Abstract
Objectives:
Sleep disturbance and aging are associated with increases in inflammation, as well as increased risk of infectious disease. However, there is limited understanding of the role of sleep loss on age-related differences in immune responses. This study examines the effects of sleep deprivation on toll-like receptor activation of monocytic inflammation in younger compared to older adults.
Design, Setting, and Participants:
Community-dwelling adults (n = 70) who were categorized as younger (25–39 y old, n = 21) and older (60–84 y old, n = 49) participants, underwent a sleep laboratory-based experimental partial sleep deprivation (PSD) protocol including adaptation, an uninterrupted night of sleep, sleep deprivation (sleep restricted to 03:00–07:00), and recovery.
Measurement and Results:
Blood samples were obtained each morning to measure toll-like receptor-4 activation of monocyte intracellular production of the inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). Partial sleep deprivation induced a significant increase in the production of IL-6 and/or TNF-α that persisted after a night of recovery sleep (F(2,121.2) = 3.8, P < 0.05). Age moderated the effects of sleep loss, such that younger adults had an increase in inflammatory cytokine production that was not present in older adults (F(2,121.2) = 4.0, P < 0.05).
Conclusion:
Older adults exhibit reduced toll-like receptor 4 stimulated cellular inflammation that, unlike in younger adults, is not activated after a night of partial sleep loss. Whereas sleep loss increases cellular inflammation in younger adults and may contribute to inflammatory disorders, blunted toll-like receptor activation in older adults may increase the risk of infectious disease seen with aging.
Citation:
Carroll JE, Carrillo C, Olmstead R, Witarama T, Breen EC, Yokomizo M, Seeman TE, Irwin MR. Sleep deprivation and divergent toll-like receptor-4 activation of cellular inflammation in aging. SLEEP 2015;38(2):205–211.
Keywords: age, inflammatory cytokines, immune, monocyte, sleep deprivation
INTRODUCTION
Chronic sleep disturbance is a pervasive problem in modern society, and is thought to contribute to increased morbidity and mortality, particularly in those at elevated risk by virtue of their older age.1,2 Although older adults are more likely to experience sleep disturbances than younger adults,3 little research to date that has examined the differential health-related effects of sleep loss in older as compared to younger individuals.
One important component to maintaining good health is proper functioning of the immune system. Sleep may play a pivotal role in immunocompetence.4 Sleep promotes anti-viral immunity by supporting the adaptive immune response,5 with evidence that experimental and naturalistic sleep loss is associated with poorer immunological memory after a vaccination,6–8 whereas poor sleep quality is associated with increased risk for developing an upper respiratory infection.9 In contrast to the adaptive immune response, inadequate sleep appears to activate the innate immune system, with elevations in both circulating and intracellular inflammatory responses after sleep loss.10–15 This pattern of inflammation if chronic is thought to contribute to risk for age-related diseases including cardiovascular disease.14,16 Whereas it is well known that older adults are more vulnerable to viral infections, have an attenuated response to vaccination,17 and show systemic rises in inflammatory markers,16 there is limited understanding of the role of sleep and sleep loss on age-related differences in immune responses.
Toll-like receptors serve to sense pathogens and activate innate immune responses.18 Ligation of the toll-like receptor-4 (TLR-4) with bacterial lipopolysaccharide (LPS) induces the production of proinflammatory cytokines by monocytes, and serves as an indicator of the innate immune system's inflammatory response19,20 A mice model showed that LPS-mediated macrophage activation was reduced in aged mice,21 suggesting that this lowered response to bacterial stimulation with age reflects an impaired immune system.
In young adult humans, experimental partial night sleep deprivation (PSD) has been found to induce marked increases the following morning in LPS/TLR-4 mediated monocytic production of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α).10–12 Although circulating cytokines are increased after repeated and total night sleep deprivation,13,15,22,23 they typically do not show similar increases after 1 night of PSD.24 Whereas older adults are more likely to have sleep disturbances and lowered immune responses,3,17,25 the effects of sleep loss on TLR-4 induced monocytic production of inflammatory cytokines in older adults is not known.
This study aims to evaluate differences in TLR-4 mediated monocyte production of proinflammatory cytokines in response to sleep loss and recovery sleep between young (25–39 y) and older adults (60–84 y). Given the existing evidence showing a lowered TLR-4 mediated monocyte responsiveness with age,21 we hypothesized that there would be a blunted activation of LPS-stimulated, monocytic intracellular production of IL-6 and TNF-α following PSD in older as compared to younger adults. An experimental model of PSD was used because it is thought that loss of sleep during part of the night, as opposed to total sleep deprivation, replicates the type of sleep loss that is ubiquitous in the general population, and resembles the reduction of sleep duration that is often found in older adults. Measures of sleep continuity (e.g., total sleep time [TST], wake after sleep onset [WASO]) and sleep architecture (e.g., slow wave sleep [SWS], rapid eye movement [REM]) were obtained at baseline, PSD, and recovery sleep to explore whether differences in stimulated monocytic production of proinflammatory cytokines in response to PSD were related to age differences in sleep continuity and architecture before and after PSD.
METHODS
Participants
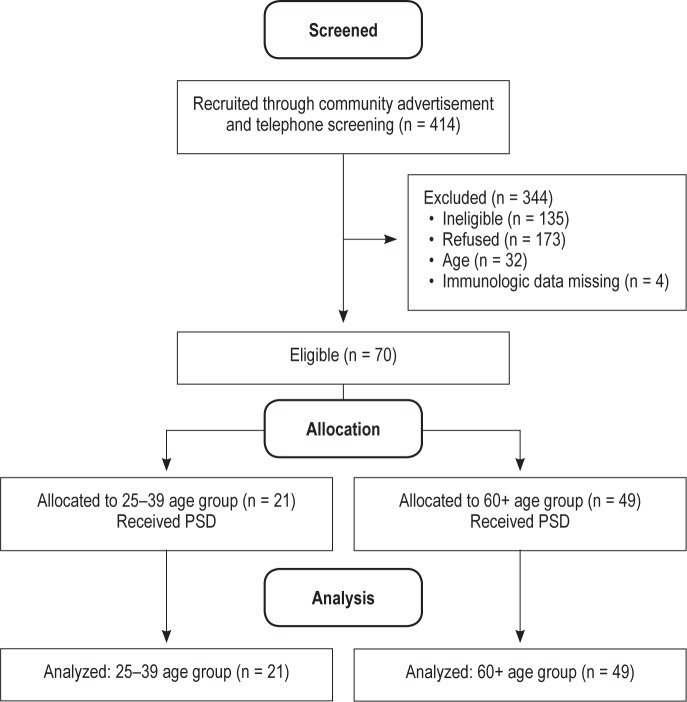
The current analysis includes participants from several ongoing laboratory studies of the effects of sleep deprivation across the life span.10 All studies were conducted by the same research group and followed standard laboratory procedures as previously described.10,26–28 A full Consolidated Standards of Reporting Trials (CONSORT) flow diagram of the sample selection can be seen in Figure 1. After preliminary telephone screening, 414 individuals were invited for additional eligibility evaluation. Of this sample, 135 were deemed ineligible (see exclusion criterion in the following paragraphs), 173 declined participation in the PSD, 32 did not meet the age criterion for the current analyses, and 4 had missing immunologic data. The current analysis includes 70 subjects who underwent the PSD protocol, and is comprised of two age categories: (1) 25–39 y (n = 21) and (2) 60 y and older (n = 49). A total of 62 subjects (ages 25–39 y, n = 20; 60 y and older, n = 42) had PSG data. All subjects gave informed consent and the University of California, Los Angeles (UCLA) institutional review board approved the protocols. Based on medical interview, physical examination, and screening laboratory tests, subjects were included in the study if they were deemed physically healthy including no past history of cancer or inflammatory disorders, were nonsmokers, and had a body mass index (BMI) < 40 (calculated as weight (kg) divided by the square of height [m]). Additional exclusion criterion was: current diagnosis of mental illness based on the Diagnostic and Statistical Manual of Mental Disorders (Editions 4 (revised) or 5); sleep apnea as identified during the night of adaptation to the sleep laboratory, chronic or acute (< 2 w) infection, and comorbid uncontrolled chronic disease. In addition, all subjects completed a 2-w sleep diary to verify their normal sleep pattern to be 7–9 h nightly between 22:30 and 07:30.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram.
Procedures
After eligibility and medical evaluation, subjects were invited to stay at the UCLA General Clinical Research Center (renamed the Clinical Translational Research Center) where they underwent experimental procedures reported previously.10 Briefly, following the adaptation night, subjects had an uninterrupted night of sleep (23:00–07:00) which serves as baseline. On a third night, sleep was deprived during the early part of the night (23:00–03:00) with the sleep period restricted to the hours between 03:00–07:00 (PSD), with awakening occurring regardless of sleep stage. The subsequent night serves as a recovery night where sleep was uninterrupted from 23:00–07:00. Blood samples were obtained each morning following baseline, PSD, and recovery nights for the assessment of LPS-stimulated TLR-4 activation of monocytic intracellular production of proinflammatory cytokines prior to any daytime activities and/or meals. Nighttime sleep patterns were monitored using polysomnography (PSG) recordings of sleep on baseline, PSD, and recovery nights.
Measures
To evaluate the presence and severity of depressive symptoms, the Beck Depression Inventory (BDI) was administered.29 Severity of sleep disturbances prior to the experimental protocol was assessed by the Pittsburgh Sleep Quality Index (PSQI).30 Sleep continuity and architecture was objectively evaluated by PSG. Briefly, electroencephalography readings were obtained during continuous polysomnography recordings from bedtime to waking (approximately 23:00–07:00) during each of the experimental nights and visually scored by trained sleep technicians, as previously described,27,28,31 and used to estimate WASO, sleep efficiency (SE), TST, sleep onset latency (SOL), SWS, and REM sleep. Eight participants did not have sleep continuity and architecture data because of acquisition errors.
CD14+ Intracellular Cytokine Production. TLR-4 stimulated monocyte intracellular production of IL-6 and TNF-α was assessed using a flow cytometric approach reported previously.10 Heparin-treated whole blood was stimulated using 100 pg/mL LPS (Sigma, St. Louis, MO, USA) and incubated for 4 h at 37°C in the presence of brefeldin A to retain newly produced cytokines within the cell. A parallel sample was incubated without addition of LPS to quantify unstimulated levels, representative of levels of in vivo activation. Red blood cell lysis was then performed. Monocytes were identified using Peridinin chlorophyll protein (PerCP)-CD14 antibodies and stained for intracellular cytokines: Phycoerythrin (PE)-IL-6 and Allophycocyanin (APC)-TNF (BD Biosciences, San Jose, CA). Approximately 80,000 total events were acquired through the flow cytometer (FACScan, BD Biosciences) and percentage of monocytes positive for TNF-α and/or IL-6 was computed using quadrant statistics on CellQuest Pro Software (BD Biosciences).
Analytic Strategy
IBM SPSS statistical software v. 22 was used to perform all statistical analyses. Independent sample T tests were performed to examine differences between groups in BMI, PSQI scores, educational attainment, and BDI scores. To test for the effect of PSD on monocyte production of inflammatory cytokines, repeated measures mixed-model analyses of variance with a 2 (age group: younger, older) × 3 (night: baseline, PSD, recovery) design, controlling for BMI and BDI scores was used. To examine differences between groups in sleep continuity and sleep architecture from baseline to recovery night, repeated measures mixed-model analyses of variance with a 2 (younger, older) × 2 (baseline, recovery) design, controlling for BMI and BDI scores, was used. The mixed model allows for values to be missing as long as they are occurring at random (i.e., missingness is not dependent on the value of unobserved variables); model estimates are unbiased under these conditions. Change in sleep continuity and architecture was computed by subtracting baseline from PSD and recovery night values. Change scores were used as an additional covariate, and missing values were replaced with group means in the model to retain sample size of the original model. Independent sample t tests were used to test differences between age groups within each night, and pairwise comparisons were used to test within-group differences between nights.
RESULTS
Demographic and clinical characteristics of the two groups are presented in Table 1. The younger age group (n = 21) had a similar distribution to the older age group (n = 49) in sex, PSQI global scores, and educational attainment, with no statistically significant mean differences at the P < 0.05 level. Mean depressive symptom scores (determined using the BDI) and average BMI were both elevated in the older age group compared to the younger age group, although not statistically different (P > 0.05). Nevertheless, to adjust for the possible contribution of depressive symptomatology and body mass on the age-related differences, subsequent analyses included depressive symptom scores and BMI as covariates in the model.
Table 1.
Demographic and clinical characteristics of participants by age group.
Sleep Deprivation and TLR-4 Activation of Inflammation
Consistent with our previous findings, there was a significant main effect of night, such that PSD increased the percentage of monocytes producing TNF-α and/or IL-6, (F(2,121.8) = 3.8, P < 0.05). Additionally, there was a significant main effect of age group on monocytic production of TNF- α and/or IL-6 (F(1,65.4) = 13.8, P < 0.001) and a significant interaction of age group × night (F(2,121.8) = 4.0, P < 0.05). After a night of PSD and recovery sleep, younger subjects (less than 40 y) showed robust increases in TLR-4 activation of monocytic production of TNF-α and/or IL-6 compared to baseline (P < 0.05), with no change in the older subjects (60 y or older) (P > 0.5) (Figure 2). Exploratory analyses examined the role of sex, and found no significant effect of gender on the age × night interaction (P > 0.50). Unstimulated production of TNF-α and/or IL-6 did not change over time nor differ between groups (data not shown).
Figure 2.
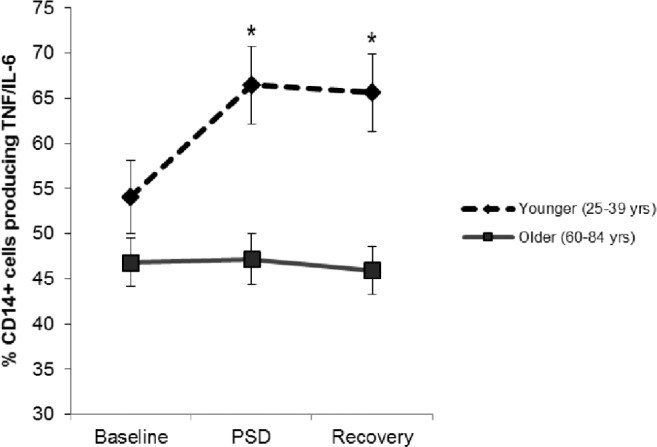
Estimated mean (standard error) percentage of monocytes expressing TNF-α and/or IL-6 the morning after a normal night of sleep (baseline), partial sleep deprivation (PSD), and a subsequent normal night of sleep (recovery) by age group. Asterisk indicates significant difference between groups, P < 0.05. TNF-α, tumor necrosis factor-α.
Age Differences in Sleep Continuity
Mixed linear model analyses, adjusting for BDI and BMI, tested differences in sleep continuity measures between age groups from baseline to recovery sleep (see Table 2 for estimated marginal means). There were no significant baseline differences in any of the four sleep continuity measures between younger and older adults (all P > 0.20). For WASO, SOL, and SE, there were no main effects of age group or night, nor were there any significant interactions of age by night (all P > 0.20). For TST overall, there was a significant main effect of age (F(1,61.6) = 6.44, P = 0.01) with less TST in older compared to younger adults. Whereas overall TST did not change from baseline to recovery sleep (F(1,62.7) = 2.43, P = 0.12), there was a significant interaction of age × night (F(1,62.68) = 4.34, P = 0.04; Figure 3), in which younger adults had longer TST at recovery night as compared to older adults (t = 2.6, P = 0.01). Covarying for change in TST from baseline to PSD or to recovery in the two age groups did not alter the effects of PSD on TLR-4 activation of monocytic production of IL-6 and/or TNF-α. We observed no significant time, age group, or time by age group interaction effects in SWS. There was a significant age group main effect in REM sleep (F(1,53.1) = 7.58, P = 0.008), with slightly higher % in REM among younger compared to older adults. There was also a main effect of time on REM (F(1,54.67) = 7.03, P = 0.01), with increases in REM from baseline to recovery in both groups. The interaction was not significant (P = 0.82). Estimated marginal means and standard errors can be found in Table 2. Change in REM from baseline to PSD and baseline to recovery nights of sleep in the two age groups did not alter the interaction effects of age with PSD on TLR-4 activation of monocytic production of IL-6 and/or TNF-α.
Table 2.
Estimated marginal means (standard error) of polysomnography recordings of sleep continuity during baseline and recovery sleep adjusted for body mass index and Beck Depression Inventory.
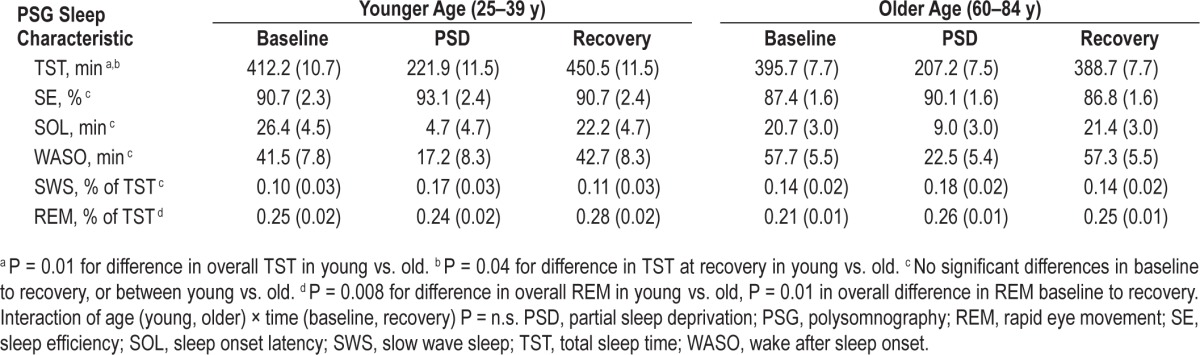
Figure 3.
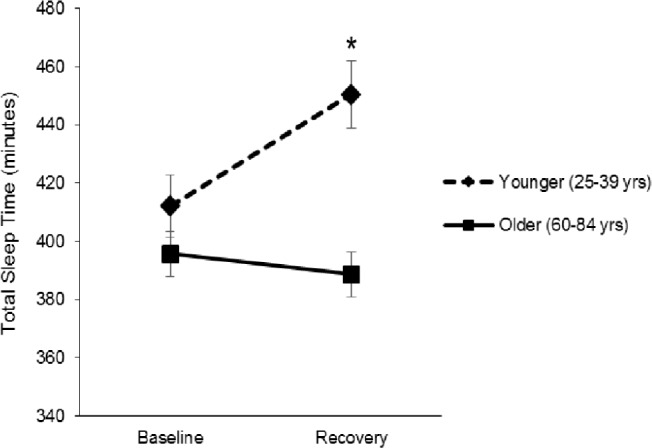
Estimated mean (standard error) total sleep time at baseline and recovery by age group. Asterisk indicates significant difference between groups, P < 0.05.
DISCUSSION
In the current analyses, 1 night of partial sleep loss caused a marked increase in TLR-4 stimulated monocytic production of proinflammatory cytokines, consistent with our previous findings.10 However, activation of cellular inflammation in response to sleep loss substantially differed by age. Whereas young adults showed robust increases in TLR-4 induced monocytic production of inflammatory cytokines after a night of PSD, older adults did not. These data provide the first evidence that older adults do not show activation of this component of innate immunity in response to one night of sleep loss seen in younger individuals.
Our results are contrasted to findings that a night of poor sleep in older adults increases a circulating marker of inflammation (IL-6).32 Whereas the current analyses assess intracellular inflammatory cytokine production after in vitro ligation of the TLR-4 receptor, circulating cytokines are a marker of systemic inflammation derived from numerous cellular sources. This difference in source likely accounts for the difference in findings, and future research should also consider age differences in systemic inflammatory cytokines after sleep loss.
In addition to the lack of TLR-4 mediated immune response to sleep loss, older age was independently associated with a general decline in responsiveness to TLR-4 activation at baseline. This is consistent with other findings that show age-related declines in immune function, such as reduced TLR production and responsiveness in monocytes and reduced T cell proliferative potential.33 Our findings showing lower baseline inflammatory response to TLR-4 activation in older adults parallels animal research, in which older mice have reduced macrophage production of proinflammatory cytokines TNF-α and IL-6 after LPS exposure. This mouse model further confirmed that this was the consequence of changes in mitogen-activated protein kinase (MAPK) expression and transcriptional regulation.21 Lowered response to bacterial stimulation indicates impaired immunity, highlighting a potential pathway through which older adults may become more vulnerable to viral and bacterial infections.
In our sample, older adults also appear to have impairment in the ability to recover sleep amounts in response to PSD. Sleep amounts typically increase during recovery sleep following a night of sleep loss.34–36 In the current analyses, younger subjects showed increases in TST averaging around 38 min. In contrast, older subjects decreased TST an average of 7 min (Table 2, Figure 3). Extension of TST during recovery sleep in older adults has been reported after total sleep deprivation or sleep fragmentation.34–37 Our selection criteria restricted the sample of older adults to healthy, normal sleepers, which may not be comparable to other older adult populations. Differences in TST in response to PSD between the two age groups did not account for the observed differences in TLR-4 stimulated monocytic production of proinflammatory cytokines. Both younger and older individuals showed significant increases in percentage of time spent in REM sleep from baseline to recovery nights, with older adults having slightly lower overall means; however, this did not account for the observed differences in proinflammatory cytokine production.
A meta-analytic study of sleep continuity and architecture across the life span reports that older individuals typically show declines in SWS and REM along with increases in WASO and stage 1 and 2 sleep.38 However, this study also noted that the effect sizes were reduced when factors such as sleep apnea and comorbidities were considered. Given our sample of older adults were only included if they exhibited regular sleep duration without phase shifting and did not have sleep apnea or inflammatory diseases, the similar sleep patterns we observed in young and older adults should be reviewed within this context.
Our finding showing that, in younger adults, sleep deprivation affects inflammatory responses to a subsequent immune challenge is similar to the findings showing that stress can prime innate immune cells to mount a greater inflammatory response to subsequent LPS challenge.39 Stress-induced priming of an inflammatory response is thought to be evolutionarily adaptive in the short term, while detrimental in the long term (e.g., inflammatory diseases of aging).39,40 Similarly, sleep loss-induced priming of inflammatory responses may also be adaptive in the short term, in that disruptions in sleep might serve as a danger signal—there is threat to survival (e.g., predation, hostile conspecific) and/or a need for protection from pathogen exposure. In other words, priming of the innate immune system for subsequent pathogen encounters would maximize fitness in an environment where imminent threat of bacterial exposure is of high probability. Indeed, this is consistent with findings showing that sleep deprivation in Drosophila prior to exposure to bacterial infection enhanced bacterial clearance and survival compared to those not sleep deprived.41 The immune enhancing effect of sleep deprivation in this study was mediated through increased nuclear factor kappa B (NFkB) signaling, a transcription factor activated by TLR-4 and a key regulator of inflammatory cytokine gene expression. This is consistent with our result showing enhanced TLR-4 mediated inflammatory cytokine expression after PSD in younger adults, which was not present in older adults.
The intracellular inflammatory response pattern to short-term sleep loss appears to be altered with age, consistent with evidence that the aged immune system has a generally compromised immune response to invading pathogens and elevated infection risk. This aging of the immune system is caused in part by a greater proportion of cells having reached a senescent (end stage with reduced functionality and an inability to replicate) or near-senescent state.42–44 Another role of the TLR-mediated activation of innate immune system response is its ability to enhance the adaptive immune defense to pathogens.19,20 Indeed, TLR stimulation is currently being proposed as an adjuvant to vaccines to activate the innate immune system, which in turn enhances the adaptive immune system response to the invading viral pathogens (e.g., vaccine).19 A declining TLR–mediated activation may put older individuals at elevated risk for poor vaccination responses. Our findings have important implications for understanding the role of aging on both innate and adaptive immune responses.
Although cortisol was not measured in the current study, there is existing evidence that sleep deprivation reduces morning cortisol levels.22,45,46 In contrast to younger adults, older adults are reported to have elevated nocturnal cortisol levels, which are associated with a lack of nocturnal rise in TNF-α.32 Given the anti-inflammatory properties of cortisol, it is feasible that the blunted TLR-4 activation in older adults is the consequence of elevated cortisol. Future research should consider this possible mechanism.47,48
In considering potential limitations to the current analyses, depressive symptom scores were elevated in older compared to younger subjects, although the difference was not statistically significant. Depression is associated with elevated inflammation49; however, our analyses control for depressive symptoms and major depression was an exclusion criterion. It therefore is doubtful that depression is contributing to the observed age differences in TLR-4 stimulated inflammation. Another limitation in the current study is a single time-point assessment of TLR-4 activated monocyte responsiveness, and future research should consider whether differences by age are present across the daytime and nighttime hours. Likewise, to minimize the influence of circadian factors on TLR activation of cellular inflammation, a constant time in bed and time of blood draw were maintained, which did not allow for extension of sleep in the morning on the recovery night. Nevertheless, subjects in both groups had TST, on average, that was far less than the available time to sleep. However, extension of available time in bed during recovery might have further magnified the TST difference evident in the younger versus older adults at recovery.
Major strengths of the current study are our considerable sample size for PSD research (n = 70), which includes a 4-night stay at a clinical research facility where subjects are monitored for sleep abnormalities and observed under controlled conditions, and a thorough screening for mental health and medical problems that might affect the immune system. Although population-based studies suggest that sleep duration and continuity is often compromised in later life,3 our sample of older adults was selected based on healthy sleep patterns, which is reflected in comparable PSQI scores between younger and older adults and similar baseline sleep continuity. Thus, the differences observed in inflammatory responses are not likely caused by differences in current average sleep quality. However, this may limit the generalizability of our observations as the current sample is less representative of the older adult population in terms of sleep characteristics.
Sleep loss has been shown to have a detrimental effect on numerous system regulators beyond immunity50; however, the majority of evidence links sleep loss to changes in these parameters in young healthy subjects, with limited research on these biological regulatory systems in older adults. Given the prevalence of sleep complaints among older adults, additional research is warranted to address the question of whether sleep loss has differential effects in older compared to younger individuals.
SUMMARY
In summary, our findings document significant increases in TLR-4 activated monocyte intracellular production of inflammatory cytokines after 1 night of partial sleep deprivation in younger (25–39 y) but not older (60–84 y) adults. These differences by age are similar to findings in animal models showing declines in TLR-4 mediated macrophage responses with age and suggest that older adults exhibit a reduced immune response that, unlike younger adults, is not activated after a night of partial sleep loss.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported in part by the National Institute of Health grants supported by R01-AG034588; R01-AG026364; R01 CA160245-01; R01-CA119159; R01 HL095799; R01 DA032922-01; P30-AG028748 to MRI; and UCLA CTSI UL1TR000124, and the Cousins Center for Psychoneuroimmunology. The manuscript preparation was supported by grant T32-MH19925, the American Sleep Medicine Foundation Bridge to K Award, and the Cousins Center for Psychoneuroimmunology, UCLA (J.E.C). Dr. Seeman served on an advisory board for a NIH funded National Health and Aging Trends study and received honoraria. She also received honoraria for giving a keynote speech at the University of Alabama, Birmingham Health Disparities conference. The other authors have indicated no financial conflicts of interest. The work was performed at the University of California, Los Angeles.
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants for their valuable contributions.
REFERENCES
- 1.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31:1087–96. [PMC free article] [PubMed] [Google Scholar]
- 2.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 3.Vaz Fragoso CA, Gill TM. Sleep complaints in community-living older persons: a multifactorial geriatric syndrome. J Am Geriatr Soc. 2007;55:1853–66. doi: 10.1111/j.1532-5415.2007.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinges DF, Douglas SD, Hamarman S, Zaugg L, Kapoor S. Sleep deprivation and human immune function. Adv Neuroimmunol. 1995;5:97–110. doi: 10.1016/0960-5428(95)00002-j. [DOI] [PubMed] [Google Scholar]
- 5.Besedovsky L, Lange T, Born J. Sleep and immune function. Eur J Physiol. 2012;463:121–37. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 7.Prather AA, Hall M, Fury JM, et al. Sleep and antibody response to hepatitis B vaccination. Sleep. 2012;35:1063–9. doi: 10.5665/sleep.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65:831–5. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–7. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 11.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–7. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pr Res Clin Endocrinol Metab. 2010;24:775–84. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–84. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- 18.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 19.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–4. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 21.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–9. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 23.Frey DJ, Fleshner M, Wright KP. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 25.Irwin MR, Levin MJ, Carrillo C, et al. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain Behav Immun. 2011;25:759–66. doi: 10.1016/j.bbi.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun. 2004;18:349–60. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Redwine L. Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom Med. 2003;65:75–85. doi: 10.1097/01.psy.0000038943.33335.d2. [DOI] [PubMed] [Google Scholar]
- 28.Redwine L. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 30.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Rechtschaffen A, Kales A. Los Angeles, CA: UCLA Brain Information Services; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 32.Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–95. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 33.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–9. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds CF, Kupfer DJ, Hoch CC, Stack JA, Houck PR, Berman SR. Sleep deprivation in healthy elderly men and women: effects on mood and on sleep during recovery. Sleep. 1986;9:492–501. doi: 10.1093/sleep/9.4.492. [DOI] [PubMed] [Google Scholar]
- 35.Bonnet MH, Rosa RR. Sleep and performance in young adults and older. Biol Psychol. 1987;25:153–72. doi: 10.1016/0301-0511(87)90035-4. [DOI] [PubMed] [Google Scholar]
- 36.Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51:632–41. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet MH. The effect of sleep fragmentation on sleep and performance in younger and older subjects. Neurobiol Aging. 1989;10:21–5. doi: 10.1016/s0197-4580(89)80006-5. [DOI] [PubMed] [Google Scholar]
- 38.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello M V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 39.Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun. 2013;33:1–6. doi: 10.1016/j.bbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo T-H, Williams JA. Acute sleep deprivation enhances post-infection sleep and promotes survival during bacterial infection in Drosophila. Sleep. 2014;37:859–69. doi: 10.5665/sleep.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murillo-Ortiz B, Albarrán-Tamayo F, López-Briones S, Martínez-Garza S, Benítez-Bribiesca L, Arenas-Aranda D. Increased telomere length and proliferative potential in peripheral blood mononuclear cells of adults of different ages stimulated with concanavalin A. BMC Geriatr. 2013;13:99. doi: 10.1186/1471-2318-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Effros RB. The role of CD8 T cell replicative senescence in human aging. Discov Med. 2005;5:293–7. [PubMed] [Google Scholar]
- 44.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19:1680–98. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Pejovic S, Basta M, Vgontzas AN, et al. Effects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performance. Am J Physiol Endocrinol Metab. 2013;305:E890–6. doi: 10.1152/ajpendo.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khapre RV, Samsa WE, Kondratov RV. Circadian regulation of cell cycle: molecular connections between aging and the circadian clock. Ann Med. 2010;42:404–15. doi: 10.3109/07853890.2010.499134. [DOI] [PubMed] [Google Scholar]
- 48.Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 2011;3:479–93. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 50.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]