Abstract
Dopaminergic (DA) neuron-like cells obtained through direct reprogramming of primary human fibroblasts offer exciting opportunities for treatment of Parkinson’s disease. A significant obstacle is the low efficiency of conversion during the reprogramming process. Here, we demonstrate that the suppression of p53 significantly enhances the efficiency of transcription factor-mediated conversion of human fibroblasts into functional dopaminergic neurons. In particular, blocking p53 activity using a dominant-negative p53 (p53-DN) in IMR90 cells increases the conversion efficiency by 5–20 fold. The induced DA neuron-like cells exhibit dopamine neuron-specific gene expression, significant dopamine uptake and production capacities, and enables symptomatic relief in a rat Parkinson’s disease model. Taken together, our findings suggest that p53 is a critical barrier in direct reprogramming of fibroblast into dopaminergic neurons.
Keywords: reprogramming, direct conversion, dopamingergic neurons, p53, Parkinson’s disease
Parkinson’s disease (PD) is a chronic and progressive movement disorder, which involves the selective loss of midbrain dopamine neurons. One of the promising approaches for PD treatment is cell replacement therapy. Generation of dopaminergic (DA) neurons cells has been achieved through the use of pluripotent cells, either embryonic stem cells or induced pluripotent stem cells (iPSCs) [1–5]. Similar to embryonic stem cells, iPSCs have the ability to permanently self-renew and also produce multiple cell types through directed differentiation. In theory, large-scale production of differentiated DA neuron cells from iPS cells can be achieved following established protocols[6, 7]. Furthermore, because iPSCs can be derived from somatic cells, it overcame the many obstacles that embryonic stem cells face. However, there remain some challenges in using iPSC-derived DA neurons for PD treatment. One obstacle is teratoma-formation in vivo [8]. Such risks need to be addressed before iPS cell-based cell replacement therapy for Parkinson’s disease can become a reality.
As an alternative to iPSC-derived DA cells, directly reprogramed DA cells derived from somatic cells have some unique advantages. With the direct reprogramming method, the use of cancer inducing factors (e.g. myc), and the intermediates steps for inducing and selecting embroyoid bodies and rosette-neural-precursors are skipped, which significantly reduces the risk of teratoma-formation. Direct reprogramming of mouse or human fibroblasts into dopaminergic neurons has been achived through ectopic expression of different transcription factors [9–13]. Vierbuchen T, et al. [14] identified a combination of three factors, Ascl1, Brn2 and Myt1l, that suffice to directly convert mouse embryonic fibroblasts (MEFs) into functional neurons in vitro. The conversion efficiency ranged from 1.8% to 7.7% in MEFs. Caiazzo, M et al. [9] demonstrated a combination of three factors, Mash1, Nurr1 and Lmx1a, that were able to generate fuctional dopaminergic neurons directly from mouse and human fibroblasts. The conversion efficiency was 18±3% when calculated as the fraction of GFP+ cells among mouse embryonic fibroblasts from TH (tyrosine hydroxylase)-GFP transgenic mice. In human IMR90 fibroblasts, the efficiency of conversion was 6±2%. In our own published study [11], we show that a combination of five transcriptional factors Mash1, Ngn2, Sox2, Nurr1, and Pitx3 could directly reprogram human fibroblasts into dopaminergic neuron cells. The overall frequency of fibroblasts to dopaminergic neurons conversion was around 1–2% of the gene-transduced IMR90 cells. Thus the conversion efficiency of human cells in to DA neurons is low taking account of different calculation methods. There is clearly a need to increase the efficiency of direct reprogramming from primary human cells into DA cells.
In our previous study, we observed that during the repogramming process, most human fibroblast (IMR90) cells died from detachment and sloghing off, which was the main reason leading to low conversion efficiency. In the presenst study, we examined the strategy of boosting direct reprogrmaming efficiency through inhibition of the tumor suppressor gene p53. Previously, it has been shown that p53 and serveral other tumor genes are significant barriers for the induction of iPSC [15–19]. We reasoned that it may play a similar role in reprogrmaming human fibroblasts into DA neurons. Thus its inhibition may lead to increased efficiency of deriving DA neurons through direct reprogramming.
1 Materials and methods
1.1 Cell culture and lentiviral vector transduction
Early passage human fibroblasts IMR90 (from fetal lung), were maintained in standard medium (MEM + non-essential amino acid + 10% bovine serum) and sub-cultured every 3–5 days as necessary. Human induced dopaminergic neuron-like cells (HiDA) were generated using an established protocol with lentiviral vectors encoding transcription factors [9, 11], and cultured in the neural induction medium consisting of Dulbecco’s modified Eagle’s medium/F12 medium, B27 supplement (Invitrogen), 25 ng/ml Sonic Hedgehog (SHH, R&D), 25 ng/ml FGF-8 (R&D), and non-essential amino acid (Fisher). The dominate negative form of p53 cDNA (p53-DN) was obtained from Addgene and cloned into the pLEX lentiviral vector (Open Biosystems, Huntsville, AL, USA) and packaged into lentivirus vectors. Lentiviral vectors encoding p53-DN were co-transduced with other reprogramming factors.
1.2 Immunofluorescence staining
Immunofluorescence staining on 35-mm glass-bottomed dishs was described previously [11]. The primary antibodies were used at dilutions recommended by the manufacturers. The antibodies (anti human in all cases) used were mouse anti-Tuj1 (Covance, 1:800), rabbit anti-TH (Pel-Freez, 1:1 000), rat anti-DAT (Chemicon, 1:5 000), rabbit anti-DDC (Chemicon, 1:500), rat anti-Serotonin (Chemicon, 1:200), goat anti-ChAT (Chemicon, 1:100), mouse anti-HN (Millipore, 1:200). The immunostaining was developed with appropriate fluorescent-tagged secondary antibodies (Invitrogen, Carlsbad, CA, USA). In all cases, cellular nuclei were stained with DAPI in VectorShield mounting medium (Vector Laboratories, Burlingame, CA, USA). Images were collected with a Ziess or Leica SP5 confocal laser-scanning microscope.
1.3 Calculation of transduction efficiency
To calculate the efficiency of neuronal induction, the average number of neuron-specific marker-positive cells present in DAPI-positive cells of 10 randomly selected ×20 visual fields was used to determine the fraction of total number of neurons present. The total numbers of cells were determined using cells cultured in parallel wells or dishes. Statistical analyses were performed using one-way ANOVA in SPSS 10.0.
1.4 Real-time PCR
Total RNA was extracted using RNAeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. RNA was then subjected to complementary DNA synthesis with random hexamer primers using Superscript III reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed using SYBR Green PCR Master Mix (Qiagen). PCR primer sequences are available in the previous paper [11].
1.5 Quantification of cellular dopamine (DA) levels
Cellular DA levels were quantified using HPLC following the previously described method [11, 20]. The [3H]DA uptake assay was also performed by liquid scintillation spectrometry using previously published methods [11, 21]. Data were presented as mean ± SEM.
1.6 Transplantation of cells into a rat model of Parkinson’s disease
We followed a previously established cellular transplant protocol [11, 22]. Sprague-Dawley rats (Taconic) were lesioned with 6-hydroxydopamine (6-OHDA) following the procedure of an established rat Parkinson’s disease model. The lesioned rats were transplanted with 3×105 IMR90 cells or reprogrammed DA cells into the middle of the striatum using stereotactic surgery (coordinates from Bregma AP + 0.5, ML + 2.5, DV – 5.0). The animals were evaluated for rotational behavior in response to D-amphetamine before and after implantation as previously described.
At the end of experiments, whole brains of the rats were fixed and sectioned in the coronal plane on a freezing microtome at 30 μm thickness and subsequently processed for immunofluorescence and immunohistochemistry. Staining of the tissue sections for tyrosine hydroxylase, and human nuclei antibodies.
2 Results
2.1 Dominant-negative p53 Improve the Efficiency of hiDA Conversion Process
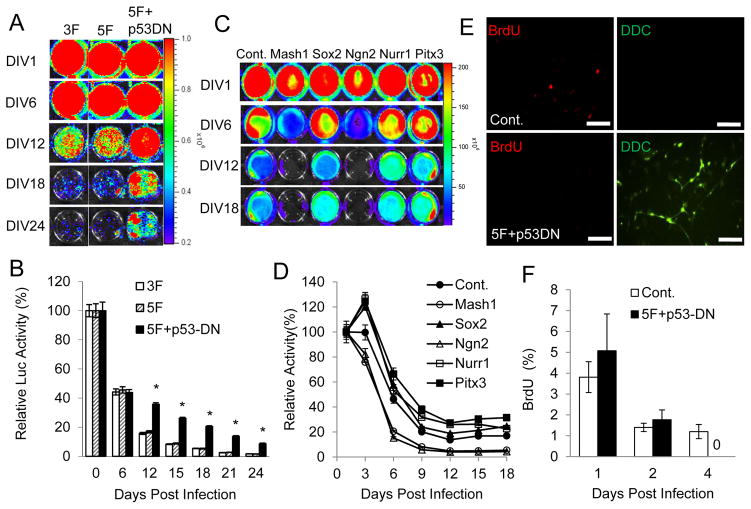
To investigate the effort of p53 in direct reprogramming fibroblasts into dopaminergic neurons, we used a lentivirus-encoded dominant-negative p53 (p53-DN) [23]. Dominant-negative p53 (p53-DN) can suppress p53 function effectively and was used to boost the efficiency of iPSC induction when using Yamanaka factors. p53-DN was introduced into IMR90 cells together with our 5-transcription factor set (Mash1, Sox2, Ngn2, Nurr1 and Pitx3, 5F) following a previously established protocol in our laboratory [11]. We also used a 3-transcription factor (Mash1, Nurr1, Lmx1a, 3F) set as a control, which was reported by Caizzo et al [9]. We monitored the number of IMR90 cells after they are transfected with 3F, 5F, 5F + p53-DN by use of a stably tranduced CMV-luc reporter (Figure 1A; Figure S1A). When co-infected with the five transcription factors, p53-DN markedly increased the number of surviving cells later in the conversion process. The survival rate was about 3–5 fold higher than that of non-p53-DN groups (3F, 5F) after 12 days in culture. The survival rates were similar using either the 3F or 5F protocol (Figure 1B). Therefore, expression of the dominant-negative P53 gene can clearly augment the survival rate of the IMR90 cells. Fig. S1B shows data demonstrating that p53-DN significantly suppressed p21 expression, indicating that it is functioning as expected.
Figure 1. Dominant-negative p53 (p53DN) increase the survival of 5F transduced IMR90 cells.
A. IMR90 cells were initially transduced with a constitutively expressed luciferase gene. They were then infected with lentiviral vectors encoding 3F (Mash1, Nurr1 and Lmx1a), or 5F (Mash1, Sox2, Ngn2, Nurr1 and Pitx3), or 5F+p53DN. Cell numbers were subsequently monitored by use of bioluminescence imaging at different times after lentiviral infection. Shown are representative images of different cell populations at different time points after infection. DIV: days in vitro.
B. Quantitative data of luciferase activities in 3F, 5F, and 5F+p53DN-transduced IMR90 cells. (*, 5F vs 5F+p53DN, p < 0.001)
C. Luciferase activities of IMR90Luc cells infected with individual lentiviral vectors encoding control (Cont.), Mash1, Sox2, Ngn2, Nurr1, and Pitx3, respectively. The amount of cells was monitored by bioluminescence imaging at different times after lentiviral infection.
D. Quantitative bioluminescence levels in various lentivirus-transduced IMR90 cells.
E. Lack of proliferation in 5F+p53DN transduced IMR90 cells. Shown are representative images of BrdU and dopa decarboxylase (DCC) staining at day 4 after sham (top panel) or 5F+p53DN (Lower panel) transduction. The scale bars represent 200μm.
F. Fraction of cells labeled with BrdU when it was added at different times after 5F+p53DN transduction.
Error bars indicate standard error of the mean (SEM, n=3).
To investigate which transcription factor contributed to the cell loss, each of the five transcription factors were individually introduced into IMR90 cells with a CMV-Luc reporter, and cultured with neuron specific medium. As shown in Figure 1C&D, cell numbers reduced for each of the transduced factor, as did for the vector control-transduced cells. Cell numbers showed significant decrease from day 3 when the cells were cultured with neuron-specific medium without the transcription factors, thereby suggesting that exposing the fibroblast cells to neuron-specific medium was the main reason leading to cell loss. In addition, compared to IMR90 celll transduced with sham vector, Mash1 and Ngn2 are the two transcription factors which could incur additional cell loss in the conversion process. Furthermore, Sox2, Nurr1, and Pitx3 attenuated cell loss induced by neuron-specific medium.
Because p53 is a tumor suppressor gene and loss of function of p53 is one of the most common molecular events in cancer, p53 inhibition may lead to increased cellular proliferation and higher cancer risk. To investigate this possibility, p53-DN was introduced into IMR90 cells together with our 5-transcription factor set. IMR90 cells were also transduced with a sham lentivirus vector as control. The treated IMR90 cells were cultured with neuron-specific medium containing thymidne analog BrdU, a common reagent used for evaluating cell proliferation. Only proliferating cells will be marked by BrdU incorporation, which may be detected using fluorescently-labeled BrdU antibody. BrdU was added to the medium at different times after gene transduction. Four days after 5F+ p53-DN transduction, no cells were labeled positive for BrdU, indicating a total lack of cell proliferation 4 days after transduction (Figure 1E&F). Thus it appeared that although p53 inhibition improved cellular survival during reprogramming, it did not cause cell proliferation.
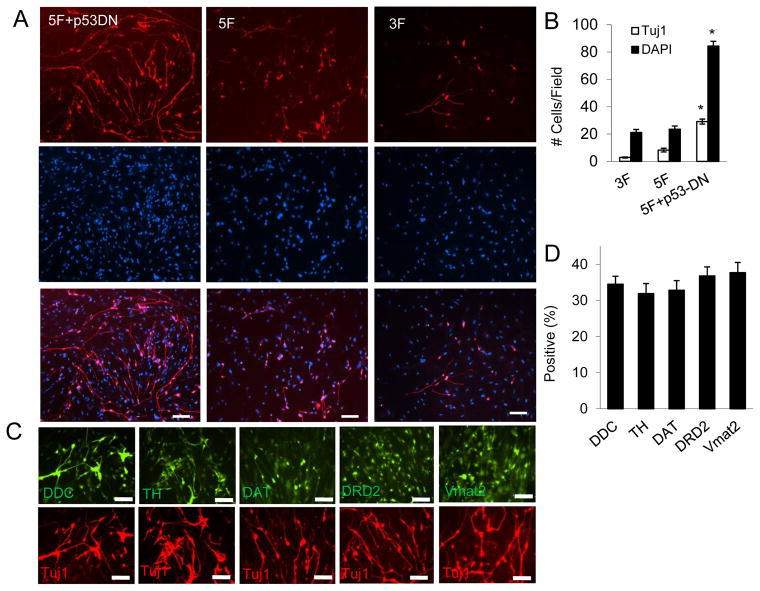
To determine the fraction of surviving cells that were successfully reprogrammed, the converted cells were stained with a neuron-specific marker, Tuj1. Our results showed a substantial increase in the number of Tuj1-positive cells in the 5F + p53-DN treated IMR90 cells (Figure 2A&B). The rates of Tuj1-positive on day 20 of 3F, 5F and 5F+ p53-DN treated IMR90 were 9.12 ± 1.04%, 34.11 ± 5.87%, and 35.53 ± 2.20%, respectively. Thus there was about 4-fold increase in the fraction of Tuj1-positve cells in 5F or 5F + p53-DN transduced IMR90 compared with that of 3F transduced cells. DAPI staining (Figure 2A) showed all surviving IMR90 cells on day 20. In addition, there was 13.7 ± 0.5% of initially plated IMR90 cells that survived in 5F+p53-DN transduced IMR90 cells, about 5-fold increase compared with that of 3F (1.78 ± 0.9%) and 5F (2.84 ± 0.7%) transduced cells. Taken into consideration of both Tuj1-positive cells and and total cell survival, the overall frequency of fibroblast to DA neuron conversion was about 20-fold higher in 5F + p53DN transduced IMR90 fibroblasts compared with that in 3F-transduced cells.
Figure 2. Dominant-negative p53 (p53DN) expression increases reprogramming efficiency of 5F transduced IMR90 cells.
A. IMR90 cells were infected with lentivirus vector encoding 5F+p53DN, 5F, and 3F. At 18 days after infection, infected cells were stained with an antibody against Tuj1(Upper panel). DAPI staining was used to identify individual cells (Middle panel). The lower panel showed the merged pictures. The scale bars represent 50μm.
B. Quantification of cells that stained positive for Tuj1 and DAPI in 10 randomly selected fields (20x) of observation. Error bars indicate standard error of the mean (SEM, n=5). (*, 5F vs 5F+p53DN, p < 0.001)
C. Representative images of of 5F+p53DN transduced cells that were stained with various antibodies against general and DA neuron-specific antibodies at 18 days after infection. The scale bars represent 200μm.
D. Fraction of cells that stained positive various antibodies in (C). Error bars indicate standard error of the mean (SEM, n=5).
We also examined the dopaminergic neurons-specific molecular markers of the 5F+ p53DN treated IMR90 cells. Immunocytochemical analysis showed that 5F + p53DN treated IMR90 cells stained positive for tyrosine hydroxylase (TH), dopamine transporter (DAT), dopamine D2 receptor (DRD2), and vesicular monoamine transporter 2 (Vmat2) (Figure 2C&D). Moreover, they stained negative for serotonin (a marker for serotogenic neurons) and ChAT (a marker for cholinergic neurons) (data not shown). Quantitative PCR of reverse transcribed mRNAs showed that 5F + p53DN treated IMR90 cells had significantly enhanced expression of DA neuron-specific genes, including DAT, DDC, DRD2, engrailed 1 (EN1), TH, Vmat2. The expression levels of these genes increased tremendously (from 200 to 4500-fold) in the reprogrammed cells when compared with control fibroblast cells (Figure 3A). Our data therefore indicated that the reprogrammed cells (which we term as human induced DA cells, or hiDA cell) showed characteristics typical of dopaminergic neurons. We also examined the expression levels of the endogenous genes for the five transcription factors through quanititative PCR (Figure 3B). Our results indicated that exogenous expression of the five transcription factors can activate the expression of their endogenous counterparts to different extents for each of the the genes. While the overall gene expression levels are high for these genes, activation of their endogenous counterparts are not as high, perhaps because the exogenous genes were expressed at such high levels already.
Figure 3. Neuron-specific gene expression in hiDA cells.
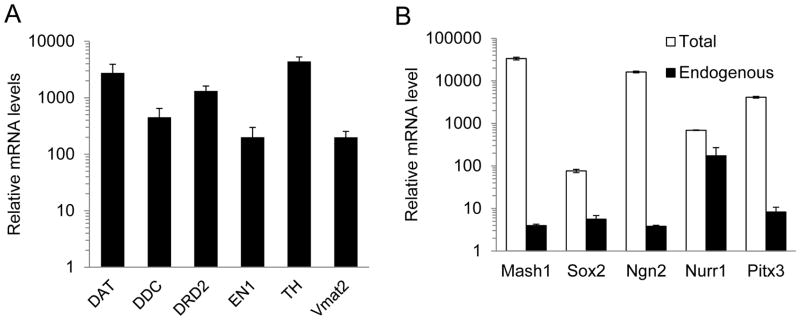
A. Quantitative PCR analysis of neural and DA specific gene expression in 5F+p53-DN transduced IMR90 cells at 20 days after infection.
B. Quantitative PCR analysis of the total and endogenous expression of five transcription factors in 5F+p53-DN transduced IMR90 cells at 20 days after infection.
The error bars represent standard error of the mean (SEM).
2.2 Functional Characteristics of hiDA
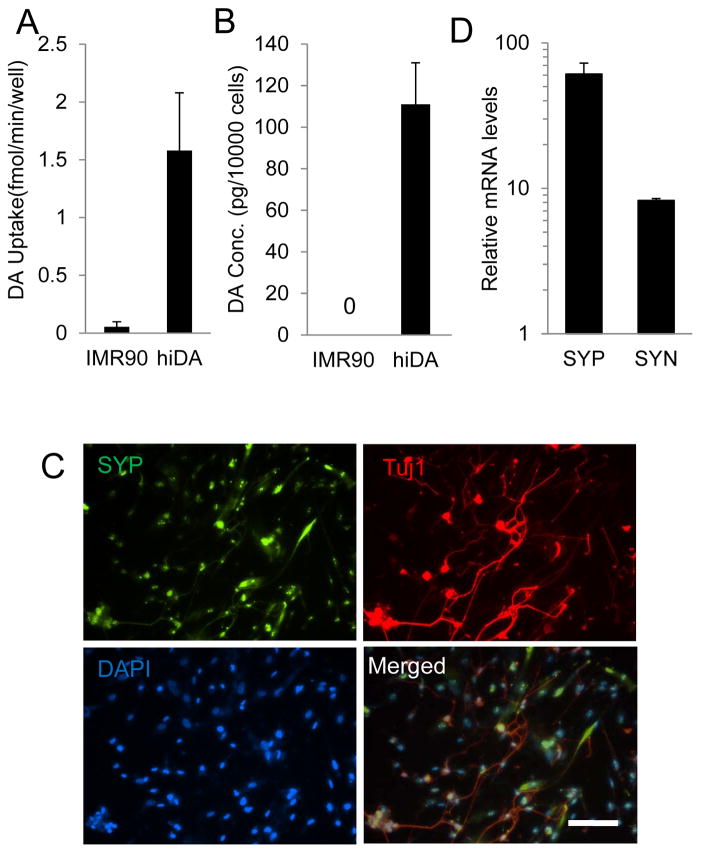
With evidence that p53 suppression increased the efficiency of direct reprogramming from fibroblasts into dopaminergic neurons, we next tested the function of the hiDA cells by determining the dopamine uptake and production in 5F+ p53-DN converted hiDA cells. Specific DAT-mediated dopamine uptake is one of the key characteristic of DA neurons. Our results indicated the specific dopamine uptake was detected in 5F+p53-DN transduced hiDA cells at day 24 after transduction (Figure 4A). Dopamine synthesis is another key property of dopamine neuron. The secreted levels of dopamine in the culturing medium of 5F+p53-DN transduced hiDA cells were measured using high-performance liquid chromatography. Our results indicated that 5F+p53-DN transduced hiDA cells produced a significant amount of dopamine, while parental IMR90 fibroblasts did not produce any dopamine (Figure 4B). Therefore our results suggested that the directly reprogrammed hiDA cells cells express dopamine-speicfic molecular markers, and show charateristic ability to take in and produce dopamine. We next determined whether reprogrammed hiDA cells could establish synaptic connections in culture. We examined the expression of a synaptic resident protein, synaptophysin (SYP) in 5F+ p53DN treated IMR90 cells. Our results indicate that hiDA cells strongly express synaptophysin (SYP) at the protein level (Figure 4C). When q-RT-PCR was used to examine gene expreesion in the same cells, our results show that the mRNA levels for SYP and SYN (α-synuclein), another DA-neuron-specific protein, were both increased (Figure 4D).
Figure 4. Functional characterization of hiDA cells.
A. Dopamine uptake of hiDA neurons. The specific [3H]DA uptake was detected 5F+p53-DN transduced IMR90 cells at day 24 after transduction. Error bars indicate standard error of the mean (SEM, n=4).
B. Dopamine release of hiDA neurons.
C. SYP (synaptophysin) co-expression with Tuj1 in 5F+p53-DN transduced IMR90 cells at 20 days after gene transduction. The scale bar represents 200 μm.
D. Quantitative PCR analysis SYP (synaptophysin) and SYN gene expression in 5F+p53-DN transduced IMR90 cells at 20 days after gene transduction.
2.3 Relief of PD sympotoms by transplantation of hiDA into a Parkinsonian Rat Model
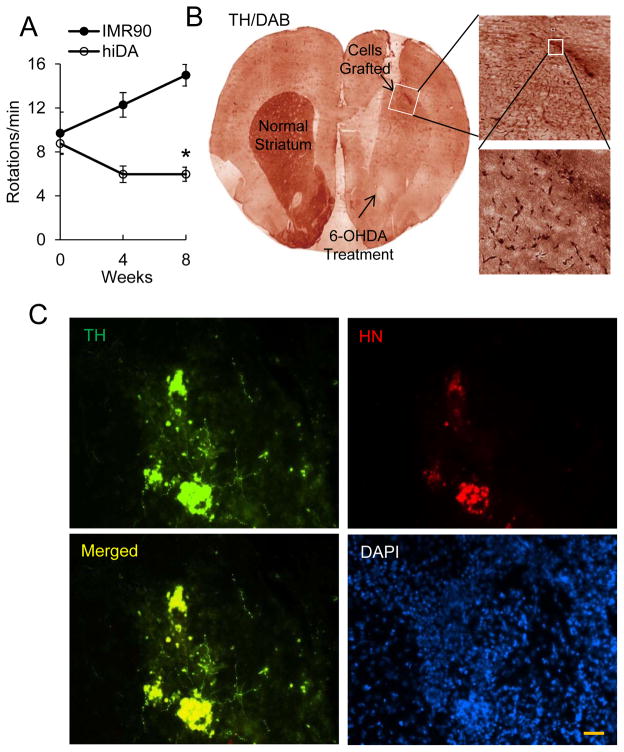
To investigate whether the reprogrammed dopaminergic neurons are functional, we transplanted the reprogrammed hiDA cells into 6-hydroxydopamine (6-OHDA)-lesioned side of a parkinsonian rat model (PD). Two days before transplantation, cyclosporine (clinical grade, diluted fresh daily in saline) were injected intraperitoneally at 10 mg/kg per day to prevent immuno-rejection. Sham-treated rats and those that received hiDA cells were evaluated by use of an amphetamine-induced rotation test. Our results indicated that the control (IMR90) cells injected PD animals showed increasingly higher rotary behavior over the course of experiment. In contrast, the PD animals transplanted with the reprogrammed hiDA cells showed significant reduction in rotational scores at both four and eight weeks after transplantation (Figure 5A, p < 0.05).
Figure 5. Symptomatic relieve in a parkinsonian rat model mediated by hiDA cell derived from 5F+p53DN transduced IMR90 cells.
A. Amphetamine-induced rotational behaviors (duration:180 min) in 6-OHDA lesioned rats before cell engraftment, and at 4 and 8 weeks after the transplantation of reprogrammed cells (about 6×105, DIV12) or IMR90 cells (control) into the middle of striatum on the lesioned side. Transplantation of reprogrammed cells significantly prevented a steady increase in amphetamine-induced rotation scores in 6-OHDA lesioned rats at both 4 and 8 weeks after transplantation (*, p<0.05). Error bars indicate standard error of the mean (SEM, n=10 for reprogrammed cells group and n=4 for control group).
B. Coronal section of rat brain showing the 6-OHDA lesion area and the site of hiDA injection. Immunohistochemical analyses with tyrosine hydroxylase antibody and diaminobenzidine (TH/DAB) staining notices the strong staining of tyrosine hydroxylasein both the lesioned striatum and injection site.
C. Expression of tyrosine hydroxylase in reprogrammed cells at the injection site. TH; tyrosine hydroxylase; HN: human nucleus protein, a marker for injected, reprogramed human hiDA cells; Nucleus were stained with DAPI. The scale bar represents 50 μm.
Eight weeks after transplantation, the rats were sacrificed and examined for the survival of grafted hiDA cells with a human cell-specific antibody. Tyrosine hydroxylase immunohistochemistry analysis showed that the grafted cells were located in coronal sections of rat brain injection sites (Figure 5B). Immunofluorescence staining further confirmed that the grafted cells (those staining positive for HN, human nuclear protein) stained positive for TH and localized around the injection area (Figure 5C). Furthermore, no Ki67 positive cells were found in any of the brain sections despite thorough examination (data not shown). This was true for all animals examined regardless of the cells being transplanted were IMR90 or hiDA cells. Our results thus indicated that transplantation of 5F+p53-DN transduced hiDA cells could relieve the PD symptoms in rat PD model, but did not lead to any visible cellular proliferation, which would suggest increased carcinogenic risk.
3 Discussion
Direct reprogramming of somatic cells into dopaminergic neurons provides an alternative source of DA neurons that significantly reduce the carcinogenic risks inherent in the iPSC-differentiation approach. However, one of the major obstacles in using the hiDA cells is the low reprogramming efficiency encountered in the published methods. Most previous studies have very low freqeuency of direct reprogramming efficiency due to cell death. A significant boost in reprogramming efficiency is needed before hiDA is to be used in treating patients.
In the present study, we show that the efficiency of successful reprogramming could be significantly evlevated by inhibition of p53 in direct reprgraming process. Importantly, we found that increased efficiency was mainly achieved through enhanced cellular survival but not cellular proliferation, thus reducing the likelihood of oncogenesis caused by p53 inhibition. Of course, the safety of such cells should be further evaluated in longer term studies to ensure their safely before any human studies are initiated.
Our finding that inhibition of p53 enhances the efficicincy of transcription factor-mediated direct reprogramming of fibroblast into dopaminergic neurons is consistent with earlier findings on the role of p53 as a “guardian of reprograming” [15, 24]. In those studies, p53 is identified as a key barrier involved in preventing the generation of mouse and human induced pluripotent cells (iPSCs) [15, 17, 18, 25]. Similar to our study, the suppression of p53 increased the efficiency of human and mouse iPS cell generation. However, despite the similarities in enhanced reprogramming efficiencies, it is not clear if p53 plays exactly the same roles in the direct reprogramming vs the iPSC induction processes: while our evidence points to a main role for p53 inhibitoin in direct reprogramming to that of boosting survival of cells placed in neuron-specific medium, it is not clear what exactly is the role of p53 inhibition in the iPSC inducing process.
Our present study demonstrates that suppression of p53 protein augments the reprogramming efficiency in direct reprogramming of fibroblast into functional dopaminergic neurons. Furthemore, we found that improved efficiency is achived through increasing cellular survival rate rather than cell proliferation. Increased dopaminergic neuron generation in this way will not only improve the feasibility of translating our discovery into the clinic but also facilitate understanding the underlying biological mechanisms associated with trans-differentiation, and thus improving efficiency of direct reprogramming of other types of potentially useful cells.
Supplementary Material
Acknowledgments
We thank Drs. Nancy Zahniser, John R Sladek, and Barbara Blanchard (University of Coloado School of Medicine) for helpful discussions and technical assistance. This study was supported in part by grants from the US National Cancer Institute (CA131408, CA136748, CA155270) (to C-Y Li).
Footnotes
DISCLOSURE OF POTENTIAL CONFLECTS OF INTEREST
The authors declare no conflict of interests in this study.
References
- 1.Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from es cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nature biotechnology. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou W, Lee YM, Guy VC, Freed CR. Embryonic stem cells with gfp knocked into the dopamine transporter yield purified dopamine neurons in vitro and from knock-in mice. Stem cells. 2009;27:2952–2961. doi: 10.1002/stem.216. [DOI] [PubMed] [Google Scholar]
- 8.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, Ogawa D, Ikeda E, Okano H, Yamanaka S. Variation in the safety of induced pluripotent stem cell lines. Nature biotechnology. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 9.Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA, Lengner CJ, Chung CY, Dawlaty MM, Tsai LH, Jaenisch R. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell stem cell. 2011;9:413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Li F, Stubblefield EA, Blanchard B, Richards TL, Larson GA, He Y, Huang Q, Tan AC, Zhang D, Benke TA, Sladek JR, Zahniser NR, Li CY. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell research. 2012;22:321–332. doi: 10.1038/cr.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng C, Zheng Q, Wu J, Xu Z, Sang L, Wang L, Guo C, Zhu W, Tong M, Liu L, Li W, Liu ZH, Zhao XY, Wang L, Chen Z, Zhou Q. Generation of dopaminergic neurons directly from mouse fibroblasts and fibroblast-derived neural progenitors. Cell research. 2012;22:769–772. doi: 10.1038/cr.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Alexanian AR. Dopaminergic-like cells from epigenetically reprogrammed mesenchymal stem cells. Journal of cellular and molecular medicine. 2012;16:2708–2714. doi: 10.1111/j.1582-4934.2012.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into ips cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure ips cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, Blasco MA, Serrano M. The ink4/arf locus is a barrier for ips cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Izpisua Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson AM, Larson GA, Zahniser NR. Low or high cocaine responding rats differ in striatal extracellular dopamine levels and dopamine transporter number. The Journal of pharmacology and experimental therapeutics. 2009;331:985–997. doi: 10.1124/jpet.109.159897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorkina T, Richards TL, Rao A, Zahniser NR, Sorkin A. Negative regulation of dopamine transporter endocytosis by membrane-proximal n-terminal residues. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:1361–1374. doi: 10.1523/JNEUROSCI.3250-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collier TJ, Gallagher MJ, Sladek CD. Cryopreservation and storage of embryonic rat mesencephalic dopamine neurons for one year: Comparison to fresh tissue in culture and neural grafts. Brain research. 1993;623:249–256. doi: 10.1016/0006-8993(93)91435-u. [DOI] [PubMed] [Google Scholar]
- 23.Kendall SD, Linardic CM, Adam SJ, Counter CM. A network of genetic events sufficient to convert normal human cells to a tumorigenic state. Cancer research. 2005;65:9824–9828. doi: 10.1158/0008-5472.CAN-05-1543. [DOI] [PubMed] [Google Scholar]
- 24.Menendez S, Camus S, Izpisua Belmonte JC. P53: Guardian of reprogramming. Cell cycle (Georgetown, Tex. 2010;9:3887–3891. doi: 10.4161/cc.9.19.13301. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Lengner CJ, Kirak O, Hanna J, Cassady JP, Lodato MA, Wu S, Faddah DA, Steine EJ, Gao Q, Fu D, Dawlaty M, Jaenisch R. Reprogramming of postnatal neurons into induced pluripotent stem cells by defined factors. Stem cells. 2011;29:992–1000. doi: 10.1002/stem.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.