Abstract
In 2007 and 2008, we published two articles reporting a tamoxifen (TM)-inducible, chondrocyte-specific gene-targeting mouse model in which the expression of CreERT2 is driven by the type II collagen promoter (Col2CreERT2). The fusion protein is specifically expressed and translocated into the nucleus upon TM administration, which in turn triggers gene recombination. Since then, this animal model has become a powerful tool to study the molecular mechanism of skeletal development and degenerative cartilage diseases, including knee joint osteoarthritis (OA), temporomandibular joint (TMJ) OA, and intervertebral disc (IVD) degeneration. In this review article, we summarise the application of Col2CreERT2 mice and discuss the potential usage of this animal model in a broad spectrum of cartilage development and molecular pathology studies.
Keywords: Animals, cartilage development, cartilage physiology, chondrocytes, gene expression
Introduction
Chondrocyte differentiation and maturation are tightly regulated by a series of signalling molecules and transcription factors. Dysregulation of the critical genes leads to degenerative and developmental diseases, such as chondrodysplasia, osteoarthritis (OA), and disc degeneration. A Cre-loxP gene-targeting technique has been widely used to dissect the function of critical genes in cartilage development and pathogenesis. However, conventional and tissue-specific gene deletion approaches can cause embryonic lethality or severe skeletal deformation, given that cartilage functions as a skeletal architect.
Most of the skeleton is formed by endochondral bone formation, a process that involves a cartilage intermediate in which chondrocytes are the sole cell type. In the adult skeleton, cartilage is gradually replaced by bone; the residual hyaline cartilage covering the extremities of bone is termed ‘articular cartilage’. Chondrocyte differentiation and maturation are orchestrated by intracellular transcription factors and extracellular signalling molecules. Human genetic studies have identified and characterised a series of genes associated with chondrodysplasia and OA. The study of the functions of these genes largely relies on mouse genetic models in which these genes have been manipulated.
Global gene knockout (KO) technology was achieved by introducing a specific gene mutation by homologous recombination into embryonic stem (ES) cells in vitro, without losing ES cell pluripotency. Upon injection of the ES cells with the gene mutation into blastocysts of foster female mice, the offspring chimeric animals are composed of both mutant and wild-type cells. If the chimeric mice possess the mutant ES cells-derived germ cells, genetic manipulation can be performed. The function of the target gene can be inferred by analysing the mouse phenotype. While global gene KO mice are becoming a powerful tool for identifying gene function, about 15 % of KO mouse strains are embryonically lethal; thus these genetically manipulated animals cannot survive to adulthood. The lethal phenotype observed in KO mice limits studies to embryonic development stages and makes it difficult to determine gene function as it relates to human diseases.
The conditional gene KO inactivates the target gene in specific tissues. This technology bypasses the limits of constitutive gene KO and the phenotype observed is more reliably associated with the target gene. The system has two components. The first is the tissue-specifically expressed Cre enzyme, an integrase from bacteriophage P, and the second is the floxed mouse, in which 34-bp repeats of loxP sites are cloned at both sides of the target gene (Nagy, 2000; Kwan, 2002; Branda and Dymecki, 2004). However, the conditional gene deletion model cannot fully overcome the limits imposed by embryonic lethality, the complex phenotype, and compensatory mechanisms when the target gene is essential for vital organ function. In some instances, a gene may serve different functions in adults from in embryos. To control the target gene precisely, a fusion protein of a mutated ligand-binding domain (G400V, M543A, and L544A) of the human oestrogen receptor (ERT2) and Cre recombinase has been generated (Feil et al., 1997; Indra et al., 1999; Metzger et al., 2005). The affinity of the mutant oestrogen receptor to endogenous oestrogen is negligible (Feil et al., 1997). In the absence of tamoxifen (TM) or 4-hydroxytamoxifen (4-OHT), the fusion protein expresses and localises in the cytoplasm. Upon TM administration to the mice expressing the CreERT2 fusion protein, the ERT2 binds to 4-OHT, a metabolic product of TM, and translocates into the nucleus to induce the deletion of genomic fragment flanked with loxP sequences (Feil et al., 1997; Indra et al., 1999). Consequently, the deletion of the target gene can be achieved at specific time points during embryonic development or in postnatal stages by TM treatment.
To overcome the embryonic lethality issue for chondrocyte-specific gene deletion, we generated a chondrocyte-specific and TM-induicible Cre transgenic mouse model (Chen et al., 2007; Zhu et al., 2008). The transgene DNA is composed of a type II collagen promoter and CreERT2. Type II collagen is a matrix protein that is detectable in hyaline cartilage, elastic cartilage and fibrocartilage. The type II collagan promoter has been used to generate chondrocyte-specific gene expression in cartilaginous tissues in various mouse models (Schipani et al., 1997; Stricker et al., 2002). In our Col2CreERT2 mouse model, the expression of CreERT2, which consists of Cre recombinase fused to a triple mutant form of the human oestrogen receptor ligand binding domain, is driven by the chondrocyte-specific 1.0 kb type II collagen promoter (Tanaka et al., 2000; Tsuda et al., 2003). The TM-inducible gene deletion system has been examined in vivo in a variety of contexts although there are some concerns about leaking if a strongly expressed promoter is used and the efficiency largely depends on the expression level of the promoter.
Col2CreERT2 Mice Target: Growth Plate Chondrocytes
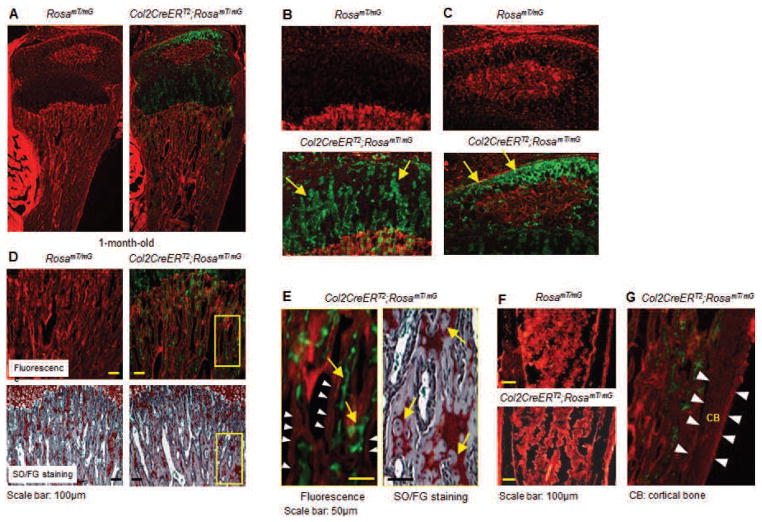
Endochondral bone formation is a well-organised process by which long bone is formed. The condensed mesenchymal cells form cartilage as the precursor of bone. Primary and secondary ossification centres are separated by a growth plate, formed by chondrocyte columns with three distinct zones, resting, proliferating and hypertrophic. The growth plate chondrocytes undergo sequential maturation processes. The TM-inducible model has been used to identify the role of critical genes in growth plate chondrocyte differentiation in both developmental and postnatal stages. The efficiency of TM-induced gene recombination was measured by crossing Col2CreERT2 mice with RosaLacZ reporter mice (Soriano, 1999). During the embryonic stage, TM was administered to the pregnant female animal (0.1 mg/g body weight of TM in corn oil). The gene recombination was demonstrated by X-gal staining. About 80 % of the chondrocytes of the hind limb were positive for X-gal staining, suggesting high efficiency of TM-induced gene recombination during embryonic development (Chen et al., 2007). High recombination efficiency of growth plate chondrocytes was also observed in the postnatal Col2CreERT2;RosamT/mG reporter mice (4 weeks-old) (Fig. 1A,B), suggesting the usefulness of the application of the animal model in postnatal skeletal development and growth.
Fig. 1.
Chondrocyte-specific Cre expression in Col2CreERT2 transgenic mice. Col2CreERT2 mice were bred with RosamT/mG reporter mice. The offspring were treated with tamoxifen (TM) at 2 weeks and sacrificed at 4 weeks. Frozen sections were prepared and analysed under fluorescent microscopy. (A–C) A Cre-recombination efficiency over 80 % is achieved in tibial growth plate chondrocytes and articular chondrocytes. The yellow arrows indicate green fluorescent protein (GFP)-positive cells. (D) Some green fluorescent signals are seen embedded in the trabecular bone matrix (upper panel) and Safranin O/Fast green (SO/FG) stained sections show a comparable amount of Safranin O-positive staining inside the trabecular bone matrix (lower panel). In contrast, no green signals are observed on the surface of the trabecular bone. The while arrow heads point to trabecular bone surface and no GFP-positive cells were found on the surface of trabecular bone. (E) Higher magnification pictures of fluorescent and SO/FG staining showed that unremoved chondrocytes are embedded in the bone matrix, suggesting that the GFP-positive cells below the growth plate could be unremoved chondrocytes. However, since the fluorescent picture and SO/FG stained picture are not same section, we could not definitely demonstrate that these GFP-positive cells are indeed unresorbed chondrocytes. Alternatively, these cells may also represent osteoblast precursor cells. The yellow arrows on the left panel indicate GFP-positive cells and the yellow arrows on the right panel indicate unremoved chondrocytes. (F) Only tomato red fluorescence is observed in bone marrow cells of the tibia (far away from the growth plate), indicating that Col2CreERT2 mice do not target osteoblasts. (G) Higher magnificent picture showed that no GFP-positive cells were found in cortical bone (CB) (Cortical bone was marked by white arrow heads). Parts A–C were adapted from Shen et al. (2013) and parts D–F were adapted from Wang et al. (2014).
The Col2CreERT2 mouse model has been demonstrated to be a powerful tool to achieve gene recombination in both embryonic and postnatal long bone development. Dao et al. investigated the role of β-catenin in embryonic cartilage developement in chondrocyte-specific gain-of-function and loss-of-function mouse models by deleting the floxed exon3 or floxed exons 2–6 of the β-catenin gene using TM-inducible Col2CreERT2 mice (Dao et al., 2012). The deletion of exon3 resulted in the production of a stable β-catenin that was resistant to GSK-3β phosphorylation mediated degradation. TM was injected into pregnant female mice to induce gene recombination at E13.5 when cartilage was just forming during embryonic development. TM induction resulted in the conditional activation and deactivation of β-catenin signalling in chondrocytes with significant development defects due to the roubst gene recombination. The activation of β-catenin leads to the premature maturation of chondrocytes coupled with enhanced perichondral bone formation. In contrast, inactivation of β-catenin leads to delay of chondrocyte maturation.
Using the same Col2CreERT2 β-cateninflox(ex3)/+ mice, Wang et al. (2014) reported that, in the postnatal stage, TM-induced chondrocyte-specific β-catenin-activated mice developed local high bone mass, attributed to a β-catenin regulatory mechanism on osteoclastogenesis. Unlike Dao’s study (Dao et al., 2012), TM was injected into postnatal 2-week-old animals and the mice were sacrificed at 3-months-old. At the postnatal stage, activation of β-catenin suppressed the expression of Rankl in chondrocytes and inhibited osteoclastogenesis; whereas mice with the conditional deletion of β-catenin in chondrocytes possessed a bone loss phenotype reversible by treatment with osteoprotegerin (OPG) or by crossing with Col2-Opg transgenic mice (Wang et al., 2014). These two studies are examples of using Col2CreERT2 mice to dissect functions of a specific gene at different developmental stages.
Shu et al. reported that a tissue-specific deletion of Bmp2, but not Bmp4, impaired embryonic chondrocyte proliferation and maturation. In their study, TM was injected into 12.5-day pregnant female mice. The deletion of Bmp2, but not Bmp4, altered proliferation and maturation of growth plate chondrocytes in long bones (Shu et al., 2011). This inducible model has also been used to study postnatal skeleton growth. Cantley et al. (2013) used this animal model to delete β-catenin in chondrocytes of 2-week-old mice and demonstrated that ablation of β-catenin caused a chondroma-like phenotype with lateral outgrowth of growth plate. Using the Col2CreERT2 mouse model, Kohn et al. (2012) studied the function of notch signalling in cartilage development and identified that both RBPjkappa-dependent and independent notch signals coordinate chondrocyte differentiation, proliferation and apoptosis.
A cartilage developmental disease, metachondromatosis, is a postnatal skeleton disorder characterised by benign bone tumours of the hands and feet. Genetic studies have revealed an association between the disease and the Shp2 gene. Because homozygous Shp2−/− mice are embryonic lethal due to multiple defects in gastrulation and mesodermal patterning, the mechanism of SHP2 in skeletal development is largely unknown. Using Col2CreERT2 mice, Kim et al. studied the function of SHP2 in Col2CreERT2;Shp2flox/flox mice. TM was injected into these mice and control mice at 4-weeks of age twice a week for 5 weeks. Loss of Shp2 in the growth plate chondrocytes during the postnatal stage resulted in the development of metachondromatosis (Kim et al., 2014). This study clearly demonstrated the causal relationship between SHP2 in chondrocytes and metachondromatosis.
Col2CreERT2 Mice Target: Articular Chondrocytes
OA is a degenerative joint disease and the leading cause of disability in the world. The pathological characteristics of OA include progressive loss of articular cartilage, osteophyte formation and changes in subchondral bone. Gross articular cartilage loss is the primary pathological feature of OA. Articular chondrocytes are the only cell type responsible for producing extracellular matrix and maintaining the homeostasis of articular cartilage. Articular chondrocytes have minimal reparative potential and degeneration is almost irreversible. The molecular mechanism of maintaining and establishing articular cartilage remains largely unknown. Several studies indicate that genetic factors play a critical role in the development of OA.
Since we characterised the gene recombination of the mouse model in the articular chondrocyte of adult animals, Col2CreERT2 mice have been widely used to dissect the functions of regulatory molecules governing the stability and integrity of articular cartilage. In 2009, we first used this animal model to determine the role of β-catenin in articular cartilage degeneration (Zhu et al., 2009). Human genetic studies showed that a single nucleotide polymorphism of sFRP3 (R324G) abolished its ability to antagonise Wnt signalling and predisposed patients to OA in weight-bearing joints (Gordon et al., 2007). Elevated β-catenin was observed in the articular chondrocytes of human OA patients (Zhu et al., 2009; Papathanasiou et al., 2010). To identify the role of β-catenin in articular cartilage homeostasis, we specifically activated β-catenin in postnatal articular chondrocytes by deletion of β-catenin exon3 using Col2CreERT2 mice (Zhu et al., 2009). The deletion of exon3 resulted in the production of a stable β-catenin resistant to GSK-3β phosphorylation-mediated degradation. At both 5- and 8-month time points, the activation of β-catenin in chondrocytes resulted in an OA-like phenotype with knee joint articular surface fibrillation, vertical clefting and osteophyte formation. Reductions in Safranin O and Alcian blue staining and articular cartilage thickness were also observed. The expression of the chondrocyte maturation markers, Aggrecan, Mmp9, Mmp13, Alp, OC, and Col10a were consistently and significantly increased. The β-catenin conditional activation study illustrated a causal relationship between elevated β-catenin in articular chondrocytes and OA.
The transforming growth factor-β (TGF-β) signalling pathway is another key signalling pathway associated with the development of OA in human patients (Yang et al., 2001; Yao et al., 2003; Valdes et al., 2010). A single nucleotide polymorphism in the Smad3 gene is correlated with OA of weight-bearing joints in a cohort of 527 patients (Valdes et al., 2010). Recent mouse genetic studies indicate that dysregulation of TGF-β signalling induces development of OA via the Smad3 signalling pathway (Li et al., 2006; Li et al., 2010). To determine the mechanism of inhibition of TGF-β signalling in OA, a conditional KO of TGF-β receptor II was generated by crossing Col2CreERT2 mice with Tgfbr2flox/flox mice (Shen et al., 2013). The gene deletion was induced by administering TM to these mice at 2-weeks. Histologic assessments revealed an OA-like phenotype with tears and clefts in articular surfaces and increased numbers of hypertrophic chondrocytes in 3-month-old mice. The expression of Mmp13, Adamts 4 and 5, Runx2, Alp, OC and Col10a1 increased, suggesting accelerated chondrocyte maturation. Interestingly, no significant phenotype was observed in the growth plate of long bones in these mice. The study dissected signalling in articular cartilage and growth plate cartilage during postnatal development, and demonstrated the usefulness of the mouse model.
Fibroblast growth factor (FGF) family members regulate cartilage homeostasis. Mice with Fgf2 deficiency showed accelerated spontaneous OA (Chia et al., 2009). The conditional deletion of Fgfr1 resulted in embryonic lethality. Weng et al. administered TM to 8-week-old Col2CreERT2;Fgfr1flox/flox mice, and found that the deactivation of Fgfr1 inhibited articular cartilage degeneration in three different OA models, aging-induced spontaneous OA (12-months of age), destabilisation of medial meniscus (DMM)-induced OA and antigen-induced OA (Weng et al., 2012).
In addition to characterising the molecules involved in OA development, Col2CreERT2 is also used to examine potential targets for OA treatment and prevention. Wang et al. (2013a) investigated the role of MMP13 in DMM-induced OA model. After treatment with TM, the Col2CreERT2;Mmp13flox/flox mice had no significant phenotypic change in either the growth plate cartilage or articular cartilage. However, Mmp13 conditional KO mice were resistant to DMM-induced OA, suggesting a therapeutic potential for a MMP13 inhibitor in OA treatment. In the same study, the authors demonstrated that a MMP13 inhibitor efficiently prevented and decelerated progression of OA. The broad application of Col2CreERT2 mice demonstrated the impact of this inducible, tissue-specific model for mechanistic research and prevention of OA.
Col2CreERT2 Mice Target: Craniofacial Cartilage
The temporomandibular joint (TMJ) is a hinge and gliding joint that connects the condyle of the mandibular bone with a concave, socket-like articular fossa of the temporal bone. It is arguably the most complex synovial joint in humans. Unlike most other synovial joints, the TMJ is bilaterally linked and consists of fibrocartilage. TMJ disorders represent an array of diverse pathological and psychosocial disorders that affect any or all structures within the joint. Among TMJ disorders, OA is the most prevalent degenerative disease arising from pathological conditions, as well as the aging process. TMJ condyle cartilage is divided into three distinct zones, fibrocartilage-like tissue in the superficial zone and hyaline cartilage tissue in the middle and lower zones. During the progression of TMJ OA, the chondrocytes undergo maturation with a phenotype of hypertrophy and apoptosis. The molecular mechanism of TMJ OA parallels knee joint OA, which is marked by the reduction of type II collagen expression and increased expression of hypertrophic markers, such as type X collagen and Runx2. Because the TMJ condyle also serves as the mandibular growth centre in postnatal development to provide a larger space for permanent teeth and larger brain capacity, conventional and conditional gene KO targeting the mandibular cartilage may cause severe developmental defects of the mandible.
TM-inducible cartilage-specific animal models have also been used to study the role of β-catenin in TMJ arthritis. Gene recombination efficiency was measured by crossing Col2CreERT2 mice with RosamT/mG reporter mice. TM was administered to 2-week-old mice and the mice were sacrificed at 1 month to assess recombination efficiency. With TM treatment, over 80% of TMJ condyle cells were positive for enhanced green fluorescent protein (EGFP), indicating high recombination efficiency (Wang et al., unpublished data). Two months after TM induction, the Col2CreERT2;β-cateninflox(ex3)/+ mice showed a progressive TMJ OA-like phenotype coupled with reduced chondrocyte numbers in the superficial zone, and increased hypertrophic chondrocytes in all three zones. The total cartilage area was decreased significantly, compared to the negative control. The expressions of β-catenin target genes, such as Mmp13 and Adamts5, were consistently elevated. When Mmp13 was deleted in the background of inducible β-catenin conditional activation, the progress of TMJ OA was significantly slower.
Another application of the inducible animal model in craniofacial cartilage development was reported by Wang et al. in Meckel’s cartilage development (Wang et al., 2013b). Meckel’s cartilage is a transient cartilage tissue supporting mandible development. Col2CreERT2 mice were crossed with pmes-caBmprI and pmes-caBmprII mice to generate animals with a constitutively active form of either type I or type II bone morphogenetic protein (BMP) receptors (BMPRI or BMPRII). With treatment with TM at E12.5, chondrocytes in Meckel’s cartilage demonstrated a strong efficacy of recombination (Wang et al., 2013b). When caBmprIa was activated in chondrocytes, enlarged Meckel’s cartilage was observed, suggesting that temporal regulation of BMP signalling is required for normal Meckel’s cartilage development.
Col2CreERT2 Mice Target: Intervertebral Disc
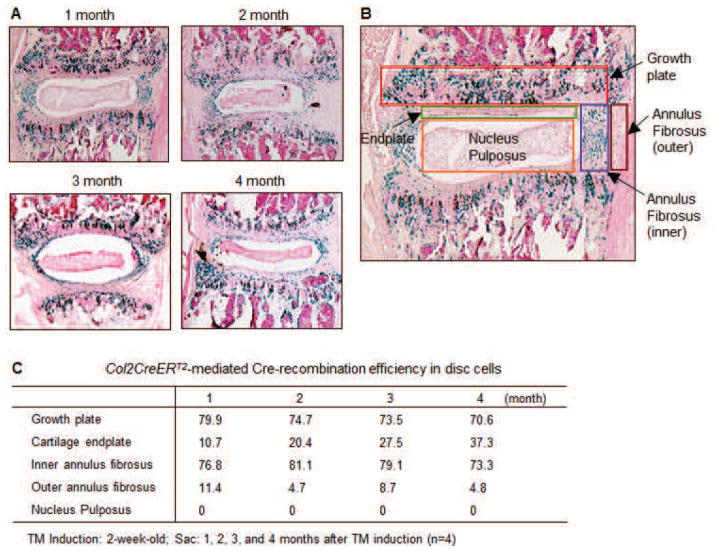
Similar to the TMJ, the intervertebral disc (IVD) is formed by fibrocartilage tissues expressing type II collagen. The IVD consists of an annulus fibrosus (AF) that encloses the nucleus pulposus (NP), a viscous gel-like tissue rich in aggrecan and other proteoglycans. Jin et al. (2011) bred Col2CreERT2 transgenic mice with RosaLacZ reporter mice to analyse tissue specificity and recombination efficiency of the IVD. TM was injected into animals at 2 weeks of age; the recombination efficiency reached over 75 % at one month and over 70 % at 4 months in both growth plate chondrocytes and the inner AF cells (Fig. 2C), suggesting slow turnover of IVD chondrocytes (Jin et al., 2011). Using this animal model, Jin et al. (2011) studied the function of TGF-β signalling in the inner AF cells of the disc tissue and surrounding growth plate chondrocytes by conditional deletion of TGF-β receptor type 2 using Col2CreERT2. The loss of TGF-β signalling in growth plate chondrocytes and in the inner AF cells led to the reduction of matrix proteins by endplate cartilage cells and abnormal growth plate cartilage morphology. Similar to the long bone chondrocyte deletion of Tgfbr2, the disc cells of the KO mice expressed high levels of Mmp13, Col10a1 and other maturation markers. Kim et al. reported the phenotype of Col2CreERT2;Shp2flox/flox mice (Kim et al., 2013). TM was injected in 4-week-old mice resulting in 85 % reduction of Shp2 expression. The Shp2-deficient animals exhibited a phenotype of scoliosis and kyphosis.
Fig. 2.
X-gal staining of Col2CreERT2;RosaLacZ mice. (A) Two-week-old Col2CreERT2;RosaLacZ double transgenic mice were injected with tamoxifen (TM) (0.1 mg/g body weight, intraperitonal injection daily for 5 days). Mice were sacrificed at ages of 1, 2, 3 and 4 months (a–d) and disc samples (L3/L4) were fixed, decalcified and processed for frozen section preparation. X-gal staining was followed by counterstaining with nuclear fast red. Col2CreERT2 targeted cells are lacZ-positive and stained blue. Recombination efficiency was evaluated by counting LacZ-positive cells divided by the total cell number in the growth plate, cartilage endplate, inner and outer annulus fibrosus (AF), and nucleus pulposus (NP) regions. High Cre-recombination efficiency in growth plate and inner AF cells is observed in Col2CreERT2 transgenic mice 1, 2, 3 and 4 months after TM induction. Relatively lower Cre-recombination efficiency is found in the cartilage endplate and outer AF cells. No lacZ-positive cells are found in the NP region. (B) Evaluation of Cre recombination efficiency. TM was administered to 2-week-old Col2CreERT2;RosaLacZ mice and mice were sacrificed 1 month after TM induction. X-gal staining was performed and sections (L3/L4) were then counterstained with nuclear fast red. Total and X-gal-positive cell numbers were counted in the growth plate, cartilage endplate, inner and outer annulus fibrosus and nucleus pulposus regions. (C) The table demonstrated Col2CreERT2-mediated Cre-recombination efficiency in different regions of disc cells. This figure was adapted from Jin et al. (2011).
Tracing the Fate of Col2-Targeting Chondrocytes
CreERT2 transgenic mice have been used for tracing cell fate (Maes et al., 2010). To determine if TM-induced Cre-mediated recombination is maintained in articular chondrocytes of adult animals, Col2CreERT2 mice were bred with RosaLacZ reporter mice to follow the course of reporter gene expression. TM was administered to 2-week-old mice and Cre-mediated recombination was examined at 1- and 6-months. The results showed that TM induced efficient Cre recombination in articular chondrocytes in both 1- and 6-months-old mice. The Cre recombination efficiency in articular cartilage of Col2CreERT2 mice was 85 % at 1-month and 82 % at 6-months (Zhu et al., 2008). These findings suggest that articular chondrocytes have very low turnover rates.
There are two major cell types in the human NP tissue, notochord-like cells and chondrocyte-like cells. The immature disc NP consists of notochordal cells. With NP maturation, notochordal cells are replaced by chondrocyte-like cells in the human spine. In mice, small percentages of notochordal cells survive in the postnatal stage. It has been reported that the transition from the notochordal to cartilaginous NP is accomplished by the postnatal migration of chondrocyte-like cells from the cartilage endplate and inner AF region into the notochordal NP region (Kim et al., 2003, 2009; Thorsten et al., 2010). A study using a rabbit model indicated that chondrocytes from the cartilage endplate can migrate toward the NP region as notochordal cells underwent apoptosis (Pazzaglia et al., 1989). Our in vivo cell tracing studies using Col2CreERT2;RosaLacZ mice demonstrated that no X-gal positive cells migrated from the cartilage endplate and AF cells to the NP region during the postnatal 4-month period (Fig. 2A,B). This suggests that, at least in early postnatal mice, chondrocyte-like cells in the NP region are not derived from the surrounding cartilage endplate and AF cells.
Targeting Non-Chondrocyte Cells
To test the targeting specificity of Col2CreERT2 mice, we examined X-gal staining in multiple organs, such as heart, kidney, liver, lung, spleen and skin from Col2CreERT2;RosaLacZ mice. We found that Col2-driving Cre activity was not detectable in these organs (Chen et al., 2007). These findings suggested that Col2CreERT2 mice have relative specificity for targeting chondrocytes.
We used another reporter mouse model, RosamT/mG mice, to investigate targeting specificity and recombination efficiency. We crossed Col2CreERT2 transgenic mice with RosamT/mG mice expressing tomato red and the membrane-targeted EGFP (mG) driven by the constitutively active Rosa26 locus. The floxed membrane-targeted tdTomato (mT) cassette with the stop codon located upstream of the EGFP coding sequence is necessary for Cre-mediated excision to allow EGFP expression. Following TM exposure, all GFP-positive cells are those cells expressing Cre recombinase, in which the tomato red sequence was removed from the genome. Those cells in which excision of the stop region does not occur express tomato red only. Cre recombination efficiency over 80 % was achieved in growth plate chondrocytes of tibiae, determined by calculation of the percentage of GFP-positive cells among all growth plate chondrocytes (Fig. 1A,B). There were some green signals found within the trabecular bone area (Fig. 1D, upper right) which appear to co-localise with Safranin O-positive staining cells (Fig. 1D, lower right and Fig. 1E, right panel). These results suggest that the green signals in the trabecular bone area may be unresorbed chondrocytes embedded in bone matrix. However, since the fluorescent picture and SO/FG stained picture are not from the same section, we could not definitely demonstrate that these GFP-positive cells are indeed unreported chondrocytes. Alternatively, these cells may also represent osteoblast precursor cells. Bone marrow cells far away from the growth plate were not targeted in Col2CreERT2 transgenic mice (Fig. 1F). Another possible reason for Col2CreERT2 targeting cells under the growth plate is that these cells represent osteoblast precursor cells. A recent report demonstrated that deletion of the von Hippel-Lindau gene (Vhl) in Col2CreERT2 targeting cells led to activation of hypoxia-inducible factor α (HIFα) signalling and increased bone mass (Weng et al., 2014); the phenotype is generally a phenocopy of mice lacking Vhl in mature osteoblasts (Zhao et al., 2012).
Other Inducible Systems Targeting Chondrocytes
Several investigators have independently generated cartilage-specific, TM-inducible mouse models. IN 2006, Nakamura et al. reported the production of Col2CreERT transgenic mice, in which TM injection induced a strong Cre-mediated gene deletion from embryonic day 11 to postnatal day 21 (Nakamura et al., 2006). Thereafter, the Cre-mediated gene recombination decreased significantly and became undetectable in 12-week-old mice. Although the same type II collagen promoter was used to achieve tissue-specific gene expression, the discrepancy between the mice reported by Nakamura et al. and ours may be due to differences in transgene integration sites and copy numbers. Hilton et al. also generated Col2CreERT transgenic mice, in which TM administration during embryogenesis induced Cre activity in chondrocytes of limbs, but not in the perichondrium (Hilton et al., 2007). These authors did not assess the recombination efficiency in postnatal cartilage. In addition to type II collagen, aggrecan is a major extracellular matrix protein in both growth plate and articular cartilage. Henry et al. knocked-in CreERT2 cDNA into the 3′-UTR of the aggrecan gene to create Agc1CreERT2 mice (Henry et al., 2009). TM-induced gene recombination in cartilage was documented up to 6-months of age. The authors speculated that Agc1CreERT2 mice could be useful for studying the gene function in adult animals because the aggrecan gene is highly expressed in articular chondrocytes in aged animals when type II collagen expression is diminished.
Summary
We combined tissue-specific and TM-inducible gene targeting technologies and generated a mouse model in which a gene can be inactivated spatiotemporally in chondrocytes. The targeting efficiency of these transgenic mice in different tissues was summarised in Table 1. This model has been used to identify the role of critical genes in embryonic and postnatal skeletal development (Shu et al., 2011; Dao et al., 2012; Kohn et al., 2012; Cantley et al., 2013; Wang et al., 2013b), the progression of arthritis of the knee joint (Zhu et al., 2009; Shen et al., 2013; Wang et al., 2013a) and TMJ (unpublished data) and IVD degeneration (Jin et al., 2011; Kim et al., 2013b). In these studies, the targeted genes include β-catenin (Zhu et al., 2009; Dao et al., 2012; Cantley et al., 2013), BMPs (Shu et al., 2011), type I and type II BMP receptors (Bmpr1 and Bmpr2) (Wang et al., 2013b), Mmp13 (Wang et al., 2013a), Shp2 (Kim et al., 2013a; Kim et al., 2013b), and Tgfbr2 (Jin et al., 2011; Shen et al., 2013).
Table 1.
Summary of the targeting efficiency of transgenic mice in different tissues
| Targeting tissue | Induction/harvest time | Targeting gene/reporter system | Recombination efficiency | References |
|---|---|---|---|---|
| Growth plate chondrocytes | E15.5/E18.5 | RosaLacZ | 80 % | Chen et al., 2007 |
| P2 weeks/3 weeks | RosaLacZ | 78 % | Chen et al., 2007 | |
| P8 weeks/9 weeks | RosaLacZ | 70 % | Chen et al., 2007 | |
| P5, 6, 7 days/P1, 2, 5, 8 weeks and 6 months | β-catenin | N/A | Cantley et al., 2013 | |
| P2 weeks/1 month | RosamT/mG | >85 % | Shen et al., 2013 | |
| E13.5/E14.5, 15.5, 16.5, 18.5 | β-catenin | N/A | Dao et al., 2012 | |
| E12.5/E14.5, 18.5 | Bmp2 | N/A | Shu et al., 2011 | |
| P4 weeks/9 weeks | Shp2 | 80 %* | Kim et al., 2013 | |
| E12.5 | RBPjκ, NICD | N/A | Kohn et al., 2012 | |
| Articular chondrocytes (knee joint) | E15.5/E18.5 | RosaLacZ | 80 % | Chen et al., 2007 |
| P2 weeks/1, 6 months | RosaLacZ | 85 % | Zhu et al., 2008 | |
| P2 weeks/5, 8 month | β-catenin/RosaLacZ | 80 % | Zhu et al., 2009 | |
| 8 weeks/14, 18 weeks, 12 months | Fgfr1 | N/A | Weng et al., 2012 | |
| P2 weeks/3, 6 months | Tgfbr2/RosaLacZ | >85 % | Shen et al., 2013 | |
| P2 weeks/4, 8, 12 weeks | Mmp13 | N/A | Wang et al., 2013 | |
| Intervertebral disc | P2 weeks/3 weeks | RosaLacZ | 75 % | Chen et al., 2007 |
| P2 weeks/1, 2, 3, 4 months | Tgfbr2/RosaLacZ | 70 % | Jin et al., 2011 | |
| P2 weeks/1, 3, 6 month | β-catenin/RosaLacZ | 75–80 % | Wang et al., 2012 | |
| P4 weeks/9 weeks | Shp2 | 85 %* | Kim et al., 2014 | |
| Meckel’s cartilage | E 12.5/E15.5 | Bmpr1/RosaLacZ | 80 % | Wang et al., 2013 |
| Temporomandibular joint | P2 weeks/1, 3, 6 months | β-catenin/RosamT/mG | 80 % | Wang et al., 2014 (unpublished data) |
Note:
recombination efficiency is evaluated by targeting gene expression level by real-time PCR
While this animal model is widely utilised, one concern is the anabolic effect of TM. TM is a selective oestrogen receptor modulator with oestrogenic and anti-oestrogenic effects. The main clinical use of TM in humans is as an antagonist of oestrogen in breast cancer. In bone, TM is a weak oestrogen agonist and is not used clinically as a selective oestrogen receptor modulator (SERM) (Li et al., 1996). In rats, daily injections of TM for 28 days (0.1 mg/kg body weight) resulted in approximately a 20 % increase in bone volume (BV/TV, %) in the tibial metaphysis (Weise et al., 2001). In our studies, TM was administered on only 5 or fewer occasions and no significant effect on bone was observed (Shu et al., 2011).
Oestrogen also has effects on cartilage and regulates growth plate closure and senescence through the oestrogen receptor. Weise et al. performed a comprehensive morphological analysis of the rabbit growth plate and found that oestrogen treatment did not qualitatively alter the growth plate, but did accelerate growth plate senescence (Weise et al., 2001). Interestingly, in rats with normal hormonal status, 6-weeks of TM administration at 0.02 mg/g body weight had no effect on skeletal growth or bone formation parameters (Weise et al., 2001). Morphometric features, including width of the growth plate, proliferating region, and hypertrophic zone were unchanged (Weise et al., 2001).
We can, therefore, conclude that in Col2CreERT2 mice (1) TM (short-term administration) does not cause growth plate senescence or closure; (2) efficient Cre-induced gene recombination can be achieved with only 5 doses of TM, or fewer; (3) data from our studies and those of other groups using Col2CreERT2 mice do not show any obvious changes in growth plate morphology with treatment with TM; and 4) TM-treated controls allow accurate interpretation of observed phenotypes.
Acknowledgments
This work was supported by grants AR055915 and AR054465 to D.C. from the National Institute of Health (NIH). The authors thank Verhonda Hearon-Eggleston for assistance in preparing the manuscript.
References
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Cantley L, Saunders C, Guttenberg M, Candela ME, Ohta Y, Yasuhara R, Kondo N, Sgariglia F, Asai S, Zhang X, Qin L, Hecht JT, Chen D, Yamamoto M, Toyosawa S, Dormans JP, Esko JD, Yamaguchi Y, Iwamoto M, Pacifici M, Enomoto-Iwamoto M. Loss of beta-catenin induces multifocal periosteal chondroma-like masses in mice. Am J Pathol. 2013;182:917–927. doi: 10.1016/j.ajpath.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O’Keefe RJ, Chen D. Generation of a transgenic mouse model with chodrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60(7):2019–2027. doi: 10.1002/art.24654. [DOI] [PubMed] [Google Scholar]
- Dao DY, Jonason JH, Zhang Y, Hsu W, Chen D, Hilton MJ, O’Keefe RJ. Cartilage-specific beta-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J Bone Miner Res. 2012;27:1680–1694. doi: 10.1002/jbmr.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Gordon A, Southam L, Loughlin J, Wilson AG, Stockley I, Hamer AJ, Eastell R, Wilkinson JM. Variation in the secreted frizzled-related protein-3 gene and risk of osteolysis and heterotopic ossification after total hip arthroplasty. J Orthop Res. 2007;25:1665–1670. doi: 10.1002/jor.20446. [DOI] [PubMed] [Google Scholar]
- Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis. 2009;47:805–814. doi: 10.1002/dvg.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Long F. Tamoxifen-inducible gene deletion reveals a distinct cell type associated with trabecular bone, and direct regulation of PTHrP expression and chondrocyte morphology by Ihh in growth region cartilage. Dev Biol. 2007;308:93–105. doi: 10.1016/j.ydbio.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basel layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible CreERT and CreERT2 recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Shen J, Wang B, Wang M, Shu B, Chen D. TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett. 2011;585:1209–1215. doi: 10.1016/j.febslet.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HKW, Feng G, Chen D, King P, Kamiya N. Targeted disruption of Shp2 in chondrocytes leads to metachondromatosis with multiple cartilaginous protrusions. J Bone Miner Res. 2014;29:761–769. doi: 10.1002/jbmr.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Aruwajoye O, Sucato D, Richards BS, Feng GS, Chen D, King PD, Kamiya N. Induction of SHP2 deficiency in chondrocytes causes severe scoliosis and kyphosis in mice. Spine (Phila Pa 1976) 2013;38:E1307–1312. doi: 10.1097/BRS.0b013e3182a3d370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine (Phila Pa 1976) 2003;28:982–990. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- Kim KW, Ha KY, Lee JS, Nam SW, Woo YK, Lim TH, An HS. Notochordal cells stimulate migration of cartilage end plate chondrocytes of the intervertebral disc in vitro cell migration assays. Spine J. 2009;9:323–329. doi: 10.1016/j.spinee.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Kohn A, Dong Y, Mirando AJ, Jesse AM, Honjo T, Zuscik MJ, O’Keefe RJ, Hilton MJ. Cartilage-specific RBPjkappa-dependent and -independent Notch signals regulate cartilage and bone development. Development. 2012;139:1198–1212. doi: 10.1242/dev.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM. Conditional alleles in mice: practical considerations for tissue-specific knockouts. Genesis. 2002;32:49–62. doi: 10.1002/gene.10068. [DOI] [PubMed] [Google Scholar]
- Li TF, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, Drissi H, O’Keefe RJ. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006;21:4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TF, Gao L, Sheu TJ, Sampson ER, Flick LM, Konttinen YT, Chen D, Schwarz EM, Zuscik MJ, Jonason JH, O’Keefe RJ. Aberrant hypertrophy in Smad3-deficient murine chondrocytes is rescued by restoring transforming growth factor beta-activated kinase 1/activating transcription factor 2 signaling: a potential clinical implication for osteoarthritis. Arthritis Rheum. 2010;62:2359–2369. doi: 10.1002/art.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Takahashi M, Kushida K, Koyama S, Hoshino H, Kawana K, Horiuchi K, Inoue T. The effect of tamoxifen on bone metabolism and skeletal growth is different in ovariectomized and intact rats. Calcif Tissue Int. 1996;59:271–276. doi: 10.1007/s002239900122. [DOI] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D, Li M, Chambon P. Targeted somatic mutagenesis in the mouse epidermis. Methods Mol Biol. 2005;289:329–340. doi: 10.1385/1-59259-830-7:329. [DOI] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreERT to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- Papathanasiou I, Malizos KN, Tsezou A. Low-density lipoprotein receptor-related protein 5 (LRP5) expression in human osteoarthritic chondrocytes. J Orthop Res. 2010;28:348–353. doi: 10.1002/jor.20993. [DOI] [PubMed] [Google Scholar]
- Pazzaglia UE, Salisbury JR, Byers PD. Development and involution of the notochord in the human spine. J R Soc Med. 1989;82:413–415. doi: 10.1177/014107688908200714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipani E, Lanske B, Hunzelman J, Luz A, Kovacs CS, Lee K, Pirro A, Kronenberg HM, Juppner H. Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide. Proc Natl Acad Sci USA. 1997;94:13689–13694. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Li J, Wang B, Jin H, Wang M, Zhang Y, Yang Y, Im HJ, O’Keefe R, Chen D. Deletion of the transforming growth factor beta receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheum. 2013;65:3107–3119. doi: 10.1002/art.38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, Tang D, Harris SE, Mishina Y, O’Keefe RJ, Hilton MJ, Wang Y, Chen D. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124:3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stricker S, Fundele R, Vortkamp A, Mundlos S. Role of Runx genes in chondrocyte differentiation. Dev Biol. 2002;245:95–108. doi: 10.1006/dbio.2002.0640. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Matsumoto Y, Nakatani F, Iwamoto Y, Yamada Y. A zinc finger transcription factor, alphaA-crystallin binding protein 1, is a negative regulator of the chondrocyte-specific enhancer of the alpha1(II) collagen gene. Mol Cell Biol. 2000;20:4428–4435. doi: 10.1128/mcb.20.12.4428-4435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thorsten G, Andreas N, Markus K. Sensitivity of notochordal disc cells to mechanical loading: an experimental animal study. Eur Spine J. 2010;19:113–121. doi: 10.1007/s00586-009-1217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Takahashi S, Takahashi Y, Asahara H. Transcriptional co-activators CREB-binding protein and p300 regulate chondrocyte-specific gene expression via association with Sox9. J Biol Chem. 2003;278:27224–27229. doi: 10.1074/jbc.M303471200. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Spector TD, Tamm A, Kisand K, Doherty SA, Dennison EM, Mangino M, Kerna I, Hart DJ, Wheeler M, Cooper C, Lories RJ, Arden NK, Doherty M. Genetic variation in the SMAD3 gene is associated with hip and knee osteoarthritis. Arthritis Rheum. 2010;62:2347–2352. doi: 10.1002/art.27530. [DOI] [PubMed] [Google Scholar]
- Wang B, Jin H, Zhu M, Li J, Zhao L, Zhang Y, Tang D, Xiao G, Xing L, Boyce BF, Chen D. Chondrocyte β-catenin signaling regulates postnatal bone remodeling through modulation of Rankl/Opg expression and osteoclast formation. Arthritis Rheum. 2014;66:107–120. doi: 10.1002/art.38195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, Chen D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013a;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zheng Y, Chen D, Chen Y. Enhanced BMP signaling prevents degeneration and leads to endochondral ossification of Meckel’s cartilage in mice. Dev Biol. 2013b;381:301–311. doi: 10.1016/j.ydbio.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li S, Xie W, Shen J, Im H-J, Holz JD, Wang M, Diekwisch TGH, Chen D. Activation of β-catenin signalling leads to temporomandibular joint defects. Eur Cells Mater. 2014;28:223–235. doi: 10.22203/ecm.v028a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise M, De-Levi S, Barnes KM, Gafni RI, Abad V, Baron J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci USA. 2001;98:6871–6876. doi: 10.1073/pnas.121180498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T, Xie Y, Huang J, Luo F, Yi L, He Q, Chen D, Chen L. Inactivation of vhl in osteochondral progenitor cells causes high bone mass phenotype and protects against age-related bone loss in adult mice. J Bone Miner Res. 2014;29:820–829. doi: 10.1002/jbmr.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JY, Wang Y, An J, Mao CM, Hou N, Lv YX, Wang YL, Cui F, Huang M, Yang X. Mutation analysis of the Smad3 gene in human osteoarthritis. Eur J Hum Genet. 2003;11:714–717. doi: 10.1038/sj.ejhg.5201034. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Shen X, Zhang W, Zhu G, Qi J, Deng L. Mice with increased angiogenesis and osteogenesis due to conditional activation of HIF pathway in osteoblasts are protected from ovariectomy induced bone loss. Bone. 2012;50:763–770. doi: 10.1016/j.bone.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen M, Lichtler AC, O’Keefe RJ, Chen D. Tamoxifen-inducible Cre-recombination in articular chondrocytes of adult Col2a1-CreERT2 transgenic mice. Osteoarthritis Cartilage. 2008;16:129–130. doi: 10.1016/j.joca.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O’Keefe RJ, Zuscik M, Chen D. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]