between depression and brain tumors. J Neurooncol. 2009;94(2):153–161. doi: 10.1007/s11060-009-9825-4. [DOI] [PubMed] [Google Scholar]
- 37.Gainotti G. Emotional behavior and hemispheric side of lesion. Cortex. 1972;8(1):41–55. doi: 10.1016/s0010-9452(72)80026-1. [DOI] [PubMed] [Google Scholar]
- 38.Irle E, Peper M, Wowra B, et al. Mood changes after surgery for tumors of the cerebral cortex. Arch Neurol. 1994;51(2):164–174. doi: 10.1001/archneur.1994.00540140070017. [DOI] [PubMed] [Google Scholar]
- 39.Pringle AM, Taylor R, Whittle IR. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery. Br J Neurosurg. 1999;13(1):46–51. doi: 10.1080/02688699944177. [DOI] [PubMed] [Google Scholar]
- 40.Klein M, Taphoorn MJB, Heimans JJ, et al. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 2001;19(20):4037–4047. doi: 10.1200/JCO.2001.19.20.4037. [DOI] [PubMed] [Google Scholar]
- 41.Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011;103(1):61–76. doi: 10.1093/jnci/djq458. [DOI] [PubMed] [Google Scholar]
- 42.Carson AJ, MacHale S, Allen K, et al. Depression after stroke and lesion location: a systematic review. Lancet. 2000;356(9224):122–126. doi: 10.1016/S0140-6736(00)02448-X. [DOI] [PubMed] [Google Scholar]
- 43.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 44.Hinz A, Krauss O, Hauss JP, et al. Anxiety and depression in cancer patients compared with the general population. Eur J Cancer Care. 2010;19(4):522–529. doi: 10.1111/j.1365-2354.2009.01088.x. [DOI] [PubMed] [Google Scholar]