Abstract
Study Objectives:
Sleep apnea (SA) is characterized by apnea during sleep and is associated with cardiovascular diseases and an increase in all-cause mortality. Chronic kidney disease (CKD) is a global health problem that has placed a substantial burden on healthcare resources. However, the relationship between SA and the incidence of CKD is not clear. This study aimed to determine whether SA is an independent risk factor for the development of CKD.
Design:
Retrospective cohort study.
Setting:
National Health Insurance Research Database (NHIRD) of Taiwan.
Patients or Participants:
A total of 4,674 adult patients (age ≥ 30 y) in whom SA was newly diagnosed from 2000 to 2010 were included, together with 23,370 non-SA patients as the comparison group. The two groups were frequency-matched for sex, age, and year of receiving medical service. Each individual was followed until 2011.
Interventions:
N/A.
Measurements and Results:
These two groups were monitored and observed for the occurrence of CKD. Patients with SA experienced a 1.94-fold increase (95% confidence interval [CI], 1.52–2.46; P < 0.001) in the incidence of CKD, which was independent of sex, age, and comorbid medical conditions. Additionally, they showed a 2.2-fold increase (95% CI, 1.31–3.69; P < 0.01) in the incidence of end-stage renal disease (ESRD).
Conclusions:
Patients with sleep apnea are at increased risk for chronic kidney disease and end-stage renal disease compared with the general population. As such, screening renal function and treatment of chronic kidney disease is an important issue in patients with sleep apnea.
Citation:
Lee YC, Hung SY, Wang HK, Lin CW, Wang HH, Chen SW, Chang MY, Ho LC, Chen YT, Liou HH, Tsai TC, Tseng SH, Wang WM, Lin SH, Chiou YY. Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. SLEEP 2015;38(2):213–221.
Keywords: chronic kidney disease, cohort study, end-stage renal disease, National Health Insurance, sleep apnea
INTRODUCTION
Sleep apnea (SA) has become a global issue in recent years.1 It is characterized by apnea resulting either from partial or complete obstruction of the upper airway during sleep, which is referred to as obstructive sleep apnea (OSA). It can also manifest as a loss of respiratory drive caused by a central neurologic problem, which is referred to as central sleep apnea (CSA). The most common form of SA is OSA, comprising more than 90% of cases, whereas CSA makes up less than 10%. SA can lead to significant hemodynamic instability, intermittent hypoxemia, and increased sympathetic activity.2 Previous studies have revealed that SA may induce an inflammatory state, increase blood pressure, and promote endothelial injury.3–7 Consequences of SA include excessive daytime somnolence, neuro-cognitive impairment, and increased risk for accidents related to sleep deprivation.8,9 In addition, SA has also been associated with a variety of cardiovascular diseases and noncardiovascular diseases, including hypertension, arrhythmia, stroke, coronary artery disease, diabetes, and chronic obstructive pulmonary disease (COPD).10,11
Chronic kidney disease (CKD) is a common condition in which there is a progressive loss of renal function over a period of months to years, eventually leading to the development of end-stage renal disease (ESRD).12 CKD is a major public health problem worldwide. In the United States, about 19 million adults have CKD and it is estimated that more than two million people will require dialysis or transplants by the year 2030.13 In Taiwan, the rapidly increasing number of persons with ESRD requiring dialysis and transplants has placed a substantial burden on healthcare resources.14
Despite this alarming increase in the incidence of CKD, to date, little is known about its correlation with SA. Although many studies have described a relationship between SA and CKD,15–32 most of these studies have been limited by cross-sectional study design, a relatively small sample size, the absence of an appropriate comparison group, and a lack of consideration of potential confounders in the regression model.
As such, the aim of our study was to determine whether SA is an independent risk factor for the development of CKD in a large-scale population-based cohort study.
METHODS
This study was designed as a retrospective cohort study. In this longitudinal observational study, we enrolled a group of people who exhibited SA within a defined period as the SA study cohort (SA group). From the same population, we also enrolled a group of people who did not exhibit SA as a comparison cohort (non-SA comparison group). Then, we followed the outcomes of both groups, including CKD and ESRD, over time.
Database
The National Health Insurance (NHI) of Taiwan is a universal health insurance plan that has been in operation since 1995. The NHI of Taiwan provides coverage to almost 97% of the nation's population of 23 million.33 The National Health Insurance Research Database (NHIRD) of Taiwan has used the claims data from the NHI to extract numerous database sets for researchers.34 The data used in this study were obtained from the Longitudinal Health Insurance Database (LHID) 2000, which includes one million insured people randomly selected from the total population covered by the NHI. This database covered NHI claims data from 1996 to 2011. There were no statistically significant differences in age, sex, and healthcare costs between the sample group and all enrollees, as reported by the NHIRD.35 This database has previously been used for epidemiological research, and information on prescription use, diagnoses, and hospitalizations has been shown to be of high quality.36–40 The identification numbers of all patients have been encrypted for personal privacy.
Study Sample
This retrospective cohort study consisted of both study and comparison groups. Diagnosis codes were assigned by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Initially, patients with diagnoses of insomnia with SA, unspecified (780.51); hypersomnia with SA, unspecified (780.53); or unspecified SA (780.57) between January 2000 and December 2010 were included as the SA group.41 In the enrollment of patients with SA, we excluded patients who already had an SA diagnosis before January 2000. We also excluded patients younger than age 30 y to focus on a high-risk population and assess its risk of developing CKD and ESRD following a diagnosis of SA. In addition, patients with a diagnosis of renal disease (ICD-9-CM codes 250.4, 274.1, 283.11, 403, 404, 405.01, 405.11, 405.91, 440.1, 442.1, 447.3, 572.4, 580–589, 642.1, and 646.2, 753.1 and 791.0) before enrollment were also excluded from the study. Finally, a total 4,674 patients in whom SA was newly diagnosed were included in this cohort study (Figure 1). Each individual was followed until 2011.
Figure 1.
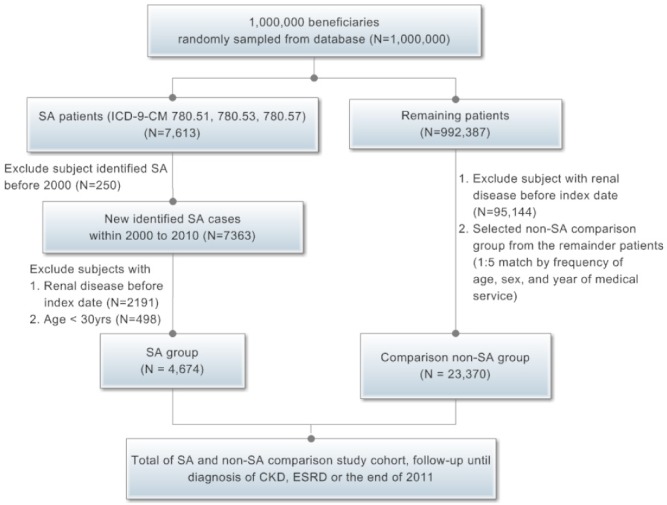
Study flow chart. CKD, chronic kidney disease; ESRD, end-stage renal disease; ICD-9-CM, International Classification of Diseases, 9th Revision Clinical Modification; SA, sleep apnea.
The comparison group was selected from the remaining population in the database. First, we excluded patients in whom SA was diagnosed during the included period (years of receiving medical service) and patients with preexisting renal disease before enrollment. Then, to ensure an equal distribution of variables between groups, we selected comparison group patients from the remaining people by frequency matching within the following strata: sex, age (three categories, 30–44, 45–60 and > 60 y) and year of receiving medical service at a ratio of 1: 5 for each patient with SA. For example, we enrolled 51 male patients (1.09 %) aged 30–44 y in the year 2000 (year of receiving medical service) in the SA group, and also selected 255 male patients (1.09 %) aged 30–44 y in the same year of receiving medical service in the non-SA comparison group. Finally, we enrolled 23,370 in the comparison group.
Matching
In our study, we used frequency matching, also known as category or group matching, to ensure an equal distribution of variables between study groups. In the current study, frequency matching was applied to ensure that the SA group and non-SA comparison group had the same distributions over strata defined by gender, age and year of receiving medical service.42–46
Potential Confounders
We used enrollee category (EC) as a proxy measure of socioeconomic status to classify people into four subgroups: EC 1 (e.g., full-time or regularly paid personnel in public schools and governmental agencies, civil servants), EC 2 (employees of privately owned enterprises), EC 3 (other employees or paid personnel, members of the farmers or fishers associations, and self-employed), and EC 4 (members of low-income families, substitute service draftees, and veterans). The payroll-related cost of health insurance was most expensive in EC 1, followed by EC 2, EC 3, and least expensive for EC 4, on average.
In addition, to evaluate the associations linking urbanization level and chronic kidney disease, the townships were classified into three categories: urban, suburban, and rural areas based on five indices: population density, percentages of residents who were agricultural workers, the number of physicians per 100,000 people, percentages of residents with college or higher education, and percentages of residents aged 65 y or older.47 In general, residents in urban and suburban areas have a higher socioeconomic status.
For all individuals in both groups, we identified potential confounding risk factors for CKD, including hypertension (HTN), diabetes mellitus (DM), atherosclerotic vascular disease (ASVD), hyperlipidemia, nephrolithiasis, chronic hepatitis, diseases of the musculoskeletal system and connective tissue (MSCT), gout, and obesity.48–54 We also adjusted for COPD in multivariate analysis because this disease is highly associated with smoking, which is regarded as a risk factor for CKD.55
Main Outcome Measure
The endpoint of the study was defined as CKD (ICD-9-CM code 585), ESRD (catastrophic illness registration cards for ESRD with ICD-9-CM code 585).56–59 In Taiwan, a patient with ESRD and who requires long-term dialysis is generally qualified by NHI to apply for a catastrophic illness card. Patients who have catastrophic illness registration cards of ESRD do not need to pay copayments of dialysis therapy.60
Validation
We validated the ICD-9-CM codes for the identification of SA and CKD by analyzing the medical records (charts) of 100 patients in E-DA Hospital, a 900-bed teaching hospital in Taiwan. We randomly selected 50 patients who had SA ICD-9-CM codes 780.51, 780.53, and 780.57; and 50 patients who had CKD ICD-9-CM code 585 from the inpatient and outpatient claims database between January 2008 and December 2010 in E-DA Hospital. The contents of this database were similar to those of the NHIRD. The clinical diagnosis of SA was ascertained by the International Classification of Sleep Disorders (ICSD) definition, which includes the apnea-hypopnea index (AHI) ≥ 15/h or an AHI ≥ 5/h with documented SA-related symptoms.61 Clinical diagnosis of CKD was determined according to the definition of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI), which includes the presence of kidney damage (pathological abnormality or markers of kidney damage) or glomerular filtration rate < 60 mL/min/1.73 m2 for more than 3 mo.49,62 Positive predictive values of both diseases were estimated. The results showed a positive predictive value of 98% (95% confidence interval [CI], 94.1–100%) for CKD and 88% (95% CI, 79.0– 97.0%) for SA.
Statistical Analysis
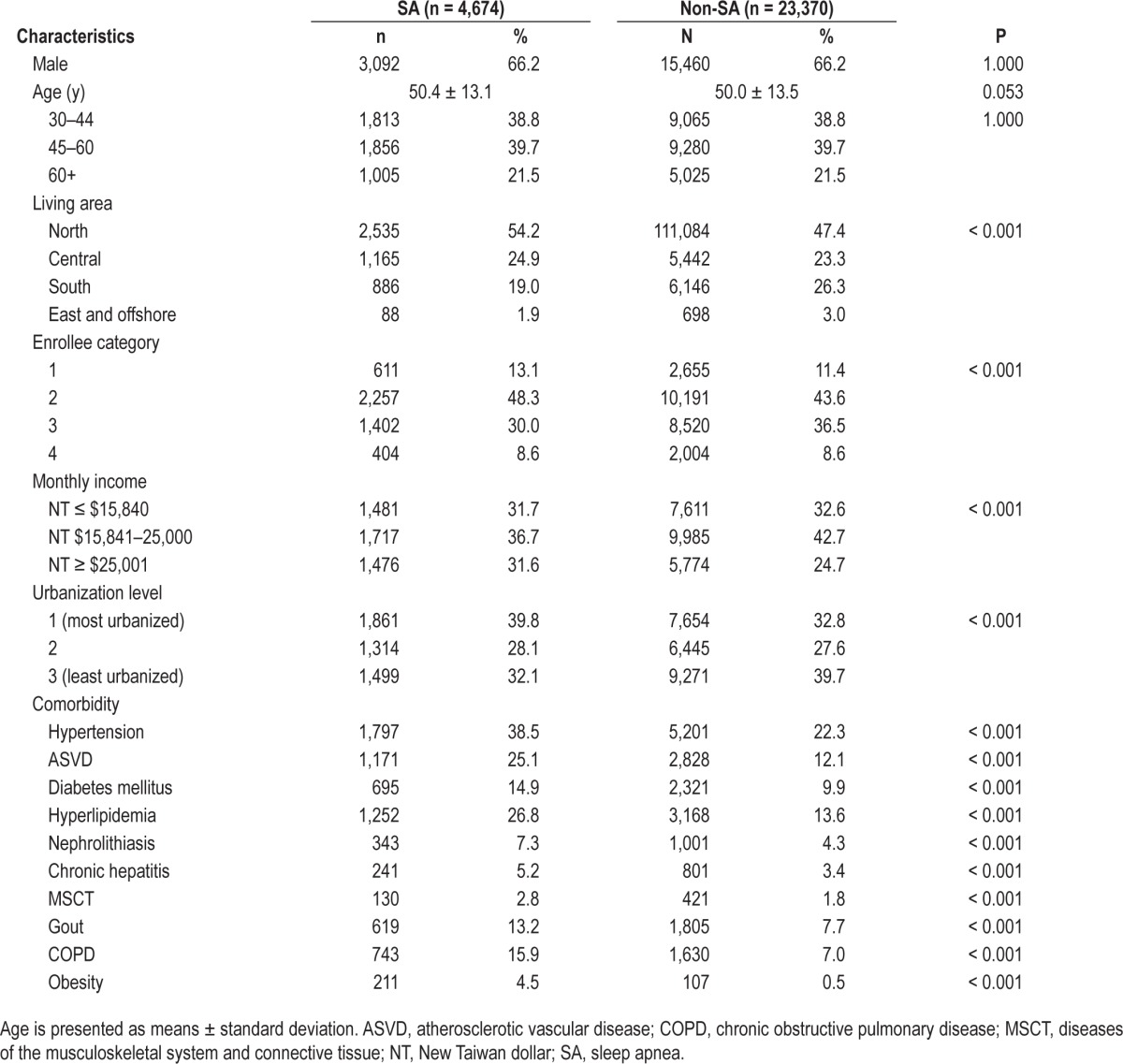
In Table 1, we used Pearson χ2 tests to compare the demographic and clinical characteristics and comorbidities between patients in the SA group and the non-SA comparison group.
Table 1.
Demographic information and premorbid comorbidities for the cohort of sampled patients.
Tables 2 and 3 show the crude and adjusted hazard ratios (HRs) of CKD and ESRD. The HRs were analyzed using Cox regression model and adjustments were made for age group, geographic locations, enrollee categories, income, urbanization level, and comorbidities, including HTN, DM, ASVD, hyperlipidemia, nephrolithiasis, chronic hepatitis, diseases of the MSCT, gout, obesity, and COPD. After testing proportional hazards (PH) assumption for a Cox regression model fit, the results showed that the sex variable does not satisfy PH assumptions. Therefore, stratified Cox regression was applied for controlling sex.63 In addition to using frequency matching, age had been also adjusted in the regression model to avoid potential confounding. We also used competing-risk models to adjusted risk of death because death may act as a competing risk for CKD and ESRD (R package “cmprsk”).64,65
Table 2.
Multivariable-adjusted Cox regression models and multivariable-adjusted competing-risk regression models hazard ratios of chronic kidney disease among the cohort of sampled patients during the follow-up years
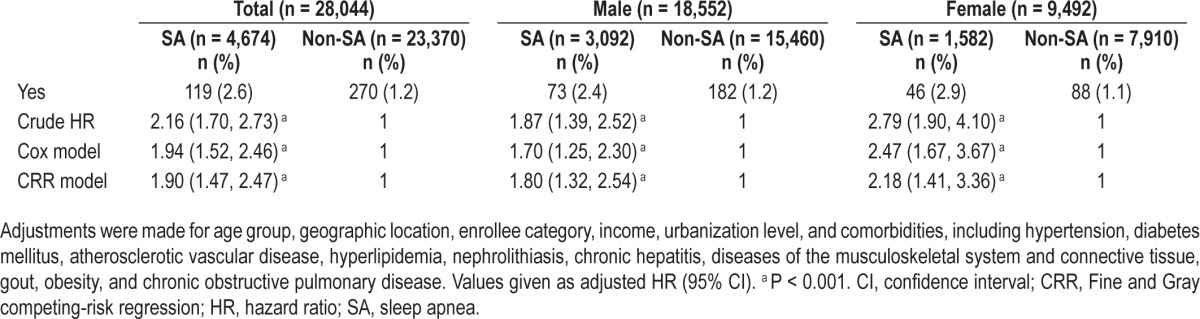
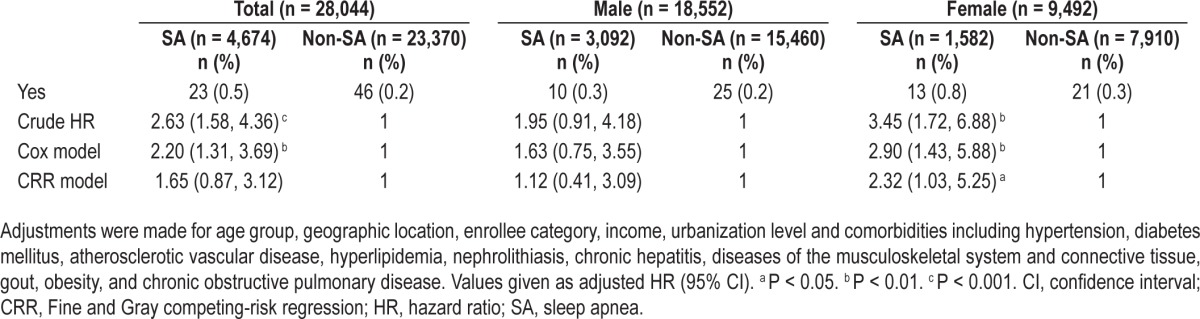
Table 3.
Multivariable-adjusted Cox regression models and multivariable-adjusted competing-risk regression models hazard ratios of end-stage renal disease among the cohort of sampled patients during the follow-up years
Tables 4 and 5 show the crude and adjusted HRs of CKD and ESRD among the different sex and age groups. These HRs were analyzed using Cox regression model and adjustments were made for geographic locations, enrollee categories, income, urbanization level, and selected comorbidities, as mentioned previously.
Table 4.
Multivariable-adjusted Cox regression models and multivariable-adjusted competing-risk regression models hazard ratios of chronic kidney disease among the cohort of sampled patients stratified by sex and age group
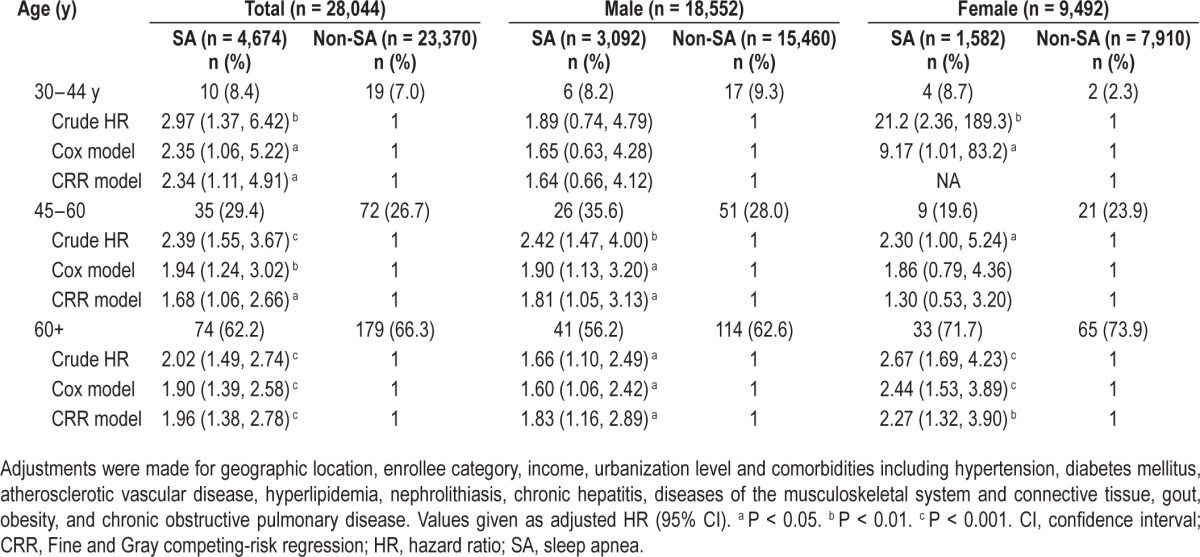
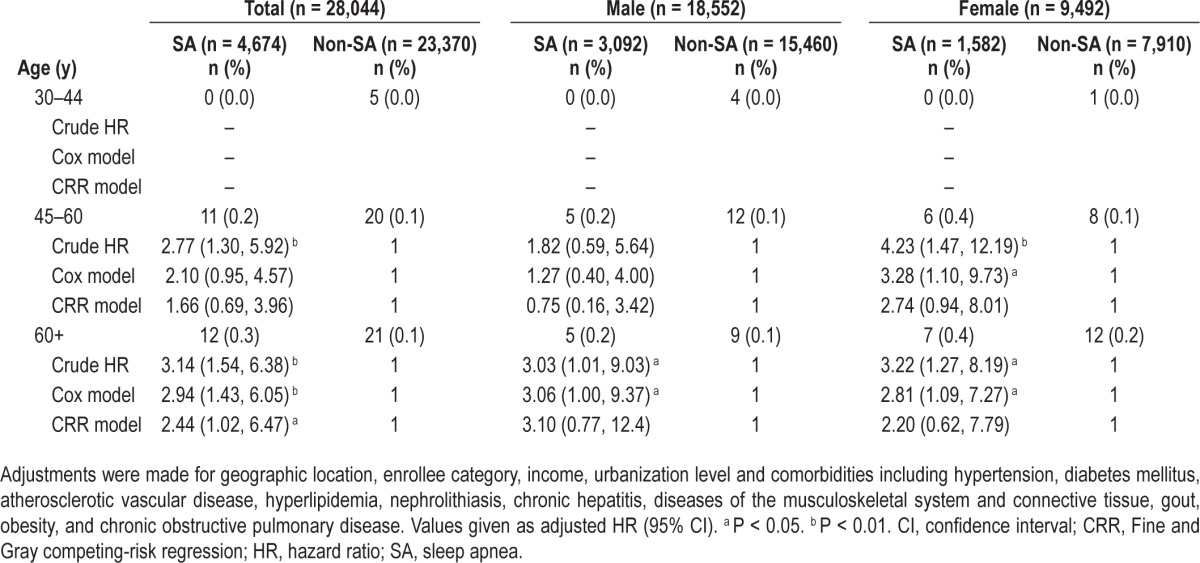
Table 5.
Multivariable-adjusted Cox regression models and multivariable-adjusted competing-risk regression models hazard ratios of end-stage renal disease among the cohort of sampled patients stratified by sex and age group.
A multicollinearity check was carried out and proved low variance inflation factor (VIF) values among all covariates. No potential multicollinearity problems were indicated. HRs and 95% CI were computed. P < 0.05 was considered to be statistically significant. All statistical analyses were conducted with SAS 9.3 statistical software (SAS Institute Inc., Cary, NC, USA).
The log-rank test also was used to compare the CKD and ESRD free survival rate distributions of the two groups (Figures 2 and 3).
Figure 2.
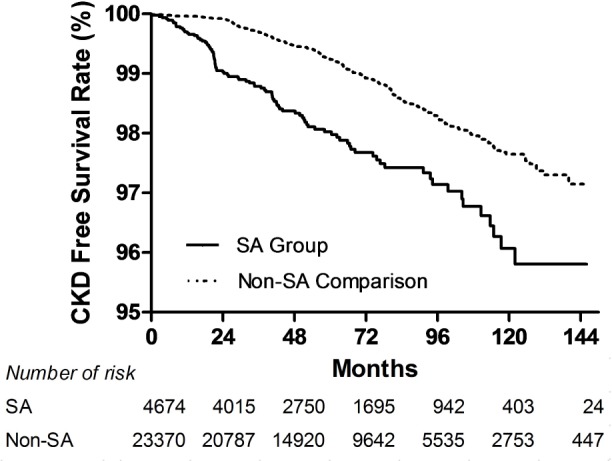
Kaplan-Meier curves of chronic kidney disease (CKD)–free survival rate in sleep apnea (SA) and non-SA comparison cohorts. (P < 0.001, unadjusted association.)
Figure 3.
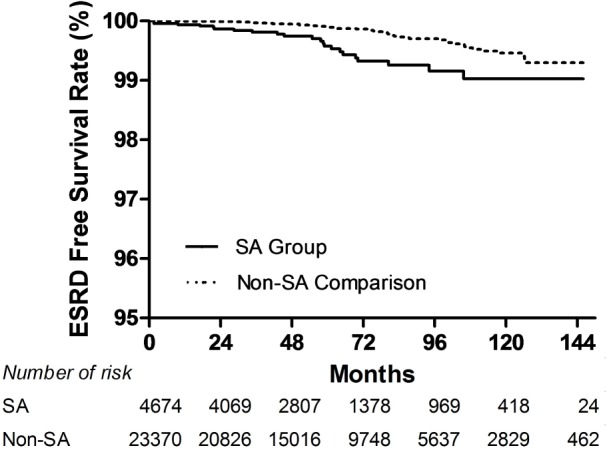
Kaplan-Meier curves of end-stage renal disease (ESRD)– free survival rate in sleep apnea (SA) and non-SA comparison cohorts. (P < 0.001, unadjusted association.)
Sensitivity Analyses
To investigate the effect of other potential residual confounding factors on the observed result, we used sensitivity analyses according to the R-package “obsSens”.66 In this analysis, we added another hypothetical unmeasured confounding factor with a similar risk effect as our disease. Then, we tested how this added factor confounded our observation with different prevalences in the SA and non-SA groups (Figures S1 and S2, supplemental material).
Ethics Statement
The study was approved by the ethics committee/institutional review board of National Cheng Kung University Hospital (IRB number: A-EX-102-009).
RESULTS
Table 1 presents the demographic characteristics and clinical comorbidities for all study patients, stratified by the presence of SA. Of the 28,044 total patients, 4,674 patients were included in the SA cohort and 23,370 patients in the non-SA comparison cohort. In the SA group, the distribution of diagnosis codes for SA were 1,732 patients (37.1%) with code 780.51, 1,693 patients (36.2%) with code 780.53, and 1,249 patients (26.7%) with code 780.57. The patients with SA had a significantly higher rate of HTN, DM, ASVD, hyperlipidemia, nephrolithiasis, chronic hepatitis, diseases of the MSCT, gout, COPD, and obesity. The average follow-up time was 61.9 ± 35.7 mo in the SA group and 65.5 ± 36.6 mo in the non-SA comparison group.
Table 2 shows the incidence of CKD during the follow-up period. Of the 28,044 total patients, CKD developed in 389 (1.3%) during the study period (119 [2.6%] of the patients with SA and 270 [1.2%] of the comparison patients). Table 2 also lists the crude and adjusted HRs of developing SA in the two groups. Patients with SA were more likely than non-SA comparison patients to develop CKD during the follow-up period (HR, 2.16; 95% CI, 1.70–2.73). After adjusting for age, sociodemographic characteristics, region of residence, and co-morbid clinical illnesses, the HR for developing CKD during the follow-up period was 1.94 (95% CI, 1.52–2.46) for patients with SA, as compared with patients in the comparison cohort. SA was found to be a significant CKD risk factor both for male and female patients; the adjusted HRs for CKD in men and women with SA were 1.70 (95% CI, 1.25–2.3) and 2.47 (95% CI, 1.67–3.67), respectively. A possible interaction effect of age and sex was tested in logistic regression with an interaction term (age × sex) and the results showed no interaction term was included. Further, if we take death as a competing risk factor for CKD, results of the competing-risk regression (CRR) model also showed the same result (P < 0.001).
Table 4 shows the results of final analysis by age group. All patients were included in the final analysis and were classified into three age groups: group 1, 30–44 y; group 2, 45–60 y; and group 3, older than 60 y. Patients with SA showed a significant risk for CKD in every age category, except in males group 1 and females group 2. However, using the Bonferroni method to adjust for multiple comparisons, only females group 3 showed a significant difference between patients in the SA group and non-SA comparison group. Results of the CRR model also showed the same result except for group 1 females because of insufficient data.
Figure 2 shows Kaplan-Meier curves of CKD free survival rate by group. The log-rank test showed that patients with SA had significantly lower 5-y CKD free survival rates than patients in the non-SA comparison group (P < 0.001).
Table 3 shows the incidence of ESRD during the follow-up period. Among all patients, ESRD developed in 69 with SA (0.2%) during the study period (23 [0.5%] and 46 [0.2%] of the non-SA comparison patients). After adjusting for age, sociodemographic characteristics, region of residence, and comorbidities, SA was associated with an HR of 2.20 (95% CI, 1.31–3.69) for ESRD. The incidence of ESRD was significantly increased in female patients but not in male patients, and the adjusted HRs were 1.63 (95% CI, 0.75–3.55) in males and 2.9 (95% CI, 1.43–5.88) in females, respectively. Further, if we take death as a competing risk factor for ESRD, the result of the CRR model indicated the incidence of ESRD was still significantly increased in the female group but not the male.
Table 5 shows the crude and adjusted HRs of ESRD in all patients stratified by sex and age group. All patients were classified into three age groups and analyzed separately. The results show that there were no ESRD patients in the SA group 1 (30–44 y). The incidence of ESRD was significantly increased in group 2 females (45–60 y) (P = 0.033) and for both males and females in group 3 (older than 60 y) (P = 0.049 for males and P = 0.033 for females). However, no significant differences were found in the aforementioned groups if Bonferroni method was used to adjust for multiple comparisons. The CRR model indicated the incidence of ESRD was still significantly increased in group 3.
Figure 3 shows Kaplan-Meier curves of ESRD free survival rate by group. The log-rank test showed that patients with SA had significantly lower 5-y ESRD free survival rates than patients in the non-SA comparison group (P < 0.001).
To investigate the effect of other potential residual confounding factors on the observed result, we also used sensitivity analysis to investigate the estimates trend of the SA group HR of CKD and ESRD on a multivariable-adjusted Cox regression model with the add-on of a residual confounding factor (Figures S1 and S2). For example, when all patients with SA had the add-on residual confounder (prevalence of the unmeasured confounder is 1.0) and none of the patients in the non-SA group had this residual confounder (prevalence of the unmeasured confounder is 0.0), the effect of SA would be a risk for CKD (HR = 9.7, the top line in Figure S1). Figure S1 shows that in almost all situations, patients who have SA had a higher risk of CKD occurrence relative to non-SA patients, even if an un-measured confounder exists. In addition, Figure S2 shows that in most situations, patients with SA had a higher risk of ESRD relative to non-SA patients.
DISCUSSION
We conducted a large-scale, retrospective cohort study using a nationwide database to examine the role of SA in the development of CKD. Our results showed that HRs of CKD in patients with SA were significantly greater than for those in the non-SA group in both the Cox and CRR models. Further, our results also showed that the HR of ESRD in patients with SA was significantly greater than for those in the non-SA group according to the Cox model. In summary, our results demonstrate that SA is independently associated with an increased incidence of CKD and ESRD.
Many previous studies have investigated the association between SA and renal function.15–32 Specifically, some cross-sectional studies have demonstrated a correlation between the urine albumin-to-creatinine ratio and the severity of SA or duration of nocturnal oxygenation desaturation.18,23,26,27 Two quasi-experi mental studies have found that continuous positive airway pressure therapy may reverse glomerular hyperfiltration in patients with SA.20,21 Most importantly, some cross-sectional studies observed a significant association between SA and CKD.15–19 Iseki et al.15 found a high prevalence of CKD among patients with sleep related breathing disorder (SRBD). That study included 1,624 patients with SRBD and 7,454 non-SRBD comparison subjects, and showed a higher prevalence of CKD in the SRBD group (30.5%) than in the comparison group (9.1%), with an adjusted odds ratio of 4.542 (P < 0.0001). However, the study did not adjust for other important confounding variables such as HTN and DM. Chou et al.18 found a high prevalence of CKD in patients with severe SA but without HTN and DM. In that study, the authors enrolled 40 patients with severe SA, and the results showed a significant positive correlation between SA severity and renal function impairment. Kanbay et al.19 enrolled 175 patients with suspected SA and without other cerebrovascular disease or lung disease with hypoxemia. These patients were classified into four groups—normal, mild, moderate, and severe SA—according to the AHI. The results showed that SA severity was related to CKD severity.19 The limitations of most of these studies were the small number of subjects and, in some studies, also a lack of adjustment for certain CKD risk factors, including hyperlipidemia, gout, and nephrolithiasis, in multivariable analysis. In addition, and most importantly, a cause-and-effect type of relationship between the two diseases could not be determined in these cross-sectional studies.
The mechanism by which SA may influence the incidence of CKD is not well understood and a multifactorial etiology is believed to be likely. There are several possible reasons why a patient with SA might have an increased risk of developing CKD. First, repetitive episodes of apnea, hypoxia, and hypercapnia in the patient with SA induce sympathetic nerve activity and increased the frequency of arterial blood pressure spikes.2,6
Second, when compared with a healthy population, patients with SA exhibit significant endothelial dysfunction due to elevated circulating levels of endothelin-1 (ET-1), combined with the increased vasoconstrictor response to ET-1.56–67 Third, many animal and human studies have demonstrated the occurrence of vascular inflammation in SA.68,69–72 Fourth, nuclear factor kappa B, a hypoxia-responsive transcription factor, has been shown to be upregulated in patients with SA.5,73,74 Also, reactive oxygen species, serum C-reactive protein, and some inflammatory cytokines such as interleukin-6, are elevated with intermittent hypoxia.72,75 Finally, vascular endothelial growth factor, which is a hypoxia-sensitive glycoprotein, is found at higher levels in patients with SA.76 In summary, SA induces sympathetic nerve activation and a corresponding increase in arterial blood pressure, endothelial dysfunction, and vascular inflammation, as well as a chronic inflammatory state, all of which may play a role in the multifactorial etiology of CKD.
In Table 4, we classified both male and female patients into three age groups, the results of which were inconsistent within each category, which is most likely caused by limited power (few events). The same explanation likely applies to the ERSD result in the Cox and CRR models, which were based on very few events (Tables 3 and 5); as such, we believe further studies are needed.
To our knowledge, this is the first cohort study that has used nationwide, population-based epidemiologic data to investigate whether SA is an independent risk factor for CKD. The strength of our study is that we enrolled large numbers of patients with SA and CKD from a nationwide database in Taiwan. The retrospective cohort design and large sample size provided considerable statistical power for detecting the differences between these two groups. Additionally, in our study, we adjusted for adequate CKD risk factors, including age and comorbid clinical illnesses such as HTN, DM, ASVD, hyperlipidemia, nephrolithiasis, chronic hepatitis, diseases of the MSCT, gout, and obesity.48–54
This study has several limitations. First, the diagnoses of SA, CKD, ESRD, and other comorbid medical conditions relied on administrative claims data and misclassification is possible. However, from previous epidemiological database studies, the quality of the NHIRD data is acceptable and we also validated the SA and CKD codes by chart review.38,77,78 Second, the clinical significance of CKD stage I (ICD-9-CM code 585.1) is much less in magnitude compared to CKD stage III-V (ICD-9-CM code 585.3–585.5). We only have code 585 to cover all CKD patients and we cannot capture codes 585.1–585.5. Therefore, we cannot know each patient's CKD stage. Third, frequent matching was used to control potential confounding, but the matching process itself may introduce bias. Fourth, certain personal information such as body mass index (BMI), medication history, and smoking status were not available in the administrative data of the NHIRD, and these factors may be important determinants in CKD progression.79–81 For example, some studies have reported that smoking and nonsteroidal anti-inflammatory drugs (NSAIDs) are risk factors for CKD, which if unmeasured may bias results if they differ between the two groups.79–81 To resolve the problem, we adjusted for gout and diseases of the MSCT in the multivariable analysis, not only because these diseases are risk factors for CKD but also because they are highly associated with NSAID use.82,83 We also adjusted for COPD because this disease is highly associated with smoking.55 In addition, we also adjusted for obesity (ICD-9-CM code 278.0) into the analysis instead of BMI. Another limitation is differences in the socioeconomic status matching process itself may introduce bias between SA and the comparison group, and there may be differences in medical care across groups, which could lead to a higher diagnosis rate of CKD because of increased surveillance in the SA group. So, in the analysis, we also adjusted for geographic locations, enrollee categories, income, and urbanization level. Also, according to the study of Kuo et al.,40 the prevalence of CKD in Northern Taiwan was significantly lower than in central and southern areas in Taiwan. However, in this study, the percentage of subjects from the northern area in the SA group was higher than that in the comparison group. Furthermore, the SA group had a higher number of EC1 and EC2 subjects with higher socioeconomic status, and previous epidemiology studies have shown that higher socioeconomic status is associated with lower CKD risk.84,85 The socioeconomic distribution between the SA and non-SA groups was different, favoring less CKD for SA. But in this study, patients with SA in fact had more CKD, thus reducing the likelihood that this socioeconomic difference affected the findings of this study.
Our study suggested that patients with SA are at an increased risk for CKD and ESRD compared with that in the general population. Until the current study, there has been no recommendation for routine renal function screening for patients with SA. Patients with SA should receive regular renal function evaluations to perhaps ameliorate CKD progression at the same time. In summary, we suggest that screening renal function and treatment of CKD is an important issue in patients with SA.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by NCKUEDA-10312 & EDAHP-103050 from Research Foundation of E-DA Hospital and National Cheng Kung University Hospital, Taiwan. The authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 167.
SUPPLEMENTAL MATERIAL
Sensitivity analysis with the add-on of an unmeasured residual confounding factor. This figure shows the estimates trend of the sleep apnea (SA) group hazard ratio of chronic kidney disease (CKD) on a multivariable-adjusted Cox regression model.
Sensitivity analysis with the add-on of an unmeasured residual confounding factor. This figure shows the estimates trend of the sleep apnea (SA) group hazard ratio of end-stage renal disease (ESRD) on a multivariable-adjusted Cox regression model.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Cric Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Dursunoglu N, Dursunoglu D. [Obstructive sleep apnea syndrome, endothelial dysfunction and coronary atherosclerosis] Tuberkuloz ve toraks. 2005;53:299–306. [PubMed] [Google Scholar]
- 3.Arnaud C, Dematteis M, Pepin JL, Baguet JP, Levy P. Obstructive sleep apnea, immuno-inflammation, and atherosclerosis. Semin Immunopathol. 2009;31:113–25. doi: 10.1007/s00281-009-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stinghen AE, Goncalves SM, Martines EG, et al. Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin Pract. 2009;111:c117–26. doi: 10.1159/000191205. [DOI] [PubMed] [Google Scholar]
- 5.Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–21. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lurie A. Hemodynamic and autonomic changes in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:171–95. doi: 10.1159/000325109. [DOI] [PubMed] [Google Scholar]
- 7.Caballo C, Palomo M, Cases A, et al. NFkappaB in the development of endothelial activation and damage in uremia: an in vitro approach. PLoS One. 2012;7:e43374. doi: 10.1371/journal.pone.0043374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez JM, Garcia AJ, 3rd, Anderson TM, et al. Central and peripheral factors contributing to obstructive sleep apneas. Respir Physiol Neurobiol. 2013;189:344–53. doi: 10.1016/j.resp.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 10.Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010;31:203–20. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg-Dotan S, Reuveni H, Tal A, et al. Increased prevalence of obstructive lung disease in patients with obstructive sleep apnea. Sleep Breath. 2014;18:69–75. doi: 10.1007/s11325-013-0850-3. [DOI] [PubMed] [Google Scholar]
- 12.Kalamas AG, Niemann CU. Patients with chronic kidney disease. Med Clin North Am. 2013;97:1109–22. doi: 10.1016/j.mcna.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–8. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 14.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 15.Iseki K, Tohyama K, Matsumoto T, Nakamura H. High Prevalence of chronic kidney disease among patients with sleep related breathing disorder (SRBD) Hypertens Res. 2008;31:249–55. doi: 10.1291/hypres.31.249. [DOI] [PubMed] [Google Scholar]
- 16.Sim JJ, Rasgon SA, Kujubu DA, et al. Sleep apnea in early and advanced chronic kidney disease: Kaiser Permanente Southern California cohort. Chest. 2009;135:710–6. doi: 10.1378/chest.08-2248. [DOI] [PubMed] [Google Scholar]
- 17.Fleischmann G, Fillafer G, Matterer H, Skrabal F, Kotanko P. Prevalence of chronic kidney disease in patients with suspected sleep apnoea. Nephrol Dial Transplant. 2010;25:181–6. doi: 10.1093/ndt/gfp403. [DOI] [PubMed] [Google Scholar]
- 18.Chou YT, Lee PH, Yang CT, et al. Obstructive sleep apnea: a stand-alone risk factor for chronic kidney disease. Nephrol Dial Transplant. 2011;26:2244–50. doi: 10.1093/ndt/gfq821. [DOI] [PubMed] [Google Scholar]
- 19.Kanbay A, Buyukoglan H, Ozdogan N, et al. Obstructive sleep apnea syndrome is related to the progression of chronic kidney disease. Int Urol Nephrol. 2012;44:535–9. doi: 10.1007/s11255-011-9927-8. [DOI] [PubMed] [Google Scholar]
- 20.Krieger J, Imbs JL, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med. 1988;148:1337–40. [PubMed] [Google Scholar]
- 21.Kinebuchi S, Kazama JJ, Satoh M, et al. Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin Sci (Lond) 2004;107:317–22. doi: 10.1042/CS20040074. [DOI] [PubMed] [Google Scholar]
- 22.Markou N, Kanakaki M, Myrianthefs P, et al. Sleep-disordered breathing in nondialyzed patients with chronic renal failure. Lung. 2006;184:43–9. doi: 10.1007/s00408-005-2563-2. [DOI] [PubMed] [Google Scholar]
- 23.Faulx MD, Storfer-Isser A, Kirchner HL, Jenny NS, Tracy RP, Redline S. Obstructive sleep apnea is associated with increased urinary albumin excretion. Sleep. 2007;30:923–9. doi: 10.1093/sleep/30.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canales MT, Lui LY, Taylor BC, et al. Renal function and sleep-disordered breathing in older men. Nephrol Dial Transplant. 2008;23:3908–14. doi: 10.1093/ndt/gfn364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markou N, Kanakaki M. Sleep-disordered breathing and reductions in renal function: are they associated in the elderly? Sleep Med. 2008;9:598–600. doi: 10.1016/j.sleep.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Tsioufis C, Thomopoulos C, Dimitriadis K, et al. Association of obstructive sleep apnea with urinary albumin excretion in essential hypertension: a cross-sectional study. Am J Kidney Dis. 2008;52:285–93. doi: 10.1053/j.ajkd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Ursavas A, Karadag M, Gullulu M, et al. Low-grade urinary albumin excretion in normotensive/non-diabetic obstructive sleep apnea patients. Sleep Breath. 2008;12:217–22. doi: 10.1007/s11325-008-0169-7. [DOI] [PubMed] [Google Scholar]
- 28.Canales MT, Paudel ML, Taylor BC, et al. Sleep-disordered breathing and urinary albumin excretion in older men. Sleep Breath. 2011;15:137–44. doi: 10.1007/s11325-010-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato K, Takata Y, Usui Y, et al. Severe obstructive sleep apnea increases cystatin C in clinically latent renal dysfunction. Respir Med. 2011;105:643–9. doi: 10.1016/j.rmed.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol. 2011;6:986–94. doi: 10.2215/CJN.05720710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakaguchi Y, Shoji T, Kawabata H, et al. High prevalence of obstructive sleep apnea and its association with renal function among nondialysis chronic kidney disease patients in Japan: a cross-sectional study. Clin J Am Soc Nephrol. 2011;6:995–1000. doi: 10.2215/CJN.08670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholl DD, Ahmed SB, Loewen AH, et al. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest. 2012;141:1422–30. doi: 10.1378/chest.11-1809. [DOI] [PubMed] [Google Scholar]
- 33.Chien IC, Chou YJ, Lin CH, Bih SH, Chou P. Prevalence of psychiatric disorders among National Health Insurance enrollees in Taiwan. Psychiatr Serv. 2004;55:691–7. doi: 10.1176/appi.ps.55.6.691. [DOI] [PubMed] [Google Scholar]
- 34.National health insurance research database. [updated 2003; cited 2013 Aug]. Available from: http://nhird.nhri.org.tw/
- 35.Longitudinal Health Insurance Database 2000. [cited 2013 Aug]. Available from: http://nhird.nhri.org.tw/en/Data_Subsets.html#S3.
- 36.Hsu YC, Lin JT, Chen TT, Wu MS, Wu CY. Long-term risk of recurrent peptic ulcer bleeding in patients with liver cirrhosis: a 10-year nationwide cohort study. Hepatology. 2012;56:698–705. doi: 10.1002/hep.25684. [DOI] [PubMed] [Google Scholar]
- 37.Hsu YC, Lin JT, Ho HJ, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 38.Tiao MM, Tsai SS, Kuo HW, Chen CL, Yang CY. Epidemiological features of biliary atresia in Taiwan, a national study 1996-2003. J Gastroenterol Hepatol. 2008;23:62–6. doi: 10.1111/j.1440-1746.2007.05114.x. [DOI] [PubMed] [Google Scholar]
- 39.Chiang CW, Chen CY, Chiu HF, Wu HL, Yang CY. Trends in the use of antihypertensive drugs by outpatients with diabetes in Taiwan, 1997-2003. Pharmacoepidemiol Drug Saf. 2007;16:412–21. doi: 10.1002/pds.1322. [DOI] [PubMed] [Google Scholar]
- 40.Kuo HW, Tsai SS, Tiao MM, Yang CY. Epidemiological features of CKD in Taiwan. Am J Kidney Dis. 2007;49:46–55. doi: 10.1053/j.ajkd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Kang JH, Lin HC. Obstructive sleep apnea and the risk of autoimmune diseases: a longitudinal population-based study. Sleep Med. 2012;13:583–8. doi: 10.1016/j.sleep.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 42.de Graaf MA, Jager KJ, Zoccali C, Dekker FW. Matching, an appealing method to avoid confounding? Nephron Clin Pract. 2011;118:c315–8. doi: 10.1159/000323136. [DOI] [PubMed] [Google Scholar]
- 43.Valery PC, Coory M, Stirling J, Green AC. Cancer diagnosis, treatment, and survival in Indigenous and non-Indigenous Australians: a matched cohort study. Lancet. 2006;367:1842–8. doi: 10.1016/S0140-6736(06)68806-5. [DOI] [PubMed] [Google Scholar]
- 44.Costanza MC. Matching. Preventive Med. 1995;24:425–33. doi: 10.1006/pmed.1995.1069. [DOI] [PubMed] [Google Scholar]
- 45.Wang HK, Lin SH, Sung PS, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012;83:1080–5. doi: 10.1136/jnnp-2012-302633. [DOI] [PubMed] [Google Scholar]
- 46.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 47.Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;14:1–22. [Google Scholar]
- 48.Kronbichler A, Mayer G. Renal involvement in autoimmune connective tissue diseases. BMC Med. 2013;11:95. doi: 10.1186/1741-7015-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 50.Fathallah-Shaykh SA, Cramer MT. Uric acid and the kidney. Pediatr Nephrol. 2014;29:999–1008. doi: 10.1007/s00467-013-2549-x. [DOI] [PubMed] [Google Scholar]
- 51.Keddis MT, Rule AD. Nephrolithiasis and loss of kidney function. Curr Opin Nephrol Hypertens. 2013;22:390–6. doi: 10.1097/MNH.0b013e32836214b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention (CDC) Prevalence of chronic kidney disease and associated risk factors--United States, 1999-2004. MMWR Morb Mortal Wkly Rept. 2007;56:161–5. [PubMed] [Google Scholar]
- 53.Pipili C, Ilonidis G, Cholongitas E. Hepatitis C virus and kidney: a strong association with different clinical aspects. Liver Int. 2011;31:1071–80. doi: 10.1111/j.1478-3231.2011.02458.x. [DOI] [PubMed] [Google Scholar]
- 54.Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. 2013;61:623–37. doi: 10.1053/j.ajkd.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 55.Ishii Y. [Smoking and respiratory diseases]. Nihon rinsho. Jpn J Clin Med. 2013;71:416–20. [PubMed] [Google Scholar]
- 56.Chen YC, Su YC, Lee CC, Huang YS, Hwang SJ. Chronic kidney disease itself is a causal risk factor for stroke beyond traditional cardiovascular risk factors: a nationwide cohort study in Taiwan. PLoS One. 2012;7:e36332. doi: 10.1371/journal.pone.0036332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuo CC, Kuo HW, Lee IM, Lee CT, Yang CY. The risk of upper gastrointestinal bleeding in patients treated with hemodialysis: a population-based cohort study. BMC Nephrol. 2013;14:15. doi: 10.1186/1471-2369-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuo CC, Lee CT, Lee IM, Ho SC, Yang CY. Risk of herpes zoster in patients treated with long-term hemodialysis: a matched cohort study. Am J Kidney Dis. 2012;59:428–33. doi: 10.1053/j.ajkd.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 59.Chen YC, Chiou WY, Hung SK, Su YC, Hwang SJ. Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: a 6-year nationwide cohort study across Taiwan. BMC Nephrol. 2013;14:187. doi: 10.1186/1471-2369-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Catastrophic Illness Patients. [updated 2011; cited 2013 Aug]. Available from: http://www.nhi.gov.tw/English/webdata/webdata.aspx?menu=11&menu_id=596&WD_ID=596&webdata_id=3180.
- 61.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 62.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 63.Kleinbaum DG, Klein M. 3rd ed. New York: Springer; 2012. Survival analysis: a self-learning text. [Google Scholar]
- 64.Fine JP GR. A proportional hazards model for the subdistribution of a competing risk. J Am Statistical Assoc. 1999;94:496–509. [Google Scholar]
- 65.Subdistribution Analysis of Competing Risks. [cited 2014 May]. Available from: http://cran.r-project.org/web/packages/cmprsk/index.html.
- 66.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–63. [PubMed] [Google Scholar]
- 67.Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37:511–5. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- 68.Nacher M, Serrano-Mollar A, Farre R, Panes J, Segui J, Montserrat JM. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol. 2007;155:93–6. doi: 10.1016/j.resp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 69.Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci. 2005;1051:340–50. doi: 10.1196/annals.1361.076. [DOI] [PubMed] [Google Scholar]
- 70.Dyugovskaya L, Lavie P, Hirsh M, Lavie L. Activated CD8+ T-lymphocytes in obstructive sleep apnoea. Eur Respir J. 2005;25:820–8. doi: 10.1183/09031936.05.00103204. [DOI] [PubMed] [Google Scholar]
- 71.Dyugovskaya L, Lavie P, Lavie L. Phenotypic and functional characterization of blood gammadelta T cells in sleep apnea. Am J Respir Crit Care Med. 2003;168:242–9. doi: 10.1164/rccm.200210-1226OC. [DOI] [PubMed] [Google Scholar]
- 72.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 73.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–71. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 74.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–30. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 75.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–84. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 76.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165:67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 77.Chiu HF, Huang YW, Chang CC, Yang CY. Use of proton pump inhibitors increased the risk of hip fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2010;19:1131–6. doi: 10.1002/pds.2026. [DOI] [PubMed] [Google Scholar]
- 78.Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology. 2009;137:1641–8. e1–2. doi: 10.1053/j.gastro.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 79.Stenvinkel P, Ikizler TA, Mallamaci F, Zoccali C. Obesity and nephrology: results of a knowledge and practice pattern survey. Nephrol Dial Transplant. 2013;28(Suppl 4):iv99–104. doi: 10.1093/ndt/gft193. [DOI] [PubMed] [Google Scholar]
- 80.Nderitu P, Doos L, Jones PW, Davies SJ, Kadam UT. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: a systematic review. Fam Pract. 2013;30:247–55. doi: 10.1093/fampra/cms086. [DOI] [PubMed] [Google Scholar]
- 81.Noborisaka Y. Smoking and chronic kidney disease in healthy populations. Nephrourol Mon. 2013;5:655–67. doi: 10.5812/numonthly.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tausche AK, Jansen TL, Schroder HE, Bornstein SR, Aringer M, Muller-Ladner U. Gout--current diagnosis and treatment. Deutsches Arzteblatt Int. 2009;106:549–55. doi: 10.3238/arztebl.2009.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramiro S, Radner H, van der Heijde DM, Buchbinder R, Aletaha D, Landewe RB. Combination therapy for pain management in inflammatory arthritis: a Cochrane systematic review. J Rheumatol Suppl. 2012;90:47–55. doi: 10.3899/jrheum.120342. [DOI] [PubMed] [Google Scholar]
- 84.Plantinga LC. Socio-economic impact in CKD. Nephrol Ther. 2013;9:1–7. doi: 10.1016/j.nephro.2012.07.361. [DOI] [PubMed] [Google Scholar]
- 85.Vart P, Gansevoort RT, Coresh J, Reijneveld SA, Bultmann U. Socioeconomic Measures and CKD in the United States and The Netherlands. Clin J Am Soc Nephrol. 2013;8:1685–93. doi: 10.2215/CJN.12521212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis with the add-on of an unmeasured residual confounding factor. This figure shows the estimates trend of the sleep apnea (SA) group hazard ratio of chronic kidney disease (CKD) on a multivariable-adjusted Cox regression model.
Sensitivity analysis with the add-on of an unmeasured residual confounding factor. This figure shows the estimates trend of the sleep apnea (SA) group hazard ratio of end-stage renal disease (ESRD) on a multivariable-adjusted Cox regression model.


