Abstract
Study Objective:
Inflammation may represent a common physiological pathway linking both short and long sleep duration to mortality. We evaluated inflammatory markers as mediators of the relationship between sleep duration and mortality in community-dwelling older adults.
Design:
Prospective cohort with longitudinal follow-up for mortality outcomes.
Setting:
Pittsburgh, Pennsylvania, and Memphis, Tennessee.
Participants:
Participants in the Health, Aging and Body Composition (Health ABC) Study (mean age 73.6 ± 2.9 years at baseline) were sampled and recruited from Medicare listings.
Measurements and Results:
Baseline measures of subjective sleep duration, markers of inflammation (serum interleukin-6, tumor necrosis factor-α, and C-reactive protein) and health status were evaluated as predictors of all-cause mortality (average follow-up = 8.2 ± 2.3 years). Sleep duration was related to mortality, and age-, sex-, and race-adjusted hazard ratios (HR) were highest for those with the shortest (< 6 h HR: 1.30, CI: 1.05–1.61) and longest (> 8 h HR: 1.49, CI: 1.15–1.93) sleep durations. Adjustment for inflammatory markers and health status attenuated the HR for short (< 6 h) sleepers (HR = 1.06, 95% CI = 0.83–1.34). Age-, sex-, and race-adjusted HRs for the > 8-h sleeper group were less strongly attenuated by adjustment for inflammatory markers than by other health factors associated with poor sleep with adjusted HR = 1.23, 95% CI = 0.93–1.63. Inflammatory markers remained significantly associated with mortality.
Conclusions:
Inflammatory markers, lifestyle, and health status explained mortality risk associated with short sleep, while the mortality risk associated with long sleep was explained predominantly by lifestyle and health status.
Citation:
Hall MH, Smagula SF, Boudreau RM, Ayonayon HN, Goldman SE, Harris TB, Naydeck BL, Rubin SM, Samuelsson L, Satterfield S, Stone KL, Visser M, Newman AB. Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: the Health, Aging and Body Composition Study. SLEEP 2015;38(2):189–195.
Keywords: epidemiology, sleep, inflammation, mortality, aging
INTRODUCTION
Sleep duration is a significant predictor of all-cause mortality.1–8 Despite some inconsistencies in the published literature,9–11 the majority of studies have reported that all-cause mortality risk is elevated in both short and long sleepers (generally defined as ≤ 6 h/night, and > 8 h/night, respectively). Findings from meta-analyses have also supported the associations of both short and long sleep with an increased risk of mortality.12,13 In general, the “U-shaped” relationship between sleep duration and mortality has been observed in adult men and women, in rural and urban settings, in different countries, and in cohorts evaluated at multiple time points.1,3–8,14,15
Inflammation may represent a common physiological pathway linking both short and long sleep duration to mortality. Chronic, low grade elevations in markers of inflammation, including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) are potent risk factors for all-cause mortality.16,17 While the evidence is somewhat mixed,18–20 a variety of sources support an independent association between sleep duration and elevated levels of some inflammatory markers. For example, observational and experimental studies suggest that sleep curtailment is associated with increases in levels of inflammatory markers.20–28 Although there are no experimental studies linking long sleep duration to increases in markers of inflammation, observational studies suggest that some markers of inflammation are elevated in long sleepers.23,29
Despite the potential that the inflammatory pathway links sleep duration and mortality, to our knowledge, there are no published investigations of this relationship. Mechanisms linking sleep duration and mortality may differ for short and long sleepers, and studying the sleep duration-mortality association in the context of inflammation, as well as lifestyle, medical and psychiatric health conditions, may add to the current understanding of pathways which underlie associations between sleep duration and mortality. We were able to evaluate these relationships in the Health, Aging, and Body Composition (Health ABC) cohort, which included data on self-reported habitual sleep duration, markers of inflammation and longitudinal assessments of mortality. We hypothesized that elevated IL-6, TNF-α, and CRP would explain part of the relationship between habitual sleep duration and all-cause mortality. Since obesity, health behaviors, and chronic health conditions also can contribute to elevations of these markers, we additionally adjusted for sociodemographic, lifestyle, and health conditions to evaluate the role of these factors.
METHODS
Study Population
The Health, Aging, and Body Composition (Health ABC) study is a prospective cohort study of the relationship between changes in body composition and functional decline. Recruitment and eligibility have been described elsewhere.30 Briefly, participants were sampled and recruited from Medicare listings in Pittsburgh, Pennsylvania, and Memphis, Tennessee. To be eligible for participation individuals had to be between 70 to 79 years of age during the recruitment period, have no difficulty with activities of daily living, no reported use of an assistive device or equipment to get around, and have no difficulty walking one-quarter mile, or climbing 10 steps without resting. Exclusion criteria were current participation in a lifestyle intervention trial, active treatment for cancer in the past 3 years, or plans to move out of the study area within 3 years at baseline. A total of 3,075 adults were enrolled in the study. For the present analysis, only those participants with complete baseline and 9-year follow-up data were included (n = 3,013). Institutional review boards at both study sites approved all protocols and all participants provided written, informed consent.
Mortality
Total mortality was assessed over 9 years, with a mean follow-up of 8.2 (standard deviation [SD] 2.3) years. Surveil-lance was conducted by in-person assessments or telephone interviews every 6 months. Hospital records, death certificates, informant interviews, and autopsy data were reviewed by committee to adjudicate immediate and underlying causes of death.
Sleep
Sleep patterns were assessed by interview-administered questionnaire at the baseline visit. The present paper focuses on reported habitual sleep duration (“How many hours of sleep do you usually get at night during a usual week?”). Participants' responses were classified as < 6 h (n = 406), 6 h (n = 768), 7 h (n = 779), 8 h (n = 872), > 8 h (n = 188) habitual sleep, per night, which is comparable to measures of sleep duration used in other population-based studies of sleep and health outcomes.
Markers of Inflammation
Baseline blood samples were drawn during the morning hours after an overnight fast. Serum aliquots were placed in cryovials, frozen and shipped to the Health ABC Core Laboratory at the University of Vermont. Thawed samples were assayed for IL-6, TNF-α, and CRP levels. IL-6 and TNF-α levels were measured in duplicate with enzyme-linked immunosor-bent assay kits (R&D Systems, Minneapolis, MN). The detection limit for IL-6 was 0.10 pg/mL (HS600 Quantikine kit), and for TNF-α was 0.18 pg/mL (HSTA50 kit). Serum levels of CRP were measured in duplicate by enzyme-linked immunosor-bent assay based on purified protein and polyclonal anti-CRP antibodies (Calbiochem, San Diego, CA). Assay sensitivity was 0.08 μg/mL and was standardized according to the World Health Organization First International Reference Standard. Blind duplicate assays collected for 150 participants yielded an average interassay coefficient of variation of 10.3% for IL-6, 15.8% for TNF-α, and 8.0% for CRP.
Covariates
Sociodemographic, lifestyle and health characteristics previously associated with sleep, markers of inflammation and mortality were considered as possible confounders of the relationships among these measures. Sociodemographic characteristics including age, race (self-designated as white, black, or other), gender, how well the participant felt their financial needs were met (poorly, fairly well, or very well) and highest level of education (less than high school, high school, post-secondary) were measured by self-report at baseline. Lifestyle characteristics including physical activity status (inactive, active lifestyle, regular exerciser), alcohol consumption status (never, former, current), weekly alcohol consumption (none, < 1/week, 1–7/ week, > 7/week), and smoker status (never, former, current) were also measured by self-report at baseline. Stadiometer and balance beam scale were used to collect anthropomorphic measurements of height and weight. Body mass index was used to categorize participants as normal weight (BMI < 25 kg/m2), overweight (BMI 25–29 kg/m2) or obese (BMI ≥ 30 kg/m2). Health characteristics were evaluated via a detailed medical history and inventory of prescription and over-the-counter medication use. Chronic medical conditions were evaluated by self-report and confirmation of treatment and medications related to chronic health conditions. Chronic disease status was defined as present or absent for hypertension; clinical cardiovascular disease (including those who had coronary artery bypass surgery, percutaneous transluminal angioplasty, insertion of pacemaker, self-report of myocardial infarction and use of anti-anginal medication); congestive heart failure and use of diuretic, vasodilator or cardiac glycoside; cerebrovascular disease (self-reported transient ischemic attack or stroke); lower-extremity peripheral arterial disease (self-report of intermittent claudication or pain in the legs or self-report of bypass or angioplasty in leg arteries); diabetes; pulmonary disease; ulcers; arthritis and osteoporosis. Depression was defined on the basis of a symptom score ≥ 16, using the Centers for the Epidemio-logic Studies of Depression (CES-D) scale.31
Statistical Analyses
Markers of inflammation were normalized using log transformations. Proportions were used to describe categorical demographic and key clinical characteristics of the study population by sleep duration categories. Means and standard deviations were used to summarize continuous characteristics and markers of inflammation. Hypotheses regarding differences in sleep duration by categorical demographics and clinical characteristics were tested using chi-square statistics. Mean levels of inflammatory markers by sleep duration category were compared using ANOVA. Unadjusted mortality rates were calculated and graphically presented as the number of deaths per 1,000 person-years.
After assessing the proportionality assumption, we used Cox proportional hazard models to evaluate associations among sleep duration, markers of inflammation, and mortality. The first model calculated hazard ratios (HR) for the association between sleep duration and mortality, adjusting for age, gender, and race (Model 1). Model 2 was adjusted for inflammatory markers as well as sociodemographics, lifestyle, chronic diseases, and medications that were related to either sleep duration or mortality. Anti-inflammatory medication use was also included because of clinical relevance, though not bivariately related to sleep duration or mortality. An intermediate model (not shown) without the inflammatory markers was also examined to determine their contribution to a model with health, lifestyle and sociodemographic factors. Hazard ratios (HR) and 95% confidence intervals (CI) are reported. We considered a reduction of greater than 10% of the HR for sleep duration and mortality as support for the hypothesis of mediation.32 All analyses were conducted with SAS version 9.3.
RESULTS
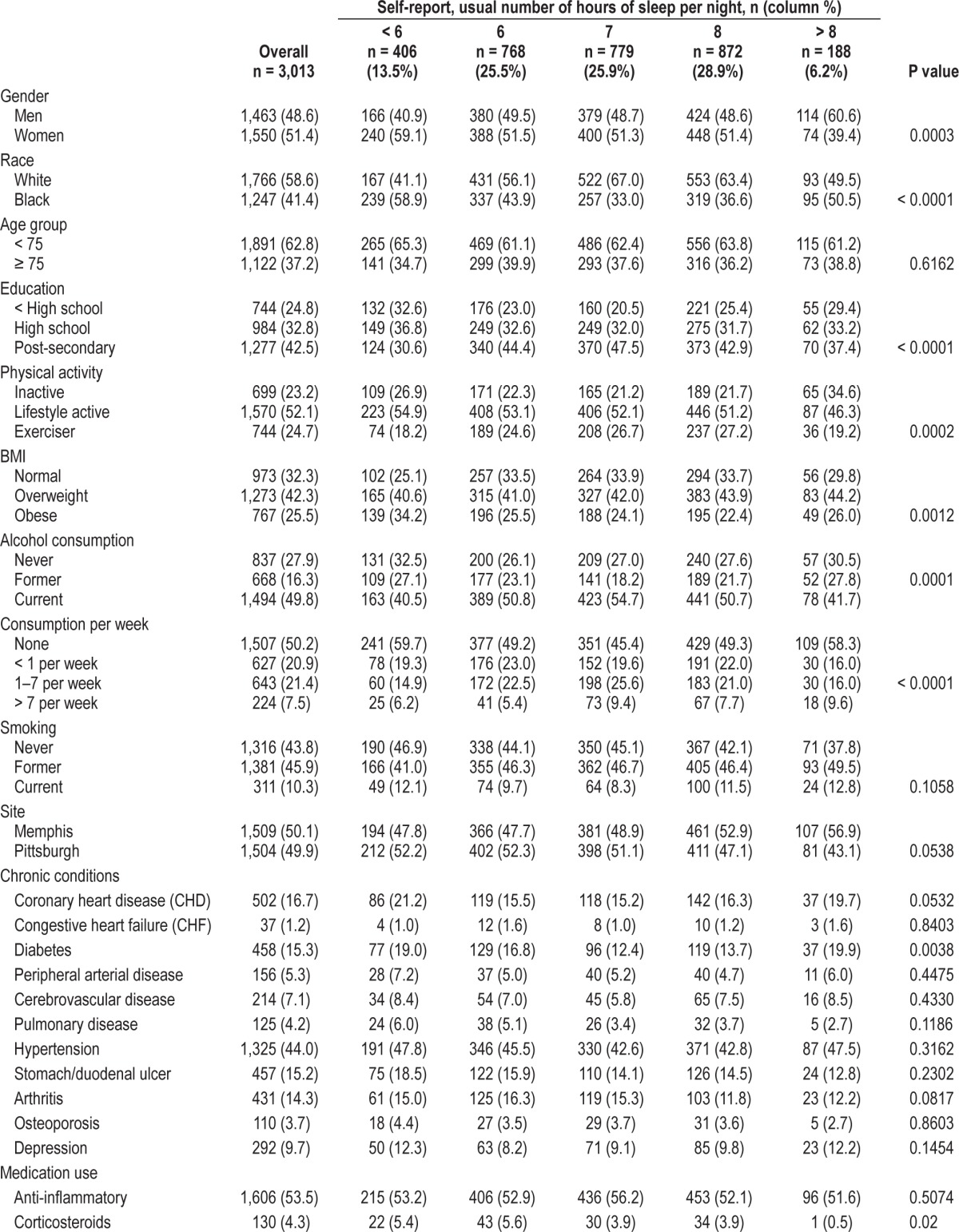
The mean sample age at baseline was 73.6 (2.9) years of age. Forty-one percent of participants were black and 49% were male. The mean reported sleep duration for the overall sample was 6.9 (1.4) hours. A number of sociodemographic, lifestyle, and health status indicators were significantly associated with sleep duration (Table 1). For example, a higher proportion of women than men slept < 6 h per night (15.5% women versus 11.3% men). A number of characteristics exhibited a “U-shaped” pattern, associated with both shorter (< 6 h) and longer (> 8 h) reported habitual sleep duration (compared to 7 h) including: black race, financial worries, low educational attainment, inactive lifestyle, obesity, abstinence from alcohol and diabetes. Corticosteroid use was more common in short sleepers (< 7 h). Reported habitual sleep duration patterns also differed by study site. A higher proportion of Pittsburgh site participants slept < 6 h, whereas a higher proportion of Memphis site participants slept > 8 hours. Significant associations were not found between reported habitual sleep duration and age group, smoker status, hypertension, clinical cardiovascular disease, congestive heart failure, cerebrovascular disease, lower-extremity peripheral arterial disease, pulmonary disease, ulcers, arthritis, osteoporosis, or depression. Significant associations were similarly absent for medication use including anti-inflammatory medications, bronchodilators, antidepressants, or diuretics (data not shown).
Table 1.
Population characteristics by reported usual sleep duration.
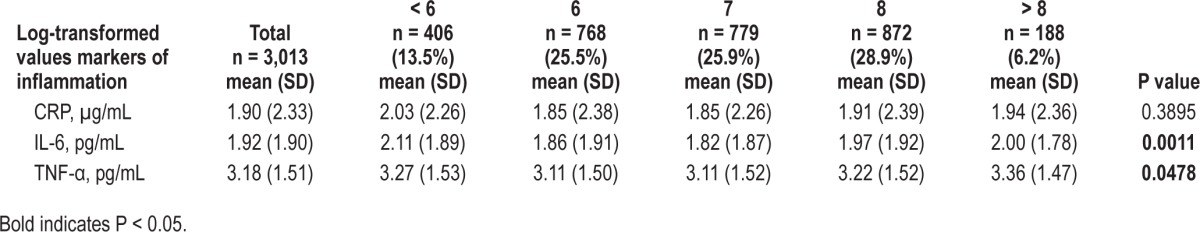
Markers of inflammation were significantly associated with reported sleep duration (Table 2). IL-6 and TNF-α levels were higher in participants with reported sleep durations < 6 or > 8 h per night (compared to 7 h). A similar but nonsignificant trend was found for CRP.
Table 2.
Markers of inflammation and sleep duration.
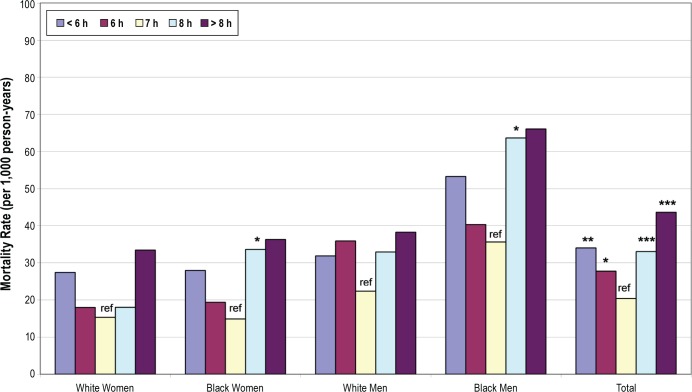
Just over 30% of participants (31.6%; N = 953) died during the 9-year follow-up. The highest crude mortality rate, per 1,000 person-years, was found among those who reported sleeping > 8 h/night (53.5), followed by those who slept < 6 h (43.4), 8 h (40.4), 6 h (36.4), and 7 h (32.8) per night (Figure 1). Crude mortality rates by race and gender illustrate the consistency of this relationship across all groups (Figure 1). All P values for interaction terms among sleep duration and race or sex were > 0.10. Survival curves by habitual sleep duration groups (Figure 2) show that the curves deviated early on and persisted over time, so that after 9 years, about 60% of those with > 8 h of sleep survived, compared to 70% of those with < 6 h and 80% of those in the referent group reporting 7 hours of sleep.
Figure 1.
Crude mortality rates by reported habitual sleep duration. Statistically significant difference from reference (ref): * P < 0.05. ** P < 0.01. *** P < 0.001.
Figure 2.
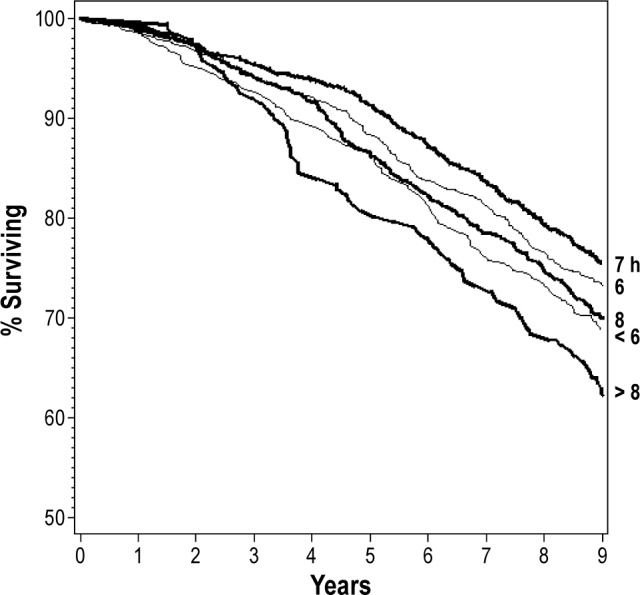
Survival curves for habitual sleep duration groups.
Proportional hazards regression was performed to evaluate relationships among reported habitual sleep duration and mortality, with 7 h of reported habitual sleep duration as the reference value (Table 3). In the first model, sleep duration was significantly associated with mortality in the short (< 6 h) and long (8, > 8 h) sleeper groups after adjusting for age, gender, and race.
Table 3.
Mortality hazard ratios by reported habitual sleep duration (n = 3,013).
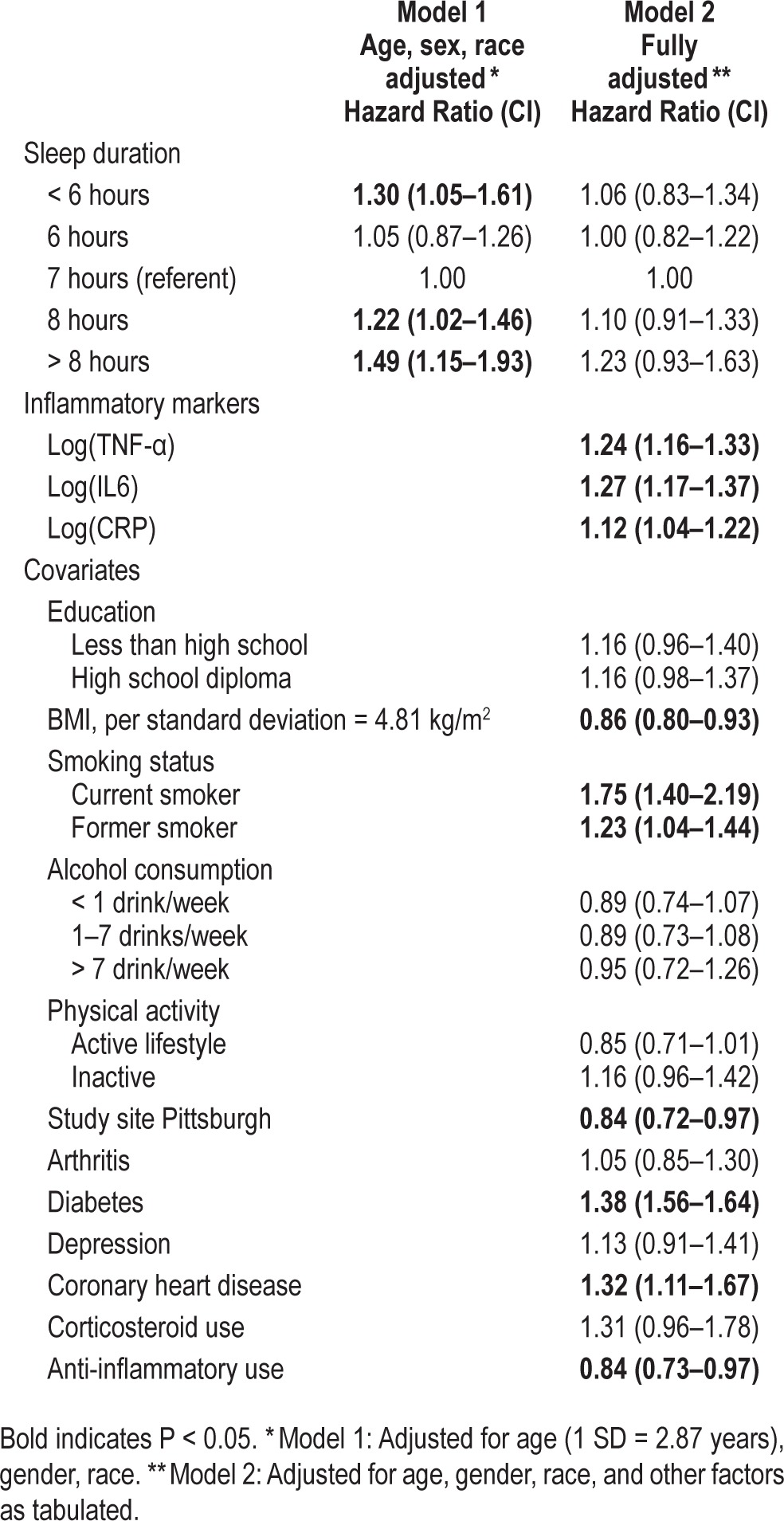
In the fully adjusted model, the associations of sleep duration with mortality were attenuated to non-significance, while the inflammatory markers remained independently associated with mortality (Table 3, Model 2). When examining the effect of the addition of inflammatory markers to health status variables, the HR for short sleep duration was reduced from 1.18 (0.95–1.47) to 1.06 (0.83–1.34; intermediate model not shown). The elevated mortality risk associated with long (> 8 h) sleep was attenuated predominantly by the health status covariates with little additional influence of inflammatory markers levels. The HR for the association of sleep duration with mortality was attenuated differently for short (< 6 h) sleep (80%) vs. long (> 8 h) sleep (53%) by the inflammatory markers and health status variables.
DISCUSSION
Both short and long sleep durations were associated with mortality and these associations were explained by inflammatory markers and health status factors. Several markers including IL-6, TNF-α, and CRP, as well as smoking, coronary disease, and diabetes were independently related to mortality. The attenuation of the associations with mortality with adjustment for these factors was relatively greater for short sleep than for long sleep.
These findings are consistent with previous studies demonstrating associations between sleep and mortality,1–8,12,13 as well as inflammation and mortality.16,17 Our study adds to the litera ture by demonstrating the complexity of these associations that can differ for short and long sleep. The potential for a direct role for inflammation in short sleepers is biological plausible, as sleep loss has been previously associated with increased blood pressure and heart rate,25,27,33 altered metabolic and en docrine,34–36 as well as catecholamine signaling,37,38 all relating to increased sympathetic activation,39,40 and a pro-inflammatory response.41
In the association of short sleep and mortality, diabetes and cardiovascular disease were also key factors, but these did not explain the associations of the inflammatory markers with mortality. These associations were especially complex in the short sleepers, where the short-sleep mortality relationship was substantially attenuated by both disease and inflammation. Because sleep, health and inflammatory markers were all measured concurrently, the causal direction of these potentially bi-directional associations cannot be determined from these analyses.
In contrast to the short sleep-mortality relationship, the mortality risk associated with long sleep duration was less strongly attenuated. The excess mortality risk associated with long (> 8 h) sleep was reduced by 53% in fully adjusted model. These findings support previous authors' suggestions that long sleep duration may serve as a proxy for underlying medical illness, major depression, and fatigue,42,43 and that the association between long sleep duration and mortality might be explained by confounding not fully accounted for in some studies, for example by unmeasured mood disorders or preclinical disease.44
It is also important to point out that obesity, one of the major risk factors for higher levels of inflammatory markers in middle-aged adults, was not a confounder in this study of older adults. In fact, higher BMI was associated with a lower risk of mortality, as has been well documented previously in this age group.45,46 Furthermore, BMI did not confound the associations of sleep duration with mortality, nor did higher BMI attenuate the role of higher levels of inflammatory markers in these associations. These findings should not be generalized to middle-aged adults in whom higher BMI is more strongly linked not only to higher levels of inflammatory markers but also higher rates of sleep apnea and mortality.
Strengths of the current study include the novel examination of sleep duration, inflammation, health status, and mortality in a single study. These findings over a long follow-up period (9 years) demonstrate the robustness of this pattern of associations. Forty percent of Health ABC participants reported habitual sleep durations of less than 7 hours, another 35% of these older adults reported sleep durations of equal to or greater than 8 hours, and during the 9-year follow-up, 32% of the sample died. These sleep duration and mortality values are similar to published statistics for American adults in this age group,47 in dicating that in these ways, this sample appears to be representative of older adults.
The current study includes a number of limitations. Due to the nature of the study sample, these results do not necessarily generalize to younger adults and some minority groups. Further, the sample size within sleep duration strata restricted our ability to evaluate cause-specific mortality; thus, it remains possible that there are cause-specific caveats to the current results. Our data is cross-sectional data with regard to inflammation, sleep duration, and chronic diseases, and thus we cannot determine which factors are most proximal in the causal pathway. Our assessment of sleep duration cannot distinguish voluntary sleep restriction from an inability to sleep due to underlying conditions such as sleep apnea or arthritis which could themselves increase inflammatory markers and directly influence sleep duration. The lack of objective sleep assessments including assessment of sleep apnea calls for cautious interpretation of the current findings. Future research should examine the extent to which these findings are supported by or might differ from those obtained by objective measures of sleep duration and in relation to primary sleep disorders such as sleep apnea and insomnia.
In conclusion, the associations of short and long sleep duration and mortality were attenuated by levels of inflammatory markers and chronic health conditions in a large, community-dwelling sample of older adults. The association of long sleep duration and mortality was more strongly attenuated by underlying comorbidity than by markers of inflammation. These findings have implications for older adults and health practitioners, namely, that attention to older adults' habitual sleep duration can provide specific, clinically relevant information. When presented with an older adult reporting short or long sleep, clinicians may initially contextualize these sleep patterns in light of a known health condition or otherwise consider the potential presence of undetected clinical or subclinical disease. Future studies investigating additional biological indicators of disease processes are needed to fully understand mechanisms linking sleep to mortality.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported by National Institute on Aging (NIA) Contracts N 01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. The authors have indicated no financial conflicts of interest. Dr. Goldman, is currently with the Vanderbilt University Medical Center, Department of Neurology, Sleep Disorders Center, Nashville, Tennessee, USA; Ms. Naydeck is currently with HM Insurance, Pittsburgh, PA, USA.
REFERENCES
- 1.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–66. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammond EC. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. Am J Public Health. 1964;54:11–23. doi: 10.2105/ajph.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002;3:305–14. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 4.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–53. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 6.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 8.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27:51–4. [PubMed] [Google Scholar]
- 9.Amagai Y, Ishikawa S, Gotoh T, et al. Sleep duration and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol. 2004;14:124–8. doi: 10.2188/jea.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10:87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan GA, Seeman TE, Cohen RD, Knudsen LP, Guralnik J. Mortality among the elderly in the Alameda County Study: behavioral and demographic risk factors. Am J Public Health. 1987;77:307–12. doi: 10.2105/ajph.77.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 14.Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Prev Med. 1980;9:469–83. doi: 10.1016/0091-7435(80)90042-0. [DOI] [PubMed] [Google Scholar]
- 15.Burazeri G, Gofin J, Kark JD. Siesta and mortality in a Mediterranean population: a community study in Jerusalem. Sleep. 2003;26:578–84. doi: 10.1093/sleep/26.5.578. [DOI] [PubMed] [Google Scholar]
- 16.Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsik C, Kazemi-Shirazi L, Schickbauer T, et al. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem. 2008;54:343–9. doi: 10.1373/clinchem.2007.091959. [DOI] [PubMed] [Google Scholar]
- 18.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP) - No association with sleep duration or sleep disordered breathing. Sleep. 2007;30:991–6. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009;23:351–4. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 22.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–7. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MA, Kandala NB, Kivimaki M, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II Study. Sleep. 2009;32:857–64. [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65:244–52. doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 25.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 26.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PloS One. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16:137–49. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21:799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–30. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 32.Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: examples from the child-clinical and pediatric psychology literatures. J Consult Clin Psychol. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- 33.Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep. 2009;32:760–6. doi: 10.1093/sleep/32.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endorinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 37.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endorinol Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 38.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 39.Zhong X, Hilton HJ, Gates GJ, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appli Physiol. 2005;98:2024–32. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 40.Dettoni JL, Consolim-Colombo FM, Drager LF, et al. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol. 2012;113:232–6. doi: 10.1152/japplphysiol.01604.2011. [DOI] [PubMed] [Google Scholar]
- 41.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best practice & research. J Clin Endorinol Metab. 2010;24:775–84. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grandner MA, Kripke DF. Self-reported sleep complaints with long and short sleep: a nationally representative sample. Psychosom Med. 2004;66:239–41. doi: 10.1097/01.psy.0000107881.53228.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez GG, Ayas NT. The impact of daily sleep duration on health: a review of the literature. Progr Cardiovasc Nurs. 2004;19:56–9. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 45.Weiss A, Beloosesky Y, Boaz M, Yalov A, Kornowski R, Grossman E. Body mass index is inversely related to mortality in elderly subjects. J Gen Intern Med. 2008;23:19–24. doi: 10.1007/s11606-007-0429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc. 2005;53:2112–8. doi: 10.1111/j.1532-5415.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 47.National Sleep Foundation. 2003 Sleep in America Poll. Washington, DC: 2003. [Google Scholar]