Abstract
Background:
The relationship between nasal surgery and its effect on continuous positive airway pressure (CPAP) device therapeutic treatment pressures and CPAP device use has not been previously systematically examined.
Study Objectives:
To conduct a systematic review and meta-analysis evaluating the effect of isolated nasal surgery on therapeutic CPAP device pressures and use in adults with obstructive sleep apnea.
Methods:
MEDLINE, Scopus, Web of Science, and The Cochrane Library were searched through July 15, 2014. The MOOSE consensus statement and PRISMA statement were followed.
Results:
Eighteen studies (279 patients) reported CPAP data after isolated nasal surgery. Seven studies (82 patients) reported preoperative and postoperative mean therapeutic CPAP device pressures and standard deviations, which reduced from 11.6 ± 2.2 to 9.5 ± 2.0 centimeters of water pressure (cwp) after nasal surgery. Pooled random effects analysis demonstrated a statistically significant pressure reduction, with a mean difference of −2.66 cwp (95% confidence intervals, −3.65 to −1.67); P < 0.00001. Eleven studies (153 patients) described subjective, self-reported data for CPAP use; and a subgroup analysis demonstrated that 89.1% (57 of 64 patients) who were not using CPAP prior to nasal surgery subsequently accepted, adhered to, or tolerated it after nasal surgery. Objective, device meter-based hours of use increased in 33 patients from 3.0 ± 3.1 to 5.5 ± 2.0 h in the short term (< 6 mo of follow-up).
Conclusion:
Isolated nasal surgery in patients with obstructive sleep apnea and nasal obstruction reduces therapeutic CPAP device pressures and the currently published literature's objective and subjective data consistently suggest that it also increases CPAP use in select patients.
Citation:
Camacho M, Riaz M, Capasso R, Ruoff CM, Guilleminault C, Kushida CA, Certal V. The effect of nasal surgery on continuous positive airway pressure device use and therapeutic treatment pressures: a systematic review and meta-analysis. SLEEP 2015;38(2):279–286.
Keywords: continuous positive airway pressure, nasal surgery, obstructive sleep apnea, sleep apnea syndromes
INTRODUCTION
Nasal continuous positive airway pressure (CPAP) was introduced by Sullivan et al.1 in 1981 as treatment for obstructive sleep apnea (OSA) and treatment has been shown to have a mortality benefit.2 Although CPAP is highly efficacious, the non-adherence rate (< 4 h of nightly use 70% of nights) is between 46–83%.3 CPAP side effects include insomnia, claustrophobia, nocturnal awakenings, irritation of the upper airway, sneezing, nasal dryness, rhinitis, rhinorrhea, laryngitis, and epistaxis.4–7 Nasal obstruction is a common complaint among CPAP users, with an estimated prevalence of 25–45%.4,7,8 Decreased cross-sectional area and reduced volume of the nasal airway is associated with patients being less compliant with CPAP therapy.9 Increased nasal resistance has also been associated with decreased CPAP therapy adherence.10 Studies demonstrate that isolated nasal surgery in patients with nasal obstruction reduces therapeutic CPAP pressures and/or improves CPAP acceptance, compliance, tolerance, adherence, or use.5,10–26 The objective of this study is to conduct a systematic review of the literature and perform a meta-analysis to evaluate the effect of isolated nasal surgery on therapeutic CPAP device pressures and CPAP use in adults with OSA.
METHODS
Search Strategy
A search of four databases (MEDLINE, Scopus, Web of Science, and The Cochrane Library) was performed from inception through September 1, 2013, with an update through July 15, 2014. Keywords, MeSH terms, and phrases searched included combinations of: “continuous positive airway pressure,” “nasal obstruction,” “nasal surgery,” “nose surgery,” “rhinoplasty,” “septoplasty,” “septorhinoplasty,” “septoturbinoplasty,” “sleep apnea,” “sleep apnea syndromes,” “surgery,” “turbinate reduction,” “turbinectomy,” and “turbinoplasty”. An example of a search on MEDLINE is: (((“Continuous Positive Airway Pressure”[MeSH]) OR (“Sleep Apnea, Obstructive”[MeSH])) AND ((“Nasal Obstruction/surgery*”[MeSH]) OR (“Nasal Septum/surgery*”[MeSH]) OR “nasal surgery” OR (“Nasal Surgical Procedures*”[MeSH]) OR “nose surgery” OR “rhinoplasty” OR (“Rhinoplasty/methods*”[MeSH]) OR “septoplasty” OR “septorhinoplasty” OR “septoturbinoplasty” OR “turbinate reduction” OR (“Turbinates/surgery”[MeSH]) OR “turbinectomy” OR “turbinoplasty”)).
The titles and abstracts for each of the results were reviewed for relevance. The full text versions of relevant articles were obtained for complete evaluation. The references of the obtained articles were also reviewed and any relevant studies in the reference lists were also obtained and included if they met criteria. As an additional step, each time a relevant article was encountered during the review of titles and abstracts, the “related citations/articles” and “cited by” features of the four databases and Google Scholar were searched to identify any additional potentially relevant articles.
Study Selection
Inclusion criteria for the studies consisted of adult patients with OSA who were treated with CPAP and underwent isolated nasal surgery. Additionally, the included studies needed to report quantitative outcomes data comparing prenasal and postnasal surgery therapeutic CPAP pressures and/or prenasal and postnasal surgery CPAP acceptance, compliance, tolerance, adherence, or use after nasal surgery, without regard to follow-up length. All languages and study designs were included. Exclusion criteria consisted of studies reporting qualitative data only and studies on children. If a study reported individual patient data for nasal surgeries and non-nasal surgeries, then the nasal surgery data were abstracted and included in this review.
Data Abstractions and Study Quality Assessment
Two authors (VC and MC) performed a literature search and screened titles and abstracts, and retrieved articles for further review. Data collected included the ages, body mass indices (BMI), therapeutic CPAP device pressures, and CPAP use data. After the data were collected, the National Institute for Health and Clinical Excellence (NICE) quality assessment tool, consisting of eight items for each study, was used to evaluate the quality of each of the included studies. Data were placed into an Excel 2013 spreadsheet. Authors of studies in which there were insufficient data reported (e.g., study means, standard deviations [SD], etc.) to include in the meta-analysis were contacted via email or phone, in an attempt to obtain these additional data. The corresponding authors were contacted for the following studies: Bican et al.,19 Friedman et al.,16 Nakata et al.,10 Park et al.,26 Pniak et al.,24 Poirier et al.,11 and Powell et al.25 The raw data were no longer available for studies by Friedman et al. or Powell et al. Means and SD for CPAP pressures and use were provided by Rotenberg (Poirier et al. study). There was no reply from corresponding authors for Park et al., Bican et al., Nakata et al., and Pniak et al.
Statistical Analysis
The statistical analysis was performed with the Statistical Package for Social Sciences software (SPSS) version 20.0 and the Cochrane Collaboration's Review Manager (REVMAN) Software version 5.2. The prenasal and postnasal surgery means, mean differences (after - before), standard deviations, and 95% confidence intervals (CI) were calculated. If a study provided individual patient data, but did not report a P value, then the data were manually entered into SPSS in order to calculate the means, SD, and mean differences. The null hypothesis before initiating the study was that there is no difference between prenasal and postnasal surgery data. When combining data, a two-tailed, paired t test was performed and P < 0.05 was considered statistically significant. The REVMAN random effects model for pooling effects was applied if heterogeneity of treatment effects was present and a fixed-effects model was used if no heterogeneity was present. Forest plots were graphically inspected and heterogeneity was assessed with the I2 statistic (low: 25%, moderate: 50% and high: 75%)27 and the Cochran Q statistic (it is generally suggested to use a heterogeneity significance level of P ≤ 0.10).28
Additionally, a sensitivity analysis was performed by removing each study from the meta-analysis to investigate the effect the individual studies on the summarized effect and heterogeneity. Visual inspections of the funnel plots were performed to assess for publication bias. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement29 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) consensus statement30 were adhered to as much as possible.
RESULTS
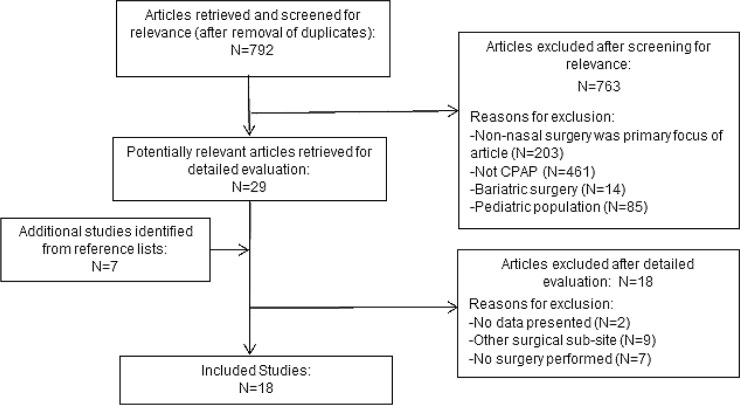
The search identified a total of 792 articles after removal of duplicates. After the preliminary review of the titles and abstracts, a total of 29 studies were agreed to as being relevant and the full text was downloaded for further evaluation. Review of the articles' reference lists identified an additional 7 articles, which were also obtained. Of these 36 articles, a total of 18 were excluded for the following reasons: no quantitative CPAP data prenasal and postnasal surgery were presented in the article,31,32 non-nasal surgeries were performed (i.e. uvulopalatopharyngoplasties, pillar implants or multilevel surgeries)33–41 or no nasal surgery was performed.4,7,9,42–45 Zonato et al.13 reported individual data for nasal surgeries and multilevel surgeries; therefore, the data for nasal surgeries could be separated and included as part of the review. A total of 18 articles were included in this study, with a total of 279 patients identified within those articles, who underwent isolated nasal surgery and had quantitative data available.5,10–25 Figure 1 demonstrates the flow diagram for the article selection. The mean patient age was 52.5 ± 8.7 y and the mean BMI was 29.5 ± 4.0 kg/m2.
Figure 1.
Literature search and study selection. CPAP, continuous positive airway pressure; N, number of studies.
Methodological Quality of the Included Studies
The studies included in this review were all either retrospective or prospective case series (Level 4 evidence), except for one study by Powell et al.25 that was a randomized, double-blind, placebo-controlled clinical trial (RCT), which is Level 1 evidence. The NICE quality assessment tool demonstrated that the included studies satisfied between three to six of the eight items, with most satisfying more than four items (presented in Table S1, supplemental material). The main limitations in general are that none of the studies were multicenter, patients were not recruited consecutively, and there was only one randomized controlled trial and the remaining studies were case series.
CPAP Results
Therapeutic CPAP Pressures
A total of 10 studies (135 patients) described therapeutic CPAP device pressures as an outcome after isolated nasal surgery.5,10–16,20,21,24 Two studies reported the mean difference (MD) of the therapeutic pressures (Balcerzak et al.20 reported a MD of −2.7 cwp and Ripberger and Pirsig21 reported a MD of −5 cwp), but lacked the SD, therefore, were excluded from meta-analysis. Friedman et al.16 reported the therapeutic CPAP means prenasal and postnasal surgery, but did not report the SD; therefore, the study was excluded from pooled meta-analysis calculations.16 The remaining 7 studies (82 patients) reported both preoperative and postoperative therapeutic CPAP device pressures and SD, which were provided in the article or were obtained by contacting the authors of the original studies.5,10–15
With regard to determination of the therapeutic CPAP device pressures, Park et al.,6 Poirier et al.,11 and Mayer-Brix et al.5 reported the therapeutic pressures based on the patients' CPAP devices, whereas in the remaining 5 studies in the meta-analysis reported therapeutic pressures based upon the in-laboratory polysomnography titrations.5,10–15 Hypopnea scoring criteria for the studies included ≥ 10 sec for the hypopnea duration, in combination with: ≥ 3% oxygen desaturation with a 50% decrease in thermistor for Sufioglu et al.,12 ≥ 3% oxygen desaturation or arousal associated with a hypopnea for Nakata et al.,10 > 4% oxygen desaturation for Nowak et al.,14 ≥ 4% oxygen desaturation with a ≥ 50% decreased effort to breathe for Katsantonis et al.,46 whereas the remaining studies did not specify which hypopnea scoring criteria was used.5,11,13,15,26
The combined data for the 7 studies demonstrated a pre-nasal and postnasal surgery therapeutic CPAP device pressure that reduced from a mean ± SD of 11.6 ± 2.2 to 9.5 ± 2.0 centimeters of water pressure (cwp), with results shown in Table 1. The pooled random effects analysis (Figure 2) demonstrated a statistically significant reduction in the mean pressures, with a MD of −2.66 cwp (95% CI, −3.65 to −1.67; overall effect: Z score = 5.27 (P < 0.00001)). The I2 (82%) and Cochran Q statistic (P < 0.00001) suggest significant heterogeneity across included studies, and therefore justified the need for a random-effects model. A sensitivity analysis showed that removing the Sufioglu et al.12 study resulted in a low level of heterogeneity (I2 = 27% and Cochran Q statistic = 0.23) but did not significantly affect the summarized effect size (MD: −3.00 cwp; 95% CI: −3.56 to −2.43, Z score = 10.39, and P < 0.00001). The study by Sufioglu et al.12 states that a postoperative CPAP titration was not performed in five patients who were cured of OSA (apnea-hypopnea index [AHI] < 5/h).12 Visual inspection of the funnel plot also demonstrated that the study by Sufioglu et al.12 was slightly outlying when compared to the remaining studies.
Table 1.
Patient demographics and pre and postnasal surgery therapeutic mean CPAP pressure data.
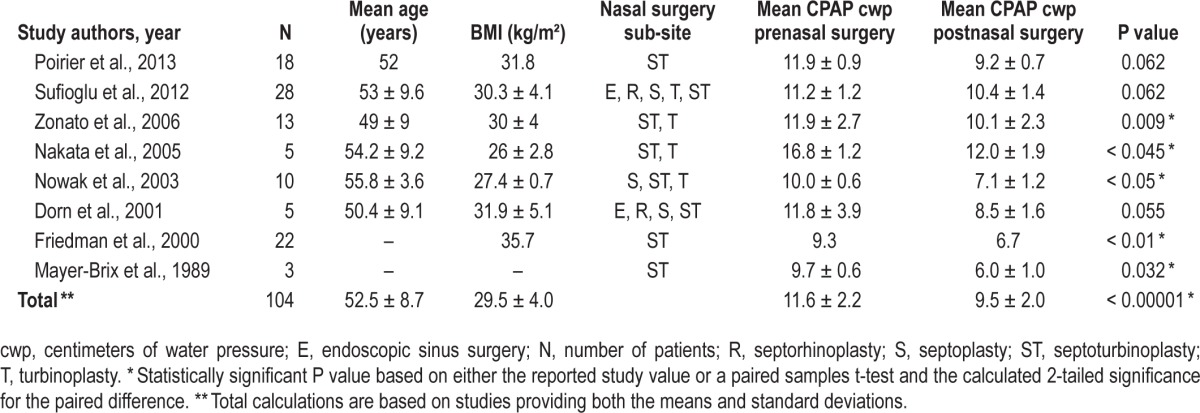
Figure 2.
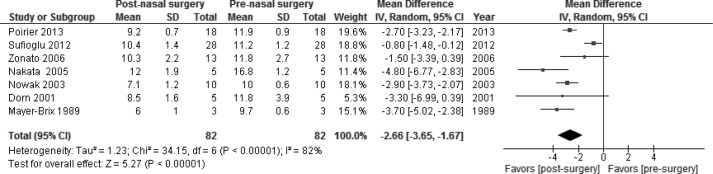
Prenasal and postnasal surgery random effects analysis. There was a statistically significant decrease in therapeutic continuous positive airway pressure device pressures with a mean difference of −2.66 cwp (95% confidence interval [CI], −3.65 to −1.67; P < 0.00001). SD, standard deviation.
A subgroup analysis was performed for studies that specifically stated in the article that nasal mask interfaces were used (studies did not specify nasal pillows versus nasal triangle masks), which resulted in the exclusion of studies by Sufioglu et al.12 and Nakata et al.10,12 When pooling the data from the remaining five studies (49 patients), the nasal CPAP pressures decreased from 11.3 ± 2.0 to 8.8 ± 1.9 cwp.5,11,13–15 There was no heterogeneity across these studies, demonstrated by an I2 of 0% and Cochran Q statistic of P = 0.54. The fixed effects analysis for this subgroup demonstrated a statistically significant decrease in the mean pressure with MD of −2.81 cwp (95% CI, −3.22 to −2.40; overall effect: Z score = 13.46, P < 0.00001 [see Figure 3]).
Figure 3.
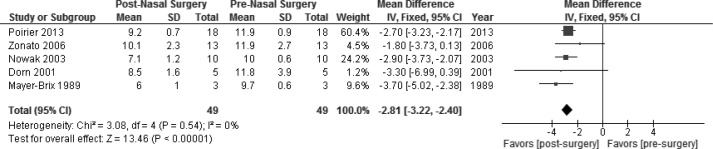
Subgroup analysis for studies in which continuous positive airway pressure (CPAP) was delivered by a nasal mask interface, prenasal and postnasal surgery. The fixed-effects analysis demonstrated a statistically significant decrease in therapeutic CPAP device pressures with a mean difference of −2.81 cwp (95% confidence interval [CI], −3.22 to −2.40; P < 0.00001). SD, standard deviation.
Individual patient therapeutic CPAP pressure data were reported for 20 patients, overall 85% (17 patients) decreased, 5% (one patient) remained the same, and 10% (two patients) increased their therapeutic CPAP pressures.5,13,15
Type of Nasal Surgery Performed and the Effect on Therapeutic CPAP Pressures
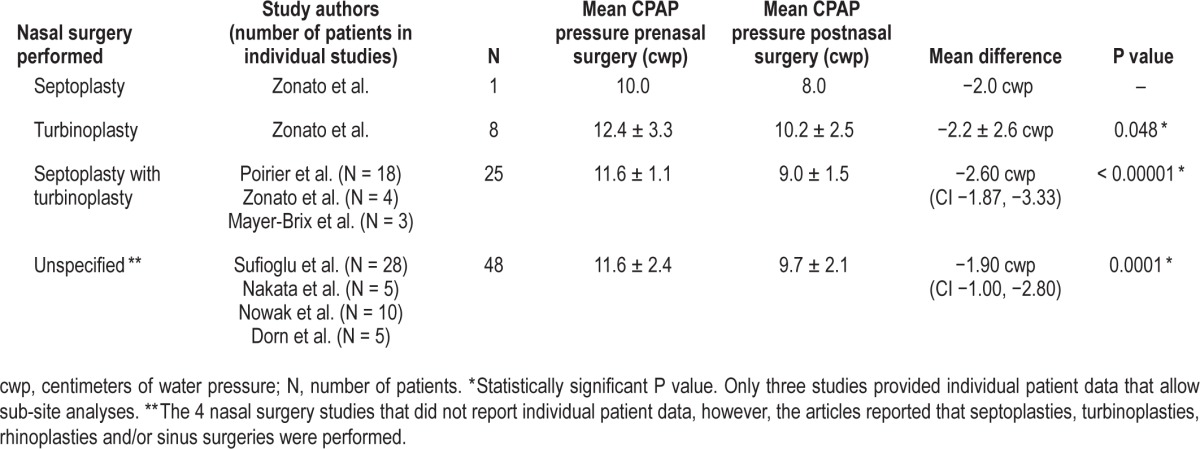
Data analyses were performed for the specific nasal surgery subsites: (1) septoplasty, (2) turbinoplasty, (3) septoplasty with turbinoplasty, and (4) unspecified; there were four studies10,12,14,15 during which multiple procedures such as septoplasties, turbinoplasties, rhinoplasties, and sinus surgeries, were performed but did not list individual patient data. Therefore the data are presented in the category of “unspecified.” Overall, 2 studies reported individual patient data and the specific sub-site,5,13 and Poirier et al.11 performed the same procedure in all patients, and therefore could be included in individual sub-site data analyses. Outcomes for the combined nasal surgery subsites are presented in Table 2. The largest MD was observed in the septoplasty with turbinoplasty group (25 patients), demonstrating a MD of −2.60 cwp (95% CI −3.33, −1.87); overall effect: Z score = 5.52, P < 0.00001. The MD for the remaining nasal surgery sub-sites included: (1) unspecified (48 patients), −1.90 cwp (95% CI −2.80, −1.00); overall effect: Z score = 2.99, P = 0.0001; (2) turbinoplasty (8 patients), −2.2 cwp; paired t test P = 0.048; and (3) septoplasty (one patient), −2.0 cwp.
Table 2.
Pre and postnasal surgery therapeutic mean CPAP pressure data stratified by nasal surgical sub-sites.
CPAP Use After Nasal Surgery
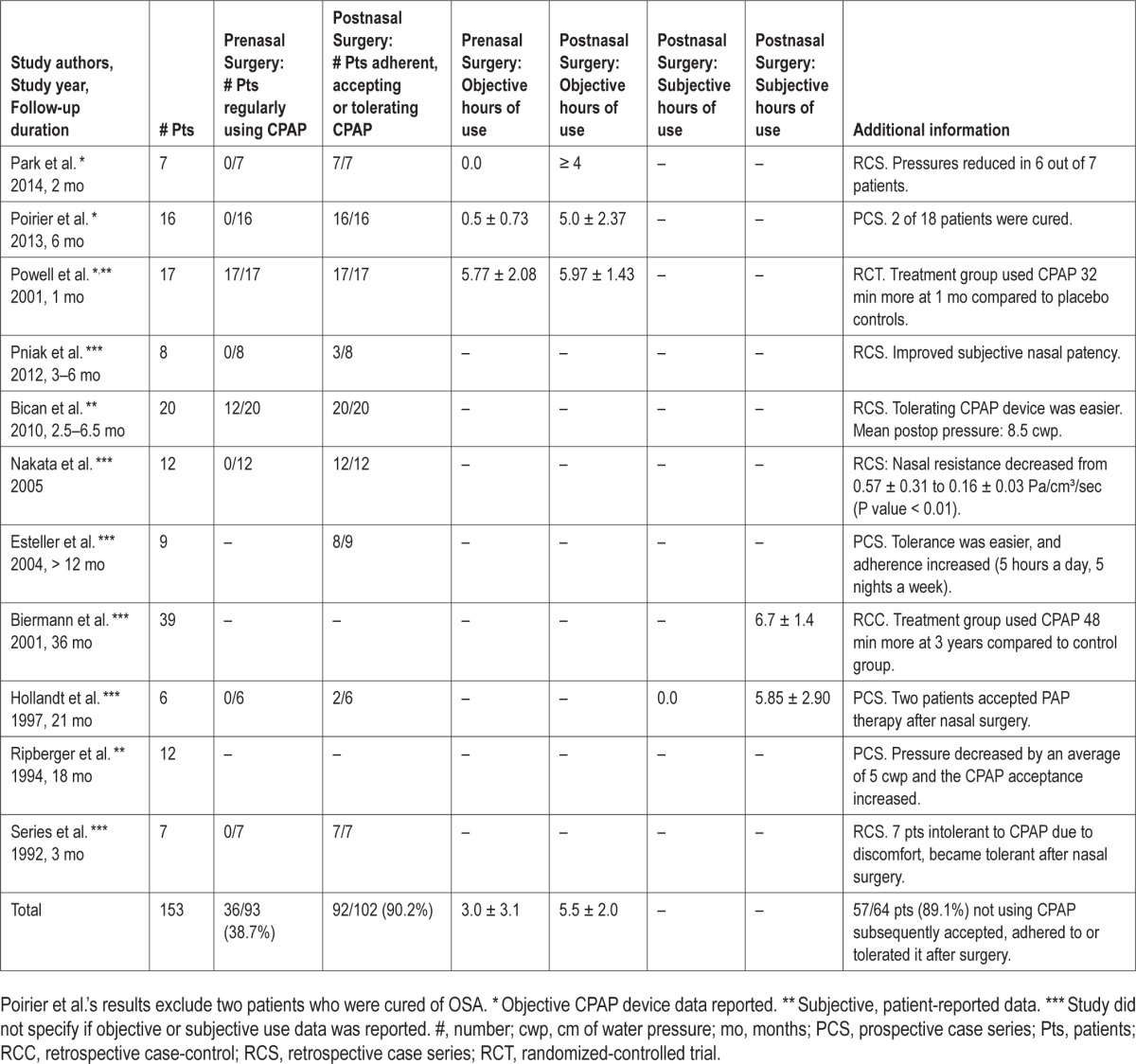
Eleven studies (153 patients) evaluated CPAP acceptance, compliance, tolerance, adherence, or use before and after nasal surgery.10,11,17–19,21–25 Three studies (n = 40) reported objective data from the CPAP devices themselves,11,25,26 three studies (n = 49) reported subjective, patient self-reported use,19,21,25 and six studies (n = 81) did not specify whether objective or subjective data was collected (Note: Powell et al. reported both objective and subjective data for all patients).10,17,18,22–24
The percentage of patients regularly using CPAP prior to nasal surgery was 38.7% (36 of 93 patients) and the percentage of patients who were adherent (synonymous with compliant), accepting, or tolerating CPAP after nasal surgery was 90.2% (92 of 102 patients). A subgroup analysis demonstrated that 89.1% (57 of 64 patients) who were not using CPAP prior to nasal surgery subsequently accepted, adhered to, or tolerated CPAP after nasal surgery. There were differences in the inclusion criteria between studies (i.e., six studies included patients not tolerating CPAP prior to nasal surgery,10,11,18,22,24,26 two studies included patients already using CPAP, but with nasal obstruction present,17,25 and three studies did not specify19,21,23). The CPAP device downloaded data for prenasal surgery hours of CPAP use mean and SD were 3.0 ± 3.1 h and postnasal surgery were 5.5 ± 2.0 h. The calculated treatment effect of nasal surgery based on Powell et al.'s RCT (difference between the treatment group and placebo controls) at 1 mo was 32 min (95% CI −21, 84), P = 0.22.25 Table 3 summarizes prenasal and postnasal surgery objective and subjective CPAP use data.
Table 3.
Data regarding prenasal and postnasal surgery acceptance, adherence, tolerance, or use.
DISCUSSION
There are five main findings in this study. First, there is a statistically significant relationship between nasal surgery and therapeutic CPAP pressures. Poiseuille's Law has demonstrated that resistance to airflow is directly proportional to the length and is inversely proportional to the fourth-power of the radius.47 Therefore, a 10% increase in the cross-sectional area of the nasal airway can result in an increase of 21% of nasal airflow.25 Thus, decreasing the size of the inferior turbinates or decreasing other sources of nasal obstruction (deviated septums, septal spurs, polyps, etc.) can provide a reduction in the nasal airway resistance, and therefore may lead to a decreased therapeutic CPAP pressure. In this meta-analysis, the pooled data for 82 patients demonstrates a MD of −2.66 cwp with a range of −0.8 to −4.8 cwp. The sensitivity analysis identified the study by Sufioglu et al.12 as the source of heterogeneity, which is possibly caused by not performing a postoperative CPAP titration in five patients who were cured of OSA (AHI < 5/h), therefore the prenasal and postnasal CPAP device pressure MD is potentially smaller (0.8 cwp) than it would have been had those cured patients undergone a postoperative CPAP titration. The remaining studies' MD decreased by at least 1.5 cwp. Additionally, the visual inspection of the funnel plot for therapeutic CPAP device pressures demonstrated that the study by Sufioglu et al.12 was slightly outlying when compared to the remaining studies. One difference in this study compared to the others is that this study reported the use of thermistors whereas the other studies did not specify whether thermistors or nasal cannulas were used.12 Despite this difference, a similar MD prenasal and postnasal surgery would be expected because the same technique was used within the same study group. Because of publication bias against negative studies, it is possible that additional studies demonstrating minimal or no difference in CPAP device pressures after nasal surgery never made it to publication and therefore the study by Sufioglu et al.12 would not be an outlier in that situation.28
Second, regardless of the nasal surgery subsite, there is at least a MD of −1.9 cwp between preoperative and postoperative CPAP device pressures (as outlined in Table 2). The greatest difference was seen in patients undergoing a combined septoplasty with turbinoplasty (MD of −2.6 cwp). It must be pointed out that only the study by Zonato et al.13 reported outcomes for isolated septoplasty (one patient) and isolated turbinoplasty (eight patients). Additionally, only three of seven studies reported both preoperative and postoperative means and SD and also had individual patient data or performed the same procedure in all patients, which allowed the subsite data to be combined (34 patients). A significant limitation in the ability to subanalyze the data for the remaining four studies (48 patients) was that multiple procedures were listed in these studies, without individual patient data. Therefore, it is possible that other procedures such as endoscopic sinus surgery for nasal polyposis or a functional rhinoplasty combined with septoplasty and turbinoplasty could be as effective or more effective in reducing CPAP device pressures; however, without stratified data, these conclusions cannot be made. Given that the currently published literature reporting outcomes for individual patient data is limited, we recommend that future researchers report outcomes for individual patients stratify the data for isolated subsites (i.e. septoplasty, turbinoplasty, etc.).
Third, overall CPAP use increased after nasal surgery and was demonstrated by 11 studies (153 patients). It should be noted that the patients in these studies were motivated enough to reat-tempt CPAP therapy. Two issues exist: first, only three studies specified that objective device data was used, whereas the remaining eight studies either reported subjective outcomes or did not specify. Second, one of the challenges in combining the “use” data is that studies refer to “use” by different terminology, such as: acceptance, compliance, adherence, tolerance, and use. We reviewed each of the study descriptions of “use” in order to organize the results into appropriate categories. The hours of use in each study reporting it varied significantly because of the differences in the inclusion criteria between studies, which ranged from including patients not tolerating CPAP prior to nasal surgery,10,11,18,22,24 to including patients already using CPAP, but with complaints of nasal obstruction significant enough to warrant surgery.17,25 Despite the differences in the inclusion criteria, there was an increase in objective and subjective CPAP use after surgery and there was a progressive increase in hours of use as the duration of follow-up from nasal surgery increased. There can be significant nasal crusting and edema after nasal surgery, and the healing phase may last 2–6 w. Therefore, studies reporting on pressure reduction and hours of use, should follow patients for 3–6 mo after surgery to ensure the healing is complete and the edema has resolved, because this can affect the results of CPAP use and therapeutic CPAP device pressures.
Fourth, in order to facilitate future research, we would like to recommend standardized terminology. Regarding the term “use”, studies have demonstrated that self-reported CPAP usage overestimates the actual usage by approximately 1 h when compared to the CPAP device download data8,48–50; therefore, we recommend reporting the device download data as “use”. The terms “adherence” and “compliance” have been used interchangeably,3,50 and have been previously defined as “using CPAP for ≥ 4 h per night.3,48,51” When reporting the percentage of nights that patients are adherent, 70% is often used as a cutoff “regularly using” CPAP therapy.48,51 The term “tolerance”, as related to CPAP and nasal surgery, has been defined by the use of a visual analog scale (10 cm) with anchors 0 cm = “unable to tolerate or use” and 10 cm = “easily tolerated and use all 7 nights per week.”25 The term “acceptance”, if used, should be defined by the authors reporting it to avoid ambiguity.
Fifth, there is a need for additional high-quality studies to allow for additional data analyses and to increase the power to draw more definitive conclusions. Most of the studies in this review were retrospective case series, with only five prospective case series and one randomized controlled trial. The study by Powell et al.,25 which was very well planned, can serve as an example for future randomized, double-blind, placebo-controlled clinical trials. Two limitations with the study by Powell et al. were that only 22 patients were included, and the follow-up was short (1 mo, which is within the window of healing). When planning a study on nasal surgery and CPAP, important data to collect includes: the total number of nights of CPAP use versus nonuse, the percentage of nights with CPAP use versus nonuse, the percentage of nights with usage ≥ 4 h per night versus < 4 h per night, the average usage on nights CPAP was used and the average usage on all nights.52 Additional important information to report includes: the 95th percentile leak, Epworth Sleepiness Scale questionnaire data,53 the effect of treatment on quality of life, inferior turbinate sizes before and after surgery,54 and information on nasal obstruction, which can be assessed with the Nasal Obstruction Symptom Evaluation (NOSE) scale questionnaire.55
CONCLUSION
Isolated nasal surgery in patients with OSA with nasal obstruction reduces therapeutic CPAP device pressures, and the currently published literature's objective and subjective data consistently suggest that it also increases CPAP use in select patients.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr Kushida has received research support from Aerial BioPharma, Pacific Medico Co., Ltd., Resmed, Apnex Medical, Impax Laboratories, Inc., and Cephalon; has consulting relationships with Apnex, Seven Dreamers Laboratories, Noven Pharmaceuticals, UCB, Philips-Respironics, and Zephyr; and has received royalties from Philips Respironics. The other authors have indicated no financial conflicts of interest. The work was performed at Stanford Hospital and Clinics, Stanford, CA. The views herein are the private views of the authors and do not reflect the official views of the Department of the Army or the Department of Defense.
ACKNOWLEDGMENTS
The authors thank Dr. Makoto Kawai for translating the Japanese article by Dr. Sato et al. into English.
SUPPLEMENTAL MATERIAL
National Institute for Health and Clinical Excellence Study Quality Assessment of cases series studies.
REFERENCES
- 1.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186:909–16. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 3.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soci. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffstein V, Viner S, Mateika S, Conway J. Treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Patient compliance, perception of benefits, and side effects. Am Rev Respir Dis. 1992;145:841–5. doi: 10.1164/ajrccm/145.4_Pt_1.841. [DOI] [PubMed] [Google Scholar]
- 5.Mayer-Brix J, Becker H, Peter JH. [Nasal high pressure ventilation in obstructive sleep apnea syndrome. Theoretical and practical otorhinolaryngologic aspects] Laryngorhinootologie. 1989;68:295–8. doi: 10.1055/s-2007-998337. [DOI] [PubMed] [Google Scholar]
- 6.Constantinidis J, Knobber D, Steinhart H, Kuhn J, Iro H. Fine-structural investigations of the effect of nCPAP-mask application on the nasal mucosa. Acta Otolaryngologica. 2000;120:432–7. doi: 10.1080/000164800750000694. [DOI] [PubMed] [Google Scholar]
- 7.Brander PE, Soirinsuo M, Lohela P. Nasopharyngeal symptoms in patients with obstructive sleep apnea syndrome. Effect of nasal CPAP treatment. Respir Int Rev Thorac Dis. 1999;66:128–35. doi: 10.1159/000029354. [DOI] [PubMed] [Google Scholar]
- 8.Pepin JL, Leger P, Veale D, Langevin B, Robert D, Levy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest. 1995;107:375–81. doi: 10.1378/chest.107.2.375. [DOI] [PubMed] [Google Scholar]
- 9.Li HY, Engleman H, Hsu CY, et al. Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea-hypopnea syndrome. Sleep. 2005;28:1554–9. doi: 10.1093/sleep/28.12.1554. [DOI] [PubMed] [Google Scholar]
- 10.Nakata S, Noda A, Yagi H, et al. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology. 2005;43:296–9. [PubMed] [Google Scholar]
- 11.Poirier J, George C, Rotenberg B. The effect of nasal surgery on nasal continuous positive airway pressure compliance. Laryngoscope. 2014;124:317–9. doi: 10.1002/lary.24131. [DOI] [PubMed] [Google Scholar]
- 12.Sufioglu M, Ozmen OA, Kasapoglu F, et al. The efficacy of nasal surgery in obstructive sleep apnea syndrome: a prospective clinical study. Eur Arch Otorhinolaryngol. 2012;269:487–94. doi: 10.1007/s00405-011-1682-z. [DOI] [PubMed] [Google Scholar]
- 13.Zonato AI, Bittencourt LR, Martinho FL, Gregorio LC, Tufik S. Upper airway surgery: the effect on nasal continuous positive airway pressure titration on obstructive sleep apnea patients. Eur Arch Otorhinolaryngol. 2006;263:481–6. doi: 10.1007/s00405-005-1018-y. [DOI] [PubMed] [Google Scholar]
- 14.Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. [Nasal obstruction and compliance to nasal positive airway pressure] Ann Otolaryngol Chir Cervicofac. 2003;120:161–6. [PubMed] [Google Scholar]
- 15.Dorn M, Pirsig W, Verse T. [Postoperative management following rhinosurgery interventions in severe obstructive sleep apnea. A pilot study] HNO. 2001;49:642–5. doi: 10.1007/s001060170062. [DOI] [PubMed] [Google Scholar]
- 16.Friedman M, Tanyeri H, Lim JW, Landsberg R, Vaidyanathan K, Caldarelli D. Effect of improved nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;122:71–4. doi: 10.1016/S0194-5998(00)70147-1. [DOI] [PubMed] [Google Scholar]
- 17.Biermann E. Nasal CPAP therapy in obstructive sleep apnea syndrome: Does functional rhinosurgery improve compliance? [Nasale CPAP-therapie beim obstruktiven schlafapnoe-syndrom: Verbessert funktionelle rhinochirurgie die compliance?] Somnologie. 2001;5:59–64. [Google Scholar]
- 18.Series F, St Pierre S, Carrier G. Effects of surgical correction of nasal obstruction in the treatment of obstructive sleep apnea. Am Rev Respir Dis. 1992;146:1261–5. doi: 10.1164/ajrccm/146.5_Pt_1.1261. [DOI] [PubMed] [Google Scholar]
- 19.Bican A, Kahraman A, Bora I, Kahveci R, Hakyemez B. What is the efficacy of nasal surgery in patients with obstructive sleep apnea syndrome? J Craniofac Surg. 2010;21:1801–6. doi: 10.1097/SCS.0b013e3181f40551. [DOI] [PubMed] [Google Scholar]
- 20.Balcerzak J, Niemczyk K, Arcimowicz M, Gotlib T. [The role of functional nasal surgery in the treatment of obstructive sleep apnea syndrome] Otolaryngologia Polska. 2007;61:80–4. doi: 10.1016/S0030-6657(07)70388-8. [DOI] [PubMed] [Google Scholar]
- 21.Ripberger R, Pirsig W. [Nasal positive pressure ventilation (nCPAP) in therapy of obstructive sleep apnea: acceptance by 50 patients] Laryngorhinootologie. 1994;73:581–5. doi: 10.1055/s-2007-997200. [DOI] [PubMed] [Google Scholar]
- 22.Hollandt JH, Kuhl S, Siegert R. [Therapy with nasal CPAP (continuous positive airway pressure) in patients with obstructive sleep apnea syndrome (OSAS). I: Long-term acceptance of nasal CPAP] Laryngorhinootologie. 1997;76:550–3. doi: 10.1055/s-2007-997477. [DOI] [PubMed] [Google Scholar]
- 23.Esteller E, Matino E, Segarra F, Sanz JJ, Adema JM, Estivill E. [Adverse effects of continuous positive airway pressure therapy and its relation to the nose] Acta Otorrinolaringologica Espanola. 2004;55:17–22. doi: 10.1016/s0001-6519(04)78477-0. [DOI] [PubMed] [Google Scholar]
- 24.Pniak T, Matousek P, Strympl P, Novak V, Kominek P. Obstructive sleep apnoea and CPAP - is it reasonable to solve nasal patency? Cesk Slov Neurol N. 2012;75:222–6. [Google Scholar]
- 25.Powell NB, Zonato AI, Weaver EM, et al. Radiofrequency treatment of turbinate hypertrophy in subjects using continuous positive airway pressure: a randomized, double-blind, placebo-controlled clinical pilot trial. Laryngoscope. 2001;111:1783–90. doi: 10.1097/00005537-200110000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Park CY, Hong JH, Lee JH, et al. Clinical effect of surgical correction for nasal pathology on the treatment of obstructive sleep apnea syndrome. PloS One. 2014;9:e98765. doi: 10.1371/journal.pone.0098765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 31.Lasters F, Mallegho C, Boudewyns A, et al. Nasal symptoms in patients with obstructive sleep apnea and their impact on therapeutic compliance with continuous positive airway pressure. Acta Clinica Belgica. 2014;69:87–91. doi: 10.1179/0001551214Z.00000000028. [DOI] [PubMed] [Google Scholar]
- 32.Sato K. [Continuous positive airway pressure therapy in multidisciplinary treatment] Nihon Jibiinkoka Gakkai Kaiho. 2005;108:150–6. doi: 10.3950/jibiinkoka.108.150. [DOI] [PubMed] [Google Scholar]
- 33.Seemann R, DiToppa JC, Holm MA, Hanson J. Combination surgical and mechanical therapy for refractory cases of obstructive sleep apnea. J Otolaryngol. 2002;31:85–8. doi: 10.2310/7070.2002.19048. [DOI] [PubMed] [Google Scholar]
- 34.Mortimore IL, Bradley PA, Murray JA, Douglas NJ. Uvulopalatopharyngoplasty may compromise nasal CPAP therapy in sleep apnea syndrome. Am J Respir Crit Care Med. 1996;154:1759–62. doi: 10.1164/ajrccm.154.6.8970367. [DOI] [PubMed] [Google Scholar]
- 35.Han F, Song W, Li J, Zhang L, Dong X, He Q. Influence of UPPP surgery on tolerance to subsequent continuous positive airway pressure in patients with OSAHS. Sleep Breath. 2006;10:37–42. doi: 10.1007/s11325-005-0041-y. [DOI] [PubMed] [Google Scholar]
- 36.Gillespie MB, Wylie PE, Lee-Chiong T, Rapoport DM. Effect of palatal implants on continuous positive airway pressure and compliance. Otolaryngol Head Neck Surg. 2011;144:230–6. doi: 10.1177/0194599810392173. [DOI] [PubMed] [Google Scholar]
- 37.Chandrashekariah R, Shaman Z, Auckley D. Impact of upper airway surgery on CPAP compliance in difficult-to-manage obstructive sleep apnea. Arch Orolaryngol Head Neck Surg. 2008;134:926–30. doi: 10.1001/archotol.134.9.926. [DOI] [PubMed] [Google Scholar]
- 38.Bertoletti F, Indelicato A, Banfi P, Capolunghi B. Sleep apnoea/hypopnoea syndrome: combination therapy with the Pillar palatal implant technique and continuous positive airway pressure (CPAP). A preliminary report. B-ENT. 2009;5:251–7. [PubMed] [Google Scholar]
- 39.Friedman M, Soans R, Joseph N, Kakodkar S, Friedman J. The effect of multilevel upper airway surgery on continuous positive airway pressure therapy in obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2009;119:193–6. doi: 10.1002/lary.20021. [DOI] [PubMed] [Google Scholar]
- 40.Lin Z, Wang T, Fan X. [Continuous positive airway pressure therapy after uvulopalatopharygoplasty] Zhonghua Er Bi Yan Hou Ke Za Zhi. 1999;34:100–2. [PubMed] [Google Scholar]
- 41.Masdon JL, Magnuson JS, Youngblood G. The effects of upper airway surgery for obstructive sleep apnea on nasal continuous positive airway pressure settings. Laryngoscope. 2004;114:205–7. doi: 10.1097/00005537-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Hollandt JH, Mahlerwein M. Nasal breathing and continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) Sleep Breath. 2003;7:87–94. doi: 10.1007/s11325-003-0087-7. [DOI] [PubMed] [Google Scholar]
- 43.Morris LG, Setlur J, Burschtin OE, Steward DL, Jacobs JB, Lee KC. Acoustic rhinometry predicts tolerance of nasal continuous positive airway pressure: a pilot study. Am J Rhinol. 2006;20:133–7. [PubMed] [Google Scholar]
- 44.Sugiura T, Noda A, Nakata S, et al. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respir Int Rev Thorac Dis. 2007;74:56–60. doi: 10.1159/000089836. [DOI] [PubMed] [Google Scholar]
- 45.Mayer-Brix J, Muller-Marschhausen U, Becker H, Peter JH. [How frequent are pathologic ENT findings in patients with obstructive sleep apnea syndrome?] HNO. 1989;37:511–6. [PubMed] [Google Scholar]
- 46.Katsantonis GP, Friedman WH, Krebs FJ, 3rd, Walsh JK. Nasopharyngeal complications following uvulopalatopharyngoplasty. Laryngoscope. 1987;97:309–14. [PubMed] [Google Scholar]
- 47.Susarla SM, Thomas RJ, Abramson ZR, Kaban LB. Biomechanics of the upper airway: changing concepts in the pathogenesis of obstructive sleep apnea. Int J Oral Maxillofac Surg. 2010;39:1149–59. doi: 10.1016/j.ijom.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 49.Engleman HM, Asgari-Jirhandeh N, McLeod AL, Ramsay CF, Deary IJ, Douglas NJ. Self-reported use of CPAP and benefits of CPAP therapy: a patient survey. Chest. 1996;109:1470–6. doi: 10.1378/chest.109.6.1470. [DOI] [PubMed] [Google Scholar]
- 50.Rauscher H, Formanek D, Popp W, Zwick H. Self-reported vs measured compliance with nasal CPAP for obstructive sleep apnea. Chest. 1993;103:1675–80. doi: 10.1378/chest.103.6.1675. [DOI] [PubMed] [Google Scholar]
- 51.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Ind J Med Res. 2010;131:245–58. [PMC free article] [PubMed] [Google Scholar]
- 52.Schwab RJ, Badr SM, Epstein LJ, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188:613–20. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–45. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 54.Camacho M, Zaghi S, Certal V, et al. Inferior Turbinate classification system, grades 1 to 4: Development and validation study. Laryngoscope. 2014 Sep 12; doi: 10.1002/lary.24923. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130:157–63. doi: 10.1016/j.otohns.2003.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
National Institute for Health and Clinical Excellence Study Quality Assessment of cases series studies.