Abstract
Despite extensive research in knee and hip osteoarthritis (OA), the underlying mechanism of temporomandibular joint (TMJ) disorder remains largely unknown. The purpose of this study was to determine whether the constitutive activation of β-catenin in the middle and deep layers of the articular cartilage can compromise the homeostasis of this tissue in the TMJ. Co12CreERT2 transgenic mice were bred with RosamT/mG reporter mice to determine Cre recombination efficiency. Co12CreERT2 mice were then crossed with β-cateninflox (ex3)/+ mice to generate β-catenin conditional activation mice, β-catenin(ex3)Co12ER. TMJ samples were harvested when the mice were 1-, 3- or 6-month-old and evaluated using histology, histomorphometry and immunohistochemistry. β-catenin(ex3)Co12ER mice were further crossed with Mmp13flox/flox and Adamts5−/− mice to generate β-catenin(ex3)/Mmp13)Co12ER and β-catenin(ex3)Co12ER)/Adamts5−/− double mutant mice to investigate the role of Mmp13 and Adamts5 in the development of TMJ disorder. High levels of Cre-recombination were seen in Co12CreERT2;RosamT/mG mice. Progressive TMJ defects developed in 1-, 3- and 6-month-old β-catenin(ex3)Co12ER mice, as revealed by histology and histomorphometry. Results further demonstrated that the defects observed in β-catenin(ex3)Co12ER mice were significantly decelerated after deletion of the Mmp13 or Adamts5 gene in (β-catenin(ex3)/Mmp13)co12ER or β-catenin(ex3)Co12ER/ Adamts5−/− double mutant mice. In summary, we found that β-catenin is a critical gene in the induction of TMJ cartilage degeneration, and over-expressing β-catenin in TMJ cartilage leads to defects assembling an OA-like phenotype. Deletion of Mmp13 and Adamts5 in β-catenin(ex3)Co12ER mice ameliorates the development of TMJ defects. This study suggests that Mmp13 and Adamts5 could be potential therapeutic targets for the treatment of TMJ disorders.
Keywords: Temporomandibular joint, osteoarthritis, β-catenin, MMP13, Adamts5
Introduction
The temporomandibular joint (TMJ) is a hinge and gliding joint that connects the condyle of the mandibular bone with the temporal articular surface composed of a concave, socket-like articular fossa and a convex eminence. Similar to the knee joint, the temporal bone and the condyle are separated and cushioned by an articular disc (Fig. 1A). The TMJ disc separates the joint space into two distinct parts. The superior joint space is bounded by the articular fossa and the articular eminence. The inferior joint space is bounded by the condyle. TMJ is one of the most frequently used joints in the human body. It is reported that about 40–70 % American adults have at least one sign of TMJ disorders and at least 33 % among those have one symptom, such as pain, limited mandibular motion, and TMJ sounds (Scrivani et al., 2008). The American Academy of Orofacial Pain classified TMJ disorder into different subtypes (de Leeuw, 2008). Among these subgroups, TMJ osteoarthritis (OA) is a highly prevalent degenerative disease that affects articular cartilage during pathological conditions, as well as during the aging process. Currently, rest therapy, pain relief, and thermotherapy are the principal clinical treatment methods, and there is no preventative treatment. Moreover, current knowledge of TMJ OA is derived primarily from clinical investigations, and many aspects of TMJ OA, especially the underlying molecular mechanisms, remain largely under-studied (Rando and Waldron, 2012).
Fig. 1.
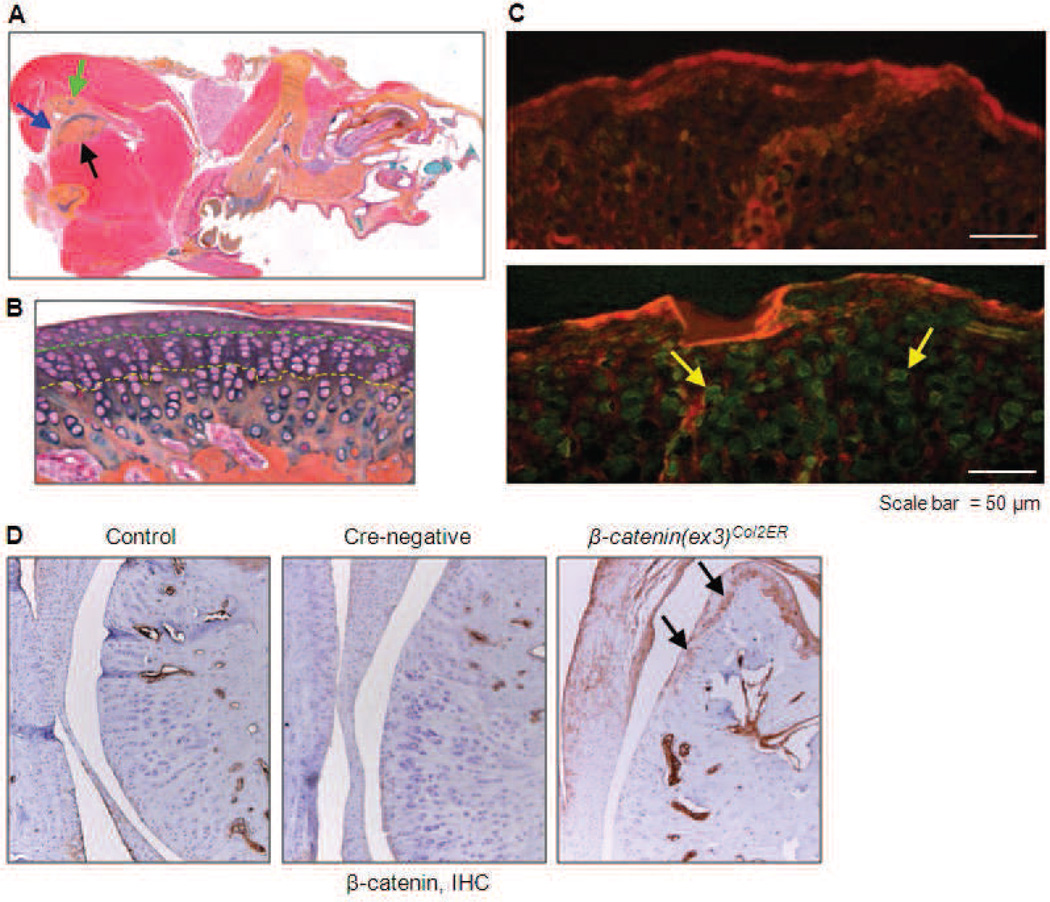
Co12CreERT2 mice efficiently target TMJ cartilage tissue. (A) Alcian blue/H&E stained sagittal skull section of adult wild-type mouse. The temporal bone (green arrow) and the mandible condyle (black arrow) are separated by an articular disc (blue arrow). TMJ condylar cartilage was stained with Alcian blue. (B) Normal TMJ cartilage can be divided into three layers: superficial layer (above green dashed line), middle layer (between green and yellow dashed lines), and deep layer (below yellow dashed line). (C) Co12CreERT2 mice were bred with RosamT/mG reporter mice. Tamoxifen was administered when the mice were 2-week-old (0.1 mg/g body weight, i.p., daily for 5 d). TMJ samples were harvested at 4-week-old. Fluorescent images showed red mT labelling in Cre-negative control mouse (upper panel) and green mG labelled chondrocytes in Cre-positive mouse (yellow arrows) (lower panel). (D) β-catenin IHC was performed and the result showed that β-catenin expression was up-regulated in the TMJ tissue of β-catenin(ex3)Co12ER mice.
Jaw function and movement result in continuous and ever changing mechanical loads exerted on the TMJ, which in turn affect the morphology and composition of its cartilages (Iwasaki et al., 2010). TMJ condylar articular cartilage is an avascular, compressible tissue comprised of dense extracellular collagen fibres and proteoglycans that protect the joint from damage during mechanical loading. The collagen fibres are well organised and aligned in an antero-posterior orientation to optimise resistance to shear stress (Singh and Detamore, 2008). Morphologically, the condylar cartilage is divided into the superficial, middle, and deep layers (Fig. 1B) (Luder et al., 1988; Mizoguchi et al., 1996). Above the middle layer the tissue is fibrocartilage-like and beneath the middle layer is hyaline-like (Kuroda et al., 2009). The principal component of the superficial layer is type I collagen (Col1), and the principal component of the middle and deep layer is type II collagen (Co12) (Hirschmann and Shuttleworth, 1976). Mandibular condylar articular chondrocytes are the only cell type embedded in TMJ cartilage, and their function includes maintenance of the cartilaginous matrix.
The pathological progression of TMJ OA parallels that of knee and hip OA and is considered to be a similar disease (Kuroda et al., 2009; de Bont et al., 1993). During the initiation stage of TMJ OA, the cartilage surface remains intact. At the cellular level, articular chondrocytes with low metabolism undergo hypertrophy, followed by apoptosis. These cellular effects result in cartilage fibrillation and subsequent progressive cartilage loss at the tissue level. Subchondral bone sclerosis and osteophyte formation happen in late-stage OA (Wang et al., 2011). At the molecular level, primary cartilage matrix proteins, such as Co12 and aggrecan, start to decrease, concomitant with gradual increases in the expression of marker genes of chondrocyte hypertrophy, such as Runx2 and Co110., as well as the genes coding for primary enzymes responsible for cartilage degeneration, such as Mmp13, Adamts4 and Adamts5 (Neuhold et al., 2001). Decreasing anabolic components, increasing metabolic components and proteolytic enzymes in the cartilage eventually tip the balance away from matrix maintenance toward degradation, resulting in complete cartilage erosion over time.
β-catenin is a key molecule in the canonical Wnt signalling pathway, which plays a critical role in bone, cartilage and joint formation, and development (Yang, 2003). Dysregulation of β-catenin causes cartilage degeneration and OA. In previous studies, we have reported that β -catenin expression was significantly up-regulated in articular cartilage in patients with knee OA (Zhu et al., 2009) and in the intervertebral disc (IVD) tissues from patients with IVD degeneration (Wang et al., 2012). Furthermore, Co12-CreERT2 driven cartilage-specific β-catenin overexpression in both knee joint and the IVD tissue leads to progressive cartilage loss and a degenerative phenotype in this mouse model (Zhu et al., 2009; Wang et al., 2012). These findings suggest that β-catenin is a critical molecule in OA pathogenesis. MMP13 and Adamts5 are key enzymes that target collagens and aggrecans for proteolytic degradation in cartilage (Glasson et al., 2005; Knäuper et al., 1996; Reboul et al., 1996; Roach et al., 2005; Wang et al., 2012, 2013). In the present study, we investigated whether the constitutive activation of β-catenin in the middle and deep layers of the articular cartilage can compromise the homeostasis of this tissue in the TMJ using a β-catenin(ex3)Co12ER mouse model.
Materials and Methods
Animals
RosamT/mG reporter mice, Mmp13flox/flox mice, and Adamts5−/− mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) (Muzumdar et al., 2007; Stickens et al., 2004; McCulloch et al., 2009a, 2009b). Co12CreERT2 transgenic mice were generated as previously described (Chen et al., 2007; Zhu et al., 2008; Jin et al., 2011; Wang et al., 2012; 2013). β-catenin(ex3)flox/flox mice were originally reported by Harada et al (1999), and we have used these mice in our previous studies (Zhu et al., 2009; Wang et al., 2012, 2014). β-catenin(ex3)Co12ER mice, (β-catenin(ex3)/Mmp13) co12ER and β-catenin(ex3)Co12ER/Adamts5−/− double mutant mice, and their Cre-negative littermate control mice were generated as shown in Tables 1–3. Tamoxifen (Sigma, St. Louis, MO, USA) was administered into 2-week-old mice by intraperitoneal (i.p.) injection (1 mg/l0 g body weight for 5 d), n = 5 in each group. Animal protocols were approved by the IACUC of the University of Rochester.
Table 1.
Breeding and production of β-catenin(ex3)Co12ER mice.
| Breeding | Desired progeny |
|---|---|
| a) β-catenin(ex3)flox/+ x β-catenin(ex3)flox/+ | β-catenin(ex3)flox/flox |
| b) Co12CreERT2 x β-catenin(ex3)flox/flox | β-catenin(ex3)Co12ER and β-catenin(ex3)flox/+ |
Table 3.
Breeding and production of β-catenin(ex3)Co12ER/Adamts5−/− mice.
| Breeding | Desired progeny |
|---|---|
| a) Co12CreERT2 x Adamts5−/− | Co12CreERT2; Adamts5+/− |
| b) Co12CreERPT2; Adamts5+/− x Adamts5−/− | Co12CreERT2; Adamts5−/− |
| c) Adamts5−/− x β-catenin(ex3)flox/flox | β-catenin(ex3)flox/+; Adamts5+/− |
|
d)
β-catenin(ex3)flox/+; Adamts5+/− x β-catenin(ex3)flox/+; Adamts5−/− |
β-catenin(ex3)flox/flox; Adamts5−/− |
|
e)
Co12CreERT2; Adamts5−/− x β-catenin(ex3)flox/flox; Adamts5−/− |
β-catenin(ex3)Co12ER/Adamts5−/− and β-catenin(ex3)flox/+/Adamts5−/− |
Histology and histomorphometry
We dissected skulls from β-catenin(ex3)Co12ER mice, β-catenin(ex3)/Mmp13)Co12ER mice, β-catenin(ex3)Co12ER/Adamts5−/− mice and their corresponding Cre-negative control mice. Samples were fixed in 10 % neutral buffered formalin (VWR, Radnor, PA, USA) for 3 d, then decalcified with formic acid (Decal Chemical Corp., Suffern, NY, USA) for 7 d. After neutralising with Cal-arrest (Decal Chemical Corp., Suffern, NY, USA), samples were processed and embedded in paraffin. Three µm thick mid-sagittal sections at 3 different levels (50 µm apart) were cut from the medial compartment of the TMJ. The sections were stained with Alcian blue/H&E (AB/ H&E) and Safranin O/Fast green (SO/FG). Quantitative histomorphometric measurement, using ImagePro 4.5 (Leeds Precision Instruments, Minneapolis, MN, USA), was used to collect data. Alcian blue-positive staining areas were outlined on projected images of each histologic section to determine articular cartilage area and thickness. Three slides per mouse, 5 mice per group were analysed in the experiment.
Microcomputed tomography (µCT)
Prior to histological processing, we evaluated formalin-fixed TMJ tissues by µCT. We used a Scanco viva CT40 cone-beam scanner (SCANCO Medical, Brüttisellen, Switzerland) with 55 kVp source and 142 µA current. We scanned the TMJ tissues at a resolution of 10.5 mm. The scanned images from each group were evaluated at the same thresholds to allow 3-dimensional structural rendering of each sample.
Cre-recombination efficiency
RosamT/mG (membrane-Tomato/membrane-Green) mice contain two loxP sites on either side of the mT cassette. Mice express red fluorescence in all cell types and tissues before Cre-recombination and green fluorescence following Cre-recombination (Muzumdar et al., 2007). Co12CreERT2 mice were bred with RosamT/mG mice to generate Co12CreERT2; RosamT/mG mice. Tamoxifen was administered into 2-week-old mice by i.p. injection (1 mg/l0 g body weight for 5 d). Skulls were dissected, fixed in 0.2 % glutaraldehyde at 4 °C for 4 d, followed by washing three times with phosphate buffered saline (PBS). Samples were decalcified in 14 % EDTAat 4 °C for 3 weeks, cryo-protected in 30 % sucrose at 4 °C for 3 d, and then embedded and processed for frozen sections. 3 µm-thick sections were imaged with a fluorescence microscope.
Immunohistochemistry
3 µm-thick paraffin sections were baked at 60 °C overnight. Slides were then deparaffinised, and rehydrated. Antigen retrieval of collagen II and collagen X was performed using 0.2 g pepsin in 50 mL of 0.01 N HC1 at 37 °C for 25 min. Antigen retrieval of Runx2 was performed using a 0.01 M citrate buffer pH 6.0 at 95 °C for 2 h. Dako endogenous blocking reagent (S2003, Dako, Carpinteria, CA, USA) was then used to quench endogenous peroxidase for 10 min. Non-specific binding sites were blocked with 1:20 normal horse/goat serum (S-2000, Vector Laboratories, Burlingame, CA, USA) for 20 min at room temperature. 1:80 dilution (2.5 µg/mL) of collagen II (MS-235-P Thermo Scientific, Rockford, IL, USA), 1:50 dilution of collagen X (2031501005, Quartett, Berlin, Germanv), or 1:100 dilution (10 µg/mL) of Runx2 (D130-3, MBL International, Woburn, MA, USA) primary antibodies were added and the slides were incubated at 4 °C overnight. Secondary biotinylated horse anti-mouse antibody (BA-2000, Vector Laboratories) or goat anti-mouse antibody (BA-9200, Vector Laboratories) at the dilution of 1:200 was added for 30 min on the second day, followed by incubation with 1:250 streptavidin (21130, Pierce, Rockford, IL, USA) for 30 min. Positive staining was detected by Romulin AEC Chromagen (Biocare Medical RAEC810L, Concord, CA, USA).
Cell proliferation and apoptosis assays
We dissected TMJs from β-catenin(ex3)Co12ER mice and Cre-negative control mice. Samples were fixed in 10 % formalin, decalcified, and embedded in paraffin. The condyles were sectioned into serial sections at 3 µm thick in an anterior-posterior direction. Cell proliferation was carried out using anti-PCNA antibody at the dilution of 1:200 (abl8197, ABCAM®, Cambridge, UK). Apoptosis assay was carried out using a TUNEL assay kit according to the manufacturer’s instructions (G3250, Promega, Madison, WI, USA).
Real-time PCR analysis
1-month-old β-catenin(ex3)Co12ER mice and their Cre-negative control mice were sacrificed. TMJ cartilage was collected from freshly sacrificed mice to obtain total RNA. After washing with sterile PBS 3 times, the TMJ cartilage tissue was digested in 1 % pronase (Roche, Basel, Switzerland) and dissolved in lx PBS for 30 min in a 37 °C water bath with continuous shaking. This was followed by washing with serum-free Dulbecco’s modified Eagle medium (DMEM) 3 times. The TMJ cartilage tissue was then digested in 1 % Collagenase A (Roche) dissolved in serum-free DMEM for 3 h in a 37 °C water bath with continuous shaking. DMEM with 10 % foetal bovine serum (FBS) was then added to stop digestion and a 40 µm cell strainer was used to filter the digestion solution. Cells were collected and RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. 1 µg aliquots of RNA were reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Real time PCR was performed using the Rotor Gene (RG 6000, Rotor Gene, Hilden, Germany) real time DNA amplification system (Corbett Research, Westborough, MA, USA) according to the manufacturer’s instructions. Reactions were performed in a 20 µL final volume containing cDNA samples, 50 µM primers of interest, and SYBR green master mix (Applied Biosystems, Foster City, CA, USA) for PCR amplification. The name and sequence of PCR primers are listed in Table 4.
Table 4.
Name and sequences of PCR primers.
| Primer Name | Sequence |
|---|---|
| Runx2 (forward) | 5’-GAGGGCACAAGT TCTATCTGGA-3’ |
| Runx2 (reverse) | 5’-GGTGGT CCGCGATGATCTC-3’ |
| Col10a1 (forward) | 5’ - ACCCCAAGGACCTAAAGGAA-3’ |
| Col10a1 (reverse) | 5’-CCCCAGGATACCCTGTTTTT-3’ |
| Mmp13 (forward) | 5’ -TTTGAGAACACGGGGAAGA-3’ |
| Mmp13 (reverse) | 5’ - ACTTTGTTGCCAATTCC AGG-3’ |
| Adamts4 (forward) | 5’ -GGCAGATGAC AAGATGGC AGCATT-3’ |
| Adamts4 (reverse) | 5’ - AGACGAGTC ACC ACCAAGTTGACA-3’ |
| Adamts5 (forward) | 5’ -CC AAATGC ACTTCAGCCACGATCA-3’ |
| Adamts5 (reverse) | 5’ - AATGTC AAGTTGCACTGCTGGGTG-3’ |
Statistical analysis
The values are presented as mean ± standard error, and error bars represent standard error. For comparison of two groups of data, unpaired Student’s t-test was performed. For comparison of multiple groups of data, one-way analysis of variance (ANOVA) was performed followed by Dunnett’s test. *p < 0.05 was considered as significant difference between groups.
Results
High Cre-recombination efficiency in TMJ cartilage
To evaluate Co12-Cre expression and recombination efficiency in the TMJ cartilage, Co12CreERT2 mice were bred with RosamT/mG reporter mice to generate Co12CreERT2;RosamT/mG mice. Tamoxifen was administered when the mice were 2-week-old and TMJ samples were harvested at 1-month-old. The red fluorescent image of TMJ cartilage in Cre-negative control mice revealed absence of Co12Cre-expressing cells (Fig. 1C, upper panel), and the green-labelled chondrocytes in Co12CreERT2 mice showed Co12Cre-expressing cells (Fig. 1C, lower panel). IHC assay showed that β -catenin overexpression was detected at the edges of TMJ cartilage (Fig. 1D). These results demonstrate that TMJ chondrocytes were targeted by Co12CreERT2 mice with high efficiency.
Conditional activation of β-catenin induces TMJ cartilage defects
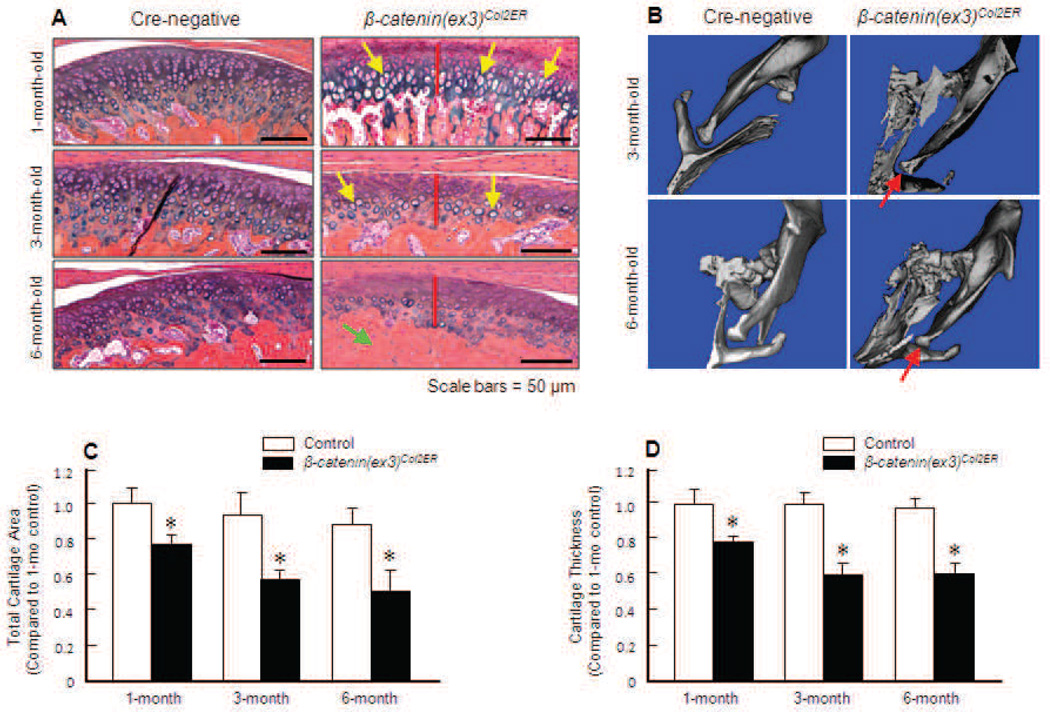
β-catenin(ex3)Co12ER mice were generated by crossing Co12CreERT2 mice with β-catenin(ex3)flox/flox mice (Table 1) and the role of β-catenin in TMJ tissue function was investigated. Tamoxifen was administered to 2-week-old mice and TMJ samples were harvested from these mice at 1, 3 and 6 months of age. One-month-old control mice showed thick and intact TMJ cartilage, which stained positive with Alcian blue. Furthermore, the chondrocytes in the control mice were well-organised in the three layers: smaller and round cells in the top superficial layer; medium-sized cells sitting in the lacuna were present in large numbers in the middle layer; and fewer, bigger, hypertrophic, mature cells in the deep layer (Fig. 2A). In contrast, 1-month-old β-catenin(ex3)Co12ER mice already presented early signs of TMJ OA: decreased chondrocyte numbers in the superficial layer accompanied with less Alcian blue staining in this area, cells in the middle and deep layers illustrated increased hypertrophy, and the cartilage was thinner. At 3 months of age, articular chondrocyte numbers in the superficial and middle layers of TMJ cartilage of β-catenin(ex3)Co12ER mice were drastically reduced; hypertrophic cells were still present in the deep layer. However, total TMJ cartilage exhibited visibly reduced Alcian blue staining, demonstrating a decrease in proteoglycan levels (Fig. 2A). At 6 months of age, increased severity of defects in TMJ tissue was observed in β-catenin(ex3)Co12ER mice. In addition to decreased cellularity of the middle and deep layers of cartilage, decreased cartilage area and subchondral bone sclerosis were also observed (Fig. 2A). Compared to middle and deep layers, the superficial area of TMJ cartilage is relatively normal. This is probably because the superficial layer of TMJ cartilage was not targeted by Co12-directed β-catenin expression. These results were further confirmed by µCT. Significant reduction in TMJ joint space was observed in 3- and 6-month-old β-catenin(ex3)Co12ER mice (Fig. 2B). Histomorphometric analysis revealed that the total cartilage area and total cartilage thickness were significantly decreased in β-catenin(ex3)Co12ER mice compared to age-matched Cre-negative control mice. The total cartilage area decreased 22 % in β-catenin(ex3)Co12ER mice compared to their Cre-negative littermate controls at 1 month of age, and decreased 41 % and 48% in 3- and 6-month-old β-catenin(ex3)Co12ER mice, respectively (Fig. 2C). These results were consistent with the quantification of total cartilage thickness. Cartilage thickness decreased 21 %, 39 % and 40 % in β-catenin(ex3)Co12ER mice at 1, 3 and 6 months of age, respectively, compared to Cre-negative controls (Fig. 2D).
Fig. 2.
Activation of β-catenin induces a TMJ OA-like phenotype. (A) Tamoxifen was administered into 2-week-old β-catenin(ex3)Co12ER mice and Cre-negative control littermates (1 mg/10 g body weight, i.p., daily for 5 d). TMJ samples were harvested when the mice were 1-, 3- or 6-month-old and Alcian blue/H&E staining was performed. Histology results showed increased cartilage degradation in 1-, 3- and 6-month-old β-catenin(ex3)Co12ER mice compared to Cre-negative control mice. Large numbers of hypertrophic chondrocytes were identified in 1- and 3-month-old β-catenin(ex3)Co12ER mice (yellow arrows). Severe subchondral sclerosis was found in 6-month-old β-catenin(ex3)Co12ER mice (green arrow). Reduction in cartilage thickness in 1-, 3- and 6-month-old β-catenin(ex3) co12ER mice is indicated by red bars. (B) µCT images of 3- and 6-month-old β-catenin(ex3)Co12ER mice showed decreased disc space in β-catenin(ex3)Co12ER mice (red arrows) compared to control mice. (C) Total TMJ cartilage area was quantified by tracing the Alcian blue-positive area. Cartilage area decreased 22 %, 41 % and 48 % in 1-, 3- and 6- month-old β-catenin(ex3)Co12ER mice compared to the same aged control mice (*p < 0.05, unpaired Student t-test, n = 5). (D) Total cartilage thickness was quantified by tracing the Alcian blue-positive thickness in the centre of the TMJ cartilage. Total cartilage thickness decreased 21 %, 39 % and 40 % in 1-, 3- and 6-month-old β-catenin(ex3)Co12ER mice compared to the same aged control mice (*p < 0.05, unpaired Student t-test, n = 5).
Histology and IHC analyses of β-catenin(ex3)Co12ER mice
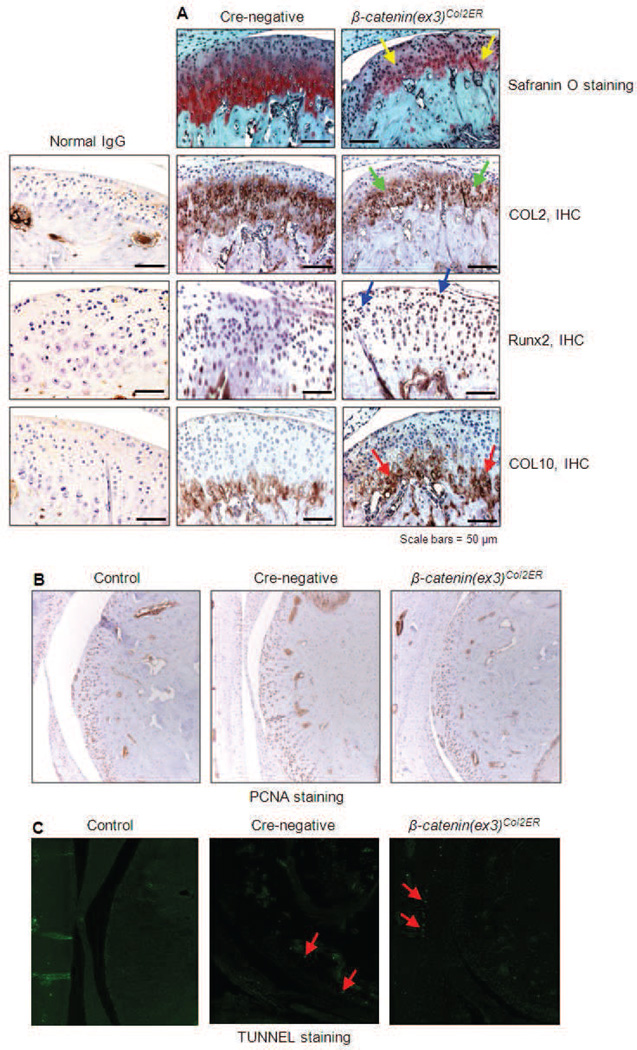
To further investigate OA related pathological alterations in β-catenin(ex3)Co12ER mice, Safranin O/Fast green (SO/ FG) staining was performed to assess changes in aggrecan levels in 3-month-old mice. Although the integrity of the TMJ articular surface was maintained, aggrecan expression as indicated by red staining was reduced in β-catenin(ex3)Co12ER mice, compared to Cre-negative controls (Fig. 3A). Immunohistochemistry (IHC) revealed decreased Co12 expression in the β-catenin(ex3)Co12ER mice. In addition, IHC results also showed significantly increased Runx2 and Co100 expression in β-catenin(ex3)Co12ER mice compared to controls, indicating the chondrocytes underwent hypertrophy at this stage (Fig. 3A). The result of PCNA staining showed that there were no significant changes in PCNA staining in TMJ tissues of β-catenin(ex3)Co12ER mice, suggesting that overexpression of β-catenin in TMJ tissue did not affect TMJ cell proliferation (Fig. 3B). Data of TUNEL staining demonstrated an increase in numbers of apoptotic cells compared to that in control mice, but did not reach statistical significance between β-catenin(ex3)Co12ER and control mice (Fig. 3C). Taken in concert, these results indicate that conditional activation of β-catenin in the TMJ induces degenerative defects that may not be due to changes in cell proliferation and apoptosis.
Fig. 3.
Alterations in matrix protein expression, cell proliferation and apoptosis in β-catenin(ex3)Co12ER mice. Tamoxifen was administered when β-catenin(ex3)Co12ER and Crenegative control mice were 2-week-old (1 mg/10 g body weight, i.p., daily for 5 d). TMJ cartilage samples were harvested at 3-month-old. (A) Safranin O/fast green staining was performed to evaluate aggrecan levels. Results showed that aggrecan levels were significantly reduced in β-catenin(ex3)Co12ER mice (yellow arrows). IHC was performed to detect changes in Co12, Runx2 and COL10 protein expression β-catenin(ex3)Co12ER mice. IHC results showed that Co12 expression was significantly decreased (green arrows), and expression of Runx2 (blue arrows) and COL10 (red arrows) was significantly increased in β-catenin(ex3)Co12ER mice. PCNA (B) and TUNNEL (C) staining was performed in TMJ tissues derived from Cre-negative control and β-catenin(ex3)Co12ER mice. Results showed that there were no significant changes in cell proliferation (PCNA staining) and apoptosis (TUNNEL staining) in β-catenin(ex3)Co12ER mice.
Gene expression analysis of β-catenin(ex3)Co12ER mice
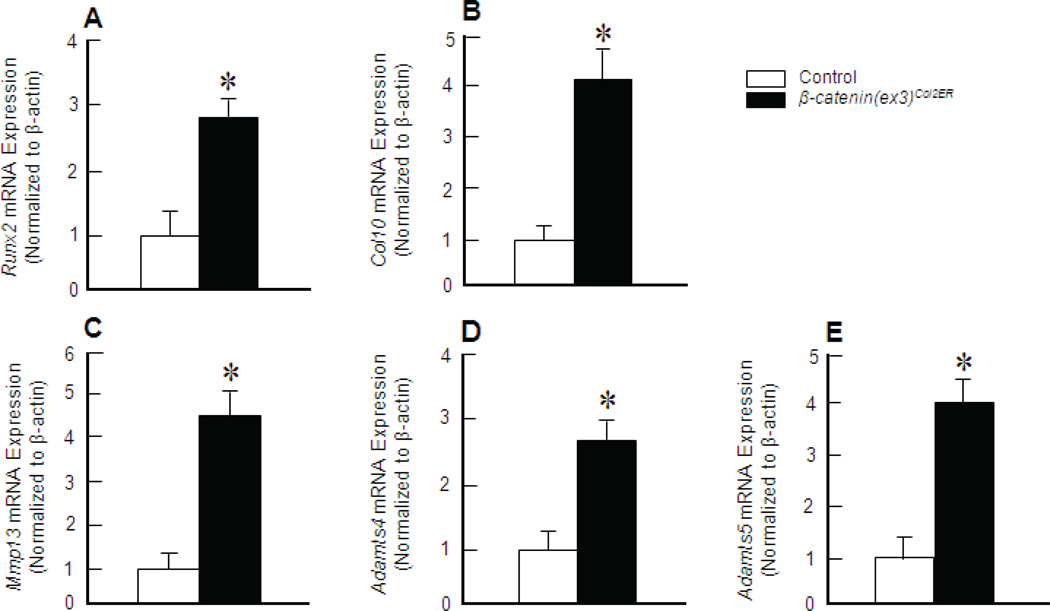
To determine changes in gene expression in the TMJ cartilage, cells were isolated from TMJ cartilage of 1-month-old mice. RT-PCR results were consistent with IHC findings. Runx2 (Fig. 4A) and Co110 (Fig. 4B) expression were increased 2.8 and 4.1 fold, respectively, in β-catenin(ex3)Co12ER mice compared to control mice. Furthermore, in β-catenin(ex3)Co12ER mice, the expression of genes encoding cartilage degrading enzymes, such as Mmp13 (Fig. 4C), Adamts4 (Fig. 4D), and Adamts5 (Fig. 4E) was increased 4.5,2.7 and 4.0 fold, respectively. These results suggest that the activation of β-catenin signalling could lead to chondrocyte hypertrophy and degenerative defects.
Fig. 4.
Alterations in gene expression in β-catenin(ex3)Co12ER mice. Total RNA was extracted from TMJ tissues from 4-week-old β-catenin(ex3)Co12ER mice and Cre-negative control mice (pooled from 4 mice) and real-time PCR analysis was performed. The results showed that expression of Runx2 (A), Co100 (B), Mmp13 (C),Adamts4 (D), and Adamts5 (E) was significantly increased 2.8, 4.1, 4.5, 2.7 and 4.0 fold, respectively, in TMJ tissues derived from β-catenin(ex3)Co12ER mice (*p < 0 05, unpaired Student t-test, n = 3).
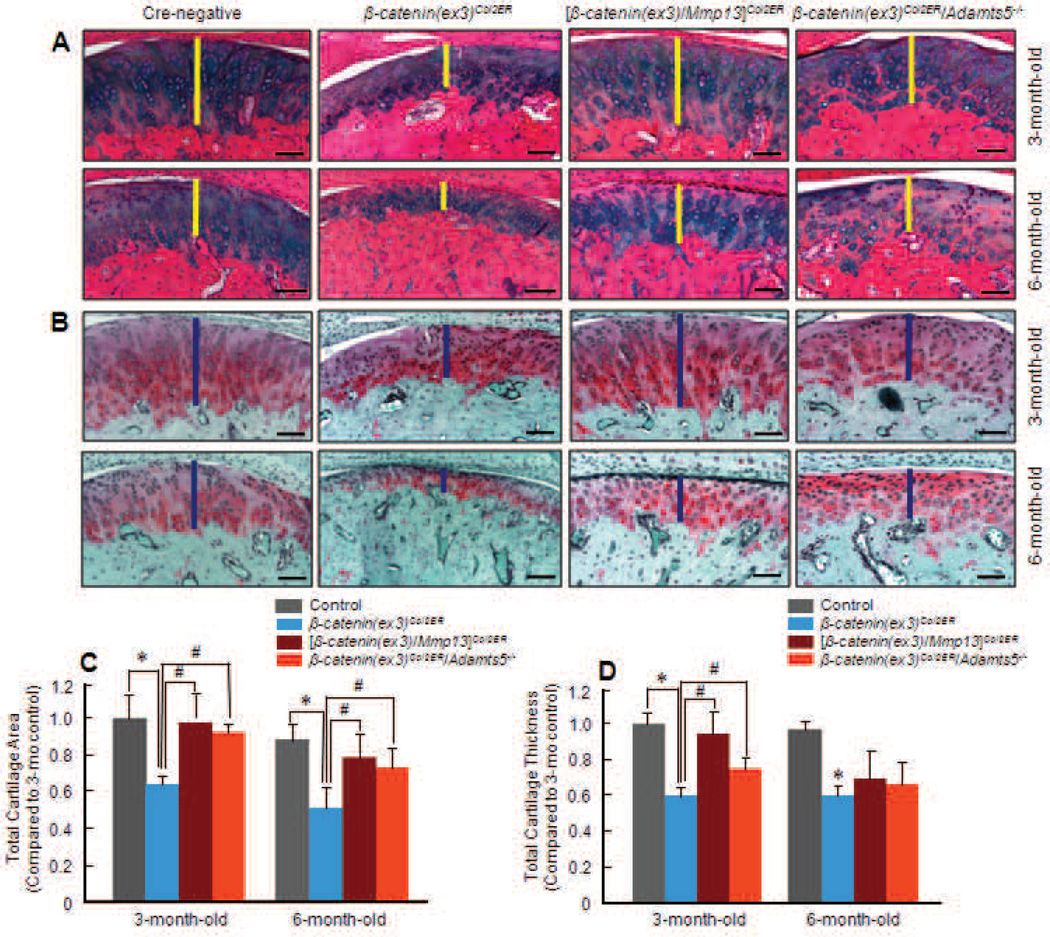
Ameliorated TMJ defects by deletion of the Mmp13 or Adamts5 gene
To assess the roles of MMP13 and Adamts5 in the development of TMJ defects, β-catenin(ex3)/Mmp13)co12ER and β-catenin(ex3)Co12ER/Adamts5−/− double mutant mice were generated (Tables 2 and 3). Tamoxifen was administered when the mice were 2-week-old and histology analysis was performed. In 3-month-old mice, TMJ defect progression was markedly decelerated in both β-catenin(ex3)/Mmp13)Co12ER and β-catenin(ex3)Co12ER/Adamts5−/− double mutant mice (Figs. 5A and B) compared to β-catenin(ex3)Co12ER mice (Fig. 2A). Histology results showed a greater amount of intact cartilage and more pronounced Alcian blue and SO/FG staining in the double mutant mice, indicating higher collagen and aggrecan content in the cartilage. Results of histomorphometry also showed that TMJ defects were decelerated after deletion of either the Mmp13 or Adamts5 gene in β-catenin(ex3)Co12ER mice (Figs. 5C and D). Compared to the 3-month-old β-catenin(ex3)Co12ER mice, the loss of cartilage area was reversed 33 and 28 % in β-catenin(ex3)/Mmp13)Co12ER and β-catenm(ex3)Co12ER/Adamts5−/− double mutant mice. Compared to the 6-month-old β-catenin(ex3)Co12ER mice, the loss of cartilage area was reversed 26 and 21 % in these double mutant mice (Table 5). Detailed quantitative analyses revealed that there was a 41 % cartilage loss in 3-month-old β-catenin(ex3)Co12ER mice. However, only a 8 and 13 % cartilage area decrease was observed in 3-month-old (β-catenin(ex3)/Mmp13)co12ER and β-catenin(ex3)Co12ER/Adamts5−/− double mutant mice compared to Cre-negative controls (Fig. 5C and Table 5). In 6-month-old mice, there was a 48 % cartilage loss in β-catenin(ex3)Co12ER mice. In contrast, only 22 and 27 % of the cartilage area decreased in (β-catenin(ex3)/Mmp13)Co12ER and β-catenin(ex3)Co12ER/Adamts5−/− double mutant mice compared to Cre-negative controls (Fig. 5C and Table 5). These results demonstrated that the deletion of either Mmp13 or Adamts5 gene can significantly reverse β-catemn activation-induced TMJ cartilage loss. Quantification of cartilage thickness showed similar results (Fig. 5D). There was a 39 % decrease in cartilage thickness in β-catenin(ex3)Co12ER mice. However, only a 6 and 25 % decrease in cartilage thickness was observed in 3-month-old double mutant mice. In 6-month-old mice, there was a 40 % decrease in cartilage thickness in β-catenin(ex3)Co12ER mice In contrast, only a 30 and 34 % decrease in cartilage thickness was observed in 6-month-old double mutant mice (Fig. 5D and Table 5). Statistical analysis results demonstrated a significant decrease in cartilage thickness in 3-month-old β-catenin(ex3)Co12ER mice. Compared to β-catenin(ex3)Co12ER mice, the reduction of cartilage thickness was significantly reversed in 3-month-old double mutant mice (Fig. 5D).
Table 2.
Breeding and production of β-catenin(ex3)/Mmp13)Co12ER mice.
| Breeding | Desired progeny |
|---|---|
| a) Co12CreERT2 x Mmp13flox/flox | Co12CreERT2;Mmp13flox/+ |
| b) Co12CreERT2;Mmp13flox/+ x Mmp13flox/flox | Mmp13Co12ER |
| c) Mmp13flox/flox x β-catenin(ex3)flox/flox | β-catenin(ex3)flox/+;Mmp13flox/+ |
|
d)
β-catenin(ex3)flox/+;Mmp13flox/+ x β-catenin(ex3)flox/+;Mmp13flox/+ |
β-catenin(ex3)flox/flox;Mmp13flox/flox |
|
e)
Co12CreERT2;Mmp13flox/flox x β-catenin(ex3)flox/flox;Mmp13flox/flox |
β-catenin(ex3)IMmp13)Co12ER and β-catenin(ex3)flox/+;Mmp13flox/flox |
Fig. 5.
OA-like phenotype was decelerated when either the Mmp13 or the Adamts5 gene was deleted in β-catenin(ex3) Co12ER mice. Tamoxifen was administered into 2-week-old mice (0.1 mg/g body weight, i.p., daily for 5 d). TMJ samples were harvested from 3- or 6-month-old mice and Alcian blue/H&E and SO/FG staining were performed. Alcian blue/H&E staining (A) and SO/FG staining (B) showed decelerated TMJ defect progression and more aggrecan contents in double mutant mice compared to those in β-catenin(ex3)Co12ER mice. (C) Total TMJ cartilage area was quantified by tracing the Alcian blue-positive area. Cartilage areas were 8 and 13 % decreased in 3-month-old (β-catenin(ex3)/Mmp13)Co12ER and β-catenin(ex3)Co12ER/Adamts5−/− double mutant mice, and were 22 and 27 % decreased in 6-month-old (β-catenin(ex3)/Mmp13)Co12ER mice and β-catenin(ex3)Co12ER/Adamts5−/− mice compared to the same age control mice (*p < 0.05, compared to Cre-negative control group; **p < 0.05, compared to β-catenin(ex3) Co12ER group; one-way ANOVA followed by Dunnett’s test, n = 5). (D) Total cartilage thickness was quantified by tracing the Alcian blue-positive thickness in the centre of the TMJ cartilage. Total cartilage thickness was decreased 6 and 25 % in 3-month-old double mutant mice and 30 and 34 % decreased in 6-month-old double mutant mice compared to the same age control mice (*p < 0.05, compared to Cre-negative control group; **p< 0.05, compared to β-catenin(ex3)Co12ER group; one-way ANOVA followed by Dunnett’s test, n = 5).
Table 5.
Deletion of the Mmp13 or Adamts5 gene reverses the TMJ OA phenotype observed in 3- and 6-month-old β-catenin(ex3)Co12ER mice.
| Mouse group | Cartilage area* | % Area recovery¶ | Cartilage thickness* | % Thickness recovery¶ |
|---|---|---|---|---|
|
β-catenin(ex3)Co12ER (3-month-old) |
59 % | 61 % | ||
| [β-catenin(ex3)/Mmp13]Co12ER (3-month-old) |
92 % | 33 % | 94 % | 33 % |
|
β-catenin(ex3)Co12ER/Adamts5−/− (3-month-old) |
87 % | 28 % | 75 % | 14 % |
|
β-catenin(ex3) Co12ER (6-month-old) |
52 % | 60 % | ||
| [β-catenin(ex3)/Mmp13]Co12ER (6-month-old) |
78 % | 26 % | 70 % | 10 % |
|
β-catenin(ex3)Co12ER/Adamts5−/− (6-month-old) |
73 % | 21 % | 66 % | 6 % |
Compared to the Cre-negative littermate controls.
Compared to β-catenin(ex3)Co12ER mice.
Discussion
Although TMJ OA is a common degenerative joint disease, the pathological mechanisms of this disease remain largely unknown. In this study, we generated a genetic mouse model and demonstrated that overexpression of β-catenin in TMJ chondrocytes leads to degenerative defects resembling an OA-like phenotype in the TMJ cartilage. Furthermore, deletion of the Mmp13 or Adamts5 gene in β-catenin(ex3)Co12ER mice significantly and partially rescued TMJ defects observed in β-catenin(ex3)Co12ER mice.
It has been shown that β-catenin protein expression was highly-upregulated in articular cartilage tissues from patients with knee OA (Zhu et al., 2009) and in intervertebral disc tissues from patients with degenerative disc disease (Wang et al., 2012). Co12-driven cartilage-specific activation of β-catenin in mice leads to a knee joint OA-like phenotype and defects in intervertebral disc tissues (Zhu et al., 2009; Wang et al., 2012). Here, we further demonstrated that Co12-driven β-catenin(ex3)Co12ER mice also showed TMJ degenerative defects. Even though articular cartilage in the knee joint is hyaline cartilage, and IVD tissue and TMJ cartilage are composed of both hyaline cartilage and fibrocartilage, the mechanisms of degeneration in these tissues are very similar. Moreover, in our study we specifically determine the role of β-catemn signalling in Co12-Cre targeting cells. Among articular cartilage, TMJ cartilage and disc tissues, it seems that knee joint articular cartilage was most efficiently targeted by Co12-Cre due to high Co12 expression in this tissue compared to IVD tissue and TMJ cartilage (Chen et al., 2007; Zhu et al., 2008; Jin et al., 2011; Fig. 1).
To determine changes in gene expression in TMJ tissues with degenerative defects in β-catenin(ex3)Co12ER mice, we isolated TMJ cartilage tissues. We found that RT-PCR results are consistent with the SO/FG and IHC results. Runx2 and Co110 are two hypertrophic markers in cartilage during OA development, and their expression levels were increased in β-catenin(ex3)Co12ER mice. The RT-PCR results also revealed increases in the expression of genes encoding collagenase (MMP13) and aggrecanases (Adamts4 and Adamts5) in β-catenin(ex3) co12ER mice. It has been shown that MMP13 and Adamts5 are the primary enzymes leading to cartilage degeneration (Neuhold et al., 2001; Glasson et al., 2005; Wang et al., 2013), and Adamts5 may play a more crucial role in cartilage degradation compared to Adamts4 (Glasson et al., 2004). We thus generated (β-catenin(ex3)/Mmp13)Co12ER and β-catenin(ex3)Co12ER/Adamts5−/− double mutant mice to determine roles of MmP13 and Adamts5 in β-catenin signalling during TMJ tissue degeneration. Our results showed decelerated TMJ defects in both of these double mutant mice. Although deletion of the Mmp13 or Adamts5 gene significantly decreased TMJ defects observed in β-catenin(ex3)Co12ER mice, normal cartilage phenotype was not completely restored by deletion of either Mmp13 or Adamts5. There was still a significant loss of proteoglycans in these double mutant mice compared to Cre-negative controls. The possible reason to account for this could be that MmP13,Adamts4 and Adamts5., as well as other catabolic factors, contribute to OA development (Stanton et al., 2005; Glasson et al., 2005; Majumdar et al., 2007), and deletion of only one of them may not be able to completely inhibit cartilage degeneration.
Moreover, our results indicate that the deletion of the Mmp13 gene in the β-catenin(ex3) Co12ER background seems more effective in preventing OA progression than deletion of the Adamts5 gene. This is probably because MMP13 preferentially cleaves the proteolysis of Co12, which is the most abundant matrix protein in the TMJ cartilage. MMP13 also targets aggrecan, types IV and IX collagens, gelatin, osteonectin and perlecan in cartilage for degeneration (Shiomi et al., 2010). In contrast, Adamts5 is an aggrecanase that is mainly responsible for aggrecan degradation. Therefore, deletion of the Mmp13 gene is likely to prevent the degradation of more substrates responsible for cartilage degeneration compared to deletion of Adamts5.
Recent reports demonstrated that β-catenin signalling is responsive to mechanical loading (Lin et al., 2009; Premaraj et al., 2013; Javaheri et al., 2014). Abnormal occlusion could lead to OA development in TMJ tissue (Zhang et al., 2013; Liu et al., 2014). We hypothesise that one of the major upstream regulatory factors for β-catemn signalling in TMJ tissue is mechanical loading. In the future, we will examine if abnormal occlusion (causing mechanical changes in TMJ tissue) will induce β-catemn signalling activation in TMJ tissue. In addition to mechanical loading, growth factors involved in inflammation, such as tumour necrosis factor-α (TNF-α) and mterleukin 1β (IL-1β) could be potentially important upstream regulators for β-catenin signalling in TMJ tissues.It has been reported that TNF-α and IL-1β regulate β-catenin signalling in other tissues (Kitazawa et al., 2011; Raymond et al., 2012; Gong et al., 2014). Regarding the mechamsm of β-catenin overexpression-induced defects in TMJ tissue, we propose that one of the major downstream mediators for β-catemn signalling in the TMJ tissue is Runx2. Activation of β-catenin causes up-regulation of Runx2, which subsequently promotes Mmp13 and Adamts5 expression in TMJ tissues (Figs. 3 and 4; Wang et al., 2012).
It has been shown that conditional activation of β-catenin in knee cartilage and intervertebral disc cartilage induce knee OA and disc tissue degeneration. β-catemn plays a key role in the activation of downstream target genes, such as Mmp13, Adamts4 and Adamts5 in cartilage, leading to the development of knee OA and disc degeneration (Wang et al., 2012). The current study is the first to demonstrate that the same signalling mechanism can also induce TMJ cartilage degradation. The potential mechanisms for β-catemn activation in patients with TMJ OA may include activating mutations of the β-catenin gene (Morin et al., 1997; Simon et al., 2005; Jamieson et al., 2004; Derksen et al., 2004), mechanical loading (Hens et al., 2005; Robinson et al., 2006; Sen et al., 2009) and inflammation (Johnson, 2009; Kaler et al., 2009). In summary, our studies suggest that β-cateninmay play a role in TMJ cartilage function and may be a mediator in TMJ OA development. Mmp13 and Adamts5 are important downstream targets of β-catenin signalling in TMJ chondrocytes during OA development.
Acknowledgments
This work was supported by Grants R01 AR055915 and R01 AR054465 to D.C. from the National Institutes of Health. The authors thank Verhonda Hearon-Eggleston for assistance in preparing the manuscript. The authors declare that no competing interests exist, and that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O’Keefe RJ, Chen D. Generation of a transgenic mouse mode wrth chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bont LG, van der Kuijl B, Stegenga B, Vencken LM, Boering G. Computed tomography in differential diagnosis of temporomandibular joint disorders. Int J Oral Maxillofac Surg. 1993;22:200–209. doi: 10.1016/s0901-5027(05)80636-8. [DOI] [PubMed] [Google Scholar]
- de Leeuw R. Orofacial Pain: Guidelines for Assessment, Diagnosis and Management. 4th. Chicago: Quintessence Publishing; 2008. [Google Scholar]
- Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, van der Neut R, Spaargaren M, Pals ST. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, Flannery CR, Kanki K, Wang E, Peluso D, Yang Z, Majumdar MK, Morris EA. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50:2547–2558. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Gong M, Liu C, Zhang L, Zhang H, Pan J. Loss of the TNFα function inhibits Wnt/β-catemn signaling, exacerbates obesity development in adolescent spontaneous obese mice. Mol Cell Biochem. 2014;391:59–66. doi: 10.1007/s11010-014-1987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro . J Bone Miner Res. 2005;20:1103–1113. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- Hirschmann PN, Shuttleworth CA. The collagen composition of the mandibular joint of the foetal calf. Arch Oral Biol. 1976;21:771–773. doi: 10.1016/0003-9969(76)90069-8. [DOI] [PubMed] [Google Scholar]
- Iwasaki LR, Crosby MJ, Marx DB, Gonzalez Y, McCall WD, Jr, Ohrbach R, Nickel JC. Human temporomandibular joint eminence shape and load minimization. J Dent Res. 2010;89:722–727. doi: 10.1177/0022034510364492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Javaheri B, Stern AR, Lara N, Dallas M, Zhao H, Liu Y, Bonewald LF, Johnson ML. Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2014;29:705–715. doi: 10.1002/jbmr.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Shen J, Wang B, Wang M, Shu B, Chen D. TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett. 2011;585:1209–1215. doi: 10.1016/j.febslet.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. TNF-induced activation of pulmonary microvessel endothelial cells: a role for GSK3beta. Am J Physiol Lung Cell Mol Physiol. 2009;296:L700–L709. doi: 10.1152/ajplung.90566.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–3902. doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Cheng D, Tsukamoto MR, Koike MA, Wes PD, Vasilevko V, Cribbs DH, LaFerla FM. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J Immunol. 2011;187:6539–6549. doi: 10.4049/jimmunol.1100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knäuper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Tanimoto K, Izawa T, Fujihara S, Koolstra JH, Tanaka E. Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthritis Cartilage. 2009;17:1408–1415. doi: 10.1016/j.joca.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- Liu YD, Liao LF, Zhang HY, Lu L, Jiao K, Zhang M, Zhang J, He JJ, Wu YP, Chen D, Wang MQ. Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis. Osteoarthritis Cartilage. 2014;22:302–312. doi: 10.1016/j.joca.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luder HU, Leblond CP, von der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 1988;182:197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, Morris EA, Glasson SS. Double- knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670–3674. doi: 10.1002/art.23027. [DOI] [PubMed] [Google Scholar]
- McCulloch DR, Goff CL, Bhatt S, Dixon LJ, Sandy JD, Apte SS. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns. 2009a;9:314–323. doi: 10.1016/j.gep.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009b;17:687–698. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi I, Takahashi I, Nakamura M, Sasano Y, Sato S, Kagayama M, Mitani H. An immunohistochemical study of regional differences in the distribution of type I and type II collagens in rat mandibular condylar cartilage. Arch Oral Biol. 1996;41:863–869. doi: 10.1016/s0003-9969(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premaraj S, Souza I, Premaraj T. Focal adhesion kinase mediates β-catemn signaling mpenodontal ligament cells. Biochem Biophys Res Commun. 2013;439:487–492. doi: 10.1016/j.bbrc.2013.08.097. [DOI] [PubMed] [Google Scholar]
- Rando C, Waldron T. TMJ osteoarthritis: A new approach to diagnosis. Am J Phys Anthropol. 2012;148:45–53. doi: 10.1002/ajpa.22039. [DOI] [PubMed] [Google Scholar]
- Raymond M, Marchbank T, Moyer MP, Playford RJ, Sanderson IR, Kruidemer L. IL-1β stimulation of CCD-18co myofibroblasts enhances repair of epithelial monolayers through Wnt-5a. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1270–G1278. doi: 10.1152/ajpgi.00458.2011. [DOI] [PubMed] [Google Scholar]
- Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes: A role in osteoarthritis. J Clin Invest. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach HI, Yamada N, Cheung KS, Tilley S, Clarke NM, Oreffo RO, Kokubun S, Bronner F. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta- catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med. 2008;35:2693–2705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem. 2009;284:34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi T, Lemaître V, D’Armiento J, Okada Y. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in nonneoplastic diseases. Pathol Intä. 2010;60:477–496. doi: 10.1111/j.1440-1827.2010.02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Grandage VL, Linch DC, Khwaja A. Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene. 2005;24:2410–2420. doi: 10.1038/sj.onc.1208431. [DOI] [PubMed] [Google Scholar]
- Singh M, Detamore MS. Tensile properties of the mandibular condylar cartilage. J Biomech Eng. 2008;130:11009–11017. doi: 10.1115/1.2838062. [DOI] [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro . Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z. Altered endochondral bone development in matrix metalloprotemase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shen J, Jin H, Im HJ, Sandy J, Chen D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;240:61–69. doi: 10.1111/j.1749-6632.2011.06258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Tang D, Shu B, Wang B, Jin H, Hao S, Dresser KA, Shen J, Im HJ, Sampson ER, Rubery PT, Zuscik MJ, Schwarz EM, O’Keefe RJ, Wang Y, Chen D. Conditional activation of β-catemn signaling leads to severe defects in intervertebral disc tissue. Arthritis Rheum. 2012;64:2611–2623. doi: 10.1002/art.34469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Sampson ER, Jin H, Li J, Im H-J, Chen D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Jin H, Zhu M, Li J, Zhao L, Zhang Y, Tang D, Xiao G, Xing L, Boyce BF, Chen D. Chondrocyte β-catemn signaling regulates postnatal bone remodeling through modulation of osteoclast formation in a murine model. Arthritis Rheumatol. 2014;66:107–120. doi: 10.1002/art.38195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Wnts and Wing: Wnt signaling in vertebrate limb development and musculoskeletal morphogenesis. Birth Defects Res C. 2003;69:305–317. doi: 10.1002/bdrc.10026. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jiao K, Zhang M, Zhou T, Liu X-D, Yu S-B, Lu L, Jing L, Yang T, Zhang Y, Chen D, Wang M-Q. Occlusal effects on longitudinal bone alterations of the temporomandibular joint. J Dent Res. 2013;92:253–259. doi: 10.1177/0022034512473482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Chen M, Lichtler AC, O’Keefe RJ, Chen D. Tamoxifen-inducible Cre-recombination in articular chondrocytes of adult Co12a1CreERT2 transgenic mice. Osteoarthritis Cartilage. 2008;16:129–130. doi: 10.1016/j.joca.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O’Keefe RJ, Zuscik M, Chen D. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]