Abstract
Study Objectives:
Attention is a cognitive domain that can be severely affected by sleep deprivation. Previous neuroimaging studies have used different attention paradigms and reported both increased and reduced brain activation after sleep deprivation. However, due to large variability in sleep deprivation protocols, task paradigms, experimental designs, characteristics of subject populations, and imaging techniques, there is no consensus regarding the effects of sleep loss on the attending brain. The aim of this meta-analysis was to identify brain activations that are commonly altered by acute total sleep deprivation across different attention tasks.
Design:
Coordinate-based meta-analysis of neuroimaging studies of performance on attention tasks during experimental sleep deprivation.
Methods:
The current version of the activation likelihood estimation (ALE) approach was used for meta-analysis. The authors searched published articles and identified 11 sleep deprivation neuroimaging studies using different attention tasks with a total of 185 participants, equaling 81 foci for ALE analysis.
Results:
The meta-analysis revealed significantly reduced brain activation in multiple regions following sleep deprivation compared to rested wakefulness, including bilateral intraparietal sulcus, bilateral insula, right prefrontal cortex, medial frontal cortex, and right parahippocampal gyrus. Increased activation was found only in bilateral thalamus after sleep deprivation compared to rested wakefulness.
Conclusion:
Acute total sleep deprivation decreases brain activation in the fronto-parietal attention network (prefrontal cortex and intraparietal sulcus) and in the salience network (insula and medial frontal cortex). Increased thalamic activation after sleep deprivation may reflect a complex interaction between the de-arousing effects of sleep loss and the arousing effects of task performance on thalamic activity.
Citation:
Ma N, Dinges DF, Basner M, Rao H. How acute total sleep loss affects the attending brain: a meta-analysis of neuroimaging studies. SLEEP 2015;38(2):233–240.
Keywords: sleep deprivation, attention, meta-analysis, fmri, fronto-parietal network, salience network, thalamus
INTRODUCTION
“Everyone knows what attention is. It is the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneously possible objects or trains of thought. Focalization, concentration, of consciousness are of its essence.”1 As observed by William James, concentration and focalization are core components of attention. Attention control allows us to behave intentionally and efficiently, avoiding irrelevant stimuli. Attention, specifically vigilance or sustained attention, which refers to the ability to maintain a consistent alertness level and behavioral response during continuous activity, is essential for many other high level cognitive processes.2,3
Chronic sleep loss, a significant health and safety concern, affects millions of people throughout the world. Many experiments have demonstrated the detrimental neurobehavioral effects of sleep deprivation on attention, working memory and other cognitive tasks, reflected in psychomotor slowing, increased errors of omission and commission, and reduced learning of cognitive tasks.4,5 A recent meta-analysis of 70 experiments and 147 cognitive tests, revealed that across six cognitive categories acute total sleep deprivation had the largest effect sizes relative to measures of simple and complex attention, as well as working memory.6
With the development of neuroimaging techniques in past decades, numerous studies have used functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) with various experimental paradigms to investigate how sleep deprivation affects the attending brain. The first neuroimaging study by Wu and colleagues7 used PET and examined brain function changes during a sustained attention task after approximately 32 h of total sleep deprivation. They showed significantly reduced absolute glucose metabolism in the frontal and temporal lobes, thalamus, basal ganglia, cerebellum, and an increased relative metabolic rate in the visual cortex. In a more recent study from the same group,8 reduced metabolism in the thalamus, basal ganglia, and frontal lobe were replicated after 24 hours of sleep deprivation. Similarly, Thomas and colleagues62 used PET and showed decreased global metabolism after short-term sleep deprivation, with larger reductions in the thalamus, prefrontal and posterior parietal cortices. Using fMRI, multiple acute sleep deprivation studies on attention also reported reduced activation in the prefrontal regions,10–12,15,49,53,91 which is consistent with PET studies. However, inconsistent findings have been reported from fMRI studies, particularly for the thalamus. For example, Portas et al.9 used fMRI and explored brain activity changes during performance on a selective attention task under different levels of arousal and found increased attention-related thalamic activation following 24 h of acute sleep deprivation, which contradicted the findings of Wu et al.7 Subsequently, some fMRI studies10,12,53,90 on attention and sleep deprivation have reported increased thalamic activation after sleep loss, while others did not report or showed no changes in thalamic activation.11,13,15,49–52 Using various selective attention paradigms in fMRI, Chee and colleagues demonstrated that total sleep deprivation not only significantly attenuated neural activation in prefrontal and parietal regions, but also reduced brain activation in other areas such as insula,10–12 parahippocampal place area (PPA),13–15 and fusiform face area (FFA).13,14 The attenuated activity in the insula and visual cortex has also been reported in some studies,49,53 but not others.50,52 These inconsistent findings may be due to variability in brain imaging techniques, experimental designs, task paradigms, statistical evaluation of imaging data, and characteristics of subject population across different studies.
Attention has been divided into four categories: selective, divided, orienting (switching), and sustained (vigilance) attention.16–18 Selective attention refers to the ability to maintain a behavioral or cognitive focus in the face of distracting or competing stimuli; divided attention is the ability to respond simultaneously to multiple tasks or multiple task demands; orienting attention refers to the ability of mental flexibility that allows individuals to shift their focus of attention and move among tasks having different cognitive requirements; and finally, sustained attention is the ability to maintain a consistent behavioral response during continuous and repetitive activity.
Although studies suggest that frontal and parietal regions are involved in almost all tasks requiring some element(s) of attention, different task paradigms may engage different brain regions to optimize attention performance. For instance, the anterior cingulate cortex (ACC) is activated in selective attention tasks,19,20 while the anterior insula, medial cingulate cortex and precuneus are involved in divided attention tasks,21 and the thalamus is recruited in sustained attention tasks.22,23 Corbetta and Shulman24 demonstrated that two brain systems are related to orienting attention tasks, one is a dorsal system including frontal eye fields (FEF) and intraparietal sulcus (IPS), which follows cues and responses for rapid control over attention, and the other is a ventral system consisting of tempo-parietal junction (TPJ) and ventral frontal cortex to identify the interrupting signal and reallocate attention.
Although various studies have undoubtedly demonstrated impaired attention performance and altered neural activation after sleep loss, it remains unclear whether acute total sleep deprivation has a common detrimental effect on brain function across different kinds of attention tasks. Moreover, large and reliable inter-individual differences in vigilance attention performance and cognitive responses to sleep deprivation have been reported, which suggests trait-like differential vulnerability.25,80 However, due to the considerable technical and practical demands for neuroimaging studies of sleep loss, most published sleep deprivation studies only employ a small number (typically 6–26) of subjects, which limits the validity and reliability of the findings. Therefore, it is important to pool existing neuro-imaging literature on sleep deprivation and attention to provide an overview of the effects of sleep deprivation on brain areas mediating various attention deficits from sleep deprivation.
To achieve this aim, the present study conducted a coordinate-based meta-analysis using the activation likelihood estimation (ALE) method26–28 in order to identify brain areas in which the reported foci of activation converge across different experiments. ALE is the most common algorithm for meta-analyses of neuroimaging literature to identify brain locations showing a consistent response across experiments, which collectively involve hundreds of subjects and numerous implementations of a particular protocol or paradigm.28,39,41 Based on the collection of peak coordinates from each study included in the meta-analysis, ALE estimates the probability that at least one of the peaks lies within a voxel. This computation is repeated at each voxel in the brain and results in an ALE map. A statistical threshold for the ALE map is computed using a nonparametric permutation test. This test identifies real activation if the null hypothesis that the activation foci are spread uniformly throughout the brain (i.e., random clustering) is rejected. This approach allows the synthesis of findings not only across different studies, but also across various task paradigms and different laboratories,29 therefore is increasingly used to integrate the knowledge accrued from a rapidly growing number of neuroimaging studies. ALE meta-analyses have been successfully applied in a number of areas to identify brain function and structural changes in both healthy populations and clinical patients. These include neurobehavioral studies of attention,30 memory,31,32 reinforcement learning,33 social perception,34,35 and sleep apnea.36 In the present study, we use ALE analysis to assess the voxel-wise correspondence of neuroimaging studies following total sleep deprivation during different types of attention tasks in order to identify the common effects of sleep deprivation on the attending brain.
METHODS
Search Criteria
We searched the PubMed (http://www.pubmed.org) and ScienceDirect (http://www.sciencedirect.com) databases from years 1990 to 2013 for neuroimaging studies investigating the effects of sleep deprivation on attention using the keywords “sleep deprivation,” “sleep loss,” or “sleep restriction” respectively with “attention.” These terms were each combined with “fMRI” or “PET” to identify relevant functional neuroimaging studies. No language restrictions were applied and the search was restricted to healthy adults tested relative to sleep deprivation. In addition, studies were only considered if they reported results of state differences between sleep deprivation and rested wakefulness (RW) from whole-brain group analysis as coordinates corresponding to a standard reference space (e.g., Talariach or MNI). For this reason, a neuroimaging study on total sleep deprivation from Portas et al.9 was excluded from the present meta-analysis because this study only reported results from individual participants (without group analyses). Moreover, due to the lack of exact coordinates in the Talariach and MNI templates, the first PET study of sleep deprivation7 and a study conducted by Chee et al.37 were also excluded. Furthermore, due to a limitation of the ALE method, which cannot examine the interaction effect between state (sleep deprivation/ rested wakefulness) and task performance, two other studies47,48 that only reported brain regions in which activation correlated with different levels of task performance were not included in the meta-analysis.
We did not find any chronic sleep deprivation studies that met our criteria. The final analyzed dataset included a total of 11 eligible acute total sleep deprivation fMRI studies using various attention tasks and a total of 185 participants, equaling 81 foci for ALE analysis. These fMRI studies were comprised from three kinds of attention tasks: divided attention (25 participants, 2 experiments, and 14 foci), selective attention (140 participants, 7 experiments, and 45 foci), and orienting attention (20 participants, 2 experiments, and 22 foci). Unfortunately, there were no neuroimaging studies investigating the effect of sleep deprivation on sustained attention included in the present meta-analysis due to the search criteria and the limitations of ALE mentioned above.
ALE Meta-Analysis
The coordinate-based meta-analysis of neuroimaging results was performed using the most common ALE approach.27,28,38 The reported coordinates were analyzed for topographic convergence using the current version of the revised ALE algorithm (http://brainmap.org/ale, Research Imaging Institute of the University of Texas Health Science Center, San Antonio, Texas). The current ALE was performed in Talariach reference space using GingerALE version 2.3.1. Coordinates originally published in MNI space were converted to Talariach reference space using the Lancaster transformation42 before the ALE analysis. The ALE procedure consisted of the following steps: first, modeling of single-study activation foci as peaks of three-dimensional Gaussian probability densities with subject-based full-width at half-maximum values28; second, summation of probability densities to produce a statistical map estimating the likelihood of activation at each voxel; third, thresholding of this ALE map based on the null hypothesis of a uniform distribution of foci39,40; fourth, correcting for multiple comparisons by permutation-based thresholding of the maximum cluster size. For all results, the significance threshold was set at P < 0.05, corrected for multiple comparisons at the cluster level. The number of permutations was 1,000 for all calculations of simple ALE maps.41
RESULTS
The Pubmed and ScienceDirect search and subsequent application of the inclusion criteria yielded a total of 11 relevant articles (see Table 1). These articles assessed the effect of total sleep deprivation on brain activation during various attention tasks, yielding 81 foci inside the brain, including 58 foci of reduced activation and 23 foci of increased activation.
Table 1.
Overview of the 11 published studies included in the meta-analysis.
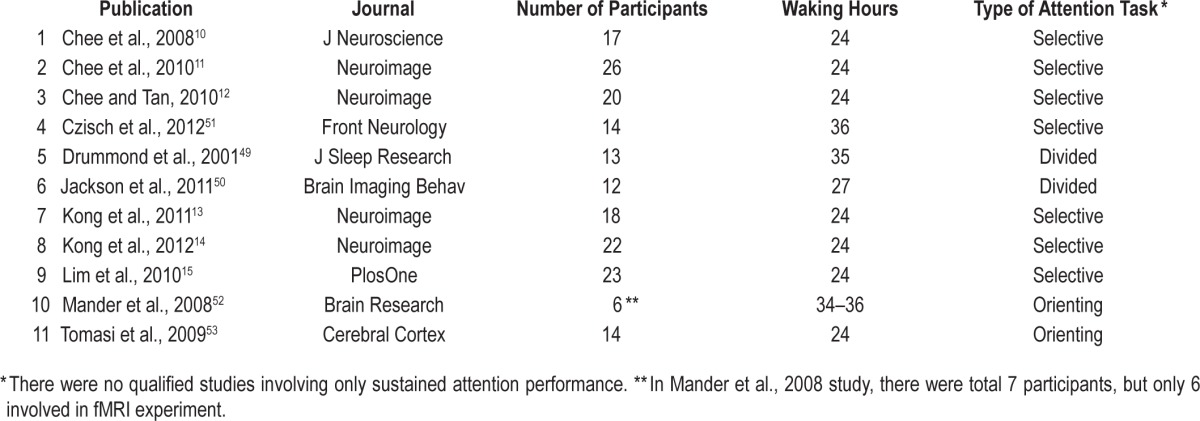
The ALE meta-analysis revealed reduced brain activation across various attention tasks following a night of total sleep deprivation compared to rested wakefulness after normal sleep, including right parahippocampal gyrus (see Figure 1 I), bilateral insula (Figure 1 II), right prefrontal cortex (PFC, Figure 1 IV), medial frontal gyrus and bilateral intraparietal sulcus (IPS, Figure 1 V). This analysis also revealed increased activation in bilateral thalamus (left lateral posterior nucleus and right ventral lateral nucleus, see Figure 1 III) after total sleep deprivation (see Table 2).
Figure 1.
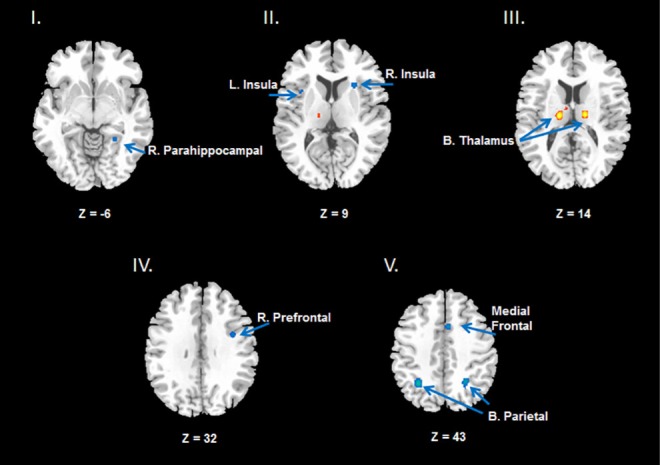
Results from the ALE meta-analysis revealed significantly reduced activation (shown in blue) in the fronto-parietal network (Prefrontal cortex and bilateral Parietal lobe), Insula, and right Parahippocampal cortex (see images at Z = −6, Z = 9, Z = 32, Z = 43, the Z values represent the horizontal coordinates from inferior to superior direction, more negative Z value, more inferior direction and vice versa), but increased activation (shown in yellow and red) in the thalamus (see image at Z = 14) across different attention tasks following total sleep deprivation compared to rested wakefulness. All activations are significant at P < 0.05 corrected for multiple comparisons using the false discovery rate. ALE, activation likelihood estimation; L, left; R, right; B, bilateral.
Table 2.
Brain activations affected by sleep deprivation.
DISCUSSION
The coordinate-based ALE meta-analysis of functional neuroimaging data has been increasingly used to integrate findings and knowledge accrued across different studies. Applying ALE meta-analysis on neuroimaging studies of attention following a night of acute total sleep deprivation, we observed significantly reduced brain activation in multiple areas, most concentrically in bilateral parietal lobule, which are key parts of the attention network in the human brain. In addition, functional enhancement was demonstrated in bilateral thalamic areas. To the best of our knowledge, this is the first neuroimaging-based meta-analysis on the effects of total sleep deprivation on brain function during attention, the cognitive domain most strongly affected by sleep loss.6 The main findings are discussed below.
Reduced Fronto-Parietal Activation Following Total Sleep Deprivation
Activation in the fronto-parietal attention network is reduced following acute total sleep deprivation compared to normal resting. The parietal lobe is divided into two major regions, the somatosensory cortex and the posterior parietal cortex.43 The posterior parietal lobe is tightly linked to attention in both healthy controls43,44 and clinical patients.45,46 Acute total sleep deprivation affects the superior parietal lobule (SPL) and inferior parietal sulcus (IPS), regardless of the type of attention tasks (simple/alert attention,47,48 divided attention,49,50 selective attention,10–12,14,15,51 orienting attention52,53) that subjects are performing. A series of experiments using a Sternberg-like memory task also showed that the IPS is crucial in modulating attention after total sleep deprivation.54–57 Collectively; these results indicate that total sleep deprivation has a detrimental effect on parietal activation during tasks requiring attention.
The right prefrontal cortex is also susceptible to total sleep deprivation. Consistent with the current meta-analysis, a number of studies have observed reduced brain activity in PFC after total sleep deprivation.10–12 However, a few studies have demonstrated no changes or increased frontal activation after sleep loss.58–60 This inconsistency may be partly due to varying degrees of task difficulty. It has been suggested that increased PFC function after sleep deprivation may be a compensatory response, which is supported by studies showing increased neural activity in PFC following sleep deprivation related to increasing working memory load.61,82,83
Salience Network and Attention
Salience network activation is also reduced following total sleep deprivation compared to normal resting. The insula is considered a node of the salience network67 and involved in multiple cognitive, affective, and regulatory functions. Insula activation likely facilitates access to attention and working memory when a salient stimulus is presented; Kerzel and Schönhammer have suggested that attention is captured by salient stimuli.68 Therefore, the attention deficits caused by sleep deprivation may be partly due to a disruption or attenuation in salience detection. However, reduced insula activation is not specific to the context of non-emotional attention tasks included in this meta-analysis and may relate to the influence of sleep deprivation on emotional appraisal or regulation.92 For example, previous studies have consistently shown that sleep deprivation significantly decreased neural activity in the insula during emotion-related attention-demanding tasks, such as food desirability choices84 and economic decision making.85,93
The meta-analysis also revealed that activation in the me-dial frontal gyrus is decreased following sleep deprivation compared to normal resting. Notably, the medial frontal region in the current findings overlapped with the ACC, which is another node of the salience network and is tightly connected to the insula.69–72 Previous study has indicated that the insula and ACC are important for identifying critical stimuli from sensory input.67 Once such a stimulus is detected, the salience network may facilitate task-related information processing in the fronto-parietal attentional network for task completion. Converging evidence suggests that recurrent activity in insula and mid-cingulate cortex is related to refocusing on attention tasks.73–75 Both structural and functional connectivity between the insula and the inferior parietal lobule have been reported,76 further supporting the involvement of insula in attention control. During sleep deprivation, the intensity for task engagement may be reduced due to lower activity in insula and ACC. Reduced activity in insula and ACC may also be related to an impaired awareness of one's internal state, a covert aspect of attention. This explanation is consistent with recent resting-state fMRI findings that the insula-ACC connection aids interoceptive awareness of body states.71 Findings from chronic sleep restriction also support this view, in which participants show a reduced awareness of the increasing cumulative cognitive deficits.88 Additional studies are needed to further investigate the association between salience network activity changes and attention after both acute and chronic sleep deprivation/restriction as well as the role of the salience network in the dissociation between objective and subjective measures of sleepiness.
Thalamic Hyperactivation
Thalamic activation is increased after total sleep deprivation compared to normal resting. The thalamus is a key node of the subcortical arousal system and cortical attention network and plays an important role in maintaining alertness and vigilant attention.63–65 Previous behavioral studies have indicated that alertness and vigilance attention are decreased after sleep deprivation5; therefore, increased thalamic activation following sleep deprivation seems contradictory. A potential explanation is that greater thalamic activation may reflect increased effort to compensate for dysfunction of the fronto-parietal attentional network after sleep loss.62 Indeed, previous study has shown greater thalamic activation for sleep deprivation than RW, and task difficulty was associated with increases in thalamic activation for the RW but not the sleep deprivation condition. Moreover, thalamic activation was inversely correlated with parietal and prefrontal activation, suggesting that increased thalamic activation may compensate for the decreased parietal activation during sleep deprivation in order to complete the task.62
Alternatively, wake maintenance during an attention task following sleep loss may simply require greater thalamic activation. For example, Coull and colleagues showed that thalamic activity was reduced under a rest control condition when the α-2 adrenoceptor agonist clonidine was applied to decrease arousal level. However, increased thalamic activity was observed when clonidine was applied during performance on a rapid visual information processing task.66 In addition, Chee and Tan examined the different effects of sleep deprivation on brain activation in subjects who were vulnerable versus those not vulnerable to sleep loss.12 They reported a trend of increased mean thalamic activity following sleep deprivation in subjects who were non-vulnerable and exhibited better performance, while significantly reduced thalamic activation was found during lapses in subjects who were vulnerable and exhibited poorer performance, suggesting a trial-to-trial modulation of thalamic activation on behavioral performance.
It is noteworthy that all studies included in this meta-analysis are based on the blood-oxygen-level dependent (BOLD) fMRI, which has limitations and the results may not be comparable to PET studies. In contrast to BOLD fMRI findings, PET studies consistently report reduced metabolic rate in thalamus after sleep deprivation.7,8,62 This inconsistency may be due to different signals measured by BOLD fMRI and PET. BOLD contrast reflects the complex interaction between changes in blood flow, blood volume, and oxygenation consumption that occurs during neural activity.94,95 Therefore, BOLD lacks absolute quantification of neural activity and can only measure relative signal changes between task and baseline conditions. This limitation makes the accurate interpretation of brain activation changes during attention tasks following sleep deprivation very difficult.96 Changes in brain activation following sleep deprivation may be due to changes in baseline neural activity, or changes in task-specific neural activity, or both. By contrast, PET provides absolute quantification of cerebral metabolic rate or blood flow, thus allowing direct comparisons of brain activity during tasks between sleep deprivation and normal resting without the contamination of potential baseline changes. Giving the opposite findings in thalamic activation from PET and BOLD fMRI studies, it is likely that the increase in relative BOLD activation in the thalamus following sleep deprivation during attention tasks is due to a complex interaction between the de-arousing effects of sleep loss and the arousing effects of task performance. Because PET requires the injection of invasive, expensive, and rapidly decaying radioactive tracers, and subjects cannot repeatedly undergo PET scans within a short time, the utility of PET is limited. Future fMRI studies using noninvasive and quantitative measures of brain activity (e.g., cerebral blood flow measured by arterial spin labeling fMRI96,97) are necessary to verify this hypothesis and dissociate the potential different effects of sleep deprivation on brain activity during task and resting baselines.
Limitations
Specific methodological limitations should also be considered with respect to the current meta-analysis. First, only 11 neuroimaging studies included in this meta-analysis reported the difference of brain activation during attention after sleep loss, thereby limiting the power to detect the potential sleep deprivation induced deficits in the human brain. Furthermore, the limited number of studies may enhance the effects of sleep deprivation on some brain areas related to the specific experimental tasks and designs used by the studies included in the meta-analysis. For example, the reduced activation in the parahippocampal gyrus observed in the current study may have been driven by the fact that several studies used object-selective attention tasks.13–15 In addition, due to the small number of neuroimaging studies included in the present meta-analysis, it is impossible to compare brain activation differences across different types of attention and/or task paradigms. Finally, because 7 of the 11 studies included in the meta-analysis focused on selective attention and many of these studies are from the same lab using a particular type of research method, it is possible that the results are biased and caution is needed when interpreting the findings. For example, the findings may overemphasize the effect of sleep deprivation on selective attention rather than a common effect among all types of attention tasks. Future studies are needed to further explore the potential different effects of sleep deprivation on different attention tasks.
Second, sustained attention is an essential component for almost all cognitive tasks, and the two neuroimaging studies that investigated the effect of acute total sleep deprivation effects on sustained attention did not meet inclusion criteria. Previous studies have consistently demonstrated that behavioral performance during sustained attention tasks and brain activations underlying sustained attention are highly impaired by sleep deprivation.62,90,91 It is difficult to dissociate the effects of sleep deprivation on selective, divided, and orienting attention from sustained attention, which are mediated by overlapping neural circuits.77–79
Third, variability in statistical approaches used in different neuroimaging studies is another potential methodological concern, particularly with respect to arbitrary thresholds selection and correction for multiple comparisons. These differences may impact rates for false positives and false negatives.30 ALE addresses this issue by weighting the findings of each peer-reviewed study equally and relying upon patterns of consistency across studies to overcome this concern.26 However, using this approach, studies with less stringent thresholds or corrections for multiple comparisons may introduce a larger number of foci, and may cause a higher impact on the final results compared to the studies which applied stricter statistical standards. Although the updated ALE method with cluster-level correction provides higher sensitivity and stringent protection against false positives than the previous FDR thresholding,41 the potential for bias cannot be fully eliminated. Future studies with larger sample sizes are essential.
Finally, meta-analytic results are often influenced by the heterogeneity of the included studies. Therefore, it is an aim of meta-analysis to statistically control for potential sources of heterogeneity. Since the ALE algorithm is based on a random effects model, it is more conservative than the fixed-effects model and incorporates both within-study and between study variance. Unfortunately, the ALE software did not allow the investigation of heterogeneity between individual studies.81 A possible solution to address the heterogeneity in meta-analysis is to use Effect-Size Signer Differential Mapping (ES-SDM), which allows for measuring between-study heterogeneity. However, this method requires t-values for peak activations, which were not reported in 6 of the 11 studies included in the current meta-analysis. Thus the ES-SDM is not feasible for this study.
Due to this limitation of ALE, we cannot completely exclude the possibility that the results are influenced by a possible heterogeneity from different studies. Although we tried to minimize the heterogeneity by establishing relatively strict inclusion criteria, many other potential moderators including the length of continuous time awake (i.e., total hours of sleep deprivation), participants' age range and gender composition, the duration of attention task performance, and within/between-participant design, may contribute variance to the current findings. However, some qualitative insight may be obtained by comparing the effects of 24-h versus 36-h total sleep deprivation on brain activation. Of the 11 studies included in the meta-analysis, 8 administered 24-h sleep deprivation and 3 administered 36-h sleep deprivation. Of the 8 studies that administered 24-h sleep deprivation, 3 reported increased thalamic activation after sleep loss. In contrast, none of the 3 studies that administered 36-h sleep deprivation reported thalamic activation changes after sleep loss. These findings suggest that 36-h sleep deprivation may have different influences on thalamic activation, possibly due to the greater severity of sleep pressure induced by 36-h sleep deprivation. Time-of-day effects may also contribute to the different influences of 36-h versus 24-h sleep deprivation on thalamic activation. In the 24-h sleep deprivation studies, subjects were scanned at the same or similar time-of-day, whereas subjects in the 36-h sleep deprivation studies were scanned at different time-of-day, which might interfere with the effect of sleep loss. For example, previous studies have shown that attention performance is more severely impaired during the morning (07:00–09:00) after 24 hours sleep deprivation, compared to evening hours after 36 hours awake (19:00–21:00) when the circadian system is promoting alertness.86,87,89
CONCLUSION
Using an ALE meta-analysis, we identified reduced activation in multiple brain regions following a night of sleep loss compared to normal resting, including bilateral inferior parietal lobe, bilateral insula, right prefrontal cortex, medial frontal cortex, and right parahippocampal gyrus, which collectively implicate the vulnerability of the fronto-parietal attention network and the salience network to acute total sleep deprivation. We also identified increased activation in bilateral thalamus, which may reflect a complex interaction between the de-arousing effects of sleep loss and the arousing effects of task performance on thalamic activity.
DISCLOSURE STATEMENT
This research was supported in part by NIH Grants R01 HL102119 (HR), R21 DA032022 (HR), National Space Biomedical Research Institute through NASA NCC 9-58 (DFD, MB), and a pilot grant from the Institute for Translational Medicine and Therapeutics (ITMAT) of the University of Pennsylvania (HR). Dr. Dinges is Editor in Chief of SLEEP and Dr. Basner is Deputy Editor of SLEEP, but they recuse themselves from all manuscripts they submit and any from members of their University or their collaborators. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Andrea Spaeth for her helpful comments during the revision of this manuscript.
REFERENCES
- 1.James W. The principles of psychology. Vol. 1. New York: Henry Holt; 1890. pp. 403–4. [Google Scholar]
- 2.Sturm W, de Simone A, Krause BJ, et al. Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 3.Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. NeuroImage. 2001;14:S76–84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- 4.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 5.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 6.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–89. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JC, Gillin JC, Buchsbaum MS, et al. The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14:155–62. [PubMed] [Google Scholar]
- 8.Wu JC, Gillin JC, Buchsbaum MS, et al. Frontal lobe metabolic decreases with sleep deprivation not total reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–92. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- 9.Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18:8979–89. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chee MW, Tan JC, Zheng H, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;21:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chee MW, Tan JC, Parimal S, Zagorodnov V. Sleep deprivation and its effects on object-selective attention. Neuroimage. 2010;49:1903–10. doi: 10.1016/j.neuroimage.2009.08.067. [DOI] [PubMed] [Google Scholar]
- 12.Chee MW, Tan JC. Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals. NeuroImage. 2010;51:835–43. doi: 10.1016/j.neuroimage.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Kong DY, Soon CS, Chee MW. Reduced visual processing capacity in sleep deprived persons. NeuroImage. 2011;55:629–34. doi: 10.1016/j.neuroimage.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 14.Kong DY, Soon CS, Chee WM. Functional imaging correlates of impaired distractor suppression following sleep deprivation. NeuroImage. 2012;61:50–5. doi: 10.1016/j.neuroimage.2012.02.081. [DOI] [PubMed] [Google Scholar]
- 15.Lim J, Tan JC, Parimal S, Dinges DF, Chee MW. Sleep deprivation impairs object-selective attention: a view from the ventral visual cortex. PLoS One. 2010;5:e9087. doi: 10.1371/journal.pone.0009087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDowd JM. An overview of attention: behavior and brain. J Neurol Phys Ther. 2007;31:98–103. doi: 10.1097/NPT.0b013e31814d7874. [DOI] [PubMed] [Google Scholar]
- 17.Posner MI, Boies SJ. Components of attention. Psychol Rev. 1971;78:391–408. [Google Scholar]
- 18.Peterson SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botvinick MM, Braveer TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 20.Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: who's in control? Nat Neurosci. 2000;3:421–3. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- 21.Nebel K, Wiese H, Stude P, de Greiff A, Diener H, Keidel M. On the neural basis of focused and divided attention. Cogn Brain Res. 2005;25:760–76. doi: 10.1016/j.cogbrainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J. Neurosci. 1998;18:7426–35. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paus T, Zatorre RJ, Hofle N, et al. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J Cogn Neurosci. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- 24.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 25.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 26.Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 28.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cog Affect Neurosci. 2007;2:150–8. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Depue BE. A neuroanatomical model of prefrontal inhibitory modulation of memory retrieval. Neurosci Biobehav Rev. 2013;36:1382–9. doi: 10.1016/j.neubiorev.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nee DE, Brown JW, Askren MK, et al. A meta-analysis of executive components of working memory. Cereb Cortex. 2013;23:264–82. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrison J, Erdeniz B, Done J. Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2013;37:1297–310. doi: 10.1016/j.neubiorev.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Shkurko AV. Is social categorization based on relational ingroup/outgroup opposition? A meta-analysis. Soc Cogn Affect Neurosci. 2013;8:870–7. doi: 10.1093/scan/nss085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–72. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 36.Weng HH, Tsai YH, Chen CF, et al. Mapping gray matter reductions in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Sleep. 2014;37:167–75. doi: 10.5665/sleep.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chee MW, Goh C, Namburi P, Parimal S, Seidl KN, Kastner S. Effects of sleep deprivation on cortical activation during directed attention in the absence and presence of visual stimuli. Neuroimage. 2011;58:595–604. doi: 10.1016/j.neuroimage.2011.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laird AR, Eickhoff SB, Kurth F, et al. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinformatics. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29:14496–505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–61. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212–7. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattingley JB. Attention, consciousness, and the damaged brain: insights from parietal neglect and extinction. Psyche. 1999;5:14–27. [Google Scholar]
- 46.Han SH, Jiang Y, Gu H, et al. The role of human parietal cortex in attention networks. Brain. 2004;127:650–9. doi: 10.1093/brain/awh071. [DOI] [PubMed] [Google Scholar]
- 47.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 48.Muto V, Bourdiec AS, Matarazzo L, et al. Influence of acute sleep loss on the neural correlates of alerting, orientating and executive attention components. J Sleep Res. 2012;21:648–58. doi: 10.1111/j.1365-2869.2012.01020.x. [DOI] [PubMed] [Google Scholar]
- 49.Drummond SPA, Gillin JC, Brown GG. Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res. 2001;10:85–92. doi: 10.1046/j.1365-2869.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 50.Jackson ML, Hughes ME, Croft RJ, et al. The effect of sleep deprivation on BOLD activity elicited by a divided attention task. Brain Imaging Behav. 2011;5:97–108. doi: 10.1007/s11682-011-9115-6. [DOI] [PubMed] [Google Scholar]
- 51.Czisch M, Wehrle R, Harsay HA, et al. On the need of objective vigilance monitoring: effects of sleep loss on target detection and task-negative activity using combined EEG/fMRI. Front Neurol. 2012;3:67. doi: 10.3389/fneur.2012.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mander BA, Reid KJ, Davuluri VK, et al. Sleep deprivation alters functioning within the neural network underlying the covert orienting of attention. Brain Res. 2008;1217:148–56. doi: 10.1016/j.brainres.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomasi D, Wang RL, Telang F, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. 2009;19:233–40. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chee MW, Chuah LYM, Venkatraman V, Chan WY, Philip P, Dinges DF. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: correlations of fronto-parietal activation with performance. NeuroImage. 2006;31:419–28. doi: 10.1016/j.neuroimage.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Lim J, Choo WC, Chee MW. Reproducibility of changes in behaviour and fMRI activation associated with sleep deprivation in a working memory task. Sleep. 2007;30:61–70. doi: 10.1093/sleep/30.1.61. [DOI] [PubMed] [Google Scholar]
- 57.Mu Q, Nahas Z, Johnson KA, et al. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28:55–67. doi: 10.1093/sleep/28.1.55. [DOI] [PubMed] [Google Scholar]
- 58.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 59.Drummond SP, Brown GG, Salamat JS, Gillin JC. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004;27:445–51. [PubMed] [Google Scholar]
- 60.Drummond SP, Meloy MJ, Yanagi MA, Off HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. 2005;140:211–23. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Choo WC, Lee WW, Venkatraman V, Sheu FS, Chee MW. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005;25:579–87. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 62.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 63.Luria AR. The working brain. Harmondsworth, England: Penguin Books; 1973. [Google Scholar]
- 64.Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–18. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 65.Parasuraman R, Warm JS, See JE. Brain systems of vigilance. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 66.Coull JT, Frith CD, Dolan RJ, Frackowiak RS, Grasby PM. The neural correlates of the noradrenergic modulation of human attention, arousal and learning. European J Neurosci. 1997;9:589–98. doi: 10.1111/j.1460-9568.1997.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 67.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kerzel D, Schönhammer J. Salient stimuli capture attention and action. Atten Percept Psychophysiol. 2013;75:1633–43. doi: 10.3758/s13414-013-0512-3. [DOI] [PubMed] [Google Scholar]
- 69.Eckert MA, Menon V, Walczak A, et al. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30:2530–41. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–49. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–45. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fischer T, Langner R, Diers K, Brocke B, Birbaumer N. Temporo-spatial dynamics of event-related EEG beta activity during the initial contingent negative variation. PLoS One. 2010;5:e12514. doi: 10.1371/journal.pone.0012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–62. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 76.Uddin LQ, Supekar K, Amin H, et al. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010;20:2636–46. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coull JT. Neural correlates of attention and arousal: insights electrophysiological, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–61. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 78.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 79.Lim J, Wu W, Wang JJ, Detre JA, Dinges DF, Rao HY. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. NeuroImage. 2010;49:3426–35. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuna ST, Maislin G, Pack FM, et al. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012;35:1223–33. doi: 10.5665/sleep.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wagner S, Sebastian A, Lieb K, Tüscher O, Tadić A. A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci. 2014;15:19. doi: 10.1186/1471-2202-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Drummond SP, Brown GG, Salamat JS, Gillin JC. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004;27:445–51. [PubMed] [Google Scholar]
- 83.Mu Q, Mishory A, Johnson KA, et al. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep. 2005;28:433–46. doi: 10.1093/sleep/28.4.433. [DOI] [PubMed] [Google Scholar]
- 84.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Venkatraman V, Huettel SA, Chuah LYM, Payne JW, Chee MW. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci. 2011;31:3712–8. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Dongen H, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clin Sport Med. 2005;24:237–49. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Vandewalle G, Maquet P, Dijk D. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–38. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 88.Van Dongen H, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 89.Basner M, Rao H, Goel N, Dinges DF. Sleep deprivation and neurobehavioral dynamics. Curr Opin Neurobiol. 2013;23:854–63. doi: 10.1016/j.conb.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Habeck C, Rakitin B, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-sample task. Cogn Brain Res. 2004;18:306–21. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 91.Asplund C, Chee MW. Time-on-task and sleep deprivation effects are evidenced in overlapping brain areas. NeuroImage. 2013;82:326–35. doi: 10.1016/j.neuroimage.2013.05.119. [DOI] [PubMed] [Google Scholar]
- 92.Gujar N, Yoo S, Hu P, Walker M. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31:4466–74. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Venkatraman V, Chuah L, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–9. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 94.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–9. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–12. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rao H. ASL imaging of brain function changes during sleep restriction. Sleep. 2012;35:1027–8. doi: 10.5665/sleep.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poudel GR, Innes CR, Jones RD. Cerebral perfusion differences between drowsy and nondrowsy individuals after acute sleep restriction. Sleep. 2012;35:1085–96. doi: 10.5665/sleep.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


