Abstract
Background
Since the initial reports of human epidermal growth factor receptor 2 (HER-2) expression as being prognostic in osteosarcoma, numerous small studies varying in the interpretation of the immunohistochemical (IHC) staining patterns have produced conflicting results. The Children’s Oncology Group therefore embarked on a prospective biology study in a larger sample of patients to define in osteosarcoma the prognostic value of HER-2 expression using the methodology employed in the initial North American study describing an association between HER-2 expression and outcome.
Procedure
The analytic patient population was comprised of 149 patients with newly diagnosed osteosarcoma, 135 with localized disease and 14 with metastatic disease, all of whom had follow up clinical data. Paraffin embedded material from the diagnostic biopsy was stained with CB11 antibody and scored by two independent observers. Correlation of HER-2 IHC score and demographic variables was analyzed using a Fisher’s exact test and correlation with survival using a Kaplan–Meier analysis.
Results
No association was found with HER-2 status and any of the demographic variables tested including the presence or absence of metastatic disease at diagnosis. No association was found between HER-2 status and either event free survival or overall survival in the patients with localized disease.
Conclusion
HER-2 expression is not prognostic in osteosarcoma in the context of this large prospective study. HER-2 expression cannot be used as a basis for stratification of therapy. Identification of potential prognostic factors should occur in the context of large multi-institutional biology studies.
Keywords: Her-2, human epidermal growth factor receptor, immunohistochemistry, osteosarcoma
INTRODUCTION
Osteosarcoma, although relatively rare, is the most common primary malignancy of bone in children and adolescents [1]. Treating localized disease with surgery and chemotherapy has been relatively successful, but metastatic disease continues to pose more of a challenge [2]. Moreover, progress in improving the survival of osteosarcoma patients over the past two decades has been limited. Many studies have been performed in an effort to identify prognostic factors in order to select patients who might benefit from more intensive therapy or be entered into studies of investigational agents. At the same time, biologic studies identifying unique features of the malignant cell could form the basis of interventions using targeted therapies [3]. Human epidermal growth factor receptor 2 (HER-2) has been a protein of interest as a potential prognostic factor as well as a therapeutic target [3,4]. In breast cancer, over-expressed HER-2 on the basis of gene amplification has been implicated in tumor growth and generally predicts a less favorable prognosis [3,5]. On the other hand, patients with breast cancer whose tumors express high levels of HER-2 have shown superior outcomes when treated with regimens containing, treatments such as trastuzumab that target HER-2 [5]. HER-2 over-expression has also been identified in other malignancies, including tumors of the bladder, pancreas and prostate, among others [6–9].
The prognostic relevance of HER-2 in osteosarcoma has been investigated, however results have varied across studies and so this issue remains controversial [10,11]. Numerous studies have attempted to determine the extent of HER-2 expression and gene amplification in osteosarcoma and whether HER-2 expression correlates with survival. Some studies have demonstrated that the protein is not over-expressed nor the gene is amplified; others that it is over-expressed but not correlated with survival and finally other studies suggest over-expression is associated with an unfavorable prognosis [10–26]. Unexpectedly, studies by Akatsuka et al. demonstrated a correlation of over-expression of HER-2 and a better survival outcome [27,28]. The majority of the studies used immunohistochemical staining to define protein expression. In these studies, variability existed in methodology, including the antibody utilized and the grading system. As HER-2 is a membrane protein in some studies the presence of any cytoplasmic staining led to evaluation of the sample as negative whereas in other studies the membrane staining was evaluated independent of the cytoplasmic staining [29].
The initial paper describing a less favorable outcome in patients whose osteosarcomas expressed HER-2 was replicated in a North American single institution patient cohort, driving further interest in this topic [14,20]. Interest in targeting HER-2 led to a phase 2 trial of trastuzumab for the treatment of osteosarcoma, which has recently been published [30]. The intent of this study was to prospectively define whether HER-2 over-expression is prognostic, utilizing samples from patients enrolled on the Children’s Oncology Group osteosarcoma biology study using the methodology described in the initial studies in which a correlation between HER-2 expression and a poor prognosis was demonstrated.
PATIENTS AND METHODS
Patients
All patients who participated in this study were enrolled on the Children’s Oncology Group osteosarcoma biology study, P9851, “Osteosarcoma Biology Studies: Companion to Intergroup Therapeutic Studies.” This study was approved by the Institutional Review Board of each site that participated in the study. All participants and/or their guardians provided written informed consent for participation in the overall study with this being one of the planned substudies. The material obtained from the patients, including the paraffin embedded tissue in the form of unstained slides or a block were sent to the Cooperative Human Tissue Network (CHTN) in Columbus, Ohio for further distribution. Slides were anonymized and sent from CHTN to the research laboratory, with the results returned to the QuadW Childhood Sarcoma Biostatistics and Annotation Office (CSBAO) at the Children’s Oncology Group for correlation with clinical data. The research laboratory had no access to any clinical data for any of the patients participating in this study. In order to permit correlation with clinical data the patient population with initially localized disease enrolled on both the P9754 clinical trial and P9851 biology study were selected for further analysis. The P9754 clinical trial, “Protocol For Patients With Newly-Diagnosed Non-Metastatic Osteosarcoma: A POG/CCG Pilot Intergroup Study” was opened September 1999 and closed February 2002 and tested intensification of doxorubicin and/or ifosfamide/etoposide. Also included in the analysis were patients enrolled on P9851, but not on P9754, for whom outcome data were available.
Methods
The methodology for staining and interpretation was the same as had been performed previously [14]. Paraffin- embedded tissue slides from the biopsies were baked at 60°C for 45 minutes and then deparaffinized with xylene and various graded alcohols. Endogenous peroxidase activity was doused with 0.3% hydrogen peroxide in methanol, and antigenic proteins were uncovered by antigen retrieval with 10mM Na-citrate buffer in a water bath of about 100°C for 20 minutes, then in room temperature for 20 minutes to cool. The tissue was then blocked for 2 hours at room temperature with 10% normal goat serum and 5% bovine serum albumin in TBS, and stained with 100µg/ml CB11 antibody (Biogenex, San Ramon, CA) diluted in 5% BSA in TBS in a humidified chamber overnight. Detection of the antibody-binding reaction was carried out with biotinylated secondary antibody paired with streptavidin-horseradish peroxidase. Avidin-biotinylated enzyme complex (Vectastain ABC system, Vector Laboratories, Burlingame, CA) was used according to the company’s directions. The tissue was then treated with 3, 3′-diaminobenzidine (Biofx Laboratories, Owings Mills, MD), to locate sites of additional antigen binding, followed by counterstain with hemotoxylin. Finally, the slides were dehydrated with alcohol, permeated with xylene, and mounted with Permount organic mounting solution (Fisher Scientific, Inc., Pittsburgh, PA) to be viewed with a Nikon Inverted Microscope ECLIPSE TE200 attached to a CCD (Diagnostic Instruments, Sterling Heights, MI) and graded.
The stained slides were scored by two independent observers. Grades of “neg,” “1+,” “2+,” “3+,” and “4+” were recorded for each of the samples; “neg” denoted no staining; 1+: 0–25% of cells stained; 2+: 26–50% of cells stained; 3+: 51–75% of cells stained; and 4+: more than 76% of cells stained. Staining was only considered positive if graded with either 3+ or 4+. Tissues with negative or little/weak staining (1+ and 2+) were considered negative.
Statistical Methods
All results were sent to a statistician who had access to the clinical data and the capability of un-blinding the laboratory results. The data were analyzed with Fisher’s exact test and survival with a Kaplan–Meier analysis. Event-free survival (EFS) was taken to be the time from enrollment until disease progression, diagnosis of a second malignant neoplasm (SMN), death, or last patient contact, whichever occurred first. A patient who experienced disease progression, SMN, or death was considered to have experienced an EFS event; otherwise the patient was considered as censored at last contact. Survival was taken to be the time from enrollment to death or last patient contact, whichever occurred first. A patient who died was considered to have experienced a death event; otherwise the patient was considered as censored at last contact. EFS and survival were compared across the demographic groups using the log-rank test, and a proportional hazards regression model using the demographics information plus HER-2 status was run to estimate hazard ratios for EFS and survival [31–33]. Each categorical demographic characteristic of interest was checked for association with the CB11 grading within the analytic population using the exact conditional test of proportions. Age at enrollment was checked in this manner as a categorical variable as well as a continuous variable using the t-test or one-way analysis of variance (ANOVA) and Tukey 95% confidence intervals (depending on whether the comparison variable had two levels or three or more levels). The analytic population was demographically compared to other patients who were eligible but not part of the population, and the rest of the patients on P9754 and P9851, to ensure that the analytic population was an accurate representation of patients. SAS 9.2 was used for the analysis.
RESULTS
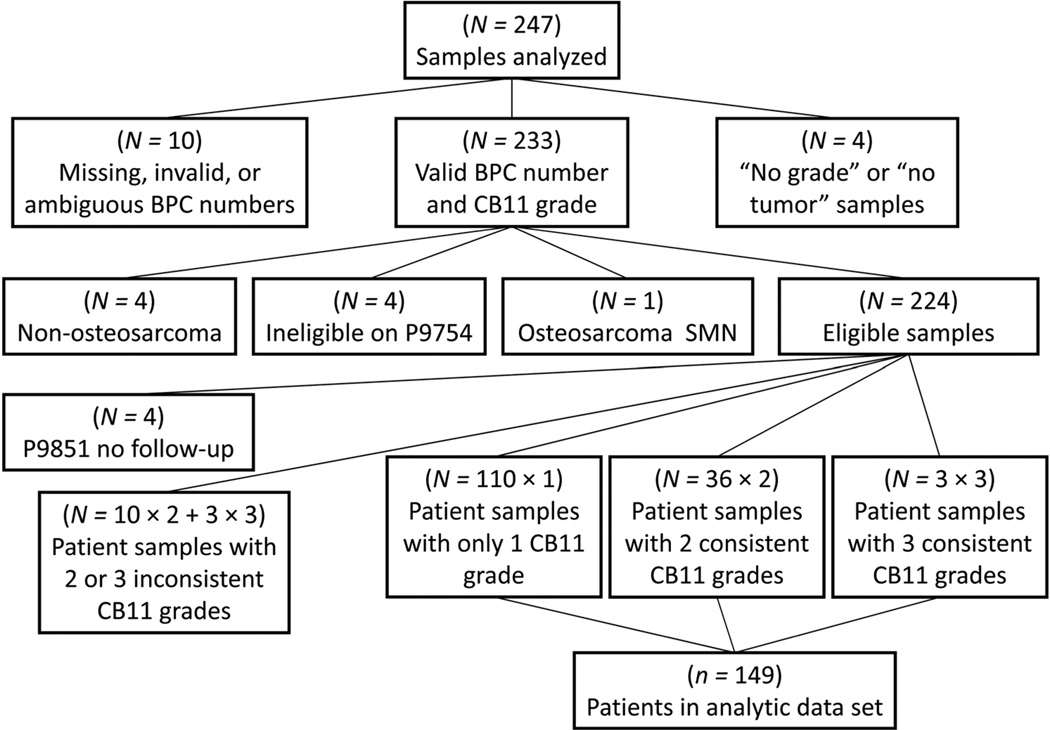
Of the 247 samples that were stained with CB11, 23 samples were excluded for the following reasons; inability to match to clinical data (n=10), technical difficulties with staining (n=4), non-osteosarcoma diagnosis (n=4), clinical trial ineligibility (n=4), and having osteosarcoma as a second malignant neoplasm (n=1; Fig. 1). An additional four samples came from patients who did not have clinical follow up and 29 samples from 13 patients were excluded due to discordant staining results (Fig. 1). The remaining 191 samples were obtained from 149 different patients with osteosarcoma, 14 with metastatic disease and the remaining 135 with localized disease, with this group making up the analytic patient population that was further assessed for correlation with demographic features and outcome in this study. The patient tree is provided in Figure 1. Of the 149 patients in the analytic population 112 were co-enrolled on the P9754 clinical trial described previously and 37 patients had follow up data available although they were only enrolled on the P9851 biology study.
Fig. 1.
Patient tree. Relationship between all samples analyzed for HER-2 expression and the analytic patient population.
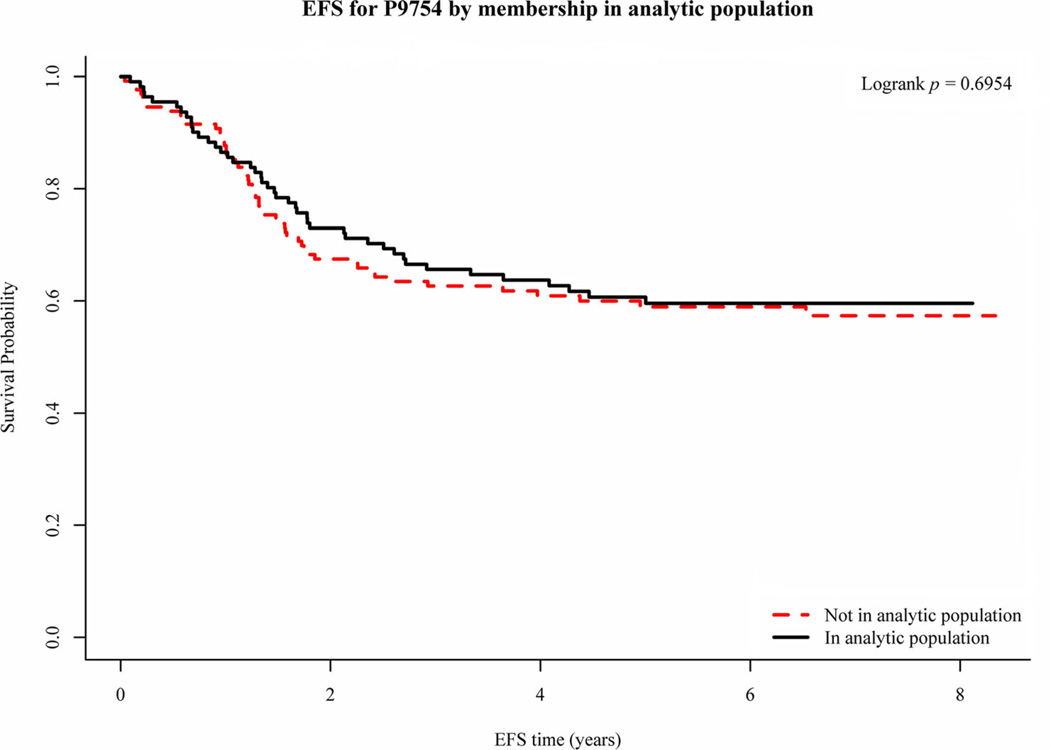
The initial characteristics of the patients in the analytic population and those enrolled on the P9754 and P9851 are summarized in Table I. For the comparison between the analytic population and the rest of P9754 and P9851, race was statistically significantly different in P9851 (P=0.0387) with a relative paucity of blacks in the analytic population and an excess of “Other” and unknown races. Although this is seen in the analytic as compared to the non-analytic populations of P9851 the overall percentage of blacks and “Other” and unknown races included on the studies appear similar. In P9851, the analytic population contained a significantly higher proportion of metastatic cases (37.8% in the analytic population vs. 19.5% in the rest of P9851, P=0.0185). Overall, however, the analytic population seems reasonably representative of the total populations of P9754 and P9851 and thus the inferences deduced in analysis on the analytic population should generalize reasonably well to all of P9754 and P9851. The EFS and OS of patients in the analytic population were similar to those enrolled on the P9754 and P9851 studies (Fig. 2 shows the EFS for P9754 for the analytic and non-analytic populations).
TABLE I.
Initial Presenting Features for the Total Analytic Population As Well As the P9754 and P9851 Populations
| Analytic population | Non-analytic P9754 population | Non-analytic P9851 population | |
|---|---|---|---|
| Gender | |||
| Male | 78 (52.3%) | 79 (60.8%) | 250 (54.2%) |
| Female | 71 (47.7%) | 51 (39.2%) | 211 (45.8%) |
| Race | |||
| White | 107 (71.8%) | 89 (68.5%) | 336 (72.9%) |
| Black | 21 (14.1%) | 20 (15.4%) | 58 (12.6%) |
| Other | 15 (10.1%) | 15 (11.5%) | 52 (11.3%) |
| Unknown | 6 (4.0%) | 6 (4.6%) | 15 (3.3%) |
| Ethnicity | |||
| Non-Hispanic | 90 (60.4%) | 97 (74.6%) | 381 (82.6%) |
| Hispanic | 15 (10.1%) | 13 (10.0%) | 59 (12.8%) |
| Unknown | 44 (29.5%) | 20 (15.4%) | 21 (4.6%) |
| Median age (min–max) | 14.02 (4.72–28.40) | 14.38 (3.85–30.50) | 14.09 (0.18–33.17) |
| <10 | 25 (16.8%) | 24 (18.5%) | 74 (16.0%) |
| 10–17 | 106 (71.1%) | 85 (65.4%) | 335 (72.7%) |
| 18+ | 18 (12.1%) | 21 (16.1%) | 52 (11.3%) |
| Primary tumor site | |||
| Extremity | 145 (97.3%) | 130 (100%) | 428 (92.8%) |
| Pelvis | 2 (1.3%) | 0 | 22 (4.8%) |
| Other | 2 (1.3%) | 0 | 11 (2.4%) |
| Disease at diagnosis | |||
| Non-metastatic | 135 (90.6%) | 130 (100%) | 371 (80.5%) |
| Metastatic | 14 (9.4%) | 0 | 90 (19.5%) |
Fig. 2.
EFS for non-metastatic patients enrolled on the P9754 study by their membership in the analytic population.
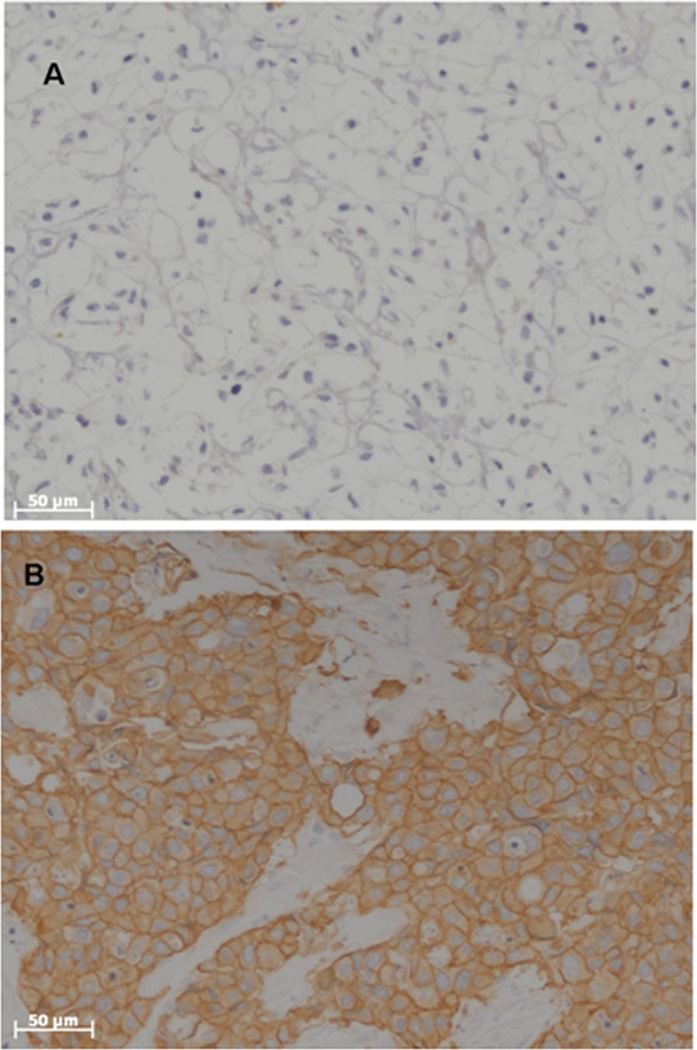
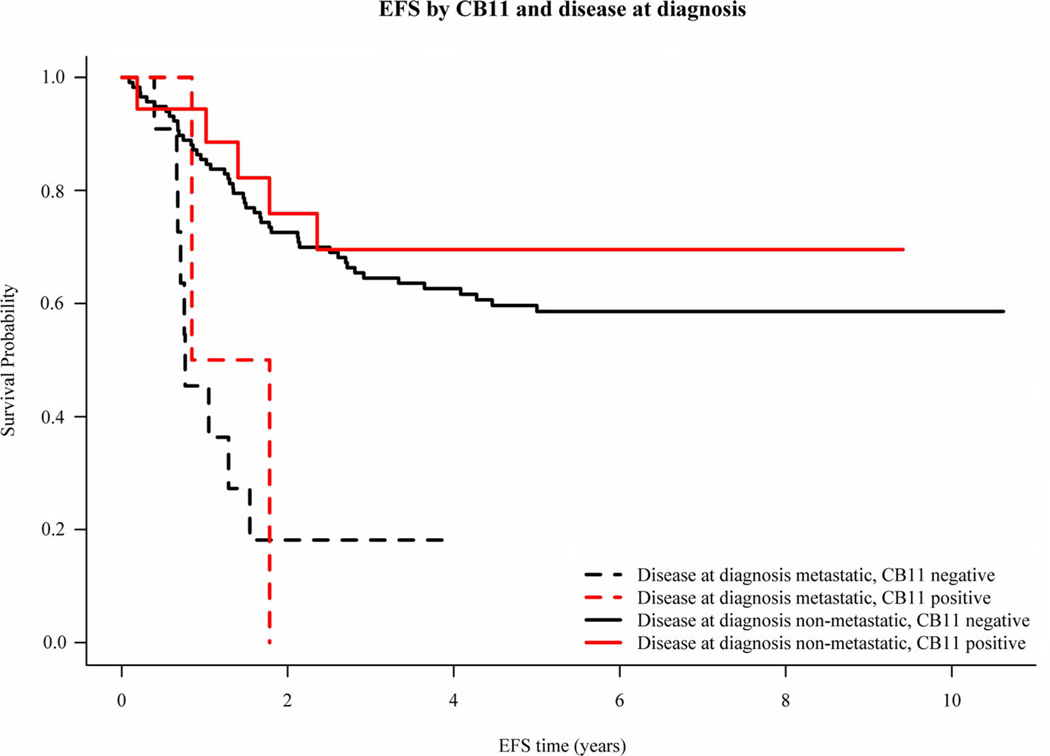
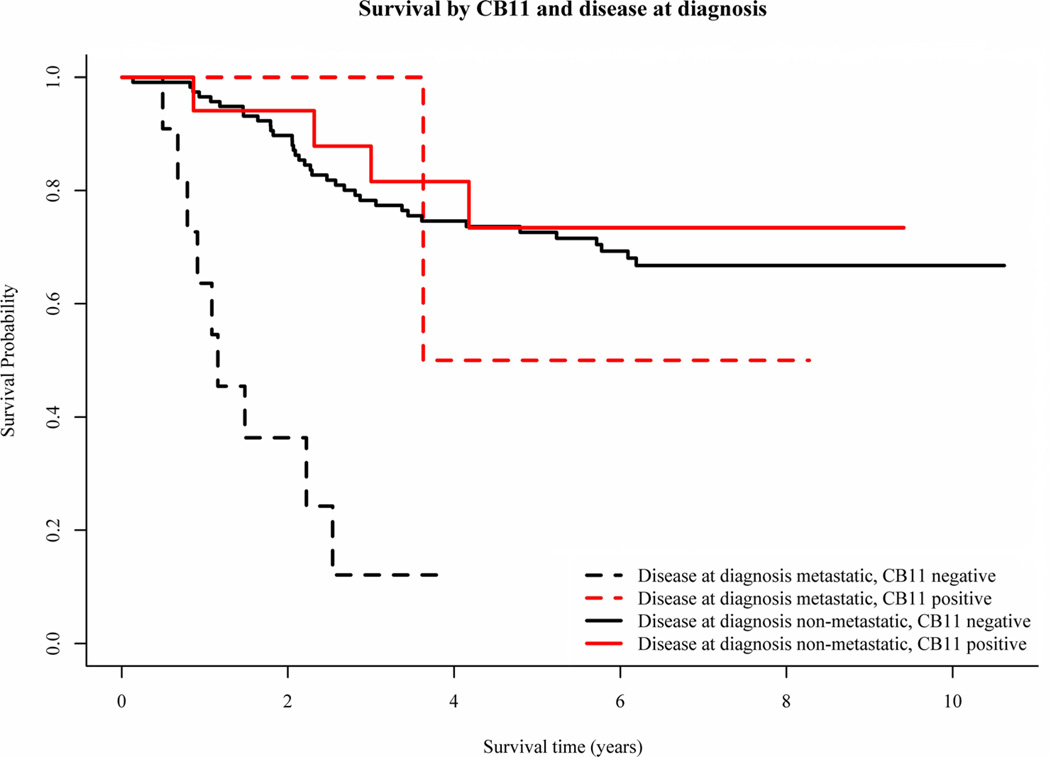
CB11 grades were concordant between the two graders 85% of the time. Samples that yielded discordant results were not included in further analyses. In several cases, samples were sent from a single specimen to the laboratory in a blinded fashion for repeat analyses. The κ statistic for agreement among the subjects with exactly two measurements was 0.24. Of the 149 analytic cases, 129 (86.6%) stained negative and 20 (13.4%) stained positive for overexpression of HER-2 with the CB11 antibody. Representative staining results are shown in Figure 3. HER-2 staining did not correlate with gender (P=0.24), race (P=0.31), ethnicity (P=0.10), age (P=0.25), or primary site (P=1.0). The presence of HER-2 overexpression as detected by CB11 staining did not correlate with EFS or OS (Figs. 4 and 5). The estimated 5-year EFS of the patients with metastatic disease who were HER-2 negative was 18.2% and HER-2 positive was 0% and patients with non-metastatic disease who were HER-2 negative was 59.7% and HER-2 positive was 69.6% (stratified logrank P=0.426). The estimated 5-year OS of the patients with metastatic disease who were HER-2 negative was 12.1% and HER-2 positive was 50.0% and patients with non-metastatic disease who were HER-2 negative was 72.6% and HER-2 positive was 73.4% (stratified logrank P=0.229). Although there appears to be a large difference in survival between CB11 grades in the portion of the population with metastatic disease, this is due to the fact that there were only two patients with metastatic disease with positive CB11 grades, and one of those two was alive at last contact more than 8 years after enrollment. Therefore, the group with metastatic disease was removed from the analysis in Figure 5.
Fig. 3.
Representative staining of osteosarcoma samples with CB11. (A) Negative (0) and (B) Positive (4+).
Fig. 4.
EFS by CB11 staining and disease at diagnosis.
Fig. 5.
OS by CB11 staining and disease at diagnosis.
DISCUSSION
This study was undertaken to resolve the ongoing controversy as to whether or not HER-2 is expressed in osteosarcoma and, if so, whether or not it is prognostic [29]. This study was performed in a blinded, prospective manner utilizing multi-institutional cooperative group osteosarcoma samples stained and interpreted with the methodology that had identified HER-2 overexpression as prognostic in prior studies [14,20]. In this study 13.4% of the 149 analytic cases stained positive for over-expression but over-expression did not correlate with either EFS or OS. Given the lack of correlation in this study, HER-2 should not be pursued further as a prognostic factor in osteosarcoma.
A critical clinical need in osteosarcoma is the identification of prognostic factors that can be assessed at diagnosis [2,34].With the exception of the presence or absence of radiographically detectable metastatic disease there is no biological or clinical prognostic factor that can be used as a basis of stratification of therapy in a newly diagnosed osteosarcoma patient [34]. In a manner quite analogous to what occurred with p-glycoprotein, initial publications suggesting the prognostic value of expression were quickly followed by contradictory reports [35,36]. Many of the studies used alternative methodologies for staining the tissue, grading the results or assessing for gene amplification rather than over-expression [10,12,15,19,24,26,29]. Although these studies may bear upon the relevance of HER-2 over-expression as fundamental to the biology of osteosarcoma or its utility as a therapeutic target, none directly refute the prognostic value observed in the initial studies. Critical to the field in the future will be standardization and replication of methodologies to enhance the likelihood results are replicated rather than generating controversies which do not move the field forward.
This study, using the same methodologies in a prospective and blinded fashion, did not replicate the results of the initial studies. When comparing this study to the prior studies, the frequency of HER-2 overexpression was markedly lower than had been reported previously [14,20]. Technical differences may account for some of the difference in results, since specimen processing and, in particular, decalcification would likely have been less uniform in a multi-institutional setting using paraffin embedded material as compared with the initial single center experience. This variability may not permit the same optimization of staining conditions and may in part account for the difference in the results. In addition, while the prior grading system was used, interpretation of immunohistochemical staining is somewhat subjective. It is possible that the interpretation of results varied between this and prior studies. It therefore remains possible that the failure to identify HER-2 as a prognostic factor may be related to technical difficulties or differences in technique, manifested in the low percentage of positive tumor samples in this study. The small size of the patient cohorts in previous studies led to a lack of power in identifying the relation between the proposed prognostic factor and outcome. The studied populations may not have accurately represented the general population of osteosarcoma patients, leading to inaccurate conclusions which cannot be reproduced in other populations of patients.
In order for a biomarker to serve as a prognostic factor that is clinically useful it needs to produce results that are sufficiently robust that similar results can be obtained in specimens obtained from multiple institutions and can demonstrate their prognostic value in a reproducible manner. In the context of osteosarcoma, single institution retrospective biology studies are not producing biomarkers which can be consistently reproduced in other institutions or cooperative group studies. This suggests that even initial assessments of biomarkers of prognosis in osteosarcoma should be performed in the context of multi-institutional cooperative studies. This will ensure that the methodology is sufficiently robust that it can be utilized in specimens with variation in their processing as well as permit the analyses to be adequately powered with larger patient sample sizes. Given the poorly understood biological heterogeneity of osteosarcoma only large studies will ensure adequate patient representation. It is only in this context that an assay has the potential to be sufficiently robust to allow the reproducibility necessary to permit use in clinical decision making. The establishment of osteosarcoma tissue banks by groups such as the Children’s Oncology Group should facilitate these initial assessments and hopefully subsequent validation of biological prognostic factors.
ACKNOWLEDGMENT
This research was supported by the Children’s Oncology Group Chair’s Grant U10 CA98543 and Human Specimen Banking Grant U24 CA114766 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. Additional support for this research was provided by a grant from the WWWW (QuadW) Foundation, Inc. (www.QuadW.org) to the Children’s Oncology Group.
Abbreviations
- HER-2
human epidermal growth factor receptor 2
- IHC
Immunohistochemical
- CHTN
Cooperative Human Tissue Network
- CSBAO
Childhood Sarcoma Biostatistics and Annotation Office
- EFS
event free survival
- ANOVA
analysis of variance
Footnotes
Conflict of Interest: Nothing to report.
REFERENCES
- 1.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill J, Ahluwalia MK, Geller D, et al. New targets and approaches in osteosarcoma. Pharmacol Ther. 2013;137:89–99. doi: 10.1016/j.pharmthera.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the her-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in her2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pegram MD, Pauletti G, Slamon DJ. Her-2/neu as a predictive marker of response to breast cancer therapy. Breast Cancer Res Treat. 1998;52:65–77. doi: 10.1023/a:1006111117877. [DOI] [PubMed] [Google Scholar]
- 6.Coogan CL, Estrada CR, Kapur S, et al. Her-2/neu protein overexpression and gene amplification in human transitional cell carcinoma of the bladder. Urology. 2004;63:786–790. doi: 10.1016/j.urology.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Dugan MC, Dergham ST, Kucway R, et al. Her-2/neu expression in pancreatic adenocarcinoma: Relation to tumor differentiation and survival. Pancreas. 1997;14:229–236. doi: 10.1097/00006676-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Agus DB, Bunn PA, Jr, Franklin W, et al. Her-2/neu as a therapeutic target in non-small cell lung cancer, prostate cancer, and ovarian cancer. Semin Oncol. 2000;27:53–63. discussion 92–100. [PubMed] [Google Scholar]
- 9.Signoretti S, Montironi R, Manola J, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 10.Fellenberg J, Krauthoff A, Pollandt K, et al. Evaluation of the predictive value of her-2/neu gene expression on osteosarcoma therapy in laser-microdissected paraffin-embedded tissue. Lab Invest. 2004;84:113–121. doi: 10.1038/labinvest.3700006. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari S, Bertoni F, Zanella L, et al. Evaluation of p-glycoprotein, her-2/erbb-2, p53, and bcl-2 in primary tumor and metachronous lung metastases in patients with high-grade osteosarcoma. Cancer. 2004;100:1936–1942. doi: 10.1002/cncr.20151. [DOI] [PubMed] [Google Scholar]
- 12.Anninga JK, van de Vijver MJ, Cleton-Jansen AM, et al. Overexpression of the her-2 oncogene does not play a role in high-grade osteosarcomas. Eur J Cancer. 2004;40:963–970. doi: 10.1016/j.ejca.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Bakhshi S, Gupta A, Sharma MC, et al. Her-2/neu, p-53, and their coexpression in osteosarcoma. J Pediatr Hematol Oncol. 2009;31:245–251. doi: 10.1097/MPH.0b013e318197947e. [DOI] [PubMed] [Google Scholar]
- 14.Gorlick R, Huvos AG, Heller G, et al. Expression of her2/erbb-2 correlates with survival in osteosarcoma. J Clin Oncol. 1999;17:2781–2788. doi: 10.1200/JCO.1999.17.9.2781. [DOI] [PubMed] [Google Scholar]
- 15.Hughes DP, Thomas DG, Giordano TJ, et al. Cell surface expression of epidermal growth factor receptor and her-2 with nuclear expression of her-4 in primary osteosarcoma. Cancer Res. 2004;64:2047–2053. doi: 10.1158/0008-5472.can-03-3096. [DOI] [PubMed] [Google Scholar]
- 16.Kilpatrick SE, Geisinger KR, King TS, et al. Clinicopathologic analysis of her-2/neu immunoexpression among various histologic subtypes and grades of osteosarcoma. Mod Pathol. 2001;14:1277–1283. doi: 10.1038/modpathol.3880474. [DOI] [PubMed] [Google Scholar]
- 17.Lee WI, Bacchni P, Bertoni F, et al. Quantitative assessment of her2/neu expression by real-time pcr and fluorescent in situ hybridization analysis in low-grade osteosarcoma. Oncol Rep. 2004;12:125–128. [PubMed] [Google Scholar]
- 18.Ma Q, Zhou Y, Ma B, et al. The clinical value of cxcr4, her2 and cd44 in human osteosarcoma: A pilot study. Oncol Lett. 2012;3:797–801. doi: 10.3892/ol.2012.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maitra A, Wanzer D, Weinberg AG, et al. Amplification of the her-2/neu oncogene is uncommon in pediatric osteosarcomas. Cancer. 2001;92:677–683. doi: 10.1002/1097-0142(20010801)92:3<677::aid-cncr1370>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Onda M, Matsuda S, Higaki S, et al. Erbb-2 expression is correlated with poor prognosis for patients with osteosarcoma. Cancer. 1996;77:71–78. doi: 10.1002/(SICI)1097-0142(19960101)77:1<71::AID-CNCR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Scotlandi K, Manara MC, Hattinger CM, et al. Prognostic and therapeutic relevance of her2 expression in osteosarcoma and ewing’s sarcoma. Eur J Cancer. 2005;41:1349–1361. doi: 10.1016/j.ejca.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Somers GR, Ho M, Zielenska M, et al. Her2 amplification and overexpression is not present in pediatric osteosarcoma: A tissue microarray study. Pediatr Dev Pathol. 2005;8:525–532. doi: 10.1007/s10024-005-0044-5. [DOI] [PubMed] [Google Scholar]
- 23.Thomas DG, Giordano TJ, Sanders D, et al. Absence of her2/neu gene expression in osteosarcoma and skeletal ewing’s sarcoma. Clin Cancer Res. 2002;8:788–793. [PubMed] [Google Scholar]
- 24.Tsai JY, Aviv H, Benevenia J, et al. Her-2/neu and p53 in osteosarcoma: An immunohistochemical and fluorescence in situ hybridization analysis. Cancer Invest. 2004;22:16–24. doi: 10.1081/cnv-120027577. [DOI] [PubMed] [Google Scholar]
- 25.Yalcin B, Gedikoglu G, Kutluk T, et al. C-erbb-2 expression and prognostic significance in osteosarcoma. Pediatr Blood Cancer. 2008;51:222–227. doi: 10.1002/pbc.21576. [DOI] [PubMed] [Google Scholar]
- 26.Zhou H, Randall RL, Brothman AR, et al. Her-2/neu expression in osteosarcoma increases risk of lung metastasis and can be associated with gene amplification. J Pediatr Hematol Oncol. 2003;25:27–32. doi: 10.1097/00043426-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Akatsuka T, Wada T, Kokai Y, et al. Erbb2 expression is correlated with increased survival of patients with osteosarcoma. Cancer. 2002;94:1397–1404. doi: 10.1002/cncr.10360. [DOI] [PubMed] [Google Scholar]
- 28.Akatsuka T, Wada T, Kokai Y, et al. Loss of erbb2 expression in pulmonary metastatic lesions in osteosarcoma. Oncology. 2001;60:361–366. doi: 10.1159/000058533. [DOI] [PubMed] [Google Scholar]
- 29.Geller DS, Gorlick R. Her-2 targeted treatment of osteosarcoma: The challenges of developing targeted therapy and prognostic factors for rare malignancies. Expert Opin Pharmacother. 2010;11:51–61. doi: 10.1517/14656560903419614. [DOI] [PubMed] [Google Scholar]
- 30.Ebb D, Meyers P, Grier H, et al. Phase ii trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the children’s oncology group. J Clin Oncol. 2012;30:2545–2551. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Hoboken, NJ: John Wiley; 2002. pp. xiii–439. [Google Scholar]
- 32.Bishop YMM, Fienberg SE, Holland PW. Discrete multivariate analysis: Theory and practice. Cambridge, MA: MIT Press; 1975. pp. x–557. [Google Scholar]
- 33.Kutner MH. Applied linear statistical models. Boston: McGraw-Hill Irwin; 2005. pp. xxviii–1396. [Google Scholar]
- 34.Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. [PubMed] [Google Scholar]
- 35.Baldini N, Scotlandi K, Barbanti-Brodano G, et al. Expression of p-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med. 1995;333:1380–1385. doi: 10.1056/NEJM199511233332103. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz CL, Gorlick R, Teot L, et al. Multiple drug resistance in osteogenic sarcoma: Int 0133 from the children’s oncology group. J Clin Oncol. 2007;25:2057–2062. doi: 10.1200/JCO.2006.07.7776. [DOI] [PubMed] [Google Scholar]