Abstract
Study Objectives:
Sleep breathing disorder (SBD) may be an important factor in age-related cognitive decline. In a cohort of healthy elderly subjects, we performed an 8-y longitudinal study to assess whether changes in cognitive function occur in untreated elderly patients with SBD and without dementia and the factors implicated in these changes.
Design:
A population-based longitudinal study.
Setting:
Clinical research settings.
Participants:
A total of 559 participants of the PROOF study aged 67 y at the study entry and free from neurological disorders were examined.
Interventions:
N/A.
Measurements and Results:
Abnormal breathing events were defined by an apnea-hypopnea index (AHI) > 15. The raw cognitive data and averaged Z-scores for the attentional, executive, and memory functions were collected at the baseline and follow-up. At baseline, AHI > 15 was found in 54% of subjects with 18% having an AHI > 30. At follow-up, the presence of abnormal breathing events was associated with a slight but significant decline in the attentional domain (P = 0.01), which was more evident in the subjects with an AHI > 30 (P = 0.004). No significant changes over time were observed in the executive and memory functions. Several indices of chronic hypoxemia, defined either as a cumulative peripheral oxygen saturation (SpO2) < 90% or a minimal SpO2, accounted for portions of the variance in the decline in attention. All observed effects were small, accounting for 4–7% of variance in multivariate models.
Conclusions:
In healthy elderly subjects, various components of sleep breathing disorder at baseline were associated with small changes in selected cognitive functions specific to the attention domain after controlling for multiple comorbidities, such as sleepiness, hypertension, diabetes, anxiety, and depression.
Clinical Trial Registration:
ClinicalTrials.gov identifiers NCT 00759304 and NCT 00766584.
Citation:
Saint Martin M, Sforza E, Roche F, Barthélémy JC, Thomas-Anterion C, PROOF study group. Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. The proof-synapse cohort. SLEEP 2015;38(2):179–187.
Keywords: cognitive functions, elderly, hypoxemia, sleep breathing disorders, sleep fragmentation
INTRODUCTION
Despite differences in research methodology and disease definition, the prevalence of sleep breathing disorders (SBD) is consistently high across different populations. In a representative sample of adults from the state of Wisconsin,1 SBD was present in 9% of women and 24% of men aged 30–60 y, with a tendency toward a greater prevalence in the elderly. Although the results of some studies in elderly subjects are conflicting,2–4 recent data on subjects aged older than 50 y5,6 provided strong evidence that the presence and severity of SBD contribute to age-related cognitive impairment, particularly in the domains of attention and vigilance,7–10 as well as executive function,11,12 memory13,14 and the measurements of global intellectual efficiency to a lesser degree based on the Mini-Mental State examination (MMS).15,16
Whether cognitive dysfunctions in elderly individuals with SBD are primarily a consequence of SBD itself or whether they coexist independently is not known. Few longitudinal studies in healthy elderly subjects have explored the effect of untreated SBD on cognitive decline. In a 4.7-y longitudinal study of osteoporotic fractures17 in 298 82-y-old women, the authors found a relationship between the presence of SBD and the risk of mild cognitive impairment or dementia. However, only the MMS and the Trail Making Test Part B (TMT-B) were used at baseline and follow-up in this study.
Although the pathophysiology of these presumed cognitive deficits remains controversial,5,18 sleep fragmentation,19,20 the degree of intermittent nocturnal hypoxemia,17,21 and excessive daytime sleepiness22–24 have been described as major contributory factors.
The aim of this prospective study is to determine whether SBD-related factors, such as hypoxemic load, autonomic sleep fragmentation, or the severity of the apnea-hypopnea index (AHI), affect the longitudinal cognitive changes using extensive cognitive testing in a homogeneous population of healthy elderly subjects without dementia. We considered the effect of covariates that potentially influence cognitive decline such as sex, educational level, blood pressure, diabetes, obesity, daytime sleepiness, and anxiety or depression. The results of this study are expected to better define the potentially independent relationship between cognitive changes and the presence of SBD.
METHODS
Population
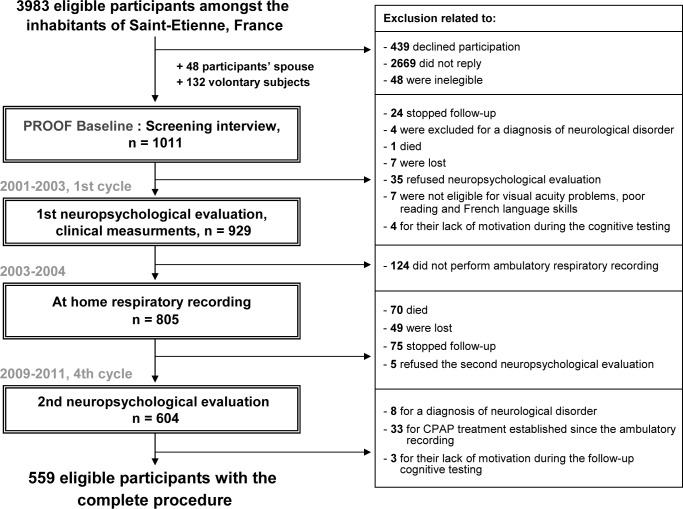
The participants consisted of a subset of individuals enrolled in a prospective study (the PROgnostic indicator OF cardiovascular and cerebrovascular events [PROOF]) to investigate the influence of the autonomic nervous system activity on cognitive functioning and cardiovascular and cerebrovascular morbidity. The details of the PROOF study have been previously described.4,25 The subjects were recruited from Saint-Etienne, France, from 2001 to 2003 and were eligible if they were 65 y old at the inclusion date. The exclusion criteria were defined as follows: previous myocardial infarction, arrhythmia, cardiac pacemaker, stroke, neurological or psychiatric disease, insulin-dependent diabetes, chronic obstructive pulmonary disease, cerebral magnetic resonance results suggesting neurological diseases or initial dementia, and current residence in institutions. Of the 3,983 eligible participants, the final study included 1,011 volunteers. Two ancillary studies addressing the association between SBD and cardiovascular and cerebrovascular events (SYNAPSE study) and the change in cognitive function (SIEMPRE study) over time were proposed to the participants. Figure 1 presents a flowchart illustrating the original and secondary studies.
Figure 1.
Diagram of the participants' inclusion procedure. CPAP, continuous positive airway pressure.
The cognitive evaluation of the SIEMPRE and PROOF population was introduced during the initial cycle (2001–2003) and repeated during cycle 4 (2009–2011). Nine hundred twenty-nine participants were enrolled for the first cognitive testing. At the fourth evaluation, 604 participants (66.4%) were reexamined. The reasons for lost follow-ups included the following: death (9.1%), ended participation in the follow-up (10.1%), moved away from the Saint-Etienne region, the current address was unavailable (8.1%), and refusal to perform the cognitive reassessment (6.3%). Of the final group that completed the follow-up, 33 participants who began continuous positive airway pressure therapy and 8 participants who received a diagnosis of dementia after the first examination were excluded.
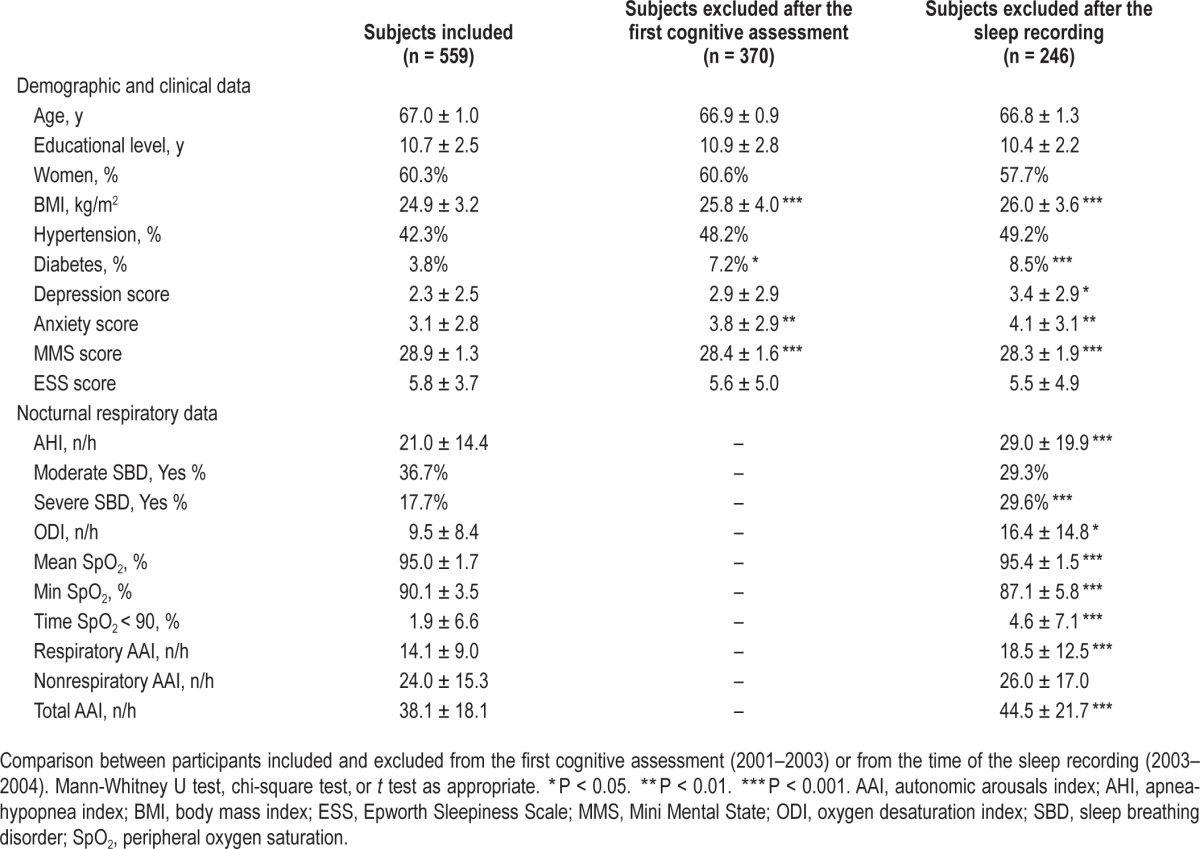
Of the original sample, 559 participants (337 women and 222 men) were eligible for this longitudinal study. Table 1 reports the clinical anthropometrics and nocturnal respiratory data for the final group and the groups of subjects who were excluded after the first evaluation or who refused follow-up testing. The final group examined in this study did not differ from the original sample in terms of age, sex, and vascular risk factors; the excluded or refusing participants differed by lower MMS scores (P = 0.002), obesity (P < 0.001) and high apnea and hypopnea indices (P < 0.001).
Table 1.
Characteristics at first evaluation for subjects included and excluded at follow-up.
The PROOF, SYNAPSE, and SIEMPRE studies were approved by the University Hospital and the IRB-IEC (CCPRB Rhône-Alpes Loire). The National Committee for Information and Liberty approved the data collection. All subjects provided written consent for participation in the study.
Neuropsychological Assessment
The measurements of cognitive performance were selected based on three considerations. First, a large battery of psycho-metric tests was administered to all subjects in identical sequence in order to broadly characterize the patients' cognitive abilities. The details of these neuropsychological tests have been previously described.26 Second, three cognitive domains were identified according to the neuropsychological testing and confirmed by a factor analysis. The “information processing speed and attentional performance domain” was assessed using the Trail Making Test Part A (TMT-A),27 the Stroop Color-Word Test (Parts I and II),28 and the Coding subtest of the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III).29 The “executive function domain” was assessed with the Trail Making Test Part B (TMT-B),27 the Stroop Color-Word Test (Part III, interference),28 a semantic fluency test using the letter “p,”30 a category fluency test using animal names,30 and the Similarities subtest of the WAIS-III.29 The “memory domain” was assessed using tests for the following subdomains: the visuospatial working memory was assessed using the Benton Visual Retention Test (BVRT form C)31 and the verbal episodic memory was evaluated with the Free and Cued Selective Reminding Test (FCSR).32 The global intellectual efficiency was assessed using the MMS.33 The results of two cognitive tasks previously mentioned in earlier papers,4,34 the Baddeley Memory Span and Tracking Dual Task test and the Digit Span Tests, were not analyzed in this longitudinal analysis, as they were not measured at follow-up.
Third, as previously described by our group,35 we constructed averaged Z-scores for each cognitive domain. The scores of each test at baseline and/or at follow-up were standardized to the Z-scores based on the distribution of the raw data at baseline. The Z-values were averaged to form composite measures of the attention, executive, and memory functions. Estimates of longitudinal change were computed for each of the three cognitive abilities by subtracting the averaged scores of cycle 4 from the averaged scores of cycle 1 (delta score, Δ).36 These values were the principal outcomes examined in this study.
At-Home Respiratory Recording
In all subjects, an unattended ambulatory nocturnal respiratory recording (HypnoPTT, Tyco Healthcare, Puritan Bennett, California, USA) was obtained, including sound, an electrocardiogram (ECG), pulse transit time (PTT), R-R timing, nasal pressure, respiratory effort, body position, and oxygen saturation (SpO2).37–41 To minimize a potential overestimation of sleep duration, the subjects completed a sleep diary in order to exclude wakefulness before lights-off from the analysis and to establish a subjective total sleep time. Hypopnea was defined as a 50% or greater reduction in the airflow from baseline lasting at least 10 sec that was associated with at least 3% oxygen desaturation. Apnea was defined as the absence of airflow on the nasal cannula lasting at least 10 sec. The AHI was established as the ratio of the number of apneas and hypopneas recorded per hour. An AHI > 15 with at least 85% of the events scored as obstructive was considered diagnostic of SBD.42 The cases were classified as mild (AHI between 15 and 30) and moderate to severe (AHI > 30).4 The indices of nocturnal hypoxemia included the mean SpO2 value, the minimal SpO2 value, the time with SpO2 < 90%, and the oxygen desaturation index (ODI) (i.e. the number of episodes of oxygen desaturation per hour of recording time during which the SpO2 decreased by 3% or more).
Definition of Autonomic Fragmentation by Pulse Transit Time Measurement
According to the manufacturer's instructions, the pulse transit time (PTT) was calculated as the time interval between the ECG R wave and a point on the pulse waveform (detected by a plethysmography finger probe) that is 50% of the height of the pulse wave. The ECG and pulse were sampled at 500 Hz. The PTT is typically approximately 250 msec and is measured to an accuracy of 2 msec. The PTT values available for every heartbeat were oversampled at 5 Hz. As previously reported,38,43–45 an autonomic arousal (AA) number and index were obtained from the continuously monitored PTT signals using the manufacturer's analysis software based on the assessment of the PTT shortening, which indicated autonomic activation. Two types of autonomic arousals were defined: the respiratory AA (RespirAA), when the shortening of the PTT occurred 2 sec before or after the end of the respiratory event; and the nonrespiratory AA (NRespirAA), when the AA occurs spontaneously without any association with snoring or respiratory event. The total AA index, the RespirAA index, and the NRespir AA index were calculated as the ratio of the number of each type of AA per hour of recording time.
Other Measurements
Clinical, Anthropometric, Vascular Measurements, Mood and Sleep Measurements at the First Evaluation
At the study entry, the clinical evaluation was assessed based on a structured interview including the history of cardiac and cerebrovascular disease, hypertension, diabetes, and neurological and psychiatric disorders. The average systolic and diastolic blood pressure (SBP and DBP) levels were measured with a 24-h ambulatory blood pressure monitor (ABPM) (Diasoft, Novavor, Rueil Malmaison, France). The subjects were defined as normotensive if they did not report a history of hypertension and antihypertensive treatment and did not have a systolic blood pressure > 130 mmHg and a diastolic blood pressure > 80 mmHg during the clinical 24-h ABPM measurement.46 The role of obesity was assessed via measurement of the body mass index (BMI), which was calculated as weight/height squared (kg/m2).
Mood Measurements
The depressive symptomatology was assessed using the Pichot (QD2A) questionnaire, a 10-item scale; a score > 7 is considered indicative of depressive symptoms.47 Anxiety was assessed using the French version of the Goldberg scale,48 a nine-item scale; a score ≥ 5 is considered indicative of anxiety.
Subjective Measures of Sleepiness
The effect of sleepiness during the day was evaluated using the Epworth Sleepiness Scale (ESS),49 and a score > 10 was considered indicative of excessive daytime sleepiness.
Statistical Analyses
The characteristics of the subjects were summarized as the mean ± standard deviation (SD) for the continuous variables and as the frequency and percentages of the sample for the categorical variables. We examined the gaussian distribution of the neuropsychological, clinical, and nocturnal respiratory variables using skew, kurtosis, and the Levene test for the equality of variances. The differences between the participants and the nonparticipants included in the study, between the men and the women, and between the three subgroups of subjects stratified according to the AHI severity were analyzed using parametric tests (t test, univariate analysis of variance [ANOVA]) or non-parametric tests (the Mann-Whitney U test, the Kruskal-Wallis test) for the continuous variables. The chi-square test was assessed for the categorical variables.
Concerning the cognitive data, we focused the analyses on the three cognitive averaged Z-scores at baseline and follow-up and on their changes at follow-up (Δ scores). First, we analyzed the Δ cognitive scores according to the AHI severity using ANOVA and a post hoc Bonferroni test. Multiple logistic regression analysis was performed for each cognitive domain, in which the dependent variable was the incidence of greater cognitive decline (the lowest 25th percentile for each Δ score) and the independent variable was the presence of mild (15 ≥ AHI ≤ 30) or moderate to severe (AHI > 30) forms of SBD. The reference group was the group with a normal AHI (AHI < 15). The final models were adjusted for demographic (sex, educational level, baseline age, number of years of follow-up) and baseline clinical data (BMI, ESS, hypertension, diabetes, anxiety, and depression).
Next, the relationship between individual measurements of hypoxia, autonomic sleep fragmentation, and disordered breathing (to overcome the multicolinearity bias) and each of the three cognitive Δ scores was examined with linear regression models. All models were adjusted for multiple demographic or clinical confounders collected at baseline.
The raw data obtained at baseline and follow-up, their Δ scores, as well as the correlation analysis with the respiratory measures are presented in Tables S1 and S2 (supplemental material).
All statistical analyses were conducted using the SPSS statistical software package (SPSS for Windows, version 17.0, SPSS, Chicago, IL, USA). After applying the Bonferroni correction for multiple comparisons, two-tailed P < 0.01 were considered statistically significant.
RESULTS
Clinical and Nocturnal Respiratory Data
The clinical, demographic, and nocturnal respiratory data obtained at the first cycle are summarized in Table 1. Five hundred fifty-nine participants (60% females) were followed for 7.8 ± 0.9 y; the participants were 66.9 ± 0.9 y old at the beginning of the study and 74.7 ± 1.0 y old at follow-up. The mean educational level of the subjects was 10.7 ± 2.5 y, the mean BMI was 24.9 ± 3.2 kg/m2, and the mean ESS score was 5.8 ± 3. Daytime sleepiness (ESS > 10) was observed in 79 subjects (14.1%). Of the participants, 42.3% had hypertension, and 3.8% had diabetes. Anxiety and depression were found in 37.9% and 4.1% of the subjects, respectively. The nocturnal respiratory data of the total sample are reported in Table 1. Overall, the mean AHI was 21.0 ± 14.4, with 36.7% of subjects having moderate abnormal respiratory events and 17.7% having an AHI > 30. The means ± SD of the indices of nocturnal hypoxemia were 9.5 ± 8.4 for the ODI, 95.0 ± 1.7% for the mean SpO2 value, 90.1 ± 3.5% for the minimal SpO2 value, and 1.9 ± 6.6 for the SpO2 < 90%. The mean RespirAAI was 14.1 ± 9.0.
The comparative analysis between men and women revealed that men were more frequently overweight (P < 0.001), and had higher blood pressure (P < 0.001), AHI (P < 0.001), ODI (P < 0.001), ESS (P < 0.001) and RespirAAI (P = 0.002) values. In contrast, women had higher anxiety (P < 0.001) and depression scores (P = 0.001). No sex differences were found for diabetes and hypertension.
The comparative analysis between the subjects with and without abnormal respiratory events (Table 2) showed that the participants with abnormal respiratory events were more frequently overweight (P < 0.001), had lower minimal SpO2 (P < 0.001), greater ODI, greater RespirAAI (P < 0.001), and higher total AAI scores (P < 0.001), and these differences were more evident in severe cases.
Table 2.
Clinical, neuropsychological, and nocturnal respiratory data at baseline according to the apnea-hypopnea index.

Cognitive Data
The means ± SD of the MMS were 28.9 ± 1.3 at the first assessment and 28.3 ± 1.7 at follow-up. Overall, the means ± SD of the averaged attentional, executive, and memory Δ Z-scores were −0.43 ± 0.71, −0.18 ± 0.46, and −0.21 ± 0.78, respectively. A more pronounced decline in the attentional Δ score was observed compared to the executive and memory Δ scores (P < 0.0001). A comparative analysis between the women and men did not reveal any significant sex differences in the scores in the attentional and executive domains, with the memory decline being significantly greater in women (women: −0.29 ± 0.82; men: −0.10 ± 0.62, P = 0.007).
Effect of the Severity of the Abnormal Respiratory Events
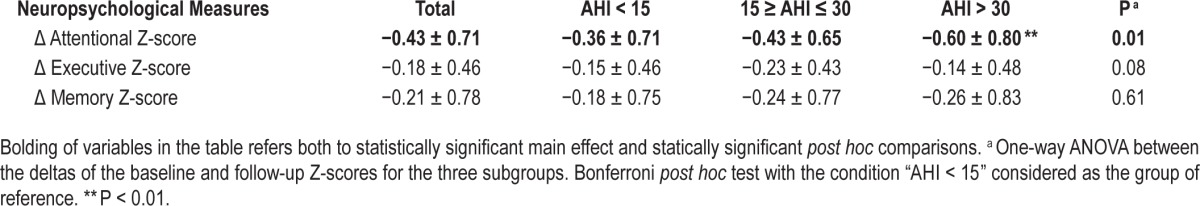
To assess the possible effect of the severity of abnormal respiratory events on cognitive changes, we compared the Z cognitive scores obtained at baseline and follow-up in the three subgroups of subjects stratified according to the AHI severity. Only a weak decline in the attention domain (P = 0.01) was observed at follow-up, and this decline was significantly higher (P = 0.004) in severe cases without significant changes in the executive (P = 0.08) and memory (P = 0.61) domains (Table 3).
Table 3.
Cognitive Z-score changes for the study population and the three subgroups of participants stratified according to the severity of the apneahypopnea index (mean ± standard deviation).
The multiple logistic regression analyses revealed that while mild cases (≥ 15 AHI ≤ 30) had no risk for attentional decline (odds ratio (OR) = 0.73, 95% confidence interval (CI) = 0.35–1.52, P = 0.40), moderate to severe cases (AHI > 30) were three times more likely to have a greater attentional decline (OR = 2.97; 95% CI = 1.45–6.10; P = 0.003).
Linear and Multivariate Regression Analyses
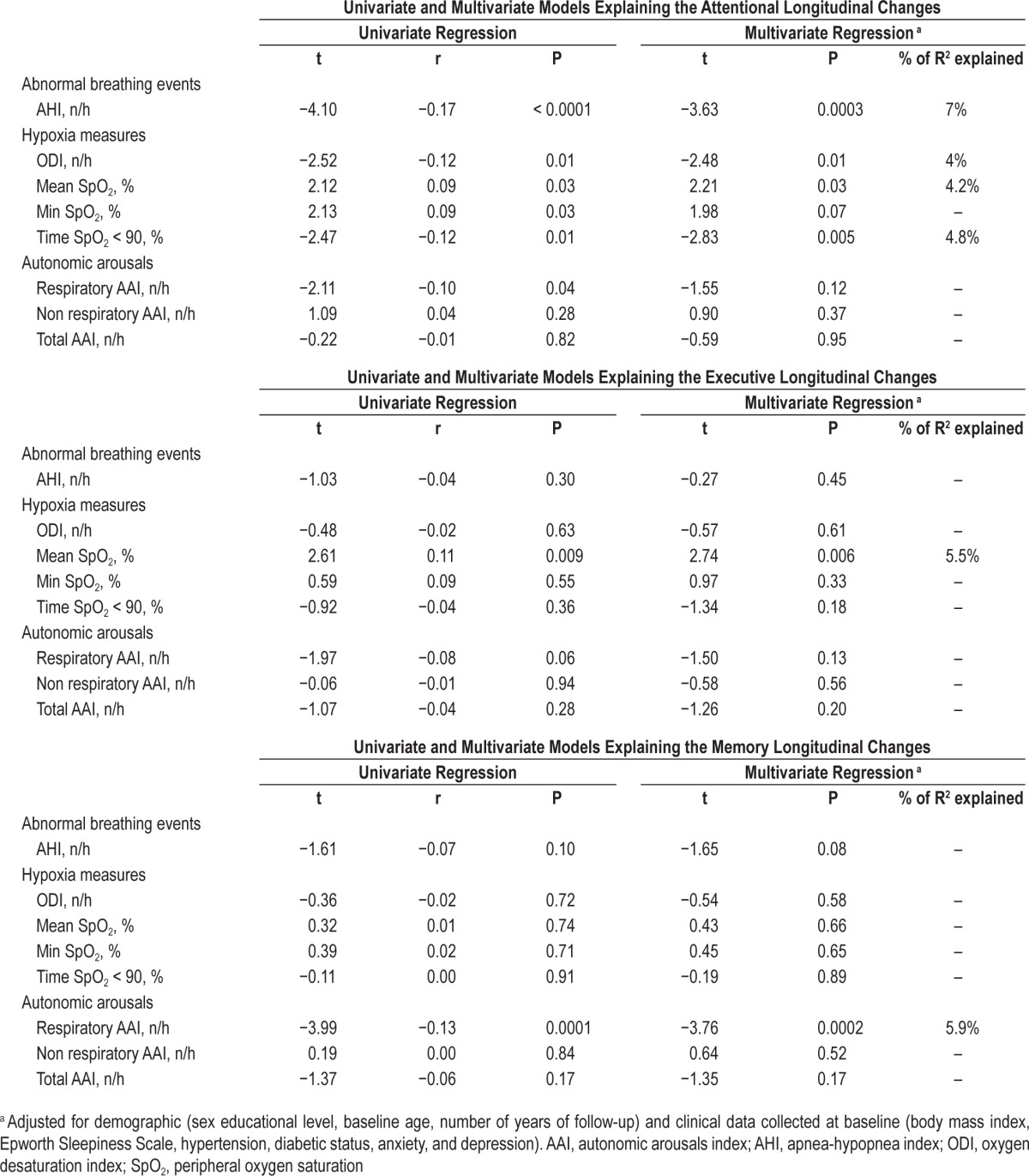
Table 4 reports the univariate and multivariate linear regression analyses for the changes in the three cognitive domains. The attentional Δ score was significantly related to the AHI (r = −0.17, P < 0.0001) and all indices of hypoxemia with a smaller effect of RespirAAI (r = −0.10, P = 0.04). After adjustment for confounders for the cognitive outcomes, the multivariate linear regression analysis showed a greater and significant effect of the AHI (P = 0.0003) and the time with oxygen desatu-ration < 90% (P = 0.005), which explained the 4.8–7% of the total variance. The executive Δ score was significantly related to the minimal SpO2 value (r = 0.11, P = 0.009) and the memory Δ score to the respiratory AA index (r = −0.13, P = 0.0001). The effect size estimates for the regression models that involve the executive and memory domains are presented only for heuristic value because the lack of statistically significant changes in these domains over time precludes the valid interpretation of such models.
Table 4.
Univariate and multivariate linear regression analyses between the nocturnal respiratory data at baseline and the longitudinal change in the three cognitive domains for the entire group.
DISCUSSION
In healthy elderly subjects without dementia, the presence of SBD was associated with a slight decline in the attention domain 8 y later without changes in the executive and memory functions. These attentional changes were related to the AHI, and to a lesser extent to hypoxia without contribution to autonomic arousal.
In contrast to data reported in middle-aged patients,3,8 the changes explained by the presence of SBD in the cognitive domains were small and limited to the attention domain. Moreover, the decline in attention (approximating 0.4 SD units) was associated with the AHI independent of other covariates known to be related to cognition, including sleepiness, hyper-tension, and diabetes.2,22,24,34 Although the changes in cognition represented small effect sizes, a role for SBD in longitudinal cognitive decline should always be considered in the clinical evaluation of elderly persons.
These results are somewhat compatible with a recent longitudinal actigraphic study50 of 700 elderly subjects aged 81.6 y that were reexamined 3.3 y later, in whom greater higher actigraphic levels of arousal were associated with an increased risk of developing Alzheimer disease. However, a recent paper on patients with mild SBD20 demonstrated that impaired memory consolidation processes were related to SBD. Using a relatively comprehensive battery of tests that encompassed executive function and memory, we could not show change over an 8-y period in these functions in our cohort. However, in the domain of attention, we found evidence that both abnormal breathing events and measures of hypoxia accounted for some of the variance in the longitudinal decline. Chronic intermittent hypoxemia,17,21,51–53 which causes both oxidative stress and inflammation, may be the major factor implicated in SBD-related cognitive dysfunction, and this finding appears to be particularly strong in middle-aged patients,11,12,54 whose SBD may reflect different pathophysiologies.51,57 The role of hypox emia in older elderly patients with SBD is controversial.16,17 Whereas Yaffe et al.17 stressed the role of hypoxemia in the dementia risk in a sample of 298 older women with SBD followed over a 3-y period, Cohen-Zion et al.16 found that changes in the MMS score were associated with the AHI and daytime sleepiness without any contribution from hypoxemia. Furthermore, a recent study of memory consolidation in middle-aged patients20 showed associations with the AHI and arousal indices without any relationship to hypoxemia. Overall, these data in the literature underline the considerable controversy in this area, especially regarding the differences between middle-aged and elderly subjects and differences between populations in the roles of abnormal respiratory events and hypoxemia severities.
Although the changes in cognition were smaller in our sample and affected the attention domain, the key role of SBD in the cognitive decline of the elderly should be considered in clinical evaluations.
Strengths and Limitations
The current study had several strengths including the sample size, the longitudinal design, and the use of extensive clinical and neuropsychological assessment, such as the use of Z-scores, which have recently been deemed to be a more accurate method of minimizing measurement error in an individual test.60,61 However, some methodological issues must be discussed. First, we examined a healthy population for which strict inclusion criteria were applied, which precluded the generalization of our data to clinical samples. Second, while the cognitive assessment was performed at baseline and follow-up, neither a clinical and medication evaluation nor ambulatory respiratory monitoring were available at the follow-up cycle. Third, some differences between the subjects examined at follow-up and those excluded after the first cognitive and/or at-home polygraphic study were found (as shown in Table 1); the AHI and indices of hypoxemia of the excluded subjects were more severe, and they differed in obesity and hypertension. These differences between the included and excluded subjects may explain the slight changes in the overall cognitive function in our population. Finally, we used an ambulatory respiratory recording during sleep and not polysomnography, which does not allow a correct estimation of the AHI severity and the EEG sleep fragmentation. However, recent studies46,62,63 have concluded that respiratory autonomic arousals might be an accurate estimate of respiratory sleep fragmentation in SBD.
In conclusion, we found that the presence of abnormal respiratory events longitudinally induces a slight decline in the attentional domain without significant effect on the memory and executive functions in healthy elderly subjects without dementia. Although these changes were smaller, they were related to the AHI severity and to some indices of nocturnal hypoxemia stressing the role of SBD in cognition. Future longitudinal studies are needed to replicate our results in large samples and to better understand the effect of SBD on the cognitive decline.
DISCLOSURE STATEMENT
This study was supported by a grant from the French Ministry of Health (Cellule Projet Hospitalier de Recherche Clinique National, Direction de la Recherche Clinique, CHU Saint-Etienne; Appel d'Offre 1998 and Appel d'Offre 2002) and by a grant from the “L'Association de Recherche SYNAPSE” (President: Michel Segura). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Mme. Delphine Maudoux and Maryse Victoire for technical assistance.
ABBREVIATIONS
- AAI
autonomic arousals index
- AHI
apnea-hypopnea index
- BMI
body mass index
- ESS
Epworth Sleepiness Scale
- FCSRT
Free and Cued Selective Reminding Test
- MMS
Mini-Mental State
- ODI
oxygen desaturation index
- PROOF cohort
the PROgnostic indicator OF cardiovascular and cerebrovascular events study
- SBD
sleep breathing disorder
- SpO2
peripheral oxygen saturation
- TMT
Trail Making Test
- WAIS
Wechsler Adult Intelligence Scale
SUPPLEMENTAL MATERIAL
Neuropsychological data at baseline and follow-up for the study population and the three subgroups of participants stratified according to the severity of the apnea-hypopnea index (mean ± standard deviation).
Correlation analysis between the changes of cognitive tests from baseline to follow-up and the clinical and respiratory data at baseline.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep disordered breathing among middle-aged adults. N Engl J Med. 1993;328:123–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26:596–9. doi: 10.1093/sleep/26.5.596. [DOI] [PubMed] [Google Scholar]
- 3.Boland LL, Shahar E, Iber C, et al. Measures of cognitive function in person with varying degree of sleep-disorder breathing: the Sleep Heart Health study. J Sleep Res. 2002;1:265–72. doi: 10.1046/j.1365-2869.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- 4.Sforza E, Roche F, Thomas-Anterion C, et al. Cognitive function and sleep-related breathing disorders in a healthy elderly population: the Synapse study. Sleep. 2010;33:515–21. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep. 2012;12:537–46. doi: 10.1007/s11910-012-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sforza E, Roche F. Sleep apnea syndrome and cognition. Front Neurol. 2012;3:1–7. doi: 10.3389/fneur.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieu A, Mazza S, Decary A, et al. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep Med. 2008;9:112–20. doi: 10.1016/j.sleep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30:1309–16. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamout K, Goldstein FC, Lah JJ, Levey AI, Bliwise DL. Neurocognitive correlates of noctural oxygen desaturation in a memory clinic population. J Clin Exp Neuropsychol. 2012;34:325–32. doi: 10.1080/13803395.2011.642849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shpirer I, Elizur A, Shorer R, Peretz RB, Rabey JM, Khaigrekht M. Hypoxemia correlates with attentional dysfunction in patients with obstructive sleep apnea. Sleep Breath. 2012;16:821–7. doi: 10.1007/s11325-011-0582-1. [DOI] [PubMed] [Google Scholar]
- 11.Ju G, Yoon IY, Lee SD, Kim TH, Choe JY, Kim KW. Effects of sleep apnea syndrome on delayed memory and executive function in elderly adults. JAGS. 2012;60:1099–103. doi: 10.1111/j.1532-5415.2012.03961.x. [DOI] [PubMed] [Google Scholar]
- 12.Hrubos-Strøm H, Nordhus IH, Einvik G, et al. Obstructive sleep apnea, verbal memory, and executive function in a community-based high-risk population identified by the Berlin Questionnaire Akershus Sleep Apnea Project. Sleep Breath. 2012;16:223–31. doi: 10.1007/s11325-011-0493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults with sleep apnea. J Psychosom Res. 2003;54:71–6. doi: 10.1016/s0022-3999(02)00548-2. [DOI] [PubMed] [Google Scholar]
- 14.O'Hara R, Schroder CM, Kraemer HC, et al. Nocturnal sleep apnea/ hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology. 2005;65:642–4. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 15.Spira AP, Blackwell T, Stone KL, et al. Sleep disordered breathing and cognition in older women. JAGS. 2008;56:45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. JAGS. 2001;49:1622–7. doi: 10.1046/j.1532-5415.2001.t01-1-49270.x. [DOI] [PubMed] [Google Scholar]
- 17.Yaffe K, Laffan AM, Harrisson SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnea: a meta-analysis review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 19.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djonlagic I, Saboisky J, Carusona A, Stickgold R, Malhotra A. Mild obstructive sleep apnea impairs sleep-dependent memory consolidation. Plos One. 2012;7:1–8. [Google Scholar]
- 21.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 22.Ohayon MM, Vecchierini M-F. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 23.Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13:886–92. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Jaussent I, Bouyer J, Ancelin ML, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35:1201–7. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barthélémy JC, Pichot V, Dauphinot V, et al. Autonomic nervous system activity and decline as prognostic indicators of cardiovascular and cerebrovascular events: the ‘PROOF’ study. Neuroepidemiology. 2007;29:18–28. doi: 10.1159/000108914. [DOI] [PubMed] [Google Scholar]
- 26.Saint Martin M, Sforza E, Barthélémy JC, Thomas-Anterion C, Roche F. Does subjective sleep affect cognitive function in healthy elderly subjects? The Proof cohort. Sleep Med. 2012;13:1146–52. doi: 10.1016/j.sleep.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Reitan RM. Tucson, AZ: Reitan Neuropsychological Laboratories Inc; 1979. Manual for administration of neuropsychological test batteries for adults and children. [Google Scholar]
- 28.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–52. [Google Scholar]
- 29.Wechsler D. San Antonio, TX: Harcourt Brace and Company; 1997. WAIS-III administration and scoring manual. [Google Scholar]
- 30.Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. Evocation lexicale formelle et sémantique chez des sujets normaux : performances et dynamiques de production en fonction du sexe, de l'âge et du niveau d'études. Acta Neurologica Belgica. 1990;90:207–17. [PubMed] [Google Scholar]
- 31.Campo P, Morales M. Reliability and normative data for the Benton Visual Form Discrimination Test. Clin Neuropsychol. 2003;17:220–5. doi: 10.1076/clin.17.2.220.16504. [DOI] [PubMed] [Google Scholar]
- 32.Van der Linden M, Coyette F, Poitrenaud F, et al. Marseille: Solal; 2004. L'épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16) L'évaluation des troubles de la mémoire, ed. [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Rouch I, Roche F, Dauphinot V, et al. Diabetes, impaired fasting glucose, and cognitive decline in a population of elderly community residents. Aging Clin Exp Res. 2012;24:377–83. doi: 10.1007/BF03325269. [DOI] [PubMed] [Google Scholar]
- 35.Saint Martin M, Sforza S, Thomas-Anterion C, Barthelemy JC, Roche F. Baroreflex sensitivity, vascular risk factors and cognitive function in a healthy elderly population. The Proof-Siempre cohort. JAGS. 2013;61:2096–102. doi: 10.1111/jgs.12548. [DOI] [PubMed] [Google Scholar]
- 36.Soubelet A, Salthouse TA. Correlates of level and change in the Mini-Mental State Examination. Psychol Assess. 2011;23:811–8. doi: 10.1037/a0023401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable monitoring task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 38.Smith RP, Argod J, Pepin JL, Levy PA. Pulse transit time: an appraisal of potential clinical applications. Thorax. 1999;54:452–7. doi: 10.1136/thx.54.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitson D, Chhina N, Knijn S, van Herwaaden M, Stradling J. Changes in pulse transit time and pulse rate as markers of arousal from sleep in normal subjects. Clin Sci. 1994;87:269–73. doi: 10.1042/cs0870269. [DOI] [PubMed] [Google Scholar]
- 40.Pepin JL, Delavie N, Pin I, et al. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest. 2005;127:722–30. doi: 10.1378/chest.127.3.722. [DOI] [PubMed] [Google Scholar]
- 41.Corral-Peñafiel J, Pepin JL, Barbe F. Ambulatory monitoring in the diagnosis and management of obstructive sleep apnea. Eur Respir Rev. 2013;22:312–24. doi: 10.1183/09059180.00004213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavlova MK, Duffy JF, Shea SA. Polysomnographic respiratory abnormalities in asymptomatic individuals. Sleep. 2008;31:241–8. doi: 10.1093/sleep/31.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pepin JL, Delavie N, Pin I, et al. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest. 2005;127:722–30. doi: 10.1378/chest.127.3.722. [DOI] [PubMed] [Google Scholar]
- 44.Chouchou F, Pichot V, Pepin JL, et al. Sympathetic overactivity due to sleep fragmentation is associated with elevated diurnal systolic blood pressure in healthy elderly subjects : the PROOF-SYNAPSE study. Eur Heart J. 2013;34:2122–31. doi: 10.1093/eurheartj/eht208. [DOI] [PubMed] [Google Scholar]
- 45.Chouchou F, Sforza E, Celle C, et al. Pulse Transit Time in Screening Sleep Disordered Breathing In An Elderly Population: The PROOF-SYNAPSE Study. Sleep. 2011;34:1051–9. doi: 10.5665/SLEEP.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickering TG, Hall JE, Appel LJ, et al. Subcommittee of professional and public education of American Heart Association Council on high blood pressure research. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of professionals and public education of the American Heart Association Council on high blood pressure research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 47.Pichot P, Brun JP. Brief self-evaluation questionnaire for depressive, asthenic and anxious dimensions. Ann Med Psychol. 1984;142:862–5. [PubMed] [Google Scholar]
- 48.Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. BMJ. 1988;297:897–9. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 50.Lim A, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer disease and cognitive decline in older persons. Sleep. 2013;36:1027–32. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front Biosci. 2012;4:1391–403. doi: 10.2741/469. [DOI] [PubMed] [Google Scholar]
- 52.Gozal D. CrossTalk proposal: the intermittent hypoxia attending severe obstructive sleep apnoea does lead to alterations in brain structure and function. J Physiol. 2013;59:379–81. doi: 10.1113/jphysiol.2012.241216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrell MJ, Twigg G. Neural consequences of sleep disordered breathing: the role of intermittent hypoxia. Adv Exp Med Biol. 2006;588:75–88. doi: 10.1007/978-0-387-34817-9_8. [DOI] [PubMed] [Google Scholar]
- 54.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: A meta-analysis. Sleep. 2013;36:1297–305. doi: 10.5665/sleep.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sforza E, Gauthier M, Crawford-Achour E, et al. A 3-year longitudinal study of sleep disordered breathing in the elderly. Eur Respir J. 2012;40:665–72. doi: 10.1183/09031936.00133011. [DOI] [PubMed] [Google Scholar]
- 56.Sforza E, Chouchou F, Collet P, Pichot V, Barthélémy JC, Roche F. Sex differences in obstructive sleep apnoea in an elderly French population. Eur Respi J. 2011;37:1137–43. doi: 10.1183/09031936.00043210. [DOI] [PubMed] [Google Scholar]
- 57.Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnea. J Sleep Res. 2009;18:397–403. doi: 10.1111/j.1365-2869.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 58.Cooke JR, Ancoli-Israel S. Normal and abnormal sleep in the elderly. Handb Clin Neurol. 2011;98:653–65. doi: 10.1016/B978-0-444-52006-7.00041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young T. Sleep-disordered breathing in older adults: is it a condition distinct from that in middle-aged adults? Sleep. 1996;19:529–30. doi: 10.1093/sleep/19.7.529. [DOI] [PubMed] [Google Scholar]
- 60.Ruddle HV, Bradshaw CM. On the estimation of premorbid intellectual functioning: validation of Nelson & McKenna's formula, and some new normative data. Br J Clin Psychol. 1982;21:159–65. doi: 10.1111/j.2044-8260.1982.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 61.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennet DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–8. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catcheside PG, Chiong SC, Orr RS, Mercer J, Saunders NA, McEvoy RD. Acute cardiovascular responses to arousal from non-REM sleep during normoxia and hypoxia. Sleep. 2001;24:895–902. doi: 10.1093/sleep/24.8.895. [DOI] [PubMed] [Google Scholar]
- 63.Trinder J, Waloszek J, Woods MJ, Jordan AS. Sleep and cardiovascular regulation. Pflugers Arch. 2012;463:161–8. doi: 10.1007/s00424-011-1041-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neuropsychological data at baseline and follow-up for the study population and the three subgroups of participants stratified according to the severity of the apnea-hypopnea index (mean ± standard deviation).
Correlation analysis between the changes of cognitive tests from baseline to follow-up and the clinical and respiratory data at baseline.