Abstract
Background
We determined the impact of genetic alterations in EGFR, ALK, or KRAS on survival after radiotherapy for brain metastases in non–small cell lung cancer (NSCLC).
Methods
Of 172 genotyped NSCLC patients treated with radiotherapy for brain metastases in 2005–2012, 54 had cancers with EGFR mutations, 12 had ALK rearrangements, 38 had KRAS mutations, and 68 were wild-type (WT). Overall survival (OS) was determined.
Results
Median follow-up was 8.6 months. Median OS was 13.6 months for patients with EGFR mutations and 26.3 months for patients with ALK rearrangements, in contrast to 5.7 months for KRAS-mutant patients and 5.5 months for WT patients (P = .001). On multivariate analysis, adjusting for receipt of targeted therapy after cranial radiotherapy, ALK rearrangements were associated with improved OS (HR, 0.31; 95% CI, 0.13–0.74; P = .008). EGFR mutations were not significantly associated with improved OS on multivariate analysis (HR, 0.71; 95% CI, 0.37–1.38; P = .3). KRAS mutations were also not associated with improved OS (HR, 0.93; 95% CI, 0.59–1.47; P = .8). Receipt of targeted therapy after cranial radiotherapy was independently associated with improved OS (HR, 0.30; 95% CI, 0.17–0.54; P < .001). Receipt of chemotherapy after cranial radiotherapy, number of brain metastases, extracranial metastases, age, and performance status were also associated with OS.
Conclusions
NSCLC patients with genetic alterations in ALK have improved survival outcomes after radiotherapy for brain metastases compared with EGFR, KRAS, or WT. Subsequent receipt of targeted therapy was associated with additional improvement in OS.
Keywords: ALK, brain metastases, EGFR, non–small cell lung cancer, radiotherapy
Non–small cell lung cancer (NSCLC) is increasingly defined by characteristic molecular changes in driver oncogenes. These include activating mutations in the epidermal growth factor receptor (EGFR)1 and Kirsten rat sarcoma viral oncogene homolog (KRAS) genes2 as well as rearrangements in anaplastic lymphoma kinase (ALK).3 An analysis of 800 tumor samples by the Lung Cancer Mutation Consortium identified mutations in 54% of samples, with KRAS mutations (22%), EGFR mutations (17%), and ALK rearrangements (7%) being most common.4
The development of targeted therapy with tyrosine kinase inhibitors (TKIs) has led to improved outcomes for patients with EGFR mutations5,6 or ALK rearrangements.7 Treatment with EGFR TKIs (eg, erlotinib, gefitinib, and afatinib) in patients harboring EGFR mutations significantly improved progression-free survival (PFS) compared with chemotherapy.8–11 Similarly, in a recent phase III trial, ALK-positive patients treated with the ALK TKI crizotinib in the second-line setting experienced improved PFS compared with standard chemotherapy.12 Despite the impact of TKIs in patients with EGFR mutations and ALK rearrangements, there are currently no targeted therapy options for patients with KRAS mutations13 or wild-type (WT) patients without a known driver mutation.
Brain metastases are common in NSCLC, occurring in 20%–40% of patients,14,15 and are associated with a poor median survival of 4–8 months.16–18 The primary treatment for brain metastases is cranial radiotherapy, delivered using whole brain radiotherapy (WBRT), involved field radiotherapy (IFRT) to a smaller region of brain,19 or stereotactic radiosurgery (SRS), with or without surgical resection.20,21 Advancements in targeted therapy have led to the use of TKIs as initial therapy for selected patients with EGFR mutations or ALK rearrangements, typically with asymptomatic brain metastases and extracranial disease. However, radiotherapy remains the standard of care for the majority of NSCLC patients, including those with EGFR or ALK genetic alterations and symptomatic brain metastases, progressive brain metastases, or larger disease burden, and all patients without EGFR or ALK genetic alterations.22,23 EGFR TKIs such as erlotinib are known to have some penetration of the blood-brain barrier.24,25 Limited data on the ALK TKI crizotinib have suggested some central nervous system (CNS) activity,26,27 and second-generation ALK TKIs with improved CNS penetration are under development.28 Thus, the use of TKIs for patients with brain metastases and genetic alterations in EGFR or ALK is an area of ongoing investigation.
Currently, little is known about the relationship between NSCLC genetic subtype and prognosis after radiotherapy for brain metastases. The purpose of this study was to determine the significance of EGFR, ALK, and KRAS genetic alterations on outcomes after radiotherapy for brain metastases in NSCLC.
Materials and Methods
Case Identification
This retrospective study was approved by our Institutional Review Board. Patients with NSCLC were included if they were treated with radiotherapy for brain metastases at Massachusetts General Hospital (MGH) from 2005–2012 and had available tumor genotyping information. Our institution is a referral-based center with ample opportunities for mutation-specific clinical trials and novel treatments, thus attracting a unique distribution of patients, many of whom sought genotype testing.
Genotyping
Genotyping has been performed for NSCLC as part of routine care at MGH since 2004, at the clinical discretion of the treating physician. EGFR mutations were assessed by analyzing the EGFR kinase domain (exons 18–24) by polymerase chain reaction (PCR) and capillary gel electrophoresis. Since 2009, the allele-specific assay SNaPshot (Versions 1–3; Applied Biosystems) has also been used to detect >50 hot-spot mutation sites in 14 cancer genes.29,30 EGFR mutations detected included G719-2155G, G719-2156G, T790-2369C, L858-2573T, L861-2582T, E746_A750-2235_2249del, and E746_A750-2236_2250del. In addition, a separate PCR was used to detect in-frame activating insertions or deletions in EGFR exons 19 and 20. KRAS mutations were detected by SNaPshot and included G12-34G, G12-35G, G13-37G, G13-38G, Q61-181C, Q61-182A, and Q61-183A. ALK rearrangements were identified by fluorescence in situ hybridization (Vysis LSI ALK [2p23] Dual Color, Break Apart Rearrangement Probe, Abbott Molecular). Samples were considered positive if more than 15% of cells showed split signals.31 The majority of patients were genotyped at the time of initial diagnosis of NSCLC, typically from intrathoracic tissue such as the primary tumor or an involved lymph node. For a minority of those who had surgical resection of a brain metastasis (6% of all patients) and did not have prior genetic testing, genotyping was performed on the brain metastasis specimen.
Outcomes
Overall survival (OS) was assessed, calculated from the last day of the initial course of cranial radiotherapy. Electronic medical records and the Social Security Death Index were reviewed to determine the dates of patients' deaths.
Statistical Analysis
Fisher' exact tests were used for descriptive analyses. Survival curves were calculated using the Kaplan-Meier method and compared by log-rank tests. Multivariable analyses were conducted using Cox proportional hazards models, with selection of variables prior to analysis based on literature review and scientific principles. All P values were 2-tailed.
Results
Demographic, Clinical, and Brain Metastases Characteristics
Of 525 NSCLC patients treated with radiotherapy for brain metastases at our institution during the study period, 172 patients (33%) underwent genotyping that identified 54 with EGFR mutations (31%), 12 with ALK rearrangements (7%), 38 with KRAS mutations (22%), and 68 WT patients (40%). Median follow-up was 8.6 months for all patients (range, 0.2–131.3 months) and 9.5 months for surviving patients (range, 1.3–29.4 months). The median age was 60 years (range, 21–86 y). The 4 subgroups were not significantly different with respect to age, sex, race, or performance status (Table 1). There were higher rates of smokers in the KRAS and WT subgroups than in the EGFR and ALK subgroups (P < .0001). Patients initially presented with stage IV NSCLC in 74% of cases, and 56% had brain metastases at diagnosis. There was no significant association between genetic alteration status and presence of brain metastases at diagnosis of NSCLC or extent of extracranial disease at diagnosis of brain metastases. The 4 subgroups were not significantly different with respect to number, neuroanatomic location, or size of brain metastases.
Table 1.
Demographic, clinical, and brain metastases characteristics
| Characteristic | All n (%) | EGFR n (%) | ALK n (%) | KRAS n (%) | WT n (%) | P value |
|---|---|---|---|---|---|---|
| Number of patients | 172 | 54 (31) | 12 (7) | 38 (22) | 68 (40) | – |
| Median age at diagnosis of BM (years) | 60 | 58 | 58 | 59 | 66 | .3 |
| Sex | .2 | |||||
| Male | 77 (45) | 21 (39) | 4 (33) | 15 (39) | 37 (54) | |
| Female | 95 (55) | 33 (61) | 8 (67) | 23 (61) | 31 (46) | |
| Ethnic origin | .06 | |||||
| Asian | 16 (9) | 11 (20) | 0 (0) | 1 (3) | 4 (6) | |
| Caucasian | 143 (83) | 38 (70) | 12 (100) | 34 (89) | 59 (87) | |
| Other | 13 (8) | 5 (9) | 0 (0) | 3 (8) | 5 (7) | |
| Smoking status | <.001 | |||||
| Never smoker | 51 (30) | 31 (57) | 9 (75) | 1 (3) | 10 (15) | |
| Current/former | 121 (70) | 23 (43) | 3 (25) | 37 (97) | 58 (85) | |
| ECOG performance status | .4 | |||||
| 0–1 | 115 (67) | 41 (76) | 9 (75) | 23 (61) | 42 (62) | |
| 2–4 | 57 (33) | 13 (24) | 3 (25) | 15 (29) | 26 (38) | |
| BM at diagnosis of NSCLC | 97 (56) | 31 (57) | 7 (58) | 20 (53) | 39 (57) | .9 |
| Extent of disease at diagnosis of BM | ||||||
| Local control of primary tumor | 55 (32) | 14 (26) | 4 (33) | 16 (42) | 21 (31) | .4 |
| Extracranial metastases | 122 (72) | 44 (81) | 9 (75) | 22 (57) | 47 (68) | .1 |
| Number of BM | .6 | |||||
| 1 | 66 (38) | 15 (28) | 5 (42) | 16 (42) | 30 (44) | |
| 2–4 | 52 (30) | 18 (33) | 2 (17) | 12 (32) | 20 (29) | |
| ≥5 | 54 (31) | 21 (39) | 5 (42) | 10 (26) | 18 (26) | |
| Neuroanatomic location if 1 BM (n = 66) | 66 | 15 | 5 | 16 | 30 | .3 |
| Supratentorial | 53 (88) | 14 (93) | 5 (100) | 12 (75) | 22 (73) | |
| Infratentorial | 13 (12) | 1 (7) | 0 (0) | 4 (25) | 8 (27) | |
| Neuroanatomic location if ≥2 BM (n = 106) | 106 | 39 | 7 | 22 | 38 | .8 |
| Supratentorial | 29 (27) | 11 (28) | 2 (29) | 7 (32) | 9 (24) | |
| Infratentorial | 6 (6) | 3 (8) | 0 (0) | 1 (5) | 2 (5) | |
| Both | 71 (67) | 25 (64) | 5 (71) | 14 (64) | 27 (71) | |
| Size of largest BM (cm) | .3 | |||||
| Median (range) | 1.5 (0.2–6.3) | 1.2 (0.4–6.3) | 1.4 (0.2–4.5) | 2.0 (0.3–3.7) | 1.8 (0.2–5.8) | |
Abbreviations: BM, brain metastases; ECOG, Eastern Cooperative Oncology Group; NSCLC, non–small cell lung cancer.
Treatment Summary
The summary of treatments received by the study population is shown in Table 2. Thirty-three percent of EGFR-mutant patients and 17% of ALK-positive patients received TKI prior to the diagnosis of brain metastases, compared with 4% of WT and 0% of KRAS-mutant patients (P = .2). Only 2 EGFR-mutant patients (4%) received TKI as initial therapy for brain metastases prior to radiotherapy; both had been on TKI prior to diagnosis of brain metastasis. Comparing the 4 subgroups, there was no difference in time from diagnosis of brain metastases to intracranial radiotherapy (median, 0.9 months). The radiation technique was WBRT in 72% of patients to a median dose of 35 Gy; 27% received SRS/IFRT to a median dose of 18 Gy. Thirty percent of EGFR-mutant and 42% of ALK-positive patients received SRS/IFRT, in contrast to 24% of WT and 26% of KRAS-mutant patients (P = .04). After radiotherapy, 83% of EGFR-mutant patients received an EGFR TKI (84% of whom received erlotinib), compared with 22% of WT, 5% of KRAS-mutant, and 0% of ALK-positive patients (P < .0001). After radiotherapy, 83% of ALK-positive patients received an ALK TKI (90% of whom received crizotinib; 50% received a second generation TKI, ceritinib), compared with no patients in the other 3 subgroups (P < .0001).
Table 2.
Treatment summary
| All n (%) | EGFR n (%) | ALK n (%) | KRAS n (%) | WT n (%) | P value | |
|---|---|---|---|---|---|---|
| TKI before diagnosis of BM | 23 (13) | 18 (33) | 2 (17) | 0 (0) | 3 (4) | .2 |
| TKI as initial therapy for BM | 2 (1) | 2 (4) | 0 (0) | 0 (0) | 0 (0) | – |
| Median time from diagnosis of BM to cranial radiotherapy (months) | 0.9 | 0.9 | 0.8 | 1.0 | 1.0 | .8 |
| Initial cranial radiotherapy | ||||||
| SRS/IFRT | 47 (27) | 16 (30) | 5 (42) | 10 (26) | 16 (24) | .04 |
| WBRT | 124 (72) | 38 (70) | 7 (58) | 28 (74) | 51 (75) | .3 |
| Surgical resection of BM | 11 (6) | 0 (0) | 0 (0) | 5 (13) | 6 (9) | .02 |
| Subsequent treatment following cranial radiotherapy | ||||||
| Additional cranial radiotherapy | 55 (32) | 16 (30) | 9 (75) | 10 (26) | 20 (29) | .02 |
| EGFR TKI | 62 (36) | 45 (83) | 0 (0) | 2 (5) | 15 (22) | < .001 |
| ALK TKI | 10 (6) | 0 (0) | 10 (83) | 0 (0) | 0 (0) | < .001 |
| Chemotherapy | 98 (57) | 30 (56) | 6 (50) | 22 (58) | 40 (59) | .9 |
Abbreviations: BM, brain metastases; IFRT, involved field radiotherapy; SRS, stereotactic radiosurgery; TKI, tyrosine kinase inhibitor.
Outcomes Following Cranial Radiotherapy
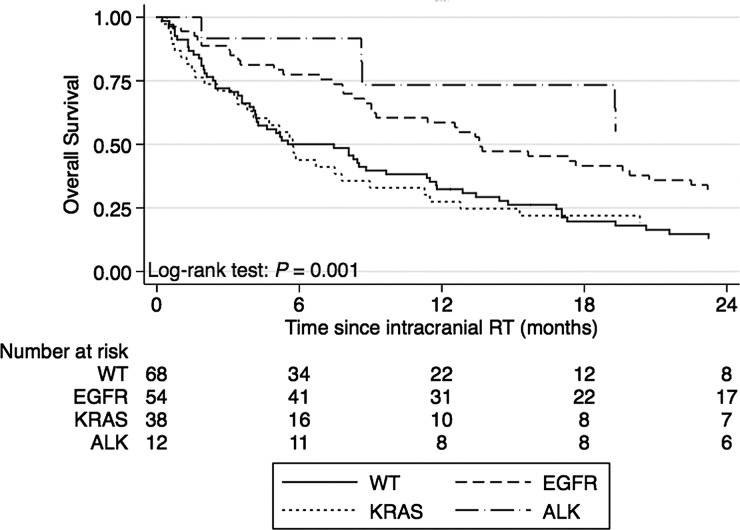
Median OS for the 4 subgroups is shown in Table 3, and the corresponding Kaplan-Meier curves are shown in Fig. 1. There was a highly significant difference in OS (P = 0.001) when comparing the 4 subgroups by log-rank test. Median OS was 13.6 months for EGFR and 26.3 months for ALK, in contrast to 5.7 months for KRAS, and 5.5 months for WT.
Table 3.
Median overall survival in months
| EGFR | ALK | KRAS | WT | P valuea | |
|---|---|---|---|---|---|
| Median OS | 13.6 | 26.3 | 5.7 | 5.5 | .001 |
aFor log-rank test.
Fig. 1.
Kaplan-Meier curves for overall survival.
Multivariate Analysis
A multivariate Cox proportional hazards model was built using genetic alteration status, receipt of targeted therapy after cranial radiotherapy, receipt of chemotherapy after cranial irradiation, number of brain metastases, presence of extracranial metastases, age, and performance status (Table 4). ALK rearrangement status was significantly associated with improved OS (HR, 0.31; 95% CI, 0.13–0.74; P = .008). EGFR mutation status was not significantly associated with improved OS (HR, 0.71; 95% CI, 0.37–1.38; P = .3). KRAS mutation status was also not associated with differences in OS (HR, 0.93; 95% CI, 0.59–1.47; P = .8).
Table 4.
Multivariate Cox proportional hazards model
| Covariate | OS |
|
|---|---|---|
| HR (95% CI) | P value | |
| EGFR | 0.71 (0.37–1.38) | .3 |
| ALK | 0.37 (0.15–0.92) | .03 |
| KRAS | 0.93 (0.59–1.47) | .8 |
| Targeted therapy | 0.32 (0.17–0.59) | <.001 |
| Chemotherapy | 0.39 (0.27–0.58) | <.001 |
| Number of brain metastases | 1.13 (1.04–1.25) | .007 |
| Extracranial metastases | 3.20 (2.02–5.07) | <.001 |
| Age | 1.02 (1.00–1.04) | .02 |
| ECOG Performance Status | 1.54 (1.07–2.23) | .02 |
Abbreviations: HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group.
Receipt of targeted therapy after cranial radiotherapy was strongly associated with improved OS (HR, 0.30; 95% CI, 0.17–0.54; P < .001), independent of genetic alteration status. Receipt of chemotherapy after radiotherapy was also strongly associated with improved OS. Number of brain metastases, presence of extracranial metastases, age, and decreased performance status were associated with worsened OS.
Impact of TKI Prior to Brain Metastasis Diagnosis
Patients with an EGFR mutation or ALK rearrangement on TKI prior to diagnosis of brain metastases (33% and 17%, respectively) had significantly worse outcomes than patients with these genetic alterations who were not on targeted therapy prior to brain metastasis diagnosis. Median OS was 9.0 versus 19.6 months (P < .001).
Discussion
The purpose of this study was to determine the impact of genetic alterations in EGFR, ALK, and KRAS on survival after radiotherapy for brain metastases in NSCLC. After adjustment for receipt of targeted therapy, receipt of chemotherapy, number of brain metastases, presence of extracranial metastases, age, and performance status, ALK rearrangements were associated with improved survival. Receipt of targeted therapy after cranial irradiation was also strongly associated with improved survival. Thus, the improved outcomes for patients with ALK genetic alterations in our study were likely due to both inherent tumor differences and the availability of targeted therapy. In contrast, with different tumor biology and no targeted therapy options, KRAS-mutant patients had similar outcomes to WT patients. EGFR mutations were significantly associated with improved OS on univariate analysis but were no longer significant after adjustment for receipt of targeted therapy, perhaps reflecting the relative importance of targeted therapy for these patients. Data suggesting that EGFR mutations were associated with improved survival in NSCLC patients with brain metastases32 may have predominantly reflected the benefit of targeted therapy. The number of EGFR-mutant patients in this study may also have been too small to detect a significant difference on multivariate analysis. There was no significant difference in time from brain metastasis diagnosis to receipt of cranial RT for patients with EGFR mutations compared with the other subgroups (median, 0.9 months, Table 2), and only 2 patients received EGFR TKIs prior to cranial RT, suggesting that there was no bias due to delay in RT or EGFR-mutant brain metastases being refractory to TKIs. However, it remains possible that there was confounding by the time of RT referral, with EGFR-mutant patients in our study having worse prognosis than in other series where patients did not receive cranial RT unless their brain metastases progressed on targeted therapy.
While other retrospective series have focused on local control and intracranial-relapse PFS in patients with brain metastases and EGFR or ALK genetic alterations,33,34 our study focused on survival as the most clinically meaningful endpoint. Due to potential unreliability in the assessment of local control and death due to brain metastases in a retrospective setting, these endpoints were not evaluated in our study. Importantly, the distribution of patients in our study may not reflect the general population of patients with NSCLC due to our referral bias for those with specific genetic alterations including EGFR mutations and ALK rearrangements.
It is currently unclear whether ALK rearrangements are a prognostic biomarker, especially in the metastatic setting. A retrospective analysis of patients with advanced NSCLC, half of whom had brain metastases, reported no difference in survival comparing crizotinib-naïve ALK-positive patients with WT controls;35 another study of stage IIIB/IV NSCLC in the pre-ALK inhibitor era also reported no difference.36 However, a third study reported improved OS in NSCLC patients with malignant pleural effusions and ALK rearrangements who did not receive targeted therapy, compared with WT controls.37 Our study is novel in that it addresses brain metastases specifically in an ALK-positive population that received targeted therapy.
In patients with early stage disease, as well as those with metastatic disease, EGFR mutations have been associated with improved survival, independent of treatment.38,39 Of note, EGFR mutations occur more frequently in women, never-smokers, adenocarcinomas, and well-differentiated cancers, which may each portend a better prognosis.40 EGFR and KRAS mutations were not associated with improved OS in a recent retrospective series of advanced NSCLC in the era of targeted therapies.40 Some studies have demonstrated that KRAS is prognostic for poor outcomes in advanced NSCLC, although the control groups in these studies were heterogeneous41,42 and specific KRAS mutations may be associated with different prognoses.43 Both ALK and EGFR are clearly predictive biomarkers, as supported in this study, where receipt of ALK TKIs or EGFR TKIs was independently associated with improved survival. KRAS is a predictive biomarker for absence of response to EGFR TKIs.44
With respect to specific sites of metastases, Doebele et al found in treatment-naïve patients with stage IV NSCLC that ALK rearrangements were associated with pericardial, pleural, and liver metastases, EGFR mutations were associated with liver metastases, and KRAS mutations were not associated with any sites compared with WT controls.45 No genetic alteration was associated with predisposition for brain, bone, adrenal, or lung metastases. Both EGFR and KRAS were not associated with incidence of brain or bone metastases in another series,46 whereas EGFR was associated with more lesions in the brain and bone compared with WT controls in a third report.47 In our study, we report the novel finding that genetic alterations in ALK, EGFR, and KRAS were not associated with significant differences in number, neuroanatomic distribution, or size of brain metastases among patients receiving cranial radiotherapy.
Targeted therapy may be used before the diagnosis of brain metastases, after diagnosis as initial therapy, and/or after diagnosis and cranial radiotherapy. Thirty-three percent of EGFR-mutant and 17% of ALK-positive patients received a TKI prior to diagnosis of brain metastases in our study. These patients had significantly worse outcomes compared with EGFR-mutant and ALK-positive patients not on TKIs prior to diagnosis of brain metastases, which was likely due to selection for resistant or more aggressive disease. The introduction of crizotinib occurred during our study period; one of the 2 ALK-positive patients who did not receive TKI before cranial radiotherapy was diagnosed with brain metastases before crizotinib was available through clinical trials at our institution or commercially. Only 2 EGFR-mutant patients received TKIs as initial therapy for brain metastases, which could reflect the relatively large size of the brain metastases in the study population (median, 1.5 cm overall; 1.2 cm for EGFR-mutant patients) as well as variations in treatment patterns during the study period. After radiotherapy, 83% of EGFR-mutant and ALK-positive patients received TKI. The strong effect of targeted therapy on improved survival after cranial radiotherapy was likely driven by the CNS activity of EGFR TKIs and the second-generation ALK TKI ceritinib on tumor cells still sensitive to these agents.
Strengths of our study include our comparison of 4 genetic subtypes of NSCLC and our multivariate model that adjusted for use of targeted therapy. Our study was inclusive of patients who received any cranial radiotherapy (with localized SRS/IFRT vs WBRT), reflecting common clinical practice. The major limitations of our study were its retrospective nature and the small sample size of some subgroups, with potential underpowering of some analyses. Pairwise comparisons were not performed in order to minimize over-analysis and multiple testing. In building the Cox proportional hazards model, we included traditional prognostic factors for survival including variables identified in the brain metastases graded prognostic assessment.48 The receipt of any targeted therapy after cranial RT was included as a binary variable in the model, instead of introducing the complexity associated with receipt of multiple TKIs at variable time points for some patients. As more patients are identified by genetic alterations and more TKIs with variable CNS activity are introduced, this model can be refined. A future question that can also be answered when larger sample sizes are available is whether specific ALK rearrangements or EGFR mutations portend different prognoses.
In conclusion, ALK rearrangements are independently associated with improved survival outcomes in NSCLC patients who receive radiotherapy for brain metastases compared with mutations in EGFR or KRAS or a WT genetic profile. Targeted therapy against ALK or EGFR after cranial radiotherapy is associated with additional survival benefit. The results of this study are encouraging for patients with brain metastases and genetic alterations in ALK or EGFR, who have targeted therapy options. As additional genetic subtypes of NSCLC are identified and targeted agents with improved blood-brain barrier penetration become available, the choreography of targeted therapy and radiotherapy will continue to evolve and likely translate into improved outcomes for more patients. The ability to apply what has been learned from the subset of patients with ALK or EGFR genetic alterations will be essential to improving outcomes in other molecularly defined subsets of NSCLC. Future prospective trials may focus on determining the optimal timing of cranial radiotherapy relative to targeted agents with activity against brain metastases, as well as the concurrent use of radiotherapy and targeted agents.
Funding
This work was supported by internal institutional funding only.
Conflicts of interest statement. Dr. Gainor is a consultant for Boehringer-Ingelheim. Dr. Sequist is an uncompensated consultant for Clovis, Boehringer-Ingelheim, AstraZeneca, and Merrimack Pharmaceuticals. Dr. Shaw is a consultant for Pfizer, Novartis, Ariad, Chugai, and Daiichi-sankyo. Dr. Shih is a consultant for Novartis. All remaining authors have declared no conflicts of interest.
References
- 1.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 2.Slebos RJ, Kibbelaar RE, Dalesio O, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990;323(9):561–565. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 4.Kris MG, Johnson BE, Kwiatkowski DJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI's Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 2011;29:477s. (suppl 415; abstr CRA7506) [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105(9):595–605. doi: 10.1093/jnci/djt072. [DOI] [PubMed] [Google Scholar]
- 7.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 9.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 10.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 11.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 13.Roberts PJ, Stinchcombe TE. KRAS mutation: should we test for it, and does it matter? J Clin Oncol. 2013;31(8):1112–1121. doi: 10.1200/JCO.2012.43.0454. [DOI] [PubMed] [Google Scholar]
- 14.Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6(9):1474–1480. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 16.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 17.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21(13):2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 18.Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70(2):510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 19.Bhangoo SS, Linskey ME, Kalkanis SN, et al. Evidence-based guidelines for the management of brain metastases. Neurosurg Clin N Am. 2011;22(1):97–104, viii. doi: 10.1016/j.nec.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 21.Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33(6):583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer (Version 2.2014) 2014. Available at http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf . Accessed 11/23/13.
- 23.Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 2012;18(4):938–944. doi: 10.1158/1078-0432.CCR-11-2529. [DOI] [PubMed] [Google Scholar]
- 24.Bartolotti M, Franceschi E, Brandes AA. EGF receptor tyrosine kinase inhibitors in the treatment of brain metastases from non-small-cell lung cancer. Expert Rev Anticancer Ther. 2012;12(11):1429–1435. doi: 10.1586/era.12.121. [DOI] [PubMed] [Google Scholar]
- 25.Weber B, Winterdahl M, Memon A, et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thorac Oncol. 2011;6(7):1287–1289. doi: 10.1097/JTO.0b013e318219ab87. [DOI] [PubMed] [Google Scholar]
- 26.Kaneda H, Okamoto I, Nakagawa K. Rapid response of brain metastasis to crizotinib in a patient with ALK rearrangement-positive non-small-cell lung cancer. J Thorac Oncol. 2013;8(4):e32–e33. doi: 10.1097/JTO.0b013e3182843771. [DOI] [PubMed] [Google Scholar]
- 27.Falk AT, Poudenx M, Otto J, et al. Adenocarcinoma of the lung with miliary brain and pulmonary metastases with echinoderm microtubule-associated protein like 4-anaplastic lymphoma kinase translocation treated with crizotinib: a case report. Lung Cancer. 2012;78(3):282–284. doi: 10.1016/j.lungcan.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Solomon B, Wilner KD, Shaw AT. Current status of targeted therapy for anaplastic lymphoma kinase-rearranged non-small–cell lung cancer. Clin Pharmacol Ther. 2013;95(1):15–23. doi: 10.1038/clpt.2013.200. [DOI] [PubMed] [Google Scholar]
- 29.Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Mod Pathol. 2008;21(Suppl 2):S16–S22. doi: 10.1038/modpathol.3801018. [DOI] [PubMed] [Google Scholar]
- 30.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2(5):146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12(11):1193–1199. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johung KL, Yao X, Li F, et al. A clinical model for identifying radiosensitive tumor genotypes in non-small cell lung cancer. Clin Cancer Res. 2013;19(19):5523–5532. doi: 10.1158/1078-0432.CCR-13-0836. [DOI] [PubMed] [Google Scholar]
- 34.Lee HL, Chung TS, Ting LL, et al. EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in non-small cell lung cancer patients with brain metastases. Radiat Oncol. 2012;7:181. doi: 10.1186/1748-717X-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12(11):1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JK, Park HS, Kim DW, et al. Comparative analyses of overall survival in patients with anaplastic lymphoma kinase-positive and matched wild-type advanced nonsmall cell lung cancer. Cancer. 2012;118:3579–3586. doi: 10.1002/cncr.26668. [DOI] [PubMed] [Google Scholar]
- 37.Wu SG, Kuo YW, Chang YL, et al. EML4-ALK translocation predicts better outcome in lung adenocarcinoma patients with wild-type EGFR. J Thorac Oncol. 2012;7:98–104. doi: 10.1097/JTO.0b013e3182370e30. [DOI] [PubMed] [Google Scholar]
- 38.Izar B, Sequist L, Lee M, et al. The impact of EGFR mutation status on outcomes in patients with resected stage I non-small cell lung cancers. Ann Thorac Surg. 2013;96:962–968. doi: 10.1016/j.athoracsur.2013.05.091. [DOI] [PubMed] [Google Scholar]
- 39.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 40.Bauml J, Mick R, Zhang Y, et al. Determinants of survival in advanced non–small-cell lung cancer in the era of targeted therapies. Clin Lung Cancer. 2013;14(5):581–591. doi: 10.1016/j.cllc.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson ML, Sima CS, Chaft J, et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer. 2013;119:356–362. doi: 10.1002/cncr.27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brugger W, Triller N, Blasinska-Morawiec M, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:4113–4120. doi: 10.1200/JCO.2010.31.8162. [DOI] [PubMed] [Google Scholar]
- 43.Keohavong P, DeMichele MA, Melacrinos AC, et al. Detection of K-ras mutations in lung carcinomas: relationship to prognosis. Clin Cancer Res. 1996;2:411–418. [PubMed] [Google Scholar]
- 44.Linardou H, Dahabreh IJ, Kanaloupiti D, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–972. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 45.Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012;118:4502–4511. doi: 10.1002/cncr.27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hendriks LE, Smit EF, Vosse BA, et al. EGFR mutated non-small-cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer. 2014;84:86–91. doi: 10.1016/j.lungcan.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Fujimoto D, Ueda H, Shimizu R, et al. Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: importance of bone metastasis. Clin Exp Metastasis. 2014;31(5):543–551. doi: 10.1007/s10585-014-9648-3. [DOI] [PubMed] [Google Scholar]
- 48.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]