Normal tissues represent complex ecological systems spanning numerous spatial and temporal scales and involving multiple distinct cell types, molecular substrates, and processes.1 The collective behavior of these open systems is defined by the interactions between their composite acellular and cellular components, which constantly change to create new homeostatic conditions. Similar to non-neoplastic tissues, brain cancers are also ecosystems composed of many interacting biotic (microglia, stem cells, astrocytes, endothelial cells, cancer cells) and abiotic (extracellular matrix [ECM], cytokines, growth factors) elements that influence the biological processes that govern overall tumor behavior (invasion, angiogenesis, cell proliferation, apoptosis). The recognition that brain tumors function as dynamic systems has galvanized a burgeoning number of researchers to investigate the roles of non-neoplastic stromal cells and signals in driving brain tumor formation, maintenance and progression.2 Additionally, these fundamental laboratory-based studies raise the intriguing possibility that future adjuvant brain cancer therapies might target cells and signals emanating from the tumor microenvironment.
The importance of the cancer microenvironment was first explored in non-nervous system tumors,3 but is now clearly appreciated to be a significant determinant of brain tumor biology. In this regard, one of the most abundant cell types in all grades of glioma is the mononuclear cell (monocyte), comprising 35–50% of the cellular mass of the tumor.4 These monocytes are further divided into microglia (resident brain macrophages) and macrophages, the latter presumably originating from peripheral sources (eg, bone marrow). Both glioma-associated macrophages and microglia can stimulate new blood vessel formation through direct and indirect effects on endothelial cells or create changes in extracellular matrix composition to modify the local tumor microenvironment. In addition, these immune system-like cells are chemokine, cytokine, and mitogen factories with the ability to provide robust growth-promoting signals that further drive neoplastic cell growth.
Over the past decade, the seminal role of microglia and macrophages in glioma formation, growth, and invasion has been revealed by several complementary types of experiments. In these studies, inhibition of microglia function using agents that (1) impact on microglia activation (eg, minocycline5, Amphoterin B6), (2) deplete microglia (eg, clodronate7), (3) genetically eliminate microglia (using CD11b-TK mice4), or (4) pharmacologically inhibit microglia signaling (eg, JNK inhibitors8) all demonstrate dramatic effects on glioma growth in vitro and in vivo. While these investigations have yielded exciting insights, it is not completely clear what molecular and cellular events regulate microglia infiltration and conversion (pro-tumoral monocyte activation) or microglia-mediated glioma growth and invasion (Fig. 1).
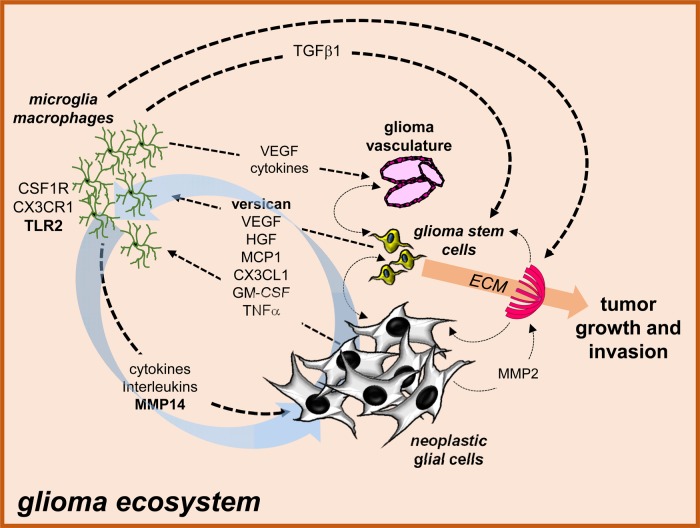
Fig. 1.
The glioma ecosystem. Numerous interdependent cellular and acellular interactions maintain the glioma ecosystem. In this regard, cancer-causing genetic changes in neoplastic glial or glioma stem cells result in the production of growth factors, chemokines, and cytokines that help establish a supportive microenvironment (“stromagens”) composed of microglia, macrophages, and endothelial cells (glioma vasculature) through chemoattraction and cell activation. These stromal cells in turn elaborate numerous other compounds that stimulate angiogenesis (eg, VEGF, cytokines), neoplastic cell growth (eg, TGFβ, cytokines, interleukins; “gliomagens”), and modify the extracellular matrix (eg, MMPs) to further promote glioma expansion and invasion. In addition, there are many additional cellular and ECM-changing interactions that further create a permissive substrate for glioma maintenance and progression. Moreover, there are also circuits established (eg, versican/TLR2/MMP14 glioma growth regulatory axis) which feed forward to continuously enhance the supportive tumor microenvironment and increase glioma growth (blue arrows).
Glioma cells are known to elaborate chemoattractants that promote the directional migration of macrophages and microglia to the developing tumor bed. Among these essential factors are CX3CL1 (fractalkine), granulocyte-macrophage colony stimulating factor (GM-CSF) and macrophage chemoattractant protein-1 (MCP1 or CCL2). In this regard, the addition of CX3CL1 increases the adhesion and migration of glioma-infiltrating microglia/macrophages in vitro,9 whereas genetic inhibition of the CX3CL1 receptor, CX3CR1, delays low-grade mouse optic glioma formation in vivo.10 In addition, GM-CSF acting on its cognate signaling receptor, the colony stimulating factor-1 receptor (CSF-1R), is responsible for glioma-mediated microglia recruitment and activation, which in turn is critical for glioblastoma maintenance and progression in vivo.11 Similarly, malignant glioma expression of MCP1 induces massive microglial infiltration to result in greater tumor growth in vivo,12 further establishing microglial recruitment and activation as critical steps in the creation of a glioma microenvironment supportive of tumor expansion.
Glioma-associated microglia and macrophages produce a large number of cytokines, interleukins, and growth factors which can either sculpt a more permissive tumor microenvironment (“stromagens”) or directly stimulate glioma cell growth and invasion (“gliomagens”). In this manner, stromagens can act on acellular (eg, matrix metalloproteinases) or cellular (eg, vascular endothelial growth factor-mediated angiogenesis) elements in the tumor microenvironment to favor glioma expansion and infiltration.7,13 These changes create a supportive soil, rich in new extracellular substrates and blood vessel-derived nutrients, that further enhance glioma growth or invasion. Similarly, tumor-associated macrophages and microglia produce a large number of gliomagens (eg, IL-6, TGFβ) that act on the neoplastic glioma cells to increase glioma stem cell or astrocytoma cell proliferation, survival, and/or invasion.14,15
The current study by Hu and colleagues (this issue) expands on the concept that gliomas maintain an intricate set of stroma-tumor interdependencies. For their model system, they employed organotypic brain slice preparations from immunocompetent C57BL/6 mice inoculated with the syngeneic GL261 high-grade murine glioma cell line. Building on previous reports from their laboratory, they elegantly describe a circuit of glioma-stroma interactions that serve to maximize tumor growth and invasion (blue arrows in Fig. 1). First, they found that both the original mouse glioma line as well as primary cultures of human glioblastoma cells express the large chondroitin sulfate glycoprotein, versican, important for normal tissue morphogenesis and maintenance. Following silencing of versican expression in glioma cells, there was a reduction in tumor size and a prolongation of mouse survival. Second, they demonstrate that glioma cell-derived versican acts on glioma-associated microglia expressing the Toll-like receptor-2 (TLR2), such that depletion of microglia abolishes the effect of versican silencing. Third, following versican binding to TLR2, microglia increase their expression of the matrix metalloproteinase protein MT1-MMP (MMP14), which further promotes glioma cell growth. These elevated levels of MMP14 subsequently increase glioma cell growth and reduce mouse survival. When TLR2 function was inhibited with neutralizing antibodies, reduced microglial MMP14 expression and glioma growth ensued. Fourth, microglia engage in a feed-forward circuit by producing soluble factors that additionally increase glioma cell-derived versican expression. Collectively, these experiments reveal a self-perpetuating circuit in which glioma cells and microglia iteratively interact to actively promote glioma expansion.
In combination with work from a number of laboratories worldwide, these new studies support the exciting notion that gliomas are complex adaptive communities in which disruptions of the innate cellular and acellular interdependencies have profound influences on tumor biology. Importantly, identifying the cellular and acellular relationships unique to the glioma ecosystem may provide unprecedented opportunities to develop effective treatments that target the special symbiotic associations that maintain these brain tumors.
Funding
This work was funded by grants from the National Cancer Institute (U01CA160882) and the James S. McDonnell Foundation.
Conflicts of interest statement. The author declares no conflicts of interest.
References
- 1.Basanta D, Anderson AR. Exploiting ecological principles to better understand cancer progression and treatment. Interface Focus. 2013;3(4):1–9. doi: 10.1098/rsfs.2013.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pong WW, Gutmann DH. The ecology of brain tumors: lessons learned from neurofibromatosis-1. Oncogene. 2011;30(10):1135–1146. doi: 10.1038/onc.2010.519. [DOI] [PubMed] [Google Scholar]
- 3.Pienta KJ, McGregor N, Axelrod R, et al. Ecological therapy for cancer:defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl. Oncol. 2008;1(4):158–164. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simmons GW, Pong WW, Emnett RJ, et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70(1):51–62. doi: 10.1097/NEN.0b013e3182032d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–1112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar S, Doring A, Zemp FJ, et al. Therapeutic activation of macrophahes and microglia to suppress brian tumor-initiating cells. Nat. Neurosci. 2014;17(1):46–55. doi: 10.1038/nn.3597. [DOI] [PubMed] [Google Scholar]
- 7.Vinnakota K, Hu F, Ku MC, et al. Toll-like receptor 2 mediates microglia/brain macrophage MT1-MMP expression and glioma expansion. Neuro Oncol. 2013;15(11):1457–1468. doi: 10.1093/neuonc/not115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daginakatte GC, Gianino SM, Zhao NW, et al. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 2008;68(24):10358–10366. doi: 10.1158/0008-5472.CAN-08-2506. [DOI] [PubMed] [Google Scholar]
- 9.Held-Feindt J, Hattermann K, Müerköster SS, et al. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs) Exp Cell Res. 2010;316(9):1553–1566. doi: 10.1016/j.yexcr.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Pong WW, Higer SB, Gianino SM, et al. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73(2):303–308. doi: 10.1002/ana.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platten M, Kretz A, Naumann U, et al. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003;54(3):388–392. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Zhang L, Zhang IY, et al. RAGE Expression in Tumor-associated Macrophages Promotes Angiogenesis in Glioma. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-14-1240. Epub Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye XZ, Xu SL, Xin YH, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol. 2012;189(1):444–453. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Sarkar S, Cua R, et al. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis. 2012;33(2):312–319. doi: 10.1093/carcin/bgr289. [DOI] [PubMed] [Google Scholar]