Abstract
Background
Accumulation and infiltration of microglia/brain macrophages around and into glioma tissue promote tumor invasion and expansion. One tumor-promoting mechanism of microglia/brain macrophages is upregulation of membrane type 1 matrix metalloprotease (MT1-MMP), which promotes the degradation of extracellular matrix. MT1-MMP upregulation is induced by soluble factors released by glioma cells activating microglial Toll-like receptor 2 (TLR2).
Methods
Versican identified by proteomics was silenced in glioma cells by short interference RNA and short hairpin RNA approaches and studied in vitro and after injection into mouse brains or organotypic brain slices.
Results
The splice variants V0/V1 of the endogenous TLR2 ligand versican are highly expressed in mouse and human glioma tissue. Versican-silenced gliomas induced less MT1-MMP expression in microglia both in vitro and in vivo, which resulted in smaller tumors and longer survival rates as compared with controls. Recombinant versican V1 induced significantly higher levels of MT1-MMP in wild-type microglia compared with untreated and treated TLR2 knockout microglial cells. Using glioma-injected organotypic brain slices, we found that the impact of versican signaling on glioma growth depended on the presence of microglia. Moreover, we found that TLR2 expression is upregulated in glioma-associated microglia but not in astrocytes. Additionally, an established TLR2 neutralizing antibody reduced glioma-induced microglial MT1-MMP expression as well as glioma growth ex vivo.
Conclusions
Our results show that versican released from glioma promotes tumor expansion through glioma-associated microglial/macrophage TLR2 signaling and subsequent expression of MT1-MMP. This signaling cascade might be a novel target for glioma therapies.
Keywords: glioma, microglia/brain macrophages, MT1-MMP, TLR2, versican
High-grade gliomas (HGGs) are the most common primary tumors of the central nervous system (CNS) with dismal prognosis despite the advantages of multimodal therapies. HGGs are heterogeneous neoplasms that contain stromal cells.1 Glioma-associated microglial cells and invaded macrophages (GAMs) make up the largest portion of tumor-infiltrating cells, contributing up to 30% of the entire glioma mass.2,3 GAMs show an immune-suppressive phenotype and promote tumor expansion.4,5 Glioma converts GAMs into a protumorigenic phenotype by glioma-released soluble factor(s). We have previously shown that these factor(s) activate Toll-like receptor 2 (TLR2) and, subsequently, p38 mitogen-activated protein kinase (MAPK) signaling pathways. This activation leads to induction of membrane type 1 matrix metalloprotease (MT1-MMP) expression, which promotes glioma invasion.6,7 Inhibition of this pathway by minocycline has therapeutic potential in experimental glioma treatment.8
TLRs are key receptors in the recognition of pathogens and thus play an important role in immunological responses.9 Besides the recognition of pathogen-associated molecular patterns, TLRs also bind endogenous ligands created by tissue injury or tumor growth (ie, damage-associated molecular patterns).10,11 We have previously identified TLR2 expressed by GAMs to be a protumorigenic factor. TLR2 is highly expressed in human glioma tissue, which contains GAMs, and its expression is inversely correlated with patient survival.7
There is an increasing list of endogenous ligands for TLRs. Versican V1 was reported as an endogenous ligand of TLR2 on macrophages.12 Versican, also known as CSPG2, is a member of large chondroitin sulfate proteoglycans that belongs to extracellular matrix components. Due to RNA splicing, 4 isoforms have been identified so far (V0, V1, V2, V3, respectively).13 Sequence analyses have revealed that different isoforms contain different glycosaminoglycan (GAG) domains. V0, the largest isoform, contains 2 alternatively spliced GAG attachment domains designated as GAG-α and GAG-β, whereas V1 contains only GAG-β, and V2 contains only GAG-α. V3 consists only of globular domains. Versican is highly expressed in tissue compartments undergoing active cell proliferation and migration, such as smooth muscle tissues and cartilage.14 Versican isoforms show distinct functions in the brain. V1 induces neuronal differentiation and promotes neurite outgrowth, while V2 is a potent inhibitor of axonal growth.15,16 Versican expression is elevated in several cancers including brain, lung, breast, and ovarian and is associated with cancer relapse and patient prognosis.12,17–19
Material and Methods
Animals
We used C57BL/6 WT mice (Charles River Laboratories) and TLR 2 knockout (KO) mice on a C57BL/6 background. The TLR2 KO mice were generated by Dr. Shizuo Akira of Osaka University, Japan, and were obtained from Oriental BioServices Inc.20 The KO efficiency has been shown previously.7 All mice were handled according to governmental (LaGeSo, G 0268/10, G 0343/10) and internal (MDC) regulations.
Human Materials
All human glioma and normal brain materials in this study were obtained from the Department of Neurosurgery and Department of Neuropathology at Charité University Hospital according to the rules of the Ethical Committee (Charité, EA4/098/11).
Cell Culture
Murine glioma cell line WT GL261 (National Cancer Institute) and human glioma cell line U373 were cultured in Dulbecco's modified Eagle's medium with supplements (200 mM glutamine, 50 units/mL penicillin, 50 μg/mL streptomycin, and 10% fetal calf serum [FCS], all from Invitrogen). EGFP and mCherry GL261 cells were generated as previously described.7 Primary human glioblastoma cells GBM1, GBM2, GBM3, and GBM4 were derived from tumor resections. The material was washed and cut into pieces. After digestion with 1 mL trypsin/DNAse, the cell suspension was filtered by a cell strainer (70 µm, BD Falcon), resuspended in culture medium, and transferred to a T25 flask. After 2 days, the cells were washed with phosphate-buffered saline (PBS), and the medium was changed. Cells were cultured adherently until confluency and then passaged. Primary microglia were prepared from neonatal C57BL/6 or TLR2 KO mice, as previously described.4,7 The CHO-K1 cells overexpressing recombinant V1 (rV1) versican (CHO-V1) used for recombinant versican production were kindly provided by Prof. R. LeBaron (Division of Life Sciences, University of Texas at San Antonio)21 and were cultured in αMEM supplemented with 10% FCS, 50 units/mL penicillin, 50 μg/mL streptomycin (Sigma-Aldrich), and Plasmocin 5 μg/mL (Invivogen). All cells were incubated at 37°C in 5% CO2. Glioma-conditioned medium (GCM) was collected as previously described7 and confirmed to be mycoplasma free. For collecting the conditioned medium from the short interference RNA (siRNA) experiments, after silencing cells by siRNA, the same number of cells from control siRNA or versican siRNA treatment groups were seeded, and GCM was collected after 24 hours.
Identification of Secreted Proteins in GCM by Shot-gun Proteomics
Proteins of serum-free GCM were concentrated and converted to peptides. Peptides were ionized and sprayed into the mass spectrometer after purification, and the recorded spectra were analyzed. (Details of the procedure are available in the Supplementary information.)
Purification of Versican V1
Full-length recombinant versican (rV1) was purified from CHO–V1-conditioned media using a combination of anion exchange and gel filtration chromatography, as previously described.22 (The modifications are outlined in the Supplementary information.)
Total RNA Isolation and Polymerase Chain Reaction
Total RNA isolation and quantitative reverse transcription (qRT-) PCR were performed as previously described.7 (The primer sequence and PCR program are listed in the Supplementary information.)
Protein Extraction and Western Blot
Protein concentration was determined as described.7 For detecting versican, protein extract was dialyzed against chondroitinase buffer (40 mM Tris, 40 mM sodium acetate, 10 mM EDTA, pH 8) and digested with chondroitinase ABC (Seikagaku; 1–2 mU/g protein) at 37°C overnight before electrophoresis. The membranes were blocked and followed by overnight incubation at 4°C with rabbit anti-V0/V1 or anti-V0/V2 antibody (a gift from Prof. Dr. Dieter R. Zimmermann, Zurich). The membranes were incubated with a secondary anti-rabbit horseradish peroxidase antibody (1:2000; Cell Signaling), developed with SuperSignal West Pico Chemiluminescence substrate kit (Thermo Fisher Scientific), and signal detected by Molecular Imager Gel Doc XR system (Bio-Rad).
Versican Knockdown with Short Interference RNA and Short hairpin RNA Approach
For transient silencing of versican expression, GL261 cells were transfected with siRNA versican (ON-TARGETplus SMARTpool, Dharmacon) and as control with scrambled nontargeted siRNA with Dharmafect4 (Dharmacon) according to the manufacture's instructions. After 24 hours of transfection, cells transfected with siRNA versican and nontargeted siRNA were seeded into 6-well plates, again with the same cell density for generating conditioned medium. siGAPDH (control SMARTpool, Dharmacon) was used as a positive control, and transfection efficiency was tested by qRT-PCR and Western blot.
For stable versican knockdown, short hairpin RNA (shRNA) was generated with pSUPER retro system (Oligoengine) according to the manufacturer's instructions. shRNA sequences are ACTGCGGAGCACACGTAAATA and GGTGGCCCAGAACGGAAATAT, and empty vector was used as a negative control. Retroviral supernatant was generated by transfection of Phoenix cells as described23 and applied to GL261 or EGFP GL261 cells to transduce versican genes. Infected cells were selected for 3 days with 2 µg/mL puromycin, and cell proliferation of versican knocked-down GL261 and control cells was analyzed by cell counting or BrdU assay according to the manufacturer's instructions (Roche).
Glioma-associated Microglia/Macrophage Isolation by Magnetic-activated Cell Sorting
GAMs were acutely isolated by magnetic-activating cell sorting (MACS), as previously described. As with any manipulation, MACS will trigger the activation of microglial cells; however, we observed a clear difference with respect to MT1-MMP expression between MACS isolated cells from normal brain versus those from glioma tissue.7
Flow Cytometry
Control brain tissue and tumor tissue were dissociated into cell suspensions, and cells were then washed and incubated with different antibodies for 30 minutes at 4°C. (Antibody information is outlined in the Supplementary information.) Data were analyzed using FlowJo software (Treestar). Flow cytometry data were quantified by median fluorescence intensity (MFI) and presented in histograms.
Organotypic Brain Slice Model and Tumor Inoculation
Organotypic brain slices were prepared, and 5000 EGFP GL261 cells were injected into slices, as described previously.4 For the treatment with a TLR2 antibody, mAb T2.5 or isotype control (both from eBioscience) was applied to the medium after the tumor implantation, and new antibody or isotype was added every other day when the medium was changed.
Glioma Implantation
Twenty-thousand WT, shcon (nontargeted control) and shver (versican silenced) GL261 cells were inoculated into mouse brains as described previously.7
Immunofluorescent Staining and Image Processing
Free-floating 40 µm thick brain sections from tumor-bearing mice were prepared as previously described.7 (Antibody information is outlined in the Supplementary material.)
Tumor Size Quantification and Survival Analysis
Two groups of wild-type (WT) C57BL/6 (at least 7 in each group) mice were injected with either shcon or shver GL261 cells. Tumor size was determined by unbiased stereology using the Stereoinvestigator software (Mbf Bioscience), and survival rate was calculated by Kaplan-Meier method with log-rank analysis.
Statistical Analysis
All data represent the average of at least 3 independent experiments. Error bars represent standard error of the mean. Datasets were analyzed statistically with SPSS11.5 software and tested for normality with the Shapiro-Wilks test. The Mann-Whitney U test was used for nonparametric analysis. Parametric testing was done with the Student t test. Comparisons between multiple groups were done using 1-way ANOVA with the Scheffé post hoc test. Statistical significance was determined at P values <.05 (*) and <.01 (**) while “n.s.” implied a nonsignificant P value.
Results
The Endogenous TLR2 Ligand Versican V0/V1 Is Expressed by Gliomas
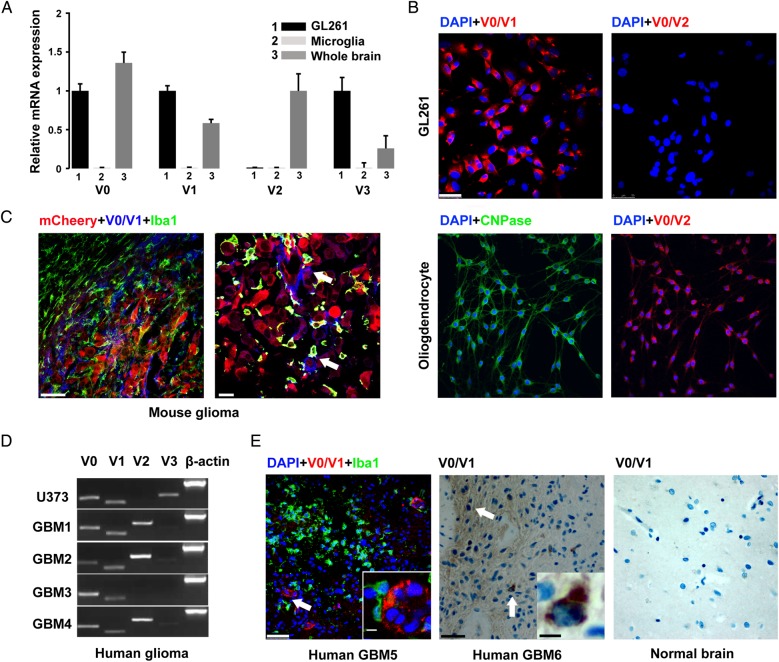
The expression of versican isoforms was analyzed in microglia, whole adult brain lysate, and GL261 cells by qRT-PCR. GL261 cells expressed predominately V0, V1, and V3 mRNA but not V2, while microglial cells did not express any versican isoforms (Fig. 1A). On the protein level, GL261 cells only expressed V0/V1 but not V2 as shown by immunostaining. (The oligodendrocyte precursor cell line Oli-neu24 was used as a positive control for V2; Fig. 1B). In mouse glioma tissue, versican V0/V1 was only expressed within tumor tissue and was mainly colabeled with mCherry-positive glioma cells (Fig. 1C, arrows). In the human glioma cell line U373 and 4 primary cultures of human GBM samples (all of them express low levels of the glioma stem cell marker CD133) versican V1 was expressed by all cells, while V2 was expressed only by some primary GBM cells (Fig. 1D). In human GBM tissue, versican V0/V1 was also expressed in 3 human samples but not in the normal brain tissue. (Fig. 1E and Supplementary Fig. S1B). Moreover, we performed mass spectrometry with conditioned medium from GL261 and could detect the presence of versican (Supplementary Table S1). These results show that versican V0/V1 is highly expressed by mouse and human gliomas compared with the normal brain tissues.
Fig. 1.
Versican is highly expressed in glioma cells but not by microglia. (A) Expression of V0, V1, V2, and V3 in cultured microglia, whole brain, and GL261 glioma cells by qRT-PCR. At least 3 adult mouse brains or 3 different preparations of microglia were analyzed in each condition. (B) Immunohistochemistry shows the expression of V0/V1, but not V0/V2, in GL261 cells. The Oli-neu cell line (stained with an oligodendrocyte marker CNPase) was used as positive control for V0/V2. Nuclei were labeled with DAPI in blue. Scale bar is 50 μm. (C) Tissue sections from a mouse inoculated with mCherry GL261 glioma cells (red) and stained with V0/V1 (blue) and Iba-1 (green). Scale bar is 50 μm in the left image and 25 μm in right image. (D) Expression of the different versican isoforms in a human glioma cell line (U373) and cells cultured from human GBM samples (GBM1-4) as determined with RT-PCR. (E) Sections from a human GBM patient stained with V0/V1 (red), Iba-1 (green), one human GBM (middle), and one normal brain tissue (right) stained with V0/V1. Scale bar is 50 μm and 10 μm for the insert.
Recombinant Versican V1 Induces Microglial MT1-MMP Expression
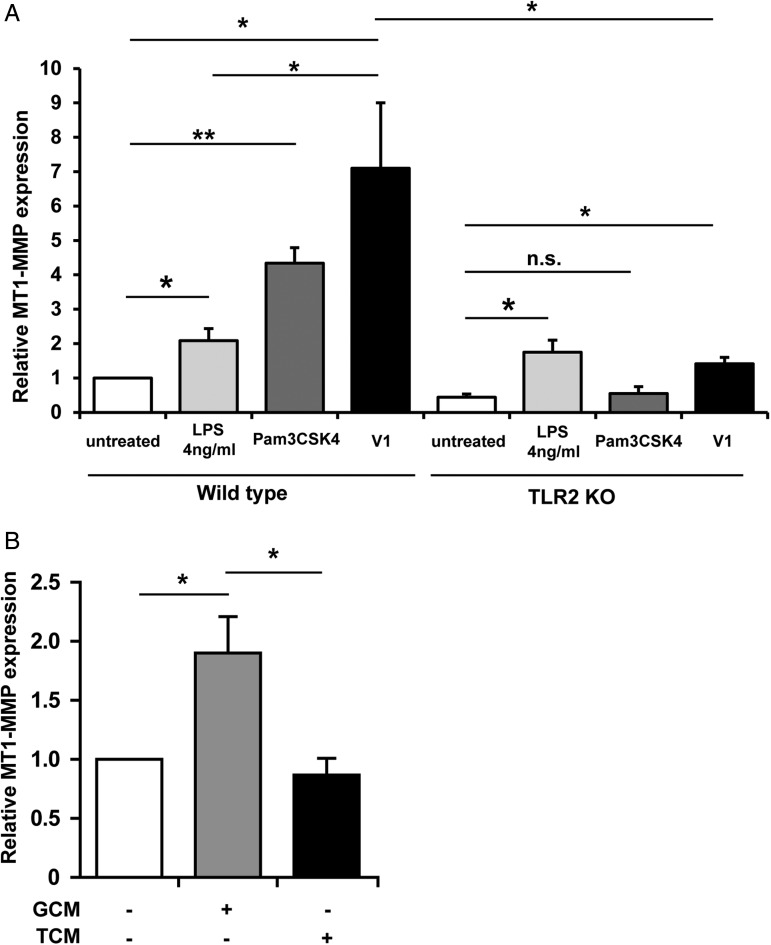
To test whether versican is responsible for MT1-MMP induction on microglia, we first treated primary cultured microglia with recombinant versican V1 and analyzed MT1-MMP expression by qRT-PCR. The TLR2 agonist heat-killed Listeria monocytogenes (HKLM; InvivoGen) was used as a positive control. Recombinant versican V1 induced MT1-MMP expression on microglia after 6 hours of stimulation (7.1 fold ± 1.9; P = .032) (Fig. 2A).To exclude the effect from endotoxin in the purified recombinant V1, we used Lipopolysaccharide (LPS) as a control. We observed a small induction of MT1-MMP with LPS (2.1 fold ± 0.35; P = .04); however, the induction was significantly less than with V1 (P = .035), indicating that the major effect was due to recombinant V1. We repeated the same treatment on microglia cultured from TLR2 KO animals. As seen in Fig. 2A, the TLR2 agonist Pam3CSK4 failed to induce MT1-MMP expression (P = .37). Although we still observed an upregulation of MT1-MMP in V1 treatment group (P = .01), it was significantly less (P = .037) compared with the WT microglia. Since the MT1-MMP induction is similar to the LPS treatment group, the V1-induced expression in the TLR2 KO microglia may be due to contaminating endotoxin.
Fig. 2.
Recombinant versican V1 induces microglial MT1-MMP expression via TLR2. (A) Wild-type and TLR2 KO microglia were stimulated with recombinant versican V1 for 6 hours, and MT1-MMP was analyzed by qRT-PCR. The internal control was 4 ng/mL LPS, and Pam3CSK4 was used as a positive control (n = 5). (B) Cultured microglia were stimulated with conditioned media from siRNA-versican (TCM) and nontarget transfected GL261 cells (glioma-conditioned medium) for 6 hours, and MT1-MMP expression was analyzed by qRT-PCR (n = 5).
Versican-silenced Gliomas Induced Less MT1-MMP Expression in Microglia, Reduced the Tumor Size, and Prolonged Survival Time of Mice Bearing Glioma
To further identify the role of versican on microglial MT1-MMP regulation, total versican expression in GL261 cells was knocked down by siRNA (Supplementary Fig. S2A), and glioma-conditioned medium was collected from both nontarget siRNA-transfected (GCM) and versican siRNA-transfected GL261 (TCM). Primary cultured microglial cells were then stimulated by both conditioned media for 6 hours, and MT1-MMP expression was analyzed by qRT-PCR. After 6 hours of stimulation, MT1-MMP expression was significantly increased in the nontarget siRNA group (GCM, 1.9 ± 0.3 fold; P = .04) while it was not increased in the versican siRNA group (TCM, 0.9 ± 0.3 fold; P = .01 compared to GCM treatment group), indicating that versican is an important factor for microglial MT1-MMP regulation (Fig. 2B).
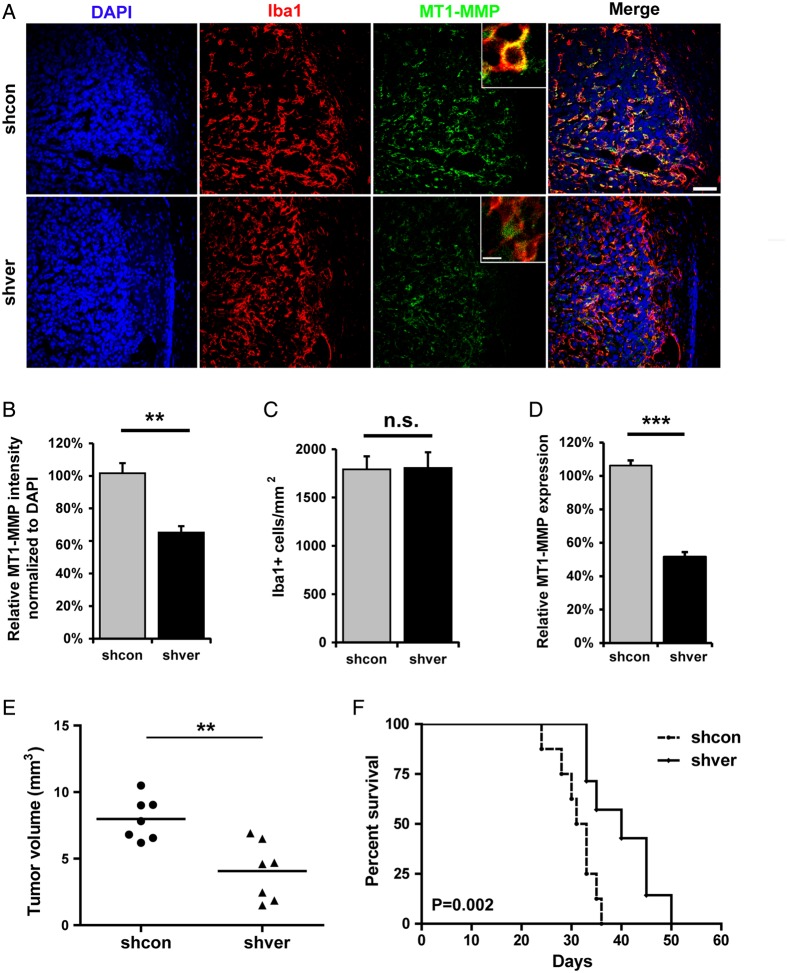
To further verify this effect in situ, we generated control and versican knocked-down GL261 cells with control shRNA (shcon) or versican shRNA (shver) (Supplementary Fig. S2A). These 2 cell lines were implanted into mouse brain (n = 7 in each group). After 2 weeks of tumor growth, mice were euthanized, and brain tissue was analyzed by immunohistochemistry. GAMs were identified by immunolabeling with Iba-1 (Fig. 3A). When MT1-MMP fluorescence intensity was analyzed by Image J and normalized to the intensity of DAPI, we observed a significant decrease in MT1-MMP immunoreactivity in GAMs in the shver group (65% ± 4%; P < .01) compared with shcon (Fig. 3B). We did not observe any difference in Iba-1 positive cell density between the 2 groups, indicating that versican is not the factor recruiting microglial/macrophages to the tumor (Fig. 3C). To further verify the role of versican in regulating MT-1MMP expression ex vivo, we implanted shcon and shver GL261 cells into the brains of WT mice. After 2 weeks of tumor growth, GAMs were isolated by MACS (CD11b+), total RNA was collected, and qRT-PCR of MT1-MMP was performed. Indeed, we observed a significant decrease of MT1-MMP expression on GAMs in shver tumor compared with shcon (51% ± 5%; P < .001, Fig. 3D). These data demonstrate that versican is a glioma-derived mediator that regulates microglial MT1-MMP expression.
Fig. 3.
Versican-silenced gliomas induced less MT1-MMP expression in microglia, reduced tumor growth, and prolonged survival. (A) Versican knocked down (shver) and control (shcon) GL261 were implanted into wild-type mice, and the glioma tissue sections were stained 14 days after inoculation with DAPI, Iba-1, and MT1-MMP. The image on the right is a merge of all 3 images. Scale bar is 50 μm and 10 μm for the insert. Images were quantified by Image J (n = 6 in each group) to show the difference in MT1-MMP expression normalized to DAPI (B) and Iba-1 positive cell density (C) comparing tissue with shver and shcon GL261 cells. (D) WT mice were injected intracerebrally with shcon or shver glioma cells, and glioma-associated microglia/macrophages were isolated 14 days after glioma cell injection by MACS from both naïve and tumor tissue. Differences in MT1-MMP expression were analyzed by qRT-PCR (n = 3 in each group). (E) shver and shcon GL261 cells were inoculated into WT mice. After 2 weeks, tumor volume was evaluated based on unbiased stereology (n = 7). (F) The Kaplan-Meier curves represent the cumulative survival of mice after shver and shcon GL261 injection (n = 8).
To investigate if ablation of versican in gliomas interferes with tumor expansion in vivo, we injected shcon GL261 and shver GL261 into mouse brains and quantified tumor volumes by unbiased stereological estimation (Cavalieri method). Tumor volumes were significantly reduced in versican shRNA GL261-implanted mice (4.07 ± 0.82 mm3) compared with shcon GL261-implanted mice (7.98 ± 0.6 mm3; P < .01, Fig. 3E). Furthermore, we did another set of tumor inoculations and found that knockdown of versican in GL261 cells led to significantly increased survival rate (Fig. 3F, n = 8; P = .002).
Depleting Microglia From Cultured Brain Slices Abolishes the Effect of Versican Silencing on Glioma Expansion
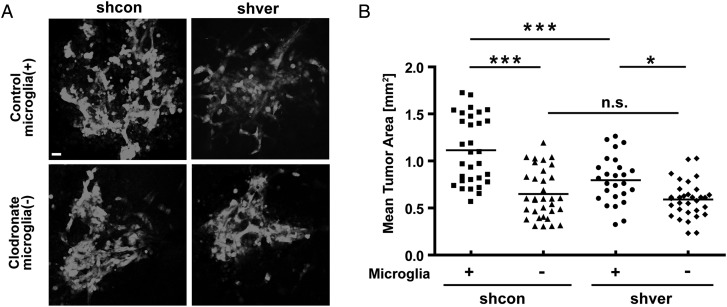
We tested whether the presence of microglia is essential for the effect of versican silencing on glioma expansion. We prepared organotypic brain slices in which we depleted microglia using liposome-encapsulated clodronate. We then injected glioma cells into the normal and microglia-depleted slices and analyzed the area occupied by glioma cells 5 days later (Fig. 4A). Versican knocked-down glioma cells resulted in significant smaller tumors (shcon-enhanced green fluorescent protein [EGFP] PBS, 1.1 ± 0.36 mm2; shver EGFP PBS; 0.79 ± 0.25 mm2; P < .001) in slices with normal density of microglia. When microglia were depleted, there was no significant difference in glioma expansion comparing the injection of shcon and shver cells (shcon cl, 0.65 ± .26 mm2; shver cl, 0.59 ± 0.2 mm2; P>.99), indicating that the role of versican in tumor growth is dependent on the presence of microglia (Fig. 4B).
Fig. 4.
Versican-silenced GL261 resulted in smaller tumors depending on the presence of microglia. Control and microglia-depleted brain slices (by treatment with clodronate-filled liposomes) from 16 day-old mice were implanted with 5000 shcon or shver EGFP-GL261 cells. The area occupied by glioma cells was measured after 5 days. (A) The representative fluorescence micrograph of EGFP-labeled glioma cells in both microglia-containing and -depleted slices were injected with shcon (left) and shver (right) cells. Scale bar is 10 μm. (B) Tumor area was quantified in microglia-containing (+) and -depleted (-) slices injected with shcon and shver glioma cells.
Conditioned Medium From Microglia but not Astrocytes Induces Glioma Versican Expression in Vitro
To investigate whether microglia or astrocytes can influence the expression of versican by glioma cells, GL261 cells were stimulated with conditioned medium from microglia and astrocytes for 24 hours. The microglia-conditioned medium increased the expression of V0, V1, and V3, but not V2. As seen in Supplementary Fig. S3A, microglial-conditioned medium (MCM) induced 1.94 (±0.1; P < .001) times higher expression in all versican isoforms: 1.5 in V0 (1.44 ± 0.07; P < .01), 1.6 in V1 (1.58 ± 0.09; P < .01), and 1.5 but not significant in V3 (1.49 ± 0.2; P > .05). V2 was undetectable. GL261 was also stimulated with astrocyte-conditioned medium. None of the versican isoforms was significantly changed after the 24 hour treatment (Supplementary Fig. S3B). These data indicate that microglia, but not astrocytes, induce versican expression in glioma and suggest a feedback loop between microglia and glioma cells.
TLR2 Is Upregulated in Glioma-associated Microglia but not Astrocytes ex Vivo
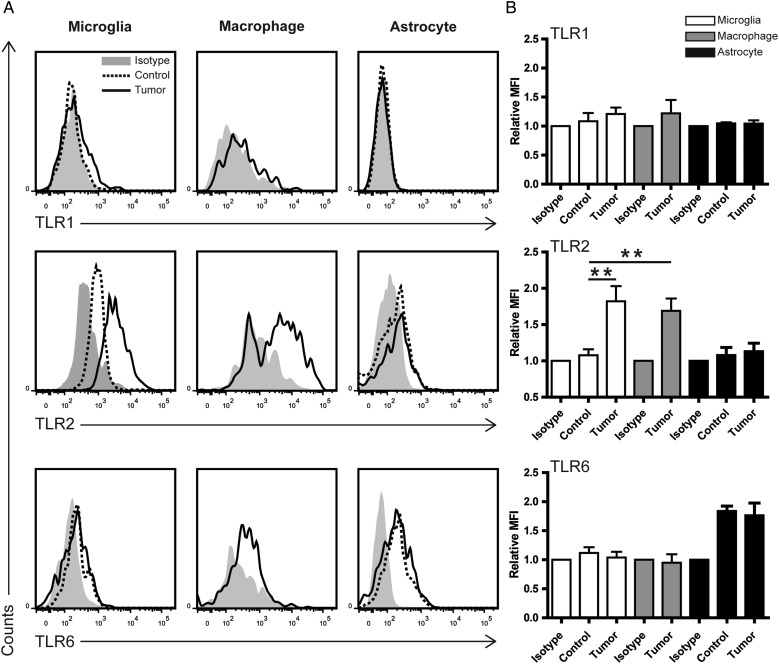
To investigate how TLRs are expressed and regulated in different cell types within gliomas, we used flow cytometry to detect TLR expression in GAMs and glioma. GL261 cells were implanted into the mouse brain; after 2 weeks, tumor and control brain tissue were dissociated and stained with different surface markers and TLR antibodies. Resident microglia and infiltrated monocytes could be distinguished by CD45 staining.25 Microglia are CD11b+CD45Low, infiltrating macrophages CD11b+CD45high, and astrocytes CD11b-CD45-GLAST+ (Supplementary Fig. S4). TLR expression was analyzed with MFI by Flowjo software in these cell subpopulations (Fig. 5). As shown in the representative histogram (Fig. 5A) of the tumor tissue, TLR2 on microglia was significantly upregulated (P = .008) compared with the naïve controls (Fig. 5B). TLR2 forms heterodimers with either TLR1 or TLR6. We observed a slight, but not significant, induction in TLR1 but not TLR6. For infiltrating macrophages, we could only demonstrate that TLR2 is expressed due to the difficulties of finding a proper control. Compared with naïve microglia, TLR2 expression is also significantly upregulated (P = .007). TLR2 and TLR6 are expressed in naïve astrocytes, but there was no difference when comparing naïve astrocytes with tumor-associated astrocytes. TLR6, but not TLR2, was expressed in GL261 cells (Supplementary Fig. S5). Taken together, TLR2 is the subtype of TLRs upregulated in GAMs but not glioma-associated astrocytes, and GAMs are the main TLR2 expressing cells in the glioma microenvironment.
Fig. 5.
TLR2 is upregulated in glioma-associated microglia/macrophages ex vivo. Mouse naïve brain or glioma tissue inoculated for 14 days was dissociated immediately after resection. Myelin was removed by Percoll gradient centrifugation, and cell pellets were further analyzed by flow cytometry. (A) Representative histograms of TLR1, TLR2, and TLR6 expression as detected by TLR subtype-specific antibodies on microglia, macrophages, and astrocytes in both control and glioma-bearing mice (left panel, n≥3). The gray label indicates isotype control. (B) Median fluorescence intensity of control and tumor was normalized to isotype and quantified by FlowJo software.
TLR2 Neutralizing Antibody Inhibits Glioma-induced Microglial MT1-MMP Expression and Reduces Tumor Growth ex Vivo
The TLR2 monoclonal neutralizing antibody T2.5 has previously been shown to functionally block TLR2 and successfully inhibit gastric cancer growth.26 We first verified that T2.5 blocks microglial TLR2 functionality in vitro. T2.5 attenuated the TLR2 agonist Pam3CSK4-induced microglial IL-6 and MT1-MMP induction (Supplementary Fig. S6). To test whether T2.5 impairs glioma-induced microglial MT1-MMP expression, microglia were stimulated with either GCM in combination with T2.5 or GCM with isotype control antibody for 6 hours. GCM treatment with isotype control resulted in an increase of 3.8 fold (±0.7; P = .03; Fig. 6A) of microglial MT1-MMP compared to the untreated cells, T2.5 treatment with GCM induced less MT1-MMP expression (1.7 ± 0.3; P = .03), which suggests that T2.5 blocked microglial TLR2 signaling.
Fig. 6.
Monoclonal antibody T2.5 inhibited glioma-conditioned medium (GCM)-driven microglial MT1-MMP expression as well as glioma growth ex vivo. (A) Primary microglial cells were stimulated with GCM, GCM together with 10 μg/mL isotype, and 10 μg/mL T2.5 for 6 hours. MT1-MMP was analyzed by qRT-PCR. (B) Organotypic brain slices obtained from 16 day-old C57BL/6 mice were inoculated with 5000 EGFP-GL261 cells and treated with isotype or mAb T2.5. The tumor area was determined 5 days after injection of glioma cells. Representative images of tumors (green) are present on top of the quantification. Scare bars are 25 μm.
We studied glioma expansion in organotypic brain slices. T2.5 or isotype control was applied into the cultured medium after EGFP GL261 cells were injected. When tumor size was quantified (Fig. 6B) after 5 days, mAb T2.5 treatment resulted in a significantly reduced tumor area (0.28 mm2 ± 0.02 mm2; P < .001) compared with isotype control (0.65 ± 0.04 mm2). When we repeated the same experiment with TLR2 KO animals, we did not see a difference between the isotype and mAb T2.5 treatment groups (Supplementary Fig. S6C).
Discussion
We, along with other groups, have previously shown that microglia are important players in glioma growth and expansion6,27 and that interfering with the GAM phenotype may provide alternative therapy for glioma treatment.8,28,29 In the present study, we identified versican as a signaling substance released from glioma cells that promotes tumor expansion via microglia/macrophages. Versican is a ligand for TLR2, and we have previously shown that TLR2 activation induces MT1-MMP expression in microglia, which activates glioma-released MMP2 and thereby promotes glioma invasion and growth.
Based on Repository for Molecular Brain Neoplasia Data (REMBRANDT), TLR2 is highly expressed in gliomas compared with nontumor tissue and is also inversely correlated to patient survival.7 These data are, however, obtained from glioma tissue that is a mix of glioma cells and GAMs. Hussain et al examined TLR expression on human GAMs by flow cytometry and found TLR2, 3, and 4.30 In our previous in vitro study, we found that TLR3 ligand Poly I:C did not induce microglial MT1-MMP expression,7 while TLR4 ligand LPS and TLR2 ligand Pam3csk4 did. However, when we inoculated glioma cells into TLR4 KO mice, we did not see a significant difference in tumor size compared with the WT control (unpublished data). Therefore, we focused on TLR2 in the present study. We showed the expression of TLR2 and its upregulation in glioma-associated microglia, as compared with control microglia; in contrast, astrocytes and glioma cells expressed only low levels of TLR2.
The endogenous agonists of TLRs can be proteins, fatty acids, proteoglycans, or nucleic acids.10 Versican is a component of the extracellular matrix and is found in a variety of tumor tissues including gliomas.17 Versican is released in lung cancer and activates TLR2 on macrophages,12 which results in macrophage TNFα and IL-6 induction and in tumor progression. When recombinant veriscan V1 was applied to macrophages, a robust induction of IL-6 and TNFα was observed, but this effect was totally abolished when the same recombinant versican was applied to macrophage from TLR2 KO mice, indicating that versican activates macrophage through TLR2.12 Moreover, V1 promoted ovarian cancer progression via macrophage TLR2 signaling and expression of hCAP18/LL-37.31
In order to identify the TLR2 ligand in the glioma context, we first screened for soluble factors released by GL261 cells using mass spectrometry. We identified 2 candidates: versican and high mobility group box 1 (HMGB1). Curtain et al demonstrated that dying gliomas, when reacting to immunotherapies, released HMGB1 to recruit dendritic cells through TLR2 signaling.32 When we applied recombinant protein HMGB1 to microglial cells, it did not induce MT1-MMP upregulation, while versican V1 significantly induced microglial MT1-MMP expression (data not shown). Due to the purification procedure, we could not eliminate endotoxin in our recombinant versican sample; thus, we used LPS as an internal control. We only detected a modest upregulation of MT1-MMP in microglia by LPS, which was significantly less when compared with the V1 treatment. We repeated the same experiments with TLR2 KO microglia and found a small residual response that was of similar magnitude as that triggered by LPS alone. Here, we confirmed that the LPS administration on microglia in our previous study induced a modest upregulation of MT1-MMP, which was not significant.7 Here we show a significant upregulation that is likely due to a larger sample size.
Our versican knockdown experiments in vitro and in vivo further demonstrated that versican is the endogenous ligand acting on TLR2 and that it upregulates MT1-MMP and promotes glioma growth. Our data on glioma-injected organotypic brain slices revealed that the impact of versican signaling on glioma growth depended on the presence of microglia. Taken together, versican is a glioma-derived endogenous TLR2 mediator that regulates microglial MT1-MMP expression for tumor expansion.
A humanized anti-TLR2 antibody (OPN-301 and OPN-305) has been shown to inhibit TLR2-mediated inflammation in a murine model of renal transplantation.33 Tye et al have recently used this neutralizing antibody to block TLR2 in a gastric cancer model that inhibited gastric tumorigenesis.26 More importantly, this antibody has already been tested in a phase I clinical trial showing its safety and tolerability on healthy subjects; a phase II study in renal transplantation is ongoing.34 Here we show that GCM-induced microglial MT1-MMP upregulation could be abolished by TLR2 antibody treatment. By using an organotypic brain slice model, we also showed that the TLR2 antibody has potential therapeutic benefits in glioma progression. Monoclonal antibodies (mAbs) have been used with increasing success against many tumors. Bevacizumab, also a humanized monoclonal antibody to vascular endothelial growth factor, was approved in 2009 by the FDA for treatment of recurrent glioblastoma in the United States.35 Thus, a mAb-targeting TLR2 is a candidate for adjuvant therapy in the treatment of glioma.
Supplementary Material
Funding
This work was supported by the China Scholarship Council stipend (CSC, China) to Feng Hu and by the Deutsche Forschungsgemeinschaft (SFB-TR 43, KE 329/30-1 SY 144/4-1), NeuroCure, and NIH Grant (U01CA160882-01A1).
Supplementary Material
Acknowledgments
We sincerely thank Prof. Dieter R. Zimmermann and Dr. María T. Dours-Zimmermann from University of Zurich for providing versican antibodies and plasmids. We thank Dr. Katyayni Vinnakota for the discussion and suggestions. We also thank Irene Haupt, Regina Piske, and Nadine Scharek for excellent technical assistance.
Conflict of interest statement. The authors declare no competing financial interests.
References
- 1.Charles NA, Holland EC, Gilbertson R, et al. The brain tumor microenvironment. Glia. 2012;60(3):502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 2.Badie B, Schartner J. Role of microglia in glioma biology. Microsc Res Tech. 2001;54(2):106–113. doi: 10.1002/jemt.1125. [DOI] [PubMed] [Google Scholar]
- 3.Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia. 2002;40(2):252–259. doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- 4.Markovic DS, Glass R, Synowitz M, et al. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64(9):754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. [DOI] [PubMed] [Google Scholar]
- 5.Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59(3):472–485. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markovic DS, Vinnakota K, Chirasani S, et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci USA. 2009;106(30):12530–12535. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinnakota K, Hu F, Ku MC, et al. Toll-like receptor 2 mediates microglia/brain macrophage MT1-MMP expression and glioma expansion. Neuro-Oncol. 2013;15(11):1457–1468. doi: 10.1093/neuonc/not115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markovic DS, Vinnakota K, van Rooijen N, et al. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun. 2011;25(4):624–628. doi: 10.1016/j.bbi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 10.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010:pii:672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matzinger P. Tolerance, danger, and the extended family. Ann Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naso MF, Zimmermann DR, Iozzo RV. Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J Biol Chem. 1994;269(52):32999–33008. [PubMed] [Google Scholar]
- 14.Wu YJ, La Pierre DP, Wu J, et al. The interaction of versican with its binding partners. Cell Res. 2005;15(7):483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Sheng W, Chen L, et al. Versican V1 isoform induces neuronal differentiation and promotes neurite outgrowth. Mol Biol Cell. 2004;15(5):2093–2104. doi: 10.1091/mbc.E03-09-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, et al. Brain derived versican V2 is a potent inhibitor of axonal growth. J Cell Sci. 2000;113(Pt 5):807–816. doi: 10.1242/jcs.113.5.807. [DOI] [PubMed] [Google Scholar]
- 17.Paulus W, Baur I, Dours-Zimmermann MT, et al. Differential expression of versican isoforms in brain tumors. J Neuropathol Exp Neurol. 1996;55(5):528–533. doi: 10.1097/00005072-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Ricciardelli C, Brooks JH, Suwiwat S, et al. Regulation of stromal versican expression by breast cancer cells and importance to relapse-free survival in patients with node-negative primary breast cancer. Clin Cancer Res. 2002;8(4):1054–1060. [PubMed] [Google Scholar]
- 19.Ween MP, Oehler MK, Ricciardelli C. Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. Int J Mol Sci. 2011;12(2):1009–1029. doi: 10.3390/ijms12021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 21.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267(14):10003–10010. [PubMed] [Google Scholar]
- 22.Ricciardelli C, Russell DL, Ween MP, et al. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J Biol Chem. 2007;282(14):10814–10825. doi: 10.1074/jbc.M606991200. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt CA, Rosenthal CT, Lowe SW. Genetic analysis of chemoresistance in primary murine lymphomas. Nat Med. 2000;6(9):1029–1035. doi: 10.1038/79542. [DOI] [PubMed] [Google Scholar]
- 24.Jung M, Crang AJ, Blakemore WF, et al. In vitro and in vivo characterisation of glial cells immortalised with a temperature sensitive SV40 T antigen-containing retrovirus. J Neurosci Res. 1994;37(2):182–196. doi: 10.1002/jnr.490370204. [DOI] [PubMed] [Google Scholar]
- 25.Karlmark KR, Tacke F, Dunay IR. Monocytes in health and disease - Minireview. Eur J Microbiol Immunol. 2012;2(2):97–102. doi: 10.1556/EuJMI.2.2012.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tye H, Kennedy CL, Najdovska M, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22(4):466–478. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–1112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- 28.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pong WW, Higer SB, Gianino SM, et al. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73(2):303–308. doi: 10.1002/ana.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain SF, Yang D, Suki D, et al. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncol. 2006;8(3):261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Wang X, Wu JL, et al. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages throug activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PloS One. 2013;8(2):e56616. doi: 10.1371/journal.pone.0056616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtin JF, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrar CA, Keogh B, McCormack W, et al. Inhibition of TLR2 promotes graft function in a murine model of renal transplant ischemia-reperfusion injury. FASEB J. 2012;26(2):799–807. doi: 10.1096/fj.11-195396. [DOI] [PubMed] [Google Scholar]
- 34.Reilly M, Miller RM, Thomson MH, et al. Randomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibody. Clin Pharmacol Ther. 2013;94(5):593–600. doi: 10.1038/clpt.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.