Abstract
Background
Patients afflicted with low-grade glioma (LGG) frequently suffer from seizures. The mechanisms of seizure initiation in these patients remain poorly understood. Tumor location has been correlated with seizure initiation. However, these correlative studies relied on dichotomized data analysis based on arbitrary lobe assignments. As a result, the lesion-symptom correlation may be incorrectly interpreted. Here, we present the first study that used a voxel-wise quantitative lesion analysis to investigate the spatial correlation between tumor location and seizure susceptibility.
Methods
We collected the medical records and magnetic resonance images of 410 LGG patients. The dataset was divided into a discovery set and a validation set. A voxel-based lesion-symptom correlative analysis was performed to determine whether tumor location was associated with seizure risk and could be related to the specific type of seizure.
Results
For all seizure types, increased seizure risks were identified for LGGs that involved the left premotor area. The LGGs that involved the posterior portion of the left inferior and middle frontal gyrus were associated with increased risk of simple partial seizures. LGGs that involved the right temporal-insular region were associated with an increased risk of complex partial seizures. LGGs that involved the left premotor area were more likely to be associated with seizures that generalize. These correlations were consistently observed in both the discovery and the validation datasets.
Conclusions
Our quantitative neuroimaging analyses support the concept that the anatomic location of an LGG is a contributing factor in tumor-related seizure.
Keywords: lesion-symptom mapping, low-grade glioma, seizure
Seizure is a frequent presenting symptom of brain tumors.1 These seizures and the side effects of the antiepileptic drugs used to treat them have a profound impact on patient quality of life.2–5 However, the underlying etiology of tumor-induced seizures is not fully understood.6 The tumor type, location, peritumoral environment, and altered expression of the genes mediating neurotransmission have been implicated as risk factors.6–11 However, the pertinence of these variables to specific brain tumor types has not been carefully scrutinized.
Diffuse low-grade glioma (LGG; World Health Organization grade II)12 is the most common type of primary brain tumor in young adults.13 Most patients with LGG experience seizure either as a presenting symptom or sometime during the course of the disease.3,7 Little is known about the etiology of these seizures. While studies have suggested a correlation between tumor location and seizure risk, there is a significant discrepancy in terms of the results.1,3,14–16 Moreover, seizure foci distant from the location of the LGG have also been documented.17 Most of these studies were limited by a retrospective study design, an MR assessment based on qualitative measures, insufficient sample sizes, and/or a lack of a validation cohort.
In recent years, voxel-based lesion-symptom mapping (VLSM) has been increasingly used for determining the relationships between brain lesions and clinical manifestations.18–22 Bates and colleagues18 first used this technique to localize the brain regions critical for speech fluency and language comprehension on a voxel level in patients with stroke. Using this approach, other studies have also measured specific neural correlates for various neurological symptoms, such as hemispatial neglect,19 postural difficulties,22 and decision-making disorder.20 Because it is based on high-resolution structural imaging rather than on a dichotomous group classification, VLSM can provide a statistical comparison of symptomatic differences between lesion and nonlesion voxels at the individual voxel level. Thus, this analysis can detect effects such as the lesion location, boundary, and volume across the entire brain without losing information.23
In this study, we present the results of a prospectively collected dataset of 410 LGG patients. To make a valid statistical inference, the study population was divided into a discovery set (n = 231) and a validation set (n = 179). A quantitative VLSM analysis was performed to determine whether tumor location was associated with seizure risk and was further related to the type of seizure. Our results indicated that LGGs that involved distinct anatomic locations were associated with differing seizure risks.
Materials and Methods
Patients
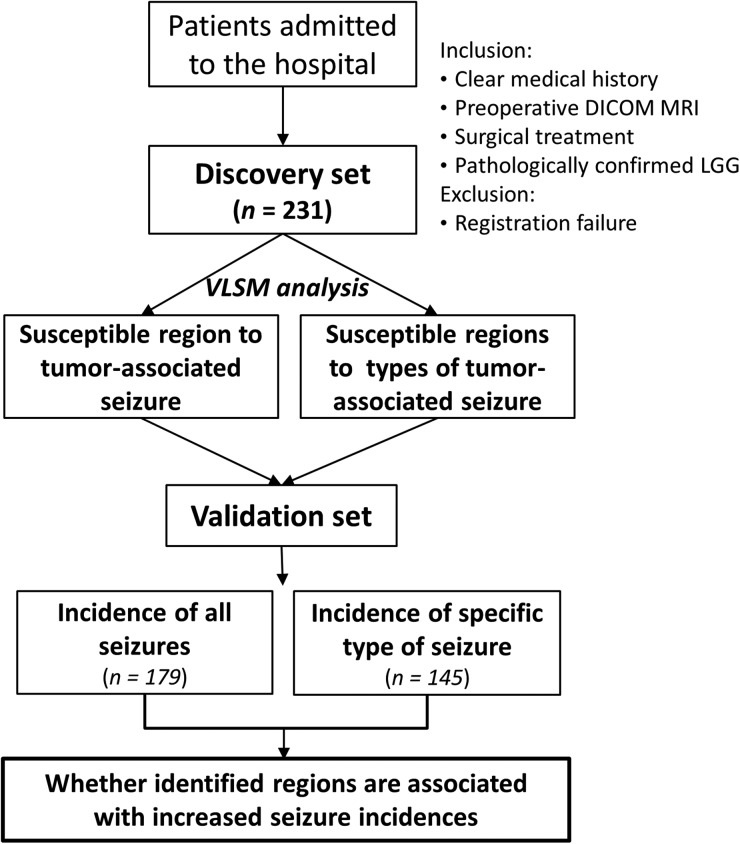
Clinical data and magnetic resonance images from a total of 410 patients with LGG were used in this study. These included a discovery set of 231 cases prospectively collected at Beijing Tiantan Hospital between September 2006 and December 2011 and an external validation set of 179 cases retrospectively collected from the China Glioma Genome Atlas (n = 145) database and The Cancer Genome Atlas (TCGA) (n = 34) database. All patients included in this study met the following criteria: pathologically confirmed World Health Organization grade II LGG,24 presurgical T2 MRIs of the brain, and no prior craniotomy or stereotactic biopsy. An additional 14 patients were excluded from the analysis due to apparent image distortion (examined by a senior neuroradiologist) after normalizing to the Montreal Neurological Institute (MNI) space. The remaining patient characteristics, including age, sex, tumor location, and seizure history, are summarized in Table 1. This study was approved by the ethics committee of Beijing Tiantan Hospital, and written informed consent was obtained from all patients in the discovery set in accordance with the 1991 Declaration of Helsinki. Our study design is illustrated in Fig. 1.
Table 1.
Clinical characteristics of 231 patients (discovery set)
| Characteristics | Seizures | No Seizures | P |
|---|---|---|---|
| Number of patients (%) | 152 (65.8) | 79 (34.2) | |
| Age, y, median (range) | 37 (15–64) | 41 (17–67) | .056a |
| Sex, M/F | 93/59 | 42/37 | .241 |
| Lesion location (% involvement) | |||
| Hemisphere, L/R | 82/70 | 42/37 | .910 |
| Frontal | 108 (71.1) | 39 (49.4) | .001 |
| Temporal | 39 (25.7) | 28 (35.4) | .120 |
| Insula | 30 (19.8) | 16 (20.2) | .926 |
| Parietal | 22 (14.5) | 12 (15.2) | .884 |
| Occipital | 3 (2.0) | 1 (1.3) | .696 |
| MRI characteristics | |||
| Tumor size, cm3, mean ± SD | 73.6 ± 53.3 | 72.3 ± 61.8 | .873a |
| Tumor pathology (%) | |||
| Oligodendroglioma | 15 (9.9) | 10 (12.7) | .517 |
| Astrocytoma | 48 (31.6) | 22 (27.8) | .558 |
| Oligoastrocytoma | 89 (58.5) | 47 (59.5) | .890 |
aResult of t-test.
Fig. 1.
Study design. The hypotheses of this study were that heterogeneous susceptibility to tumor-associated seizure exists between brain regions and that tumors involving specific brain regions could be associated with increased seizure risk. To test these hypotheses, we first prospectively examined a discovery set cohort by performing a regression-model based analysis to locate the tumor-associated seizure-susceptible regions. These VLSM-identified regions were subsequently validated by an external dataset to examine whether the identified regions were associated with increased seizure incidence in tumor patients. DICOM, Digital Imaging and Communications in Medicine (file format).
Evaluation of Tumor-related Seizure
A patient with tumor-related seizure was defined as having a history of at least one seizure with the presence of an enduring alteration (ie, LGG) in the brain.25 A history of seizure(s) and seizure types (generalized, simple partial, complex partial) in the patients was evaluated at the time of discovery by an epileptologist based on the presentation of the seizures according to the classification and terminology of the International League Against Epilepsy26 (Table 2). The types of seizures were identified for 152 and 66 patients with a history of seizure(s) in the discovery and validation sets, respectively, based on the consistent criteria referenced above. Information about seizure type was not available in the database of TCGA.
Table 2.
Characteristics of 3 seizure types (discovery set)
| Characteristics | Generalized | Simple Partial | Complex Partial | P |
|---|---|---|---|---|
| Number of patients (%) | 107 (46.3) | 17 (7.4) | 28 (12.1) | |
| Age, median (range) | 37 (15–64) | 39 (15–61) | 38.5 (21–64) | .551a |
| Sex, M/F | 65/42 | 12/5 | 16/12 | .659 |
| Hemisphere, L/R | 61/46 | 7/10 | 14/14 | .428 |
| Tumor size, cm3, mean ± SD | 69.1 ± 49.4 | 84.9 ± 61.6 | 82.2 ± 60.8 | .292a |
| Tumor pathology (%) | .099 | |||
| Oligodendroglioma | 11 (10.3) | 2 (11.8) | 2 (7.1) | |
| Astrocytoma | 29 (27.1) | 4 (23.5) | 15 (53.6) | |
| Oligoastrocytoma | 67 (62.6) | 11 (64.7) | 11 (39.3) |
Seizure classification based on 1981 Classification of Epileptic Seizures (International League Against Epilepsy).
aResult of t-test.
Brain Imaging and Tumor Masking
MRI scans of the majority of the patients (n = 189) were performed on a Magnetom Trio 3T (Siemens) with an 8-channel receive-only head coil. The T2 image parameters included: repetition time = 5800 ms; echo time = 110 ms; flip angle = 150 degrees; 24 slices; field of view = 240 × 188 mm2; voxel size = 0.6 × 0.6 × 5 mm3; matrix = 384 × 300. Other clinical structural images were acquired on a Magnetom Verio 3T (Siemens) or HD 1.5T (GE Medical System) scanner. Tumors were traced directly on the brain MRIs using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron). Masks of the brain tumors were drawn on each patient's T2 image in native space by 2 board-certified neurosurgeons, who were blinded to the patients' clinical information. The areas that produced abnormal hyperintensive signals on the T2 images of the patients were identified as tumor areas. The tumor masks were combined when there was less than a 5% discrepancy between the individual masks identified by the 2 neurosurgeons, and the masks we used were determined by a senior neuroradiologist when the individual masks from the 2 neurosurgeons were inconsistent (>5%). The T2 image and tumor mask for each patient were registered to the MNI template using the standard nonlinear spatial normalization algorithm provided by SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Subsequently, the registered tumor masks were again manually checked by the senior neuroradiologist.
Lesion Analysis
A VLSM analysis18,23 was used in 231 patients (discovery set) divided into 2 groups (left and right hemispheres) based on the primary location of their tumors. VLSM is based on applying the general linear model (GLM) (Y = βX + ε) to each voxel independently. For each voxel, Y represents tumor involvement (1 indicates that a voxel is inside the masked tumor area, while 0 indicates that it is outside) in each and every patient. X represents the symptom matrix, which consists of several columns corresponding to the classification of different symptoms in all patients and one constant column. Each row in X represents one patient. β represents the model parameter to be estimated, and ε is the estimated residual. GLM fitting provides the t-statistic value for β, showing the sensitivity of the voxel to the respective symptom (seizure in this case).
First, to statistically analyze each group, we applied VLSM analyses to determine the general neural correlates of all the tumor-related seizures. The symptom matrix X consisted of one symptom column and one constant column. For each row in X, 1 indicated patients with seizures, 0 indicated no seizures. Second, we fitted the GLM with 3 variables representing different symptoms (types of preoperative seizure). The symptom matrix X consisted of 3 symptoms and one constant column. For each row in X, [1 0 0] indicated patients with simple partial, [0 1 0] indicated patients with complex partial, and [0 0 1] indicated patients with generalized seizures. Then for each voxel, the t-statistic values for each symptom were calculated separately. The statistical threshold of each VLSM test was determined based on permutation testing (n = 1000).27 The t-value of the voxels that were greater than the t-values in >95% of permutations was retained in the VLSM results (alpha set at .05, power >0.823). The “seizure-susceptible area” was defined as the largest cluster of significant voxels acquired by this method.
Evaluation of VLSM-identified Regions
The VLSM-localized seizure-susceptible brain areas (as defined by their peak voxels [PVs]) were validated by examining the incidence of seizure following tumor involvement in the validation set. We chose to use the PV of each VLSM-identified area in order to eliminate tumors that only touched the edge of the area. Thus, involvement/noninvolvement was defined by whether a masked tumor area contained the VLSM-identified PV in MNI brain space. A chi-square test was used to compare the incidence of tumor-related seizure between subgroups with LGGs that involved or did not involve the PV. The same statistical analysis was then performed for each type of tumor-related seizure. Therefore, each of the VLSM-identified PVs (PV1, PV2, and PV3 for simple partial, complex partial, and generalized seizures, respectively) was examined to determine whether LGGs were located or not located in these areas.
Results
Demographic and Clinical Data
The main clinical and pathological characteristics of all 231 patients (discovery set) are listed in Table 1. Of the 231 patients, 124 (53.7%) had left-sided tumors and 107 had right-sided tumors; 152 (65.8%) had a history of preoperative seizures before being admitted to the hospital. A total of 179 patients (the validation set) were gathered from 2 external sources. Most of these were obtained from the China Glioma Genome Atlas database (145 cases) and the rest came from the open database of TCGA (34 cases). In the validation set, 88 cases had a history of preoperative seizures, and 66 of them for whom seizure symptoms were available were categorized into 3 subgroups according to seizure type (Supplementary Table S1).
Radiographic Characteristics
An overlap map was constructed to present an overview of all lesions in the discovery cohort of the 231 LGG patients (Fig. 2). This map shows that several brain regions, including the bilateral frontal lobes, insula, and temporal lobes, have large numbers of overlapping tumor masks. In addition, a power map23 was computed to show the distribution of voxels with adequate statistical power to detect effects in the VLSM analysis (P < .05). In this study, only the results located in regions with sufficient power (>0.8) were considered. The power map shows that the majority of areas in both hemispheres were highly reliable for statistical analysis (Fig. 2).
Fig. 2.
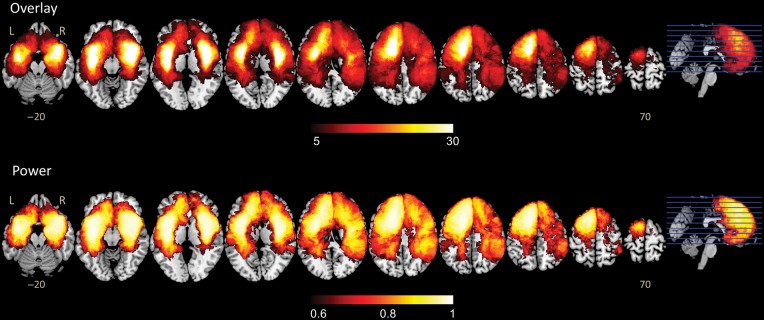
Tumor overlap and power map. (A) Overlaps of the brain lesions for all 231 patients. The color range indicates the proportion of overlap from dark (overlap of 5 cases) to light (overlap of ≥30 cases). (B) Power distribution map (0.6–1.0) over all brain regions. Only voxels with high power values (>0.8) were included in the voxel-based lesion-symptom analysis.
VLSM Analysis Findings
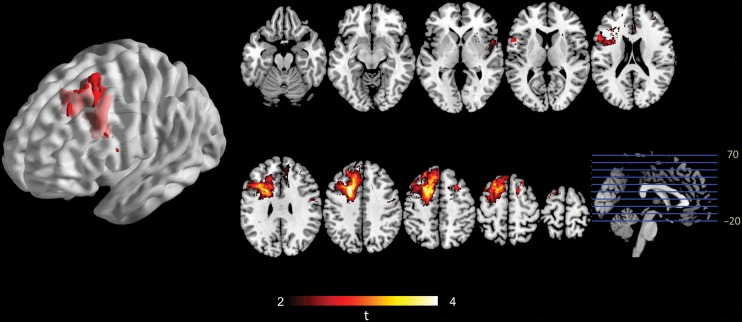
The VLSM results from the discovery cohort were mapped onto the brain structure template in MNI space. The voxels that significantly correlated to tumor-related seizure were primarily located in the left premotor area, with a small portion in the left cingulate cortex. The PV (X = 114, Y = 141, Z = 116, tmax = 4.60) of this cluster had the highest correlation with tumor-related seizure according to the method we used in this study (Fig. 3).
Fig. 3.
VLSM analysis showing seizure-susceptible brain regions associated with LGG. A voxel-wise comparison was performed between patients with and without seizures. The left premotor area is significantly susceptible to tumor-related seizures. The color range indicates the level of t-values from dark to light (least to most significant). Only significant voxels are rendered based on a critical threshold determined by permutation testing (n = 1000, P < .05).
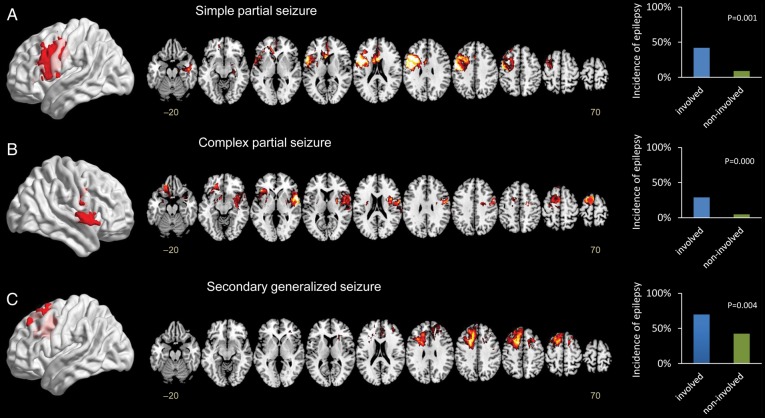
Using a symptom-based classification of seizures, the t-maps obtained using each of the symptom contrast vectors in the VLSM analysis are presented in Fig. 4. Notably, the VLSM results from the 3 subgroups revealed unique distributions of the seizure-susceptible regions that correspond to different seizure types. For the simple partial seizure group, the significant seizure-related region was located in the posterior portion of the left inferior and middle frontal gyrus (PV1, X = 136, Y = 140, Z = 99, tmax = 6.16), involving Broca's area and the primary motor area. For complex partial seizures, the region with the greatest effect was located in the right temporal-insular area (PV2, X = 35, Y = 138, Z = 70, tmax = 5.16). For cases presenting with generalized seizures, the left premotor area was identified as the critical seizure-related region (PV3, X = 114, Y = 141, Z = 116, tmax = 4.37).
Fig. 4.
VLSM analyses showing the symptom-specific seizure-susceptible brain regions associated with LGG. A voxel-wise comparison was performed between patients with a specific type of seizure (A–C) and those without. Significant clusters (P < .05) were located in the posterior portion of the left inferior and middle frontal gyrus for simple partial seizures, the right temporal-insular region for complex partial seizures, and the left premotor area for generalized seizures. The color range indicates the level of t-values from dark to light (least to most significant). Only significant voxels are shown based on a critical threshold determined by permutation testing (n = 1000, P < .05). The graphs on the right show the incidence of tumor-associated seizure in each group by different seizure types (A–C) between lesions that involved or did not involve the PVs.
Validation of VLSM-classified Seizure-susceptible Areas
Overall, 93.9% (31/33) of the patients in the discovery set and 84.2% (16/19) of the patients in the validation set had seizures (including all types) when their tumors involved the VLSM-classified PV region. In contrast, 61.4% (121/197) of the patients in the discovery set and 45.6% (72/158) of the patients in the validation set suffered from seizures (including all types) when the tumor was not located in the PV region (see Supplementary Table S1). In this study, 73.5% (108/147) of the LGGs located in the frontal lobe caused seizures when we used a typical dichotomous categorization.
Notably, significant statistical differences in the incidence of each seizure type were identified between tumors that were located in susceptible areas and those that did not involve the susceptible areas. In the discovery set, 42.1% (8/19) of patients experienced simple partial seizures when gliomas included the PV1 (X = 136, Y = 140, Z = 99) region. However, only 9.4% (20/212) of patients with tumors that did not involve this region had simple partial seizures (P = .001, chi-square test). A total of 29.2% (7/24) of patients with tumors that involved the PV2 region presented with complex partial seizures versus 4.8% (10/207) of those without PV2 involvement (P = .000, chi-square test). A total of 69.7% (23/33) of patients presented with generalized seizures when their tumors involved the PV3 region, whereas only 42.4% (84/198) had generalized seizures when their tumors had no involvement with this region (P = .004, chi-square test). Moreover, a statistical comparison using the validation set showed the same significant differences in seizure incidence between tumors that did and did not involve the seizure type–specific susceptible regions classified by VLSM (see Supplementary Table S1).
Discussion
This is the largest prospective study to explore the correlation between LGG location and seizure risk by seizure subtype (simple partial, complex partial, and seizures that generalized). It is the first to employ a discovery/validation design to ensure the generalizability of the results. Moreover, the study utilized a voxel-based quantitative analysis of MRIs as the basis for the correlation. Our study revealed that LGG location is strongly associated with seizure risk. Specifically, we found that LGGs that involved the posterior portion of the left inferior and middle frontal gyrus (Broca's area and inferior primary motor area) were associated with increased risk of simple partial seizures; LGGs that involved the right temporal-insular region were associated with increased risk of complex partial seizure; and LGGs that involved the left premotor area were more likely to be associated with seizures that generalize. By demonstrating that the specific brain structures affected by LGGs are associated with increased seizure risk, our results indicate that the anatomic location of an LGG is an influential factor in the initiation of seizure, even those that start in areas distant from the tumor.
The specific locations of the regions of the cerebrum that are at risk for seizure as a result of LGGs suggest that LGG-induced seizure may be related to the seizure susceptibility of the region involved by a tumor. Previous studies have suggested that brain regions harbor differential seizure risks, with the frontal lobe at a higher risk of containing seizure foci.3,9,15,17 This study further suggested that the left premotor area is specifically associated with increased seizure susceptibility in LGG patients. One possible explanation is that the physiologic stress associated with the LGG exacerbated an inherently lowered seizure threshold of a region. Similarly, increased seizure risk has been associated with tumor lesions in the right temporal-insular region.14 The role of dominant hemispheric eloquent areas (the left inferior frontal) in seizure initiation has also been supported by intraoperative electrophysiological evidence.28,29 In this context, it is likely that LGGs contribute to seizure initiation, at least in part by compromising the tumor-associated seizure susceptibility.
Categorizing patients according to tumor involvement in a single brain area might cause researchers to overlook important information in 2 ways. First, lesion volume is ignored. Thus, tumors that invaded more than one lobe may have been counted repeatedly. Tumors that are larger in size have a higher possibility of involving a specific susceptible seizure-initial zone. Second, the extent (number of voxels) of a lesion involved in a specific brain region can rarely be assessed. Since different subregions in a single brain lobe might have a nonuniform potential for causing seizures, both lesion volume and extent in the brain regions of interest should be considered in localizing tumor-related seizure. Inconsistent incidences of seizure (38.5% to 85.7%) were identified by previous studies for LGGs involving the frontal lobe.1,3,9 Remarkably, the involvement of VLSM-identified regions accounted for 93.9% of the seizures in this cohort of patients, a finding that suggests that anatomic specificity may exist for tumor-related seizures.
The mechanism underlying tumor-related seizure initiation has yet to be clarified. The identified correlation between tumor locations with seizure in this study does not imply that the seizure-initial foci were located inside the tumors. When a tumor was located in the identified seizure-susceptible areas, the patient was more likely to have seizures that could be in brain regions that were inside, adjacent to, or distant from the tumor area. Meanwhile, LGGs that were not located in the VLSM-identified regions still have a possibility of inducing seizures, since location is one of the significant factors rather than the only factor in causing seizure.6,8 Areas that showed abnormal hyperintensive signals on the T2 images of the patients may contain tumor cells, show evidence of gliosis, be caused by edema, or contain other abnormal histologic components. These areas could initiate seizures and were thus included in the regions that we identified as tumor areas.
There are several limitations to the current study, including the nonrandomized nature of the study design, the lack of electroencephalography examination, and the diagnosis of seizure based on patient/witness reports and clinical criteria, as well as inherent challenges such as distortions of the brain structure in the radiographic assessment of LGGs on MRI scans. Potential differences among different types of MRI scanners in the presentation of LGGs have not yet been investigated and were not considered in this study. Because of this potential heterogeneity, the masking process and the segmentation process were manually performed and supervised by independent neuroradiologists. In addition, heterogeneity of seizure susceptibility within a T2 signal abnormality was not considered in this study. Despite the above limitations, the results reported here were highly reproducible between the 2 distinct study cohorts, including a discovery set with prospectively collected data. Further, statistics-based quantitative analysis was employed to achieve objective MR interpretations. For these reasons, we consider the associations reported here to be robust and generalizable. The effect of location on the frequency of tumor-related seizure is another interesting issue that needs further investigation.
In summary, our prospective study demonstrated correlation between LGG location and seizure risk. The findings were highly reproducible between 2 independent patient cohorts. The identification of seizure-susceptible regions furthers our understanding of the etiology of LGG-related seizure. Additionally, these findings provided new evidence that may eventually be useful for customized seizure management, but currently further evidence is needed.
Supplementary Material
Funding
This study was supported by the National 973 Program (grant no. 2011CB707804) and the National Natural Science Foundation of China (grant no. 81301112).
Supplementary Material
Acknowledgments
We would like to thank Cissy Yang (Stanford University School of Medicine, USA) for her assistance in preparing this manuscript. We also thank Drs Edmund F. and Rhoda E. Perozzi for content and English editing assistance.
Conflict of interest statement. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Lynam LM, Lyons MK, Drazkowski JF, et al. Frequency of seizures in patients with newly diagnosed brain tumors: a retrospective review. Clin Neurol Neurosurg. 2007;109(7):634–638. doi: 10.1016/j.clineuro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Hildebrand J, Lecaille C, Perennes J, et al. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005;65(2):212–215. doi: 10.1212/01.wnl.0000168903.09277.8f. [DOI] [PubMed] [Google Scholar]
- 3.Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108(2):227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 4.Taphoorn MJ. Neurocognitive sequelae in the treatment of low-grade gliomas. Semin Oncol. 2003;30(6 Suppl 19):45–48. doi: 10.1053/j.seminoncol.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Maschio M, Dinapoli L. Patients with brain tumor–related epilepsy. J Neurooncol. 2012;109(1):1–6. doi: 10.1007/s11060-012-0867-7. [DOI] [PubMed] [Google Scholar]
- 6.Weller M, Stupp R, Wick W. Epilepsy meets cancer: when, why, and what to do about it? Lancet Oncol. 2012;13(9):e375–e382. doi: 10.1016/S1470-2045(12)70266-8. [DOI] [PubMed] [Google Scholar]
- 7.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 8.de Groot M, Reijneveld JC, Aronica E, et al. Epilepsy in patients with a brain tumour: focal epilepsy requires focused treatment. Brain. 2012;135(Pt 4):1002–1016. doi: 10.1093/brain/awr310. [DOI] [PubMed] [Google Scholar]
- 9.Liigant A, Haldre S, Oun A, et al. Seizure disorders in patients with brain tumors. Eur Neurol. 2001;45(1):46–51. doi: 10.1159/000052089. [DOI] [PubMed] [Google Scholar]
- 10.You G, Huang L, Yang P, et al. Clinical and molecular genetic factors affecting postoperative seizure control of 183 Chinese adult patients with low-grade gliomas. Eur J Neurol. 2012;19(2):298–306. doi: 10.1111/j.1468-1331.2011.03509.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang L, You G, Jiang T, et al. Correlation between tumor-related seizures and molecular genetic profile in 103 Chinese patients with low-grade gliomas: a preliminary study. J Neurol Sci. 2011;302(1–2):63–67. doi: 10.1016/j.jns.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Scheithauer BW, Fuller GN, VandenBerg SR. The 2007 WHO classification of tumors of the nervous system: controversies in surgical neuropathology. Brain Pathol. 2008;18(3):307–316. doi: 10.1111/j.1750-3639.2008.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist. 2006;11(6):681–693. doi: 10.1634/theoncologist.11-6-681. [DOI] [PubMed] [Google Scholar]
- 14.Lee JW, Wen PY, Hurwitz S, et al. Morphological characteristics of brain tumors causing seizures. Arch Neurol. 2010;67(3):336–342. doi: 10.1001/archneurol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffau H, Capelle L, Denvil D, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98(4):764–778. doi: 10.3171/jns.2003.98.4.0764. [DOI] [PubMed] [Google Scholar]
- 16.Pallud J, Audureau E, Blonski M, et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 2014;137(Pt 2):449–462. doi: 10.1093/brain/awt345. [DOI] [PubMed] [Google Scholar]
- 17.Ruda R, Bello L, Duffau H, et al. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14(Suppl 4):iv55–iv64. doi: 10.1093/neuonc/nos199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 19.Verdon V, Schwartz S, Lovblad KO, et al. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. 2010;133(Pt 3):880–894. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- 20.Glascher J, Adolphs R, Damasio H, et al. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci USA. 2012;109(36):14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thieme A, Thurling M, Galuba J, et al. Storage of a naturally acquired conditioned response is impaired in patients with cerebellar degeneration. Brain. 2013;136(Pt 7):2063–2076. doi: 10.1093/brain/awt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rousseaux M, Honore J, Vuilleumier P, et al. Neuroanatomy of space, body, and posture perception in patients with right hemisphere stroke. Neurology. 2013;81(15):1291–1297. doi: 10.1212/WNL.0b013e3182a823a7. [DOI] [PubMed] [Google Scholar]
- 23.Kimberg DY, Coslett HB, Schwartz MF. Power in voxel-based lesion-symptom mapping. J Cogn Neurosci. 2007;19(7):1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- 24.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 26.Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22(4):489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 27.Medina J, Kimberg DY, Chatterjee A, et al. Inappropriate usage of the Brunner-Munzel test in recent voxel-based lesion-symptom mapping studies. Neuropsychologia. 2010;48(1):341–343. doi: 10.1016/j.neuropsychologia.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szelenyi A, Joksimovic B, Seifert V. Intraoperative risk of seizures associated with transient direct cortical stimulation in patients with symptomatic epilepsy. J Clin Neurophysiol. 2007;24(1):39–43. doi: 10.1097/01.wnp.0000237073.70314.f7. [DOI] [PubMed] [Google Scholar]
- 29.Sartorius CJ, Berger MS. Rapid termination of intraoperative stimulation-evoked seizures with application of cold Ringer's lactate to the cortex. Technical note. J Neurosurg. 1998;88(2):349–351. doi: 10.3171/jns.1998.88.2.0349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.