Abstract
Inhibition of the mammalian target of rapamycin (mTOR) signaling pathway has become an attractive target for human cancer therapy. Hyperactivation of mTOR has been reported in both sporadic and syndromic (hereditary) brain tumors. In contrast to the large number of successful clinical trials employing mTOR inhibitors in different types of epithelial neoplasms, their use to treat intracranial neoplasms is more limited. In this review, we summarize the role of mTOR activation in brain tumor pathogenesis and growth relevant to new human brain tumor trials currently under way using mTOR inhibitors.
Keywords: glioblastoma, meningioma, mTORC1, mTORC2
Regulation and Function of mTOR Signaling Pathways
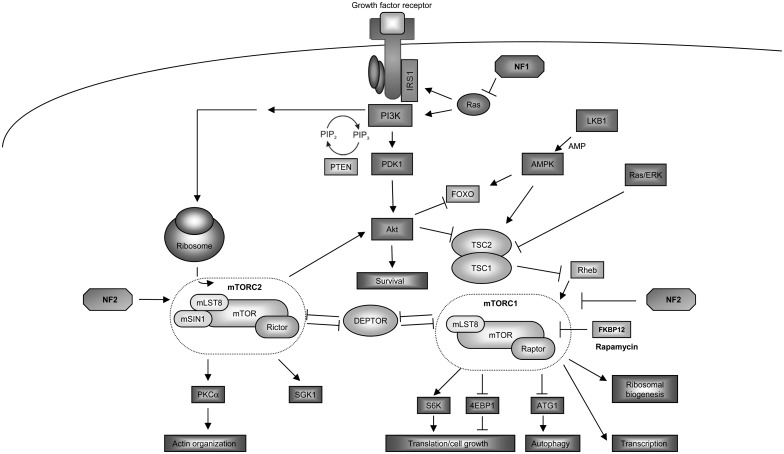
In a soil sample from the Easter Island (former Rapa Nui), a bacterial strain was isolated in the 1970s called Streptomyces hygroscopicus, which produces a macrolide subsequently found to inhibit the growth of yeast.1 The macrolide was purified and named rapamycin after the place of its discovery. In yeast (Saccharomyces cerevisiae), mutations in the target of rapamycin genes (TOR1 and TOR2) contribute to resistance to the growth-inhibitory effects of rapamycin.2 The eukaryote TOR protein belongs to the phosphatidylinositol kinase–related kinase family, which encodes large proteins (∼280 kDa) containing a carboxyl-terminal serine/threonine protein kinase domain.3 In many mammalian cell types, the mammalian TOR (mTOR) protein forms 2 distinct multimolecular complexes, termed mTORC1 and mTORC2, which differ with respect to their protein composition, substrate specificity, and mechanism of growth regulation. The mTORC1 complex consists of mTOR, regulatory-associated protein of mTOR (RAPTOR), 40 kDa proline-rich Akt substrate (PRAS40), DEP (dishevelled, Egl-10, and Pleckstrin)-domain-containing mTOR (DEPTOR) interacting protein, and mammalian lethal with SEC13 protein 8 (mLST8).4 The mTORC2 complex is composed of mTOR, rapamycin-insensitive companion of mTOR (RICTOR), proline-rich protein 5, mLST8, mammalian stress activated protein kinase interacting protein 1, and DEPTOR (Fig. 1).3,5–7
Fig. 1.
Intracellular signaling of mTORC1 and mTORC2.
In a diverse number of distinct cell types, mTORC1 and mTORC2 have been shown to regulate different cellular processes. Rapamycin-sensitive mTORC1 is a nutrient and energy sensor, important for responding to changes in amino acid and nutrient levels, redox states, and growth factor availability as well as regulating ribosomal biogenesis and nutrient transport.8–11 In contrast, the relatively rapamycin-insensitive mTORC2 is involved in actin cytoskeleton organization and cell survival. In addition, mTORC2 phosphorylates and activates Akt, serum- and glucocorticoid-inducible kinase (SGK1), and protein kinase C alpha (PKCα), which in turn control cell survival, cell cycle progression, and anabolism.12–14
Mammalian TORC1 is activated by a plethora of mechanisms. One mechanism involves phosphorylation-driven inactivation of the tuberous sclerosis complex-2 protein (TSC2, tuberin), which functions as a GTPase-activating protein (GAP) for the small GTPase Ras homolog enriched in brain (Rheb). Rheb promotes cell growth in a TOR- and S6 kinase (S6K)–dependent manner.15,16 Tuberin stimulates the intrinsic GTPase activity of Rheb, thereby accelerating the conversion of active Rheb-GTP to inactive Rheb-GDP.17 As such, cells lacking the tuberin-hamartin complex function exhibit increased Rheb and mTORC1 activation.18
In addition to growth factor–mediated activation via PI3-Kinase (PI3K), phosphoinositolphosphate (PIP) and phosphoinositid-dependend kinase 1 (PDK1), mTORC1 is activated by high amino acid levels, especially leucine and arginine.19 The GTPase Rag functions as an amino acid–specific regulator of mTORC1, independent of TSC/Rheb function.20,21 Low intracellular energy levels can inactivate mTORC1 in a manner dependent on activating adenosine monophosphate protein kinase (AMPK) and the transcription factor forkhead box O (FOXO). Activated AMPK controls energy-consuming anabolic pathways and can regulate mTORC1 either directly through phosphorylation of RAPTOR22 or indirectly by phosphorylating tuberin at residues Thr1227 and Ser1345.23,24
Following activation, mTORC1 regulates ribosomal translation and protein biosynthesis by phosphorylating key components of the protein synthesis machinery, including ribosomal protein S6K1/2 and 4E-binding protein 1 (4E-BP1). Upon 4E-BP1 phosphorylation, the translation initiation factor eIF4E is released and stimulates cap-dependent RNA translation.25 S6K1 and S6K2 regulate translation initiation factors during protein biosynthesis and coordinate ribosome biogenesis to drive efficient mRNA translation.26
Recent studies have revealed a negative feedback loop in the insulin receptor pathway involving the insulin receptor substrate 1 (IRS-1). Activation of mTORC1 promotes inhibitory IRS-1 phosphorylation, such that Akt can be activated following rapamycin treatment.27,28 This Akt activation is dose dependent, with lower rapamycin doses potentiating Akt activation, and vice versa.29,30 The presence of this feedback loop explains the paradoxical Akt activation and increased growth observed in tumor cells following therapeutic rapamycin-mediated mTOR inhibition.
The mTORC1 complexes is involved in controlling the cellular responses to changes in nutrient availability (ie, starvation). One of the fundamental processes regulated by mTORC1 following nutrient starvation is autophagy. In this respect, mTORC1 is considered a negative regulator of autophagy.31 Autophagy is the controlled self-degradation of damaged, supernumerous, or dangerous cellular components in response to starvation. During periods of low extracellular nutrient levels, cellular autophagy provides substrates for energy production. Mammalian TORC1 depends on the Rag and Rheb GTPases for activation and subsequent inhibition of autophagy in response to limiting amino acid availability.3,21 As such, inhibition of mTORC1 activity induces autophagy.11,32 In Saccharomyces cerevisiae, TOR-dependent phosphorylation of the protein autophagy-related 13 (Atg13) disrupts the Atg1-Atg13-Atg17 complex, which triggers autophagosome formation. ATG13 and Unc-51–like kinase 1 (ULK1) are the mammalian homologs of the yeast proteins Atg13 and Atg1.33 These proteins bind to the 200-kDa large focal adhesion kinase family kinase-interacting protein and the mammalian-specific homolog ATG101. Mammalian TOR phosphorylates ATG13 and ULK1 to block the initiation of the autophagosome.34 In addition, the mTORC1- and mTORC2-associated protein DEPTOR can induce autophagy through suppression of mTORC1 activity.35,36 DEPTOR is negatively regulated by mTORC1 and mTORC2, and depletion of DEPTOR activates mTORC1 and mTORC2 pathways with increased phosphorylation of S6K and Akt, respectively.4 Mammalian TORC1 has additional important functions as a key regulator of cellular metabolism. It can stimulate glucose uptake, metabolic flux through glycolysis and the oxidative arm of the pentose phosphate pathway, and production of acetyl-CoA with subsequent increase in lipid and sterol synthesis (reviewed in37).
While regulators and substrates of mTORC1 are well understood, the function of mTORC2 is less well elucidated. Mammalian TORC2, similar to yeast TORC2, is involved in actin cytoskeleton reorganization and cell migration. Genetic silencing of expression of mTOR, RICTOR, and mLST8 (but not RAPTOR) results in decreased activation of Ras-related C3 botulinum toxin substrate 1 (Rac1) upon serum restimulation and leads to defective actin reorganization.38,39 Rac1 belongs to a family of Rho GTPase molecules.40 Following growth-factor stimulation, Rac1 associates with both mTORC1 and mTORC2. It binds directly to mTORC1/2 independently of the GTP-bound state of Rac1 and mediates the localization of mTOR to specific membrane compartments.41 The mechanism underlying Rac1 control of actin cytoskeleton reorganization is incompletely understood but may involve recruitment and activation of Rac1 at the plasma membrane to increase synthesis of phosphatidylinositol (3,4,5)-triphosphate and result in actin cytoskeleton rearrangement.41 Additionally, PKCα is involved in mTORC2-dependent cell migration due to mTORC2 phosphorylation of PKCα.39 Consistent with a role in cell migration, glioma cell lines with increased RICTOR expression and mTORC2 activity exhibit elevated integrin β1 and β3 expression and enhanced motility. This increased expression of RICTOR correlates with higher levels of PKCα.42 Moreover, mTORC2 is also required for hydrophobic motif site (Ser422) phosphorylation of SGK1.14 SGK1 is stimulated by growth factors and osmotic stress.43 SGK1 phosphorylation requires protor-1 expression, such that cells lacking protor-1 are unable to activate SGK1.44
Accumulating evidence also points to a role for mTORC2 in protein synthesis and maturation, processes that have been previously attributed to mTORC1. In this regard, mTORC2 associates with ribosomal proteins,45 where it directly interacts with the 60S ribosomal subunit. In addition, RICTOR specifically binds to the L23a and L26 ribosomal proteins positioned at the exit tunnel.45,46 Together, these findings suggest that mTORC2 may control cotranslational processing or the maturation of nascent polypeptides. For example, Akt can be phosphorylated by mTORC2 at Thr450 of the turn motif and at Ser473 of the hydrophobic motif.13,47–49 The phosphorylation of the turn motif is a one-shot event, which only occurs during the synthesis of nascent Akt, when the polypeptide is still attached to the ribosome.45 However, phosphorylation at Ser473 of the hydrophobic motif is a posttranslational modification, induced by growth factors and hormones, which allosterically activates Akt to increase its activity toward many substrates.7,48,50 As such, both mTORC1/2 complexes are involved in the regulation of different members of the family of signal transducer and activator of transcription factors.51 Finally, mTORC2 may also function as a regulator of the nuclear factor-kappaB transcription factor, thus promoting chemoresistance in epidermal growth factor receptor (EGFR)–mutant glioblastoma.52
The Role of mTOR in Inherited Brain Tumor Predisposition Syndromes
The importance of mTOR in tumorigenesis has been revealed by studies focusing on familial cancer predisposition syndromes characterized by mutations in negative regulators of the mTOR pathway. Germline mutations in the phosphatase and tensin homolog gene (PTEN), for example, predispose to several disorders that exhibit diverse overlapping clinical features, collectively classified as PTEN hamartoma tumor syndrome, including Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome, and Proteus syndrome.53–55 Somatic PTEN inactivation with Akt hyperactivation occurs in many human tumors, including glioblastoma.56,57 Moreover, patients with germline PTEN mutations are at risk for the development of numerous cancers, including thyroid and breast cancer.58 Homozygous PTEN deletion in mice results in embryonic lethality, while heterozygous deletion is associated with increased cancer incidence.59
Mutations in either the TSC1 or TSC2 gene cause TSC hamartomatous syndrome.60 Loss-of-function TSC1/TSC2 mutations result in mTOR hyperactivation with increased S6K1, 4E-BP1, and ribosomal S6 phosphorylation.61 Discovering the connection between TSC and the mTOR pathway provided the first link between cancer and increased mTOR activity. In the brain of children with TSC develop subependymal nodules and subependymal giant cell astrocytomas (SEGAs).62,63 SEGAs are characterized by high expression levels of activated (phosphorylated) S6K,64 and these tumors are exquisitely responsive to treatment with the mTORC1 inhibitor everolimus.65–67
Neurofibromatosis type 1 (NF1) is a common inherited tumor predisposition syndrome affecting 1 in 2500–3000 individuals.68 Individuals with NF1 are prone to developing both benign and malignant tumors of the peripheral and central nervous systems.69 Importantly, 15%–20% of children with NF1 develop low-grade gliomas involving the optic pathway,70 while adults are at increased risk for high-grade gliomas.71,72 The human NF1 gene is located on chromosome 17q11.2 and encodes the protein neurofibromin, which functions as a GAP for the Ras small GTPase molecule.73,74 Loss of neurofibromin expression results in increased Ras activity and cell growth.75–77 Consistent with increased Ras pathway activity in NF1-deficient cancer cells, high levels of activated Akt/mTOR and Raf/mitogen-activated protein kinase (MAPK)–extracellular signal-regulated kinase (ERK) were observed.78,79 Moreover, studies by The Cancer Genome Atlas have revealed that mutations in the NF1 gene are among the most frequently occurring mutations found in glioblastoma multiforme (GBM), along with mutations in the TP53, PTEN, EGFR, and RB genes.80
Peutz-Jeghers syndrome is another familiar cancer disorder, which is caused by mutations in the serine/threonine protein kinase 11 (or liver kinase B1 [LKB1]) tumor suppressor gene, whose protein product directly activates AMPK.81 In cells lacking LKB1, mTORC1 remains active due to defective AMPK regulation.82 In contrast to the above-mentioned inherited cancer conditions, brain tumor development has been only rarely described in this syndrome.83
Mammalian TOR Hyperactivation in Gliomas and Nonglial Brain Tumors
Based on a detailed characterization of mTORC1 signaling and function, several reports have demonstrated activation of members of the mTORC1 pathway in brain tumors.
The largest group of brain tumors includes gliomas, a histologically highly heterogeneous group of tumors classified by the World Health Organization (WHO) according to malignancy grade and histological subtype. Among these glial neoplasms, GBM is the most devastating type.84 The importance of mTORC1 signaling to brain tumor formation and growth is underscored by the observation that several of the key genetic alterations described in gliomas result in increased mTORC1 activity.
In GBM, alterations of the EGFR gene are frequently found.80 EGFR gene amplification in GBM results in activation of phosphatidylinositol-3 kinase (PI3K) in about 45% of cases.85 Activating mutations or amplification of PIK3CA, the gene encoding the p110α subunit of PI3K, or of PIK3R1, which encodes for the subunit p85, has been found in ∼15% of GBM.80,86,87 In addition, ∼40% of patients with GBM display loss of function of PTEN due to mutation, chromosomal deletion, or epigenetic gene silencing, which is associated with poorer overall survival.88,89 Moreover, S6K has been reported to be activated in GBM90–92 such that PI3K inhibition in PTEN-deficient GBM suppresses S6K activity and reduces tumor growth.93
By comparing primary low-grade tumors and high-grade recurrences, recently it was demonstrated that development of high-grade glioma (ie, glioblastoma) in these cases might be driven by different genetic alterations than the ones responsible for tumor initiation. Using exome sequencing, Johnson et al94 observed that in 43% of cases half of the mutations present in the original low-grade tumor were undetected at recurrence. Moreover, they found that certain mutations activating the Akt-mTORC1 signaling pathway are closely associated with temozolomide treatment. This suggests that mTORC1 hyperactivation in malignant gliomas might represent a therapy-induced oncogenic transformation.94
While less well studied, the role of mTORC2 in gliomas is restricted to analyses of RICTOR and N-myc downstream regulated gene 1 (NDRG1). As such, RICTOR is overexpressed in GBM samples compared with normal brain. In addition, NDRG1, a downstream target of mTORC2 activity, is often increased in expression or phosphorylated in GBM.52 In a Drosophila glioma model with constitutive coactivation of EGFR-Ras and PI3K, it was shown that mTORC2-related genes like dSIN1 and dRICTOR are required to generate malignant gliomas.95
Similarly, the activation of this pathway by EGFR engagement is an important factor potentially underlying chemotherapy resistance to alkylating agents.52,96 The molecular mechanism for this negative effect of mTORC2 on GBM therapy is likely mediated by binding and stabilization of O6-DNA methylguanine-methyltransferase.96
Taken together, there is compelling evidence for activation of mTORC1 in human GBM, thus providing a strong rationale for the clinical use of mTORC1 inhibitors as adjuvant therapies for primary or recurrent GBM (Table 1).
Table 1.
Current clinical studies using mTOR inhibitors for the treatment of common brain tumors
| Substance | Tumor Type | Phase | Combination | Study Number | Status | Results | Ref. |
|---|---|---|---|---|---|---|---|
| Everolimus | Low-grade glioma (P)/NF1 | II | – | NCT01158651 | Recruiting | – | |
| Low-grade glioma (R) | II | – | NCT00823459 | Recruiting | – | ||
| Low-grade glioma (R/P) | II | – | NCT00782626 | Completed | ? | – | |
| Low-grade glioma (R/P) | II | – | NCT00831324 | Recruiting | – | ||
| Glioblastoma (R) | II | – | NCT00515086 | Completed | Terminated | – | |
| Giant cell astrocytoma (TSC) | I/II | – | NCT00411619 | Active | – | ||
| Glioblastoma (P/R/N) | I | Temozolomide | NCT00387400 | Completed | ? | – | |
| Glioblastoma | I | Temozolomide | NCT00553150 | Ongoing | 132 | ||
| Malignant glioma (R) | I/II | Sorafenib | NCT01434602 | Recruiting | – | ||
| Glioblastoma (P) | I/II | Gefitinib | NCT00085566 | Completed | ? | – | |
| Glioblastoma | I/II | Temozolomide | NCT01062399 | Ongoing | – | ||
| Glioblastoma (R) | I/II | AEE788 | NCT00107237 | Completed | ? | – | |
| Temsirolimus | Malignant glioma | I/II | – | NCT00022724 | Completed | ? | – |
| Glioblastoma (MGMT unmethylated) | II | – | NCT01019434 | Ongoing | – | ||
| Glioblastoma (R) | II | Bevacizumab | NCT00800917 | Completed | No effect | 137 | |
| Glioblastoma | I | Temozolomide | NCT00316849 | Completed | ? | – | |
| Glioblastoma (R) | I/II | Sorafenib | NCT00335764 | Ongoing | – | ||
| Glioblastoma (R/P) | I/II | Perifosine | NCT01051557 | Ongoing | – | ||
| Malignant glioma (R) | I/II | Erlotinib | NCT00112736 | Completed | No effect | 138 | |
| Glioblastoma (R) | I/II | Sorafenib | NCT00329719 | Ongoing | – | ||
| Glioblastoma (R) | I/II | Sorafenib | NCT00335764 | Completed | No effect | 136 | |
| Sirolimus | Glioblastoma | I/II | – | NCT00047073 | Completed | – | |
| Malignant glioma (R) | I/II | Erlotinib | NCT00509431 | Completed | ? | 139 | |
| Glioblastoma (R) | I | Vandetanib | NCT00821080 | Ongoing | – | ||
| AZD8055 | Malignant glioma (R) | I | – | NCT01316809 | Ongoing | – |
Abbreviations: P, progressive; R, recurrent; ?, no results published; MGMT, O6-DNA methylguanine-methyltransferase.
In addition to high-grade malignancies, pilocytic astrocytomas (PAs) are WHO grade I glial neoplasms, which occur predominantly in childhood and adolescence.84 Until recently, very little was known about the genetic alterations underlying this tumor. However, it is now clear that one of the responsible growth control pathways is hyperactivation of MAPK/ERK signaling.97–100 In this regard, loss of neurofibromin in NF1-associated gliomas leads to Ras- and PI3K-dependent hyperactivation of mTOR signaling.101–103 Using NF1 genetically engineered mouse glioma models, rapamycin-mediated inhibition of mTOR hyperactivation resulted in attenuated tumor proliferation. However, the combination of rapamycin with temozolomide in this mouse model did not increase the treatment efficiency.104 This might be partially caused by rapamycin-dependent Akt activation.105 Recently, in cell lines derived from pediatric low-grade gliomas, some antitumor effects of the rapalog ridaforolimus were demonstrated.102
While most sporadic PA tumors lack NF1 gene inactivation, they are instead characterized by a signature fusion event in which the BRAF kinase domain is fused to the amino terminus of the KIAA1549 gene.106 In cerebellar neural stem cells, fusion BRAF expression leads to MAPK-dependent mTOR activation and the formation of glioma-like lesions in vivo.103 Recent immunohistochemical data have similarly demonstrated activation of mTORC1 and mTORC2 in PAs.102 Because both NF1-associated and sporadic PAs in children share mTOR hyperactivation, the use of rapalogs as another treatment option than operation for these low-grade pediatric brain tumors is reasonable.
Primary CNS lymphoma is an aggressive brain tumor, the majority of which are diffuse B-cell lymphomas.107 In most B-cell lymphomas, the PI3K pathway is hyperactivated.108,109 Although the expression and activation of the mTORC1/2 pathway has not been studied in tumor samples, rapamycin and temsirolimus exhibit potent antitumor activity against a variety of lymphoma cell lines in vitro.109 Clinical trials using mTOR inhibition as a strategy to treat CNS lymphoma have been recently initiated.
Meningiomas are the second most common adult brain tumors, originating from the meningeal coverings of the brain and the spinal cord.110 While the majority of tumors are benign WHO grade I meningiomas, ∼20% of meningiomas are atypical (grade II) or anaplastic (grade III) tumors with significantly increased morbidity and mortality.111 One of the most common genetic alterations observed in meningioma is inactivation of the NF2 tumor suppressor gene. The NF2 gene encodes a protein called merlin or schwannomin, a member of the ezrin, radixin, moesin family of membrane-cytoskeleton linker proteins.112 Merlin regulates cytoskeleton remodeling, cell motility, and cell proliferation in response to extracellular signals.113 Of the many intracellular signaling pathways regulated by merlin, constitutively activated mTORC1 has been identified in merlin-deficient meningioma cells.114 Tumors from NF2 patients, as well as from NF2-deficient mouse embryonic fibroblasts, display elevated mTORC1 activation, which is consistent with a role for merlin in the regulation of mTORC2 function.115 As such, meningioma samples have been shown to express high levels of mTORC1 and S6K, implicating mTORC1 as a relevant signaling pathway in meningiomas.116,117
Vestibular schwannomas are another type of primary benign intracranial tumor characterized by frequent NF2 alterations. Surprisingly, data regarding the expression of mTOR-related proteins in schwannomas are rare. Only one paper described expression and phosphorylation of mTOR proteins in schwannomas, but vestibular schwannomas were not included.118
Clinical Trials Using mTOR Inhibitors to Treat Brain Tumors
The ability of rapamycin and its analogs to inhibit mTORC1 function has prompted the initiation of several clinical trials that aim to block the progression of tumors characterized by mTOR hyperactivation. Unlike kinase inhibitors that bind to the catalytic ATP-binding side, rapamycin and its derivatives are relatively specific for mTORC1, because they target FK506-binding protein 12 (FKBP12).119 When rapamycin enters the cell, it binds to the intracellular receptor FKBP12, which binds the FKBP-rapamycin binding domain in mTOR to abrogate mTOR kinase activity of mTORC1 in vitro and in vivo3 allosterically.
The development of rapamycin analogs with more favorable pharmacokinetic profiles than the parental molecule has provided new opportunities for anticancer clinical trials. These “rapalogs” include temsirolimus (Pfizer), everolimus (Novartis), and ridaforolimus (Ariad), which are slightly different in terms of their metabolism, formulation, and administration schedules. Temsirolimus is administered in a once-weekly schedule intravenously, similar to ridaforolimus.120,121 Everolimus is an orally available mTOR inhibitor, typically administered on a continuous daily schedule.122 Phase III temsirolimus trials for patients with solid tumors showed that weekly infusions of the drug in doses of 7.5 to 220 mg/m² in patients with advanced cancer resulted in mild toxicity and evidence of antitumor activity.123–125 Rapalogs also exhibit efficacy in the treatment of TSC-associated SEGAs in patients who are not candidates for surgical intervention.126 A randomized, placebo-controlled phase III trial demonstrated a 50% reduction of tumor volume in 35% of the treated SEGA patients.66 Even long-term treatment with everolimus of TSC patients suffering from SEGAs has been proven to be safe and effective.127 Furthermore, treatment with everolimus effectively reduces seizure frequency.67 Currently, a phase III trial is testing everolimus as adjunctive therapy in patients with TSC and refractory partial-onset seizure (NCT01713946). These data convincingly demonstrate that everolimus treatment in general represents a useful therapy option for slowly growing benign intracranial tumors.
Treatment of high-grade gliomas with rapalogs has recently been more intensely studied. A phase II trial for patients with recurrent GBM revealed that 36% of the subjects treated had evidence of radiographic improvement following temsirolimus administration.128 In addition, the majority of the patients showed an improvement of symptom status. However, another study reported only short-term stabilization of disease in 50% of patients with recurrent glioblastoma treated with temsirolimus.129 In a phase I trial it was proven that short-term treatment with rapamycin in patients with recurrent PTEN-deficient glioblastomas reduced tumor cell proliferation in a substantial number of cases. Moreover, the inhibition of tumor cell proliferation correlated well with the magnitude of mTOR inhibition. An activation of Akt was found in some rapamycin-treated patients, and this feedback loop was associated with shorter time to progression during postsurgical maintenance rapamycin therapy.130 In a phase II trial using temsirolimus in children and adolescents with high-grade glioma, neuroblastoma, and rhabdomyosarcoma, in some patients a prolonged stable disease following weekly administered temsirolimus 75 mg/m² was observed.131 Another phase I trial studied the combination of radiotherapy and temozolomide with everolimus in newly diagnosed glioblastoma.132 This scheme was well tolerated, and a subsequent phase II trial is still ongoing (NCT00553150). Other studies for newly diagnosed glioblastomas show similar beneficial results (RTOG0913,133 NCIC CTG134). However, it should be mentioned that combination of radiotherapy with temozolomide and temsirolimus revealed increased risk for infections.135
Other combined treatment schemes with temsirolimus have been evaluated, but the combination with neither sorafenib,136 bevacizumab,137 nor erlotinib138,139 has been proven to be effective in recurrent GBM. For newly diagnosed glioblastoma, a large phase II trial was initiated by the European Organisation for Research and Treatment of Cancer (EORTC). In this trial, patients lacking hypermethylation of the O6-methylguanine-methyltransferase promoter were treated with radiation therapy combined with temsirolimus (experimental arm) or temozolomide (control arm). The study is closed, and results are expected to be published soon.
Besides mTORC1, mTORC2 has been increasingly recognized as a promising candidate target for therapeutic inhibition in human cancer. Consequently, a number of FKBP12-independent adenosine triphosphate (ATP)–competitive mTOR kinase inhibitors (eg, Torin1, PP242, PP30) have been generated that target both mTOR complexes at similarly low half-maximal inhibitory concentration values. In contrast to rapamycin and its derivatives, newly developed ATP-binding inhibitors target mTOR-kinase activity by competing with ATP to the kinase domain in mTOR. Similarly, mTORC2-specific inhibitors are currently under development.119 Based on studies in prostate cancer models with reduced PTEN expression,140 brain tumors with elevated PI3K activity might be reasonable candidates for mTORC2 inhibitors or dual mTOR/PI3K inhibitors. Dual mTOR/PI3K inhibitors were originally developed in programs screening for new PI3K inhibitors. However, they were found to be effective inhibitors of mTORC1 and mTORC2 as well.141,142 When the half-maximal inhibitory concentration for mTORC1 and mTORC2 inhibition is significantly lower than that for PI3K inhibition, they are called pan-mTOR-inhibitors.143 Currently, there is one interventional study listed in the National Institutes of Health clinical trial database that explores the value of XL765, a dual PI3K/mTOR inhibitor, but no results have been published (NCT01240460). The same drug has been tested in a phase I study in combination with temozolomide (NCT00704080), but no study results are available.
No other malignant brain tumors (eg, primary CNS lymphoma, anaplastic oligodendroglioma) have been treated with mTORC1 inhibitors thus far. Regarding low-grade tumors, the efficacy of temsirolimus in SEGAs is well established (see above). Limited data are available for vestibular schwannomas from recent studies. It has been reported that treatment with temsirolimus in NF2-deficient vestibular schwannomas might have only limited effects,144 while another group showed at least in animal models that mTORC1 inhibition can be effective.145 Moreover, a study of a single patient with recurrent ependymoma and remarkable response to temsirolimus was published.146 No meningioma studies have been reported so far, but preclinical data indicate that meningiomas might represent a suitable target.117
Certain cancer types are at high risk to develop brain metastases during the course of the disease. Malignant melanoma, breast, lung, kidney, and gastrointestinal cancers are especially prone to spread to the brain.147 Rapalogs are able to cross the blood–brain barrier148–150 and have been designed for long-term use,151 qualifying them as interesting candidates for brain metastases treatment.
In triple-negative breast cancer metastatic to the brain, considerable effects of rapamycin and temsirolimus treatment have been recently demonstrated in vitro and in vivo.152 Interestingly, low-dosage rapamycin showed good efficacy in reducing the invasion of brain metastatic cells, while high-dosage treatment was less effective due to activation of the MAPK signaling pathway. However, combination of temsirolimus with the MAP/ERK inhibitor SL325 was able to overrun MAPK activation, with prominent inhibition of perivascular tumor cell invasion. In a currently recruiting phase II trial, patients with Her2+ brain-metastatic breast cancer will be treated with everolimus in combination with trastuzumab and vinorelbine (NCT01305941). The mTOR pathway is also activated in malignant melanoma.153 Brain metastases are frequent in melanoma, but so far only preclinical data are available. Combined treatment of brain-metastatic melanoma cell lines with the BRAF inhibitor vemurafenib and temsirolimus showed encouraging results in at least one melanoma cell line.154 Unfortunately, a phase II trial exploring the combination of temsirolimus with sorafenib and tipifarnib in untreated metastatic melanoma did not include patients with brain metastases.155 No clinical data are available regarding lung cancer metastatic to the brain, while mTOR signaling is activated in non–small cell lung cancer and may present a mechanism of acquired resistance to EGFR–tyrosine kinase inhibitors.156 Moreover, no clinical studies are under way treating brain metastases from kidney or gastrointestinal cancer.
In summary, despite the obvious activation of mTORC1 in malignant gliomas, currently the clinical value of single or combined treatment of primary or recurrent glioblastoma is unclear. In contrast, mTORC1 seems to be a reasonable target in benign intracranial tumors such as SEGA and meningioma.
Acknowledgments
We thank the Deutsche Krebshilfe (grant #108987), the Wilhelm Sander-Stiftung (grant #2010.017.1 and #2010.017.2), and the Deutsche Kinderkrebsstiftung (grant #DKS 2013.04) for their generous research support.
References
- 1.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 2.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 3.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Peterson TR, Laplante M, Thoreen CC, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall MN. mTOR—what does it do? Transplant Proc. 2008;40(10 Suppl):S5–S8. doi: 10.1016/j.transproceed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Sancak Y, Thoreen CC, Peterson TR, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25(6):903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Inoki K, Ikenoue T, et al. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes and Development. 2006;20(20):2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin D, Colombi M, Moroni C, et al. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 9.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273(7):3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 11.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal. 2009;21(6):827–835. doi: 10.1016/j.cellsig.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Facchinetti V, Ouyang W, Wei H, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27(14):1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416(3):375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 15.Patel PH, Thapar N, Guo L, et al. Drosophila Rheb GTPase is required for cell cycle progression and cell growth. J Cell Sci. 2003;116(Pt 17):3601–3610. doi: 10.1242/jcs.00661. [DOI] [PubMed] [Google Scholar]
- 16.Saucedo LJ, Gao X, Chiarelli DA, et al. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5(6):566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Gao X, Saucedo LJ, et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5(6):578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 18.Weber JD, Gutmann DH. Deconvoluting mTOR biology. Cell Cycle. 2012;11(2):236–248. doi: 10.4161/cc.11.2.19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Proud CG. mTORC1 signaling: what we still don't know. J Mol Cell Biol. 2011;3(4):206–220. doi: 10.1093/jmcb/mjq038. [DOI] [PubMed] [Google Scholar]
- 20.Kim E, Goraksha-Hicks P, Li L, et al. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10(8):935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sancak Y, Bar-Peled L, Zoncu R, et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Zhou BP. Kinases meet at TSC. Cell Res. 2007;17(12):971–973. doi: 10.1038/cr.2007.106. [DOI] [PubMed] [Google Scholar]
- 25.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103(2):253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 26.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 27.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Research. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y, Yan H, Frost P, et al. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4(10):1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 29.Phung TL, Ziv K, Dabydeen D, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10(2):159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoeltzing O, Meric-Bernstam F, Ellis LM. Intracellular signaling in tumor and endothelial cells: the expected and, yet again, the unexpected. Cancer Cell. 2006;10(2):89–91. doi: 10.1016/j.ccr.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7(12):961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamada Y, Funakoshi T, Shintani T, et al. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150(6):1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung CH, Jun CB, Ro SH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20(7):1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao D, Inuzuka H, Tan MK, et al. mTOR drives its own activation via SCF(betaTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol Cell. 2011;44(2):290–303. doi: 10.1016/j.molcel.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Xiong X, Sun Y. DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(betaTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell. 2011;44(2):304–316. doi: 10.1016/j.molcel.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yecies JL, Manning BD. mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med (Berl) 2011;89(3):221–228. doi: 10.1007/s00109-011-0726-6. [DOI] [PubMed] [Google Scholar]
- 38.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6(11):1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 39.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 40.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 41.Saci A, Cantley LC, Carpenter CL. Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol Cell. 2011;42(1):50–61. doi: 10.1016/j.molcel.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masri J, Bernath A, Martin J, et al. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Research. 2007;67(24):11712–11720. doi: 10.1158/0008-5472.CAN-07-2223. [DOI] [PubMed] [Google Scholar]
- 43.Lang F, Bohmer C, Palmada M, et al. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86(4):1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 44.Pearce LR, Sommer EM, Sakamoto K, et al. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J. 2011;436(1):169–179. doi: 10.1042/BJ20102103. [DOI] [PubMed] [Google Scholar]
- 45.Oh WJ, Wu CC, Kim SJ, et al. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO J. 2010;29(23):3939–3951. doi: 10.1038/emboj.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zinzalla V, Stracka D, Oppliger W, et al. Activation of mTORC2 by association with the ribosome. Cell. 2011;144(5):757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280(49):40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 48.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 49.Ikenoue T, Inoki K, Yang Q, et al. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. Embo J. 2008;27(14):1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frias MA, Thoreen CC, Jaffe JD, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16(18):1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Delgoffe GM, Pollizzi KN, Waickman AT, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka K, Babic I, Nathanson D, et al. Oncogenic EGFR signaling activates an mTORC2-NF-kappaB pathway that promotes chemotherapy resistance. Cancer Discovery. 2011;1(6):524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241(2):184–196. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 54.Orloff MS, Eng C. Genetic and phenotypic heterogeneity in the PTEN hamartoma tumour syndrome. Oncogene. 2008;27(41):5387–5397. doi: 10.1038/onc.2008.237. [DOI] [PubMed] [Google Scholar]
- 55.Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008;27(41):5443–5453. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 56.Bonneau D, Longy M. Mutations of the human PTEN gene. Hum Mutat. 2000;16(2):109–122. doi: 10.1002/1098-1004(200008)16:2<109::AID-HUMU3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 58.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22(14):2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki A, de la Pompa JL, Stambolic V, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8(21):1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 60.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412(2):179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mizuguchi M, Takashima S. Neuropathology of tuberous sclerosis. Brain Dev. 2001;23(7):508–515. doi: 10.1016/s0387-7604(01)00304-7. [DOI] [PubMed] [Google Scholar]
- 63.Borkowska J, Schwartz RA, Kotulska K, et al. Tuberous sclerosis complex: tumors and tumorigenesis. Int J Dermatol. 2011;50(1):13–20. doi: 10.1111/j.1365-4632.2010.04727.x. [DOI] [PubMed] [Google Scholar]
- 64.Chan JA, Zhang H, Roberts PS, et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol. 2004;63(12):1236–1242. doi: 10.1093/jnen/63.12.1236. [DOI] [PubMed] [Google Scholar]
- 65.Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59(3):490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 66.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 67.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 68.Gutmann DH, Parada LF, Silva AJ, et al. Neurofibromatosis type 1: modeling CNS dysfunction. J Neurosci. 2012;32(41):14087–14093. doi: 10.1523/JNEUROSCI.3242-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278(1):51–57. [PubMed] [Google Scholar]
- 70.Chen YH, Gutmann DH. The molecular and cell biology of pediatric low-grade gliomas. Oncogene. 2013;33(16):2019–2026. doi: 10.1038/onc.2013.148. [DOI] [PubMed] [Google Scholar]
- 71.Gutmann DH, Rasmussen SA, Wolkenstein P, et al. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1) Neurology. 2002;59(5):759–761. doi: 10.1212/wnl.59.5.759. [DOI] [PubMed] [Google Scholar]
- 72.Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68(5):1110–1118. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin GA, Viskochil D, Bollag G, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63(4):843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- 74.Xu GF, Lin B, Tanaka K, et al. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990;63(4):835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- 75.Basu TN, Gutmann DH, Fletcher JA, et al. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356(6371):713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- 76.Bollag G, Clapp DW, Shih S, et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet. 1996;12(2):144–148. doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]
- 77.DeClue JE, Papageorge AG, Fletcher JA, et al. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992;69(2):265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- 78.Dasgupta B, Yi Y, Chen DY, et al. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Research. 2005;65(7):2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- 79.Johannessen CM, Reczek EE, James MF, et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102(24):8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 83.Resta N, Lauriola L, Puca A, et al. Ganglioglioma arising in a Peutz-Jeghers patient: a case report with molecular implications. Acta Neuropathol. 2006;112(1):106–111. doi: 10.1007/s00401-006-0084-6. [DOI] [PubMed] [Google Scholar]
- 84.Louis D, Ohgaki H, Wiestler O, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 86.Broderick DK, Di C, Parrett TJ, et al. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 2004;64(15):5048–5050. doi: 10.1158/0008-5472.CAN-04-1170. [DOI] [PubMed] [Google Scholar]
- 87.Gallia GL, Rand V, Siu IM, et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006;4(10):709–714. doi: 10.1158/1541-7786.MCR-06-0172. [DOI] [PubMed] [Google Scholar]
- 88.Masica DL, Karchin R. Correlation of somatic mutation and expression identifies genes important in human glioblastoma progression and survival. Cancer Res. 2011;71(13):4550–4561. doi: 10.1158/0008-5472.CAN-11-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koul D. PTEN signaling pathways in glioblastoma. Cancer Biol Ther. 2008;7(9):1321–1325. doi: 10.4161/cbt.7.9.6954. [DOI] [PubMed] [Google Scholar]
- 90.Riemenschneider MJ, Betensky RA, Pasedag SM, et al. AKT activation in human glioblastomas enhances proliferation via TSC2 and S6 kinase signaling. Cancer Res. 2006;66(11):5618–5623. doi: 10.1158/0008-5472.CAN-06-0364. [DOI] [PubMed] [Google Scholar]
- 91.Annovazzi L, Mellai M, Caldera V, et al. mTOR, S6 and AKT expression in relation to proliferation and apoptosis/autophagy in glioma. Anticancer Res. 2009;29(8):3087–3094. [PubMed] [Google Scholar]
- 92.Pelloski CE, Lin E, Zhang L, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006;12(13):3935–3941. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- 93.Gruber Filbin M, Dabral SK, Pazyra-Murphy MF, et al. Coordinate activation of Shh and PI3K signaling in PTEN-deficient glioblastoma: new therapeutic opportunities. Nat Med. 2013;19(11):1518–1523. doi: 10.1038/nm.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Read RD, Cavenee WK, Furnari FB, et al. A drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS genetics. 2009;5(2):e1000374. doi: 10.1371/journal.pgen.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weiler M, Blaes J, Pusch S, et al. mTOR target NDRG1 confers MGMT-dependent resistance to alkylating chemotherapy. Proc Natl Acad Sci U S A. 2014;111(1):409–414. doi: 10.1073/pnas.1314469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Forshew T, Tatevossian RG, Lawson AR, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218(2):172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 98.Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19(3):449–458. doi: 10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones DT, Gronych J, Lichter P, et al. MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sci. 2012;69(11):1799–1811. doi: 10.1007/s00018-011-0898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jentoft M, Giannini C, Cen L, et al. Phenotypic variations in NF1-associated low grade astrocytomas: possible role for increased mTOR activation in a subset. Int J Clin Exp Pathol. 2010;4(1):43–57. [PMC free article] [PubMed] [Google Scholar]
- 102.Hutt-Cabezas M, Karajannis MA, Zagzag D, et al. Activation of mTORC1/mTORC2 signaling in pediatric low-grade glioma and pilocytic astrocytoma reveals mTOR as a therapeutic target. Neuro Oncol. 2013;15(12):1604–1614. doi: 10.1093/neuonc/not132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaul A, Chen YH, Emnett RJ, et al. Pediatric glioma-associated KIAA1549:BRAF expression regulates neuroglial cell growth in a cell type-specific and mTOR-dependent manner. Genes Dev. 2012;26(23):2561–2566. doi: 10.1101/gad.200907.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hegedus B, Banerjee D, Yeh TH, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68(5):1520–1528. doi: 10.1158/0008-5472.CAN-07-5916. [DOI] [PubMed] [Google Scholar]
- 105.Banerjee S, Gianino SM, Gao F, et al. Interpreting mammalian target of rapamycin and cell growth inhibition in a genetically engineered mouse model of Nf1-deficient astrocytes. Mol Cancer Ther. 2011;10(2):279–291. doi: 10.1158/1535-7163.MCT-10-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ichimura K, Bolin MB, Goike HM, et al. Deregulation of the p14ARF/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G1-S transition control gene abnormalities. Cancer Res. 2000;60(2):417–424. [PubMed] [Google Scholar]
- 107.Rubenstein J, Ferreri AJ, Pittaluga S. Primary lymphoma of the central nervous system: epidemiology, pathology and current approaches to diagnosis, prognosis and treatment. Leuk Lymphoma. 2008;49(Suppl 1):43–51. doi: 10.1080/10428190802311441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dutton A, Reynolds GM, Dawson CW, et al. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase and mTOR. J Pathol. 2005;205(4):498–506. doi: 10.1002/path.1725. [DOI] [PubMed] [Google Scholar]
- 109.Wlodarski P, Kasprzycka M, Liu X, et al. Activation of mammalian target of rapamycin in transformed B lymphocytes is nutrient dependent but independent of Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase, insulin growth factor-I, and serum. Cancer Res. 2005;65(17):7800–7808. doi: 10.1158/0008-5472.CAN-04-4180. [DOI] [PubMed] [Google Scholar]
- 110.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–i56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99(3):379–391. doi: 10.1007/s11060-010-0342-2. [DOI] [PubMed] [Google Scholar]
- 112.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72(5):791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- 113.Curto M, McClatchey AI. Nf2/Merlin: a coordinator of receptor signalling and intercellular contact. Br J Cancer. 2008;98(2):256–262. doi: 10.1038/sj.bjc.6604002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.James MF, Han S, Polizzano C, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29(15):4250–4261. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.James MF, Stivison E, Beauchamp R, et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012;10(5):649–659. doi: 10.1158/1541-7786.MCR-11-0425-T. [DOI] [PubMed] [Google Scholar]
- 116.Surace E, Lusis E, Haipek C, et al. Functional significance of S6K overexpression in meningioma progression. Ann Neurol. 2004;56(2):295–298. doi: 10.1002/ana.20201. [DOI] [PubMed] [Google Scholar]
- 117.Pachow D, Andrae N, Kliese N, et al. mTORC1 inhibitors suppress meningioma growth in mouse models. Clin Cancer Res. 2013;19(5):1180–1189. doi: 10.1158/1078-0432.CCR-12-1904. [DOI] [PubMed] [Google Scholar]
- 118.Dobashi Y, Sato E, Oda Y, et al. Significance of Akt activation and AKT gene increases in soft tissue tumors. Hum Pathol. 2014;45(1):127–136. doi: 10.1016/j.humpath.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 119.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2(67):pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 120.Hidalgo M, Rowinsky EK. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19(56):6680–6686. doi: 10.1038/sj.onc.1204091. [DOI] [PubMed] [Google Scholar]
- 121.Mita MM, Mita AC, Chu QS, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26(3):361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 122.O'Donnell A, Faivre S, Burris HA, 3rd, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26(10):1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 123.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27(23):3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 124.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 125.Raymond E, Alexandre J, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22(12):2336–2347. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 126.Curran MP. Everolimus: in patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatr Drugs. 2012;14(1):51–60. doi: 10.2165/11207730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 127.Krueger DA, Care MM, Agricola K, et al. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80(6):574–580. doi: 10.1212/WNL.0b013e3182815428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23(23):5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 129.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23(4):357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 130.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Medicine. 2008;5(1):e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Geoerger B, Kieran MW, Grupp S, et al. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur J Cancer. 2012;48(2):253–262. doi: 10.1016/j.ejca.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sarkaria JN, Galanis E, Wu W, et al. North Central Cancer Treatment Group phase I trial N057 K of everolimus (RAD001) and temozolomide in combination with radiation therapy in patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;81(2):468–475. doi: 10.1016/j.ijrobp.2010.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chinnaiyan P, Won M, Wen PY, et al. RTOG 0913: a phase 1 study of daily everolimus (RAD001) in combination with radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2013;86(5):880–884. doi: 10.1016/j.ijrobp.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mason WP, Macneil M, Kavan P, et al. A phase I study of temozolomide and everolimus (RAD001) in patients with newly diagnosed and progressive glioblastoma either receiving or not receiving enzyme-inducing anticonvulsants: an NCIC CTG study. Invest New Drugs. 2012;30(6):2344–2351. doi: 10.1007/s10637-011-9775-5. [DOI] [PubMed] [Google Scholar]
- 135.Sarkaria JN, Galanis E, Wu W, et al. Combination of temsirolimus (CCI-779) with chemoradiation in newly diagnosed glioblastoma multiforme (GBM) (NCCTG trial N027D) is associated with increased infectious risks. Clin Cancer Res. 2010;16(22):5573–5580. doi: 10.1158/1078-0432.CCR-10-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee EQ, Kuhn J, Lamborn KR, et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02. Neuro Oncol. 2012;14(12):1511–1518. doi: 10.1093/neuonc/nos264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lassen U, Sorensen M, Gaziel TB, et al. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013;33(4):1657–1660. [PubMed] [Google Scholar]
- 138.Wen PY, Chang SM, Lamborn KR, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02. Neuro Oncol. 2014;16(4):576–578. doi: 10.1093/neuonc/not247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol. 2010;96(2):219–230. doi: 10.1007/s11060-009-9950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guertin DA, Stevens DM, Saitoh M, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15(2):148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9(5):341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125(4):733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Apsel B, Blair JA, Gonzalez B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008;4(11):691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karajannis MA, Legault G, Hagiwara M, et al. Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2014;16(2):292–297. doi: 10.1093/neuonc/not150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Giovannini M, Bonne NX, Vitte J, et al. mTORC1 inhibition delays growth of neurofibromatosis type 2 schwannoma. Neuro Oncol. 2014;16(4):493–504. doi: 10.1093/neuonc/not242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bowers DC, Kucejova B, Margraf L, et al. mTORC1 activation in childhood ependymoma and response to sirolimus. J Neurooncol. 2011;103(3):797–801. doi: 10.1007/s11060-010-0455-7. [DOI] [PubMed] [Google Scholar]
- 147.Johnson JD, Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7(3):337–344. [PubMed] [Google Scholar]
- 148.Kwon CH, Zhu X, Zhang J, et al. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci U S A. 2003;100(22):12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 150.O'Reilly T, McSheehy PM, Kawai R, et al. Comparative pharmacokinetics of RAD001 (everolimus) in normal and tumor-bearing rodents. Cancer Chemother Pharmacol. 2010;65(4):625–639. doi: 10.1007/s00280-009-1068-8. [DOI] [PubMed] [Google Scholar]
- 151.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2(3):222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 152.Zhao H, Cui K, Nie F, et al. The effect of mTOR inhibition alone or combined with MEK inhibitors on brain metastasis: an in vivo analysis in triple-negative breast cancer models. Breast Cancer Res Treat. 2012;131(2):425–436. doi: 10.1007/s10549-011-1420-7. [DOI] [PubMed] [Google Scholar]
- 153.Karbowniczek M, Spittle CS, Morrison T, et al. mTOR is activated in the majority of malignant melanomas. J Invest Dermatol. 2008;128(4):980–987. doi: 10.1038/sj.jid.5701074. [DOI] [PubMed] [Google Scholar]
- 154.Daphu I, Horn S, Stieber D, et al. In vitro treatment of melanoma brain metastasis by simultaneously targeting the MAPK and PI3K signaling pathways. Int J Mol Sci. 2014;15(5):8773–8794. doi: 10.3390/ijms15058773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Margolin KA, Moon J, Flaherty LE, et al. Randomized phase II trial of sorafenib with temsirolimus or tipifarnib in untreated metastatic melanoma (S0438) Clin Cancer Res. 2012;18(4):1129–1137. doi: 10.1158/1078-0432.CCR-11-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fumarola C, Bonelli MA, Petronini PG, et al. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol. 2014;90(3):197–207. doi: 10.1016/j.bcp.2014.05.011. [DOI] [PubMed] [Google Scholar]