Abstract
Study Objectives:
Gamma-hydroxybutyrate (GHB) was originally introduced as an anesthetic but was first abused by bodybuilders and then became a recreational or club drug.1 Sodium salt of GHB is currently used for the treatment of cataplexy in patients with narcolepsy. The mode of action and metabolism of GHB is not well understood. GHB stimulates growth hormone release in humans and induces weight loss in treated patients, suggesting an unexplored metabolic effect. In different experiments the effect of GHB administration on central (cerebral cortex) and peripheral (liver) biochemical processes involved in the metabolism of the drug, as well as the effects of the drug on metabolism, were evaluated in mice.
Design:
C57BL/6J, gamma-aminobutyric acid B (GABAB) knockout and obese (ob/ob) mice were acutely or chronically treated with GHB at 300 mg/kg.
Measurements and Results:
Respiratory ratio decreased under GHB treatment, independent of food intake, suggesting a shift in energy substrate from carbohydrates to lipids. GHB-treated C57BL/6J and GABAB null mice but not ob/ob mice gained less weight than matched controls. GHB dramatically increased the corticosterone level but did not affect growth hormone or prolactin. Metabolome profiling showed that an acute high dose of GHB did not increase the brain GABA level. In the brain and the liver, GHB was metabolized into succinic semialdehyde by hydroxyacid-oxoacid transhydrogenase. Chronic administration decreased glutamate, s-adenosylhomocysteine, and oxidized gluthathione, and increased omega-3 fatty acids.
Conclusions:
Our findings indicate large central and peripheral metabolic changes induced by gamma-hydroxybutyrate (GHB) with important relevance to its therapeutic use.
Citation:
Luca G, Vienne J, Vaucher A, Jimenez S, Tafti M. Central and peripheral metabolic changes induced by gamma-hydroxybutyrate. SLEEP 2015;38(2):305–313.
Keywords: antioxidant, brain, GHB, liver, metabolomics
INTRODUCTION
Gamma-hydroxybutyrate (GHB), a short fatty acid derivative of gamma-aminobutyric acid (GABA), is an inhibitory neurotransmitter naturally present in the mammalian brain.2,3 In sleep medicine, sodium oxybate, a sodium salt of GHB, is used for the treatment of narcolepsy with cataplexy.4 GHB is an agonist of GABAB receptors but its mode of action and the range of effects are not well understood.5 Originally introduced as an anesthetic in early 1960s,2 GHB was soon proposed to produce a physiological sleep with increased slow wave sleep (SWS).6 However, studies in animals suggested that the GHB-induced changes mimicked the state of absence seizure with abnormal electroencephalogram (EEG).7,8 More recently, we extensively studied the effects of GHB on the EEG and vigilance states in mice and humans.5,9 GHB dose-dependently increased EEG slow waves but at higher doses the EEG and behavior were very similar to classic anesthetics.5 We also showed that in both humans and mice, the GHB-induced behavioral changes were different from physiological sleep. Finally, we showed that exogenous GHB acts solely through GABAB receptors because GHB had no behavioral and/or EEG effects in GABAB knockout (KO) mice.5
An increase in growth hormone, simultaneously with the first slow wave sleep episode, was reported in healthy young volunteers10 and in patients with narcolepsy treated with sodium oxybate.11 The increase in growth hormone is believed to be the reason why GHB was commonly abused by bodybuilders.12 GHB is also abused as a recreational drug and was associated with euphoria, behavioral disinhibition, dizziness, myoclonus, retrograde amnesia (date rape drug), nausea and vomiting, confusion, and coma.13 GHB overdose, both in fatal and nonfatal cases, presents with respiratory depression, bradycardia, and hypotension.14
Given the major behavioral effects of GHB, central actions of GHB have been studied most often, although GHB is also present outside of the brain and might have several actions. For instance, one of the side effects observed in patients with GHB-treated narcolepsy,1 fibromyalgia,16 and binge eating disorder17 is weight loss, suggesting a metabolic effect that remains ill defined. Early studies on GHB central mode of action indicated a dose-dependent reduction of cerebral glucose utilization in the rat brain,6,18,19 which was interpreted as an adaptation to decreased brain metabolic needs. GHB was also tested for potential protective properties in animal models of stroke and hypoxia,20,21 head trauma,22 and transient global cerebral ischemia.23 Also, GHB administration induced hypothermia in rats by decreasing metabolic heat production. Nevertheless, it is not understood how these metabolic effects are produced. It was proposed that GHB shifts the intermediary metabolism toward the pentose-phosphate pathway (PPP) by activating glucose-6-phosphate dehydrogenase.24 Similar to most other central depressants, GHB does not increase glucose supply to the brain but decreases its use by widespread neuronal inhibition but whether the same happens at periphery is not known.
Thus, the aim of the current study was to determine which metabolic pathways are affected by acute and chronic administration of GHB and how chronic administration of the drug affects body weight as well as brain and body composition in different mouse strains.
METHODS
Animals
All experiments were performed according to the protocols approved by the State of Vaud Veterinary Office, Switzerland. All mice were male and kept in individual polycarbonate cages under 12 h light/dark cycles, at 25°C. GABAB KO mice (lacking either GABAB1 or GABAB2 subunit, generated in BALB/c background) were generously provided by Dr. Bernard Bettler (University of Basel, Switzerland) and were generated as previously described.5,25 Both GABAB1 and GABAB2 −/− mice do not have functional GABAB receptors. Obese (ob/ob) mice (C57BL/6J background, leptin deficient because of a spontaneous obese “Ob” mutation) were purchased from Charles River Laboratory (L'Arbresle, France).
Experimental Design
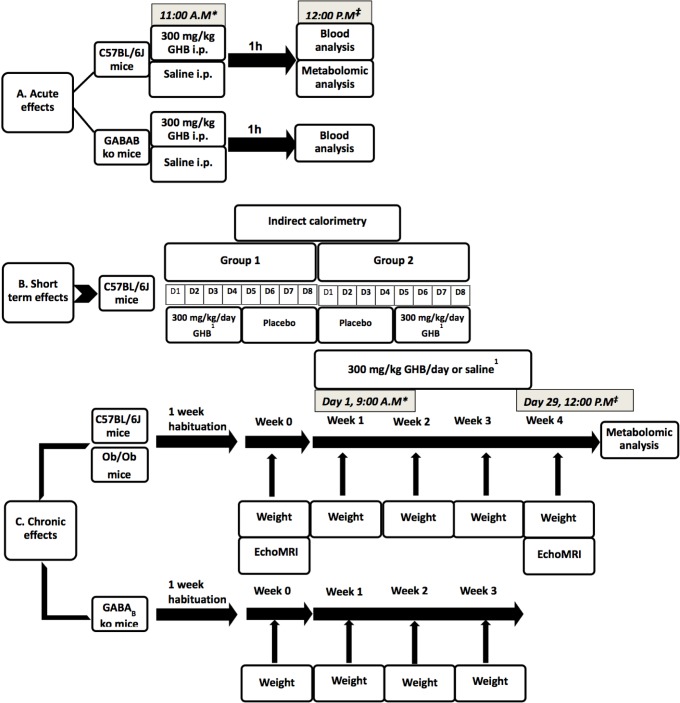
To evaluate the metabolic effects of GHB, we designed three different experiments to assess, separately, the acute, short-term, and chronic metabolic effects of the drug (Figure 1). We chose three different genotypes: C57BL/6J mice as wild type (and the strain commonly used for metabolic studies), GABAB KO mice (because previous research showed these mice are behaviorally insensitive, in terms of sleep and locomotor activity, to GHB5) and ob/ob mice to test if the effects found in the previous two genotypes are leptin-related.
Figure 1.
Study design presenting experiments to determine acute, short-term, and chronic metabolic effects of gamma-hydroxybutyrate (GHB). 1Drug administration in the drinking water. * Start of the drug administration. ‡ End of drug administration and sampling. GABA, gamma-aminobutyric acid; i.p., intraperitoneal; ob/ob, obese.
Experiment 1: Acute Effects of GHB Administration
To test the acute metabolic changes, blood and tissue metabolites were assessed 1 h after the drug administration. C57BL/6J mice (N = 16, age 12 w) were divided into two groups: treatment and placebo. Drug (300 mg/kg of Xyrem, oral solution, 500 mg/mL, UCB-Pharma SA, Basel, Switzerland, hereafter “GHB”) or placebo (NaCl 0.9%, B. Braun Medical AG, Emmenbrücke, Switzerland) were administrated intraperitoneally (ip) with a volume of 5 mL/kg body weight. The dose and the duration were based on previous experiments performed in our laboratory,5 which showed that the maximum effects were obtained at 300 mg/kg and lasted for approximately 1 h. Blood and tissues were collected 1 h after GHB/placebo administration. Blood cholesterol, triglyceride, glucose, and free fatty acids were measured. The brain and liver were removed, stored at −80°C and used for metabolome analysis.
To evaluate the effects of the drug on corticosterone, prolactin, and growth hormone we used the same protocol but in two genotypes: C57BL/6J and GABAB KO mice (lacking either GABAB1 or GABAB2), age 12–13 w. One hour after ip injection of 300 mg/kg of GHB, animals were rapidly decapitated and trunk blood was collected, centrifuged at 1,000 g for 15 min at 4°C and the supernatant stored at −80°C. Corticosterone was quantified by an enzyme immunoassay kit (Enzo Life Sciences AG, Lausen, Switzerland) according to the manufacturer instructions. Test samples (duplicates) were diluted 40 times in the provided buffer, and optical density was measured (λ = 405 nm). Prolactin and growth hormone were measured by enzyme-linked immunoassay (ELISA) kits (mouse prolactin ELISA kit, Sigma Aldrich, St. Louis, MO, USA and rat/mouse growth hormone ELISA kit, EMD Millipore, St. Charles, MO, USA). Test samples (duplicates) were diluted 1:10 in provided buffer and absorbance (λ = 450 nm for prolactin and the difference between λ = 450 nm and 590 nm for growth hormone) was recorded.
Experiment 2: Effects of Subchronic Administration of GHB
In a second step, mice were studied in the calorimetric chambers for 8 days: 4 baseline days and 4 days under drug administration. After 1 day of habituation, 10 C57BL/6J, 12 w old, were recorded for two sessions of 96 h in calorimetric chambers (Oxymax, Columbus Instruments, Columbus, OH, USA). The first group of five mice received 300 mg GHB/kg/day in drinking water, starting the first evening of testing. The second group of five mice received the same dose of drug in the same manner, from hour 97 of the experiment (balanced design). Placebo (water) was administered on the opposite order. At the beginning of the experiment mice were weighed, and measured parameters were adjusted for each mouse weight. Energy expenditure (EE) was calculated as: 3.815*VO2 + 1.232*VCO2.26 Locomotor activity, oxygen consumption (VO2) and CO2 production (VCO2), respiratory exchange ratio (RER), food and water intake, and heat production were calculated by Oxymax software.
Experiment 3: Effects of Chronic GHB Administration
For chronic metabolic changes, mice were studied 4 w under placebo or 300 mg GHB/kg/day. Twenty C57BL/6J mice and 16 ob/ob mice were divided into two groups, balanced for their age (12 w old at the beginning of the experiment) and weight. One week before the experiment all mice were habituated to drink water only during the light (sleep) period to mimic the administration of the drug in humans; water bottles were removed at 21:00 and reinstalled at 09:00; and the daily quantity of water was measured. After 1 w of habituation, mice were weighed and divided into two groups. The treated group received in the drinking water 300 mg GHB/kg/day, diluted in 4 mL of water. All mice consumed this quantity of water. Control mice received water only during the light period. Lean mass, fat mass, and water content were assessed on isoflurane-anesthesized animals using an EchoMRI (Houston, TX, USA) whole-body composition analyzer. Mice were evaluated for body composition at the beginning of the experiment and after 28 days of treatment. Weight was monitored weekly using an electronic scale. To avoid the effects of isoflurane on metabolites analysis, after the second EchoMRI measurement drug/water administration continued for another 24 h. At the end of the experiment, mice were sacrificed by cervical dislocation. The brain and the liver of C57BL/6J mice were rapidly sampled, stored at −80°C and used for metabolome analysis.
In addition, 300 mg GHB/kg/day, diluted in 4 mL of water, was also administered for 4 w, during the light period, to four GABAB1 and five GABAB2 KO mice and placebo to four GABAB1 and three GABAB2 KO mice, matched for age (12–13 w) and weight at the beginning of the experiment. Before starting the experiment mice were habituated for 1 w to have access to water only during the light period. Weight was monitored weekly.
For metabolome analysis, the cortex was dissected from the frozen brains. A sample of 100–140 mg from frozen liver was also collected. All samples were stored at −80°C until processing. Sample preparation and analysis were performed by Metabolon (Durham, NC, USA), as previously described.27
Statistical Analyses
To test the effects of the treatment on blood biochemistry, calorimetric variables, and body mass composition, paired t test or Wilcoxon signed-rank test were used when appropriate. Weekly changes in weight were analyzed by One-way repeated-measures analysis of variance (ANOVA). To identify the effect of light/dark variability, effects of treatment, order of treatment, and their interaction, two-way ANOVA was used. Evaluation of the relationship between body mass composition and weight changes was analyzed by Deming regression (errors-in-variable model), and then the slopes were compared by t test. Calorimetric data were normalized to the first 4 h of the experiment. Metabolomics data were analyzed by Welch t test; fold change (log2(treated/control)) and significance level (−log10(P value)) were calculated.
RESULTS
Experiment 1
Corticosterone is Increased by GHB
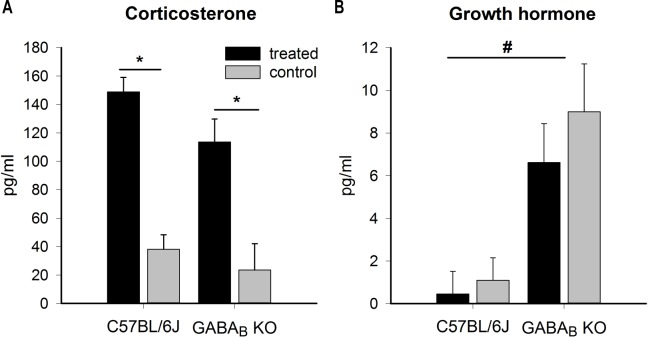
One hour after drug administration, no statistical differences were found in plasma concentrations of glucose, cholesterol, triglyceride, and free fatty acids. Corticosterone levels were significantly increased in both C57BL/6J and GABAB KO treated mice (almost fourfold), independent of genotype (twoway ANOVA, P < 0.001 for factor “treatment,” nonsignificant for genotype and interaction, C57BL/6J = 17, GABAB KO = 7) (Figure 2A). Prolactin was not affected by GHB administration, in any of the genotypes (C57BL/6J = 18, GABAB KO = 6). Growth hormone was slightly (though not significantly) decreased by GHB in both genotypes (C57BL/6J = 20, GABAB KO = 7). Nevertheless, GABAB KO mice had significantly higher values compared to C57BL/6J, independent of the treatment (Figure 2B).
Figure 2.
Effects of acute intraperitoneal administration of 300 mg gamma-hydroxybutyrate (GHB)/kg on corticosterone (A) and growth hormone (B) in C57BL/6J and gamma-aminobutyric acid B (GABAB) knockout (KO) mice. Corticosterone was significantly elevated in treated C57BL/6J and GABAB KO mice (two-way analysis of variance followed by Holm-Sidak post hoc test for “treatment,” P < 0.001; “genotype,” P > 0.05; and their interaction, P > 0.05, N = 20 for C57BL/6J and N = 7 for GABAB KO mice). Growth hormone was not affected by treatment (P = 0.36), but GABAB KO mice had significantly higher levels compared with C57BL/6J mice (P < 0.001 for “genotype,” P > 0.05 for “treatment” and their interaction; N = 17 for C57BL/6J and N = 7 for GABAB KO mice). * P < 0.05 between the treated and the control group; # P < 0.05 between genotypes.
Acute Metabolic Effects of GHB
Metabolomics was used to evaluate the effect of GHB administration on central (cortex) and peripheral (liver) biochemical processes involved in the metabolism of the drug, as well as the effects of the drug on metabolism. Overall, 226 metabolites could be identified in the cortex and 314 in the liver. Table S1 (supplemental material) shows all detected metabolites with those that display significant differences between treated and control groups highlighted.
One hour after administration, GHB was found at over 300-fold higher levels in the brain and nearly 200-fold in the liver. The major degradation pathway activated was GHB oxidation to succinic semialdehyde catalyzed by hydroxyacid-oxoacid transhydrogenase.28 The rate of catabolism was higher in the liver than in the brain (2-hydroxyglutarate was elevated 29-fold in the liver but only threefold in the brain), but surprisingly, glycolates as the final breakdown products were sevenfold in the brain and fivefold in the liver. Interestingly, GABA levels were not changed despite the large increase in GHB, strongly suggesting that GHB is not converted to GABA even at such high doses.
Glucose and many of the glycolysis metabolites glucose-6-phospahate, fructose-6-phosphate, fructose 1,6,-diphosphate/ glucose 1,6-diphosphate, were significantly increased in the cortex of GHB-treated mice. In GHB-treated mice there was a significant increase in pyruvate and acetyl coenzyme A (acetyl CoA), but metabolites of the tricarboxylic acid (TCA) cycle, citrate and malate, were significantly decreased and, although not statistically significant, fumarate levels were also reduced. The limiting factor for acetyl-CoA entrance into the TCA cycle is oxaloacetate, which was not measured in these samples.
A surprising finding was a large increase in lysolipids in the cortex of GHB-treated mice. Whether this indicates an increased membrane remodeling or an inhibition of lipid reacylation is difficult to disentangle. Long-chain acylcarnitines were also significantly increased but the ketone body 3-hydroxybutyrate that is made during β-oxidation was actually decreased.
As opposed to the cortex, most detected metabolites in the liver were decreased in mice treated acutely for GHB. With few exceptions, corticosterone was found at fourfold higher levels after GHB administration. A major pathway affected by GHB in the liver was the bile acid metabolism, with almost all detected metabolites significantly decreased in GHB-treated mice. Although this finding may suggest a reduced lipid processing, no major changes in lipids were detected, except for an increase in docosapentaenoate (22:5n6). There was also a general decrease in amino acids (except for the branched-chain amino acids “BCAAs”), peptides, and carbohydrates metabolism.
Experiment 2
Respiratory Ratio is Reduced by GHB
For the light period during the calorimetry, the group of C57BL/6J mice who received the treatment first did not show any significant differences between the treatment and control sessions. For the second group (which received the placebo first and then the drug), a significant decrease in heat production (paired t test, t = −2.51, P = 0.01), CO2 elimination (VCO2) (paired t test, t = −2.78, P = 0.006), O2 consumption (VO2) (paired t test, t = −2.92, P = 0.004) and EE (paired t test, t = −2.91, P = 0.003) was observed. Because of proportional decrease in CO2 and O2, RER was not significantly different.
During the dark period, a significant decrease for Z axis activity (exploratory and possibly drinking, paired t test, t = −2.75, P = 0.007) and RER was observed for the first group, whereas in the second group, there was a significant decrease in feeding (Wilcoxon signed-rank test, W = 2153, P < 0.001) and RER (paired t test, t = −2.63, P = 0.009). There was no significant reduction in locomotor activity between the two conditions. Despite between-group differences for locomotor activity on the Z axis, RER, and feeding, analysis of the effect of order, treatment, and their interaction for the dark period indicated that the only variable that changed was RER (two-way repeated-measures ANOVA with factors “treatment” P < 0.001, “order,” P = 0.36, and “treatment × order,” P = 0.79).
Calculated over 24 h, paired t test (Wilcoxon signed rank) for the first group showed no differences between treatment and control conditions for activity, feeding, heat production, VO2, or VCO2. The only parameter that decreased significantly was again RER (Wilcoxon signed-rank test, W = 4833, P = 0.003), and this decrease was independent of food intake. For the second group, feeding, VO2 (paired t test, t = −2.569, P = 0.01), VCO2 (t = −3.594, P < 0.001) and RER (paired t test, t = −1.997, P = 0.04) were decreased. Again, when taking into account both “treatment” and “order” effects, the decrease in RER was influenced by the treatment only (two-way repeated-measures ANOVA with factors “treatment” P = 0.04, “order” P = 0.4, and “order by treatment” P = 0.79). The RER changes over 96 h of the experiment are reported in Figure 3. No changes in weight were observed. All calorimetric results are shown in Table S2 (supplemental material).
Figure 3.
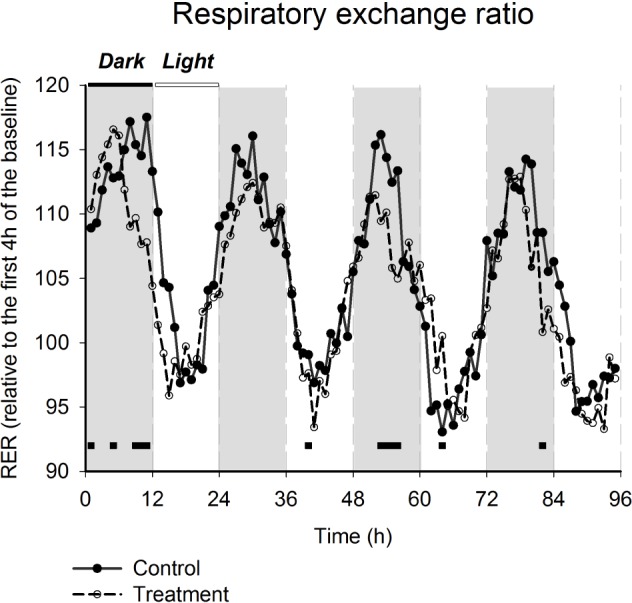
Effects of gamma-hydroxybutyrate administration on respiratory exchange ratio (RER). Data are presented as percentage of the mean of the first 4h of the baseline. Two-way repeated-measures analysis of variance for factors “treatment,” “time-point,” and their interaction, followed by Holm-Sidak post hoc test, P < 0.001 for “treatment” and “time-point”; black lines connect significant different time-points between treated and control mice, P < 0.05).
Experiment 3
Body Composition is Changed by GHB
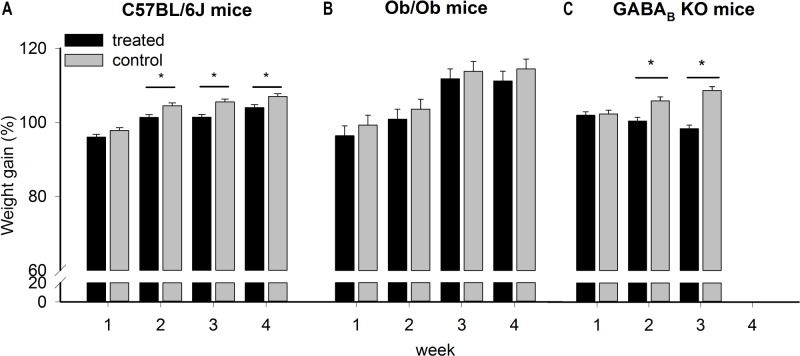
There were no significant differences between treated and control groups for fat mass, lean mass, or water content evaluated by EchoMRI after 4 w of treatment. Statistical tests were performed on both absolute and relative values (calculated as percentages as compared to baseline). Nevertheless, the regression analysis followed by t test showed a more balanced distribution of fat mass and lean mass in treated compared to control mice (t = 9.30, P < 0.001). The higher the lean mass increase, the lower was the fat content. This suggests that in the treated group weight loss is determined by initial fat content; body fat content is a determinant of the relative proportion of lean tissue loss, but the weight change is inversely correlated with relative increase in lean mass (tests for slopes equal 0 and 1, respectively; P < 0.005 in treated mice and nonsignificant in control mice). A similar experiment performed in ob/ob mice showed a comparable trend, although not significant (because of a smaller change relative to their weight).
Slower Weight Gain with GHB
During chronic (4 w) administration of GHB, C57BL/6J treated mice gained less weight than matched controls (Figure 4). Note that at this age, weight curve is still increasing in both groups (two-way repeated-measures ANOVA with factors “treatment” P = 0.001, “week,” P = 0.002, and “treatment × week,” P = 0.27) (Figure 4A). The same treatment in ob/ob mice showed a similar trend but nonsignificant (t test, P = 0.27, Figure 4B). To test if the decrease in weight observed in treated C57BL/6J mice was related to a reduction in activity level or food intake, we administrated the drug in GABAB KO mice, who are behaviorally insensitive to GHB in terms of activity and sleep-wake parameters.5 The analysis was performed after 3 w of treatment in 11 mice (six treated and five controls, only 3 w are considered because 5 mice died during the fourth week from seizures, common in these mice). A significant decrease in weight was observed in treated mice (two-way repeated-measures ANOVA with factors “treatment” P = 0.002, “week,” P = 0.20, and “treatment × week,” P < 0.001) (Figure 4C). The weight loss observed in GABAB KO mice suggests that the effect is not centrally mediated through GABAB receptors.
Figure 4.
Relative weight change calculated as a percentage of the initial weight, in gamma-hydroxybutyrate-treated and control groups (black and gray bars, respectively). (A) Two-way repeated-measures analysis of variance for factors “treatment,” “week” and their interaction, followed by Holm-Sidak post hoc test showed that the weight gain by C57BL/6J mice in the treated group was significantly less compared to the control group; * P < 0.05, N = 20. Lines connect weeks for which significant differences were found. (B) The weight gain in obese (ob/ob) treated group was lower compared to the control group, but not significantly different between the treated and the control group (N = 16). (C) GABAB KO treated mice lost weight compared with the control mice; * P < 0.05, N = 11. GABAB, gamma-aminobutyric acid B; KO, knockout mice.
Chronic Metabolic Effects of GHB
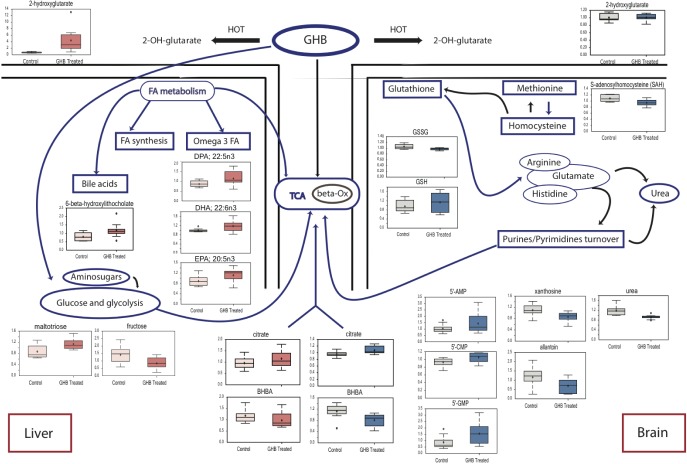
Overall, chronic GHB administration showed a different metabolic effect. As opposed to the acute administration, GHB levels were only marginally increased (Table S3, supplemental material) both in the cortex and the liver whereas 2-hydroxyglutarate (intermediate metabolite) was still increased by sixfold in the liver. Also, no changes were observed either in the glucose or the BCAA metabolism or their metabolites. This finding was anticipated because of the very short half-life of GHB (10–20 min) and because animals undergoing acute treatment were injected with a bolus of 300 mg/kg, while they consumed the same dose in their drinking water over the 12 h light period during the 4 w of the experiment. Although most lysolipids were unaffected by chronic GHB, 1- and 2-linoleoglycerophosphocho-lines were still significantly increased in the cortex (as in the acute experiment) while significantly decreased in the liver. As in the acute experiment, long-chain acylcarnitines tended to be increased in the cortex, whereas the ketone body 3-hydroxybutyrate was decreased both in the cortex and the liver (Figure 5).
Figure 5.
Main metabolic changes in the liver (left) and the brain (right) after chronic administration of gamma-hydroxybutyrate. Box plots for main significant changes (P < 0.05) are presented in red for the liver and in blue for the brain. 5'-AMP, adenosine 5'-monophosphate; beta-Ox, beta-oxidation; BHBA, 3-hydroxybutyrate; 5'-CMP, cytidine 5'-monophosphate; 5'- FA, fatty acids; GMP, guanosine 5'- monophosphate; GSH, glutathione, reduced; GSSG, glutathione oxidized; HOT, hydroxyacid-oxoacid transhydrogenase; TCA, tricarboxylic acid cycle.
S-adenosylhomocysteine was significantly decreased in the cortex (fold change = 0.87, P < 0.006) and tended to be also decreased in the liver (fold change = 0.91, P < 0.11). This might have important consequences in terms of methionine and cysteine availability. Interestingly, oxidized glutathione was significantly reduced together with riboflavin, whereas reduced glutathione tended to increase in the cortex of GHB-treated mice. This finding indicates that GHB might have some anti-oxidant effects, as already suggested.
GHB treatment increased nucleotide turnover in the cortex, with a significant increase in purinic/pyrimidinic nucleotides formation (inosine 5'-monophosphate, guanosine 5'- mono-phosphate, and cytidine 5'-monophosphate) together with an increase in N-acetylmethionine. Low levels of allantoin, xanthine, and xanthosine support the hypothesis that the increase in nucleotides is not resulting from intense catabolism, but rather from increased turnover.
As opposed to the acute condition, the primary bile acids, conjugated or not, were unchanged in the liver after 4 w of GHB treatment. However, the levels of conjugated secondary bile acids (taurodeoxycholate and 6-β-OH-lithocholate) tended to increase (fold changes from 1.22 to 1.50, P = 0.2–0.08), suggesting a role of transformation of biliary acids in the intestines and their reuptake for transport to the bile. Chronic GHB treatment led to the elongation of fatty acids in the liver (higher stearidonate, important for polyunsaturated fatty acids formation, as well as docosadienoate, and 12,13-hydroxyoctadec-9(Z)-enoate), and lower phosphopantetheine, suggestive of an increased fatty acids synthesis. Interestingly, treated mice had an increased level of omega-3 fatty acids, with known beneficial effects. An increase in oligosaccharides metabolism (high maltotriose), especially galactose, and a decrease in fructose were also observed. Tagatose, involved in the regulation of glycemic status and contributing to weight loss, also had higher levels in treated mice. An alternative energy pathway might be activated with the use of glycerate.
DISCUSSION
Evaluation of multiple variables by indirect calorimetric measurements indicated a significant decrease in RER, a good marker for substrate utilization for energy production. RER is calculated as the ratio between VCO2 and VO2, and is used to estimate the relative proportion of different substrates used to produce energy. Expected values of this parameter range between 0.7 and 1, with 1 representing pure carbohydrate use and 0.7 pure lipids use. RER values higher than 1 are associated with lipid synthesis and values lower than 0.7 are indicative of carbohydrates synthesis or ketone body metabolism.29 Small changes in RER cover significant changes in substrate used: at 0.95, there are 82.9% carbohydrates and 17.1% fat use, whereas at 0.90, carbohydrates represent 65.9% and fat 34.1%.30
The decrease in RER was most significant during the dark period when the mice are active. The differences between the treatment and placebo were bigger in the second group (placebo given before GHB). The RER decreased progressively over the 4 days of drug administration and recovered also progressively during the 4 days of placebo administration, leading to a more pronounced effect when the drug was administered after the placebo. The presence of a metabolic effect after drug administration seems to be independent of locomotor activity and feeding. Decreased heat production observed in the second group may also be a consequence of increase in sleep time. Overall, these results suggest that fatty acids are preferred to glucose as energy substrate under GHB. An increase in lipolysis and a reduction in endogenous glucose production after 3 mo were also described in patients with narcolepsy treated with GHB.31
As expected for the age range of the mice, both GHB-treated and control animals followed a normal ascending growth curve, but treated animals gained significantly less weight. Body composition evaluation was a continuation of the calorimetric experiment, because differences in energy expenditure can be explained by difference in lean mass.32 Treated mice showed a more balanced distribution of body composition, and the differences in weight gain were not caused by reduced fat mass, but by increased lean mass. The relationship between lean mass and weight change may indicate involvement of growth hormone in the changes in body composition. GHB in normal patients and patients with Parkinson disease increased levels of growth hormone,10,33,34 and this effect was mediated by cholinergic mechanisms.34 However, growth hormone was not modified in our experiments, as also not reported by others in studies of rats and dogs.35 This discrepancy might be because of the dose (ranging from 10 mg to more than 1 g), the timing of the measure after drug administration (30 min or 1 h), the technique (radioimmunoassay or ELISA), or to an unknown between-species difference. Interestingly, GABAB KO mice had constitutively higher growth hormone levels, suggesting that these receptors might somehow control the growth hormone production and/or release. Flumazenil (GABAA/benzodiazepine antagonist) and metergoline (serotonin receptor antagonist) were also shown to reduce or block growth hormone stimulation by GHB in humans, suggesting a GABAergic-serotonergic mechanism.36,37 The differences in weight progression between treated and control mice showed a similar trend in ob/ob mice, whereas a significant difference in weight was observed in GABAB KO mice, strongly suggesting that weight changes induced by GHB might not be centrally mediated through growth hormone, leptin, or GABAB receptors. Also, we showed here that even high doses of GHB do not increase GABA that could act through GABAA receptors. The lack of significant changes in leptin-deficient mice may be caused by a ceiling effect (given the morbid obesity in these mice, the GHB doses used here might have reached the maximum effect) or because these mice might require longer GHB administration to show significant changes. Recent human studies also suggest that metabolic effects of GHB are not leptin or ghrelin mediated38 and that GHB treatment does not alter the levels of these two hormones either in patients with narcolepsy or in healthy controls.
The large increase in lysolipids and acylcarnitines may suggest membrane remodeling and increased β-oxidation. Nevertheless, unlike in most tissues where acylcarnitines function in β-oxidation, in the brain they are believed to act as a sink for reacylation of phospholipids and triglycerides. The increase in acylcarnitines could be a result of released fatty acids from the phospholipids, and explain why there was no increase in overall fatty acids in the brains of GHB-treated mice. They also may suggest that the proper balance between acyl-CoA, acyl-CoA synthetase, and reacylating enzymes are somehow altered in GHB-treated mice. Also supporting an inhibition of reacylation was the significant increase in monodiglycerides and diacylglycerides.
Metabolomics analysis also revealed interesting pathways both in GHB catabolism and affected acutely or chronically by GHB administration. The catabolism of GHB is not well understood. GHB can be metabolized through β-oxidation or converted to succinic semialdehyde either by GHB dehydrogenase or by the more recently identified hydroxyacid-oxoacid transhydrogenase (HOT).28 HOT transformation of GHB into succinic semiahdehyde is coupled to the conversion of α-ketoglutarate to 2-hydroxyglutarate and final conversion of succinic semiahdehyde to 4,5-dihydroxyhexanoate, whereas succinic semialdehyde produced by GHB dehydrogenase is transformed into succinate that enters the TCA cycle. Note that both pathways are connected to the TCA cycle through α-ketoglutarate and succinate. Although we found changes in all three pathways, the most consistent one was the large increase in 2-hydroxyglutarate, strongly suggesting that the major catabolic pathway is mediated by HOT.
The most impressive change after a single dose of GHB was a dramatic increase in peripheral corticosterone. Most metabolic changes found after acute administration of GHB may be mediated by the elevated corticosterone level. Although we did not measure the activity of the hypothalamic-pituitary-adrenal axis (especially the adrenocorticotropic hormone levels), several results suggest that the effect might not be centrally mediated. Corticosterone levels were increased both in C57BL/6J and GABAB KO mice after acute i.p. GHB administration. Also, other pituitary hormones, growth hormone and prolactin, were not affected by GHB. As opposed to growth hormone and prolactin, which are stimulated in humans but not in other species, cortisol and corticosterone are reliably stimulated by GHB in all species.39,40 Whether changes in cortisol induced by GHB may also contribute to the occurrence of depression in susceptible subjects41 deserves further investigations.
Overall, GHB induced different metabolic changes after acute and chronic administration. Nevertheless, as originally proposed6,18 glucose utilization did not seem to be favored either after acute or chronic GHB administration. The hypothesis that GHB is shifting the energy metabolism to pentose phosphate pathway24 is only partially supported by our findings. The glucose availability was confirmed by the presence of very low levels of 1,5-anhydroglucitol, a good indicator of glucose levels, which was proposed as a marker for glycemic control in patients with diabetes.42 GHB by inducing a global central inhibition reduces glucose utilization (but not availability), similar to other anesthetics. Normal or increased lactate levels and decreased ketone bodies in both acute and chronic experiments suggest that the cortex has no deficit in adenosine triphosphate.
The acute changes in BCAA and lysolipids are difficult to explain, although these changes may reflect BCAA utilization as energy substrate and transient activation of phospholipase A2 or inhibition of lipid reacylation. One interesting finding was a general decrease in bile acid metabolism after acute GHB administration. This might suggest a transient reduction in lipid processing resulting in lipid waste. Detailed metabolomics analysis of the serum might be necessary to verify if lipid reabsorption and processing is acutely altered by GHB. One possibility is that acute administration of a high dose (with possible anesthetic-like effects on biliary duct and on the receptors from the intestine) blocks the feedback of biliary acids over the short term.
Given the short half-life of GHB, chronic administration resulted in a few different metabolic changes as compared to the acute situation. Decreased glutamate, s-adenosylhomocysteine, oxidized glutathione, and increased omega-3 fatty acids suggest reduced oxidative stress and improved fatty acid metabolism. GHB was originally developed as an antioxidant agent and early works suggested that GHB protects against severe hypoxia, radiation, and experimental diabetes.43–45 Although these observations could be replicated, not only for the brain protection but also for peripheral tissues,46 contradictory results were also reported, the major source being the large range of GHB doses used in various studies. In general, when GHB was used at or below 300 mg/kg, protective effects were observed whereas higher doses (500 mg/kg or higher) suggested oxidative stress.47 Nevertheless, a recent study where a single high dose (1 g/kg) of GHB was administered to rats, reported increased expression of genes with neuroplasticity and metabolic effects was similar to our findings.48
In conclusion, GHB has a large and complex metabolic effect with different central versus periphery and acute versus chronic profiles. Overall, changes reported here not only exclude any major toxic effects but instead suggest that GHB might have antioxidant, antiaging, and potentially antiobesity properties.
DISCLOSURE STATEMENT
This work was funded by an unrestricted research grant from UCB Pharma SA, University of Lausanne, and partially by a grant from Marie Curie Actions (Neuroendocrine Immune Networks in Ageing Project). Dr. Luca was supported by Marie Curie Actions. Dr Tafti received compensation from private and publicly owned organizations for serving as an advisory or scientific board member or as an invited speaker. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Mr Yann Emmenegger for technical assistance.
Main indirect calorimetric results of 4 days of GHB administration.
REFERENCES
- 1.Romanelli F, Smith KM. X, Liquid E, and Special K - The abuse of drugs at clubs and raves. Am J Pharm Educ. 2002;66:197–200. [Google Scholar]
- 2.Laborit H, Jouany JM, Gerard J, Fabiani F. [Generalities concerning the experimental study and clinical use of gamma hydroxybutyrate of Na] Agressologie. 1960;1:397–406. [PubMed] [Google Scholar]
- 3.Bessman SP, Fishbein WN. Gamma-hydroxybutyrate, a normal brain metabolite. Nature. 1963;200:1207–8. doi: 10.1038/2001207a0. [DOI] [PubMed] [Google Scholar]
- 4.A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25:42–9. [PubMed] [Google Scholar]
- 5.Vienne J, Bettler B, Franken P, Tafti M. Differential effects of GABAB receptor subtypes, {gamma}-hydroxybutyric Acid, and Baclofen on EEG activity and sleep regulation. J Neurosci. 2010;30:14194–204. doi: 10.1523/JNEUROSCI.3145-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laborit H. Sodium 4-hydroxybutyrate. Int J Neuropharmacol. 1964;3:433–51. doi: 10.1016/0028-3908(64)90074-7. [DOI] [PubMed] [Google Scholar]
- 7.Snead OC, 3rd, Yu RK, Huttenlocher PR. Gamma hydroxybutyrate. Correlation of serum and cerebrospinal fluid levels with electroencephalographic and behavioral effects. Neurology. 1976;26:51–6. doi: 10.1212/wnl.26.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Snead OC., 3rd Pharmacological models of generalized absence seizures in rodents. J Neural Transm Suppl. 1992;35:7–19. doi: 10.1007/978-3-7091-9206-1_2. [DOI] [PubMed] [Google Scholar]
- 9.Vienne J, Lecciso G, Constantinescu I, et al. Differential effects of sodium oxybate and baclofen on EEG, sleep, neurobehavioral performance, and memory. Sleep. 2012;35:1071–83. doi: 10.5665/sleep.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cauter E, Plat L, Scharf MB, et al. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men. J Clin Invest. 1997;100:745–53. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donjacour CEHM, Aziz NA, Roelfsema F, et al. Effect of sodium oxybate on growth hormone secretion in narcolepsy patients and healthy controls. Am J Physiol Endocrinol Metab. 2011;300:E1069–75. doi: 10.1152/ajpendo.00623.2010. [DOI] [PubMed] [Google Scholar]
- 12.Snead OC., 3rd Gamma hydroxybutyrate. Life Sci. 1977;20:1935–43. doi: 10.1016/0024-3205(77)90171-0. [DOI] [PubMed] [Google Scholar]
- 13.Wong CG, Gibson KM, Snead OC., 3rd From the street to the brain: neurobiology of the recreational drug gamma-hydroxybutyric acid. Trends Pharmacol Sci. 2004;25:29–34. doi: 10.1016/j.tips.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Morse BL, Vijay N, Morris ME. γ-Hydroxybutyrate (GHB)-induced respiratory depression: combined receptor-transporter inhibition therapy for treatment in GHB overdose. Mol Pharmacol. 2012;82:226–35. doi: 10.1124/mol.112.078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Husain AM, Ristanovic RK, Bogan RK. Weight loss in narcolepsy patients treated with sodium oxybate. Sleep Med. 2009;10:661–3. doi: 10.1016/j.sleep.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Spaeth M, Bennett RM, Benson BA, Wang YG, Lai C, Choy EH. Sodium oxybate therapy provides multidimensional improvement in fibromyalgia: results of an international phase 3 trial. Ann Rheum Dis. 2012;71:935–42. doi: 10.1136/annrheumdis-2011-200418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McElroy SL, Guerdjikova AI, Winstanley EL, et al. Sodium oxybate in the treatment of binge eating disorder: an open-label, prospective study. Int J Eating Disord. 2011;44:262–8. doi: 10.1002/eat.20798. [DOI] [PubMed] [Google Scholar]
- 18.Wolfson LI, Sakurada O, Sokoloff L. Effects of γ-Butyrolactone on local cerebral glucose utilization in the rat. J Neurochem. 1977;29:777–83. doi: 10.1111/j.1471-4159.1977.tb10718.x. [DOI] [PubMed] [Google Scholar]
- 19.Artru AA, Steen PA, Michenfelder JD. gamma-Hydroxybutyrate: cerebral metabolic, vascular, and protective effects. J Neurochem. 1980;35:1114–9. doi: 10.1111/j.1471-4159.1980.tb07866.x. [DOI] [PubMed] [Google Scholar]
- 20.MacMillan V. Effects of gamma-hydroxybutrate and gamma- butyrolactone on cerebral energy metabolism during exposure and recovery from hypoxemia-oligemia. Stroke. 1980;11:271–7. doi: 10.1161/01.str.11.3.271. [DOI] [PubMed] [Google Scholar]
- 21.Sadasivan S, Maher TJ, Quang LS. Gamma-hydroxybutyrate (GHB), gamma-butyrolactone (GBL), and 1,4-butanediol (1,4-BD) reduce the volume of cerebral infarction in rodent transient middle cerebral artery occlusion. Ann N Y Acad Sci. 2006;1074:537–44. doi: 10.1196/annals.1369.054. [DOI] [PubMed] [Google Scholar]
- 22.Yosunkaya A, Ak A, Bariskaner H, Üstün ME, Tuncer S, Gürbilek M. Effect of gamma-hydroxybutyric acid on lipid peroxidation and tissue lactate level in experimental head trauma. J Trauma Acute Care Surg. 2004;56:585–90. doi: 10.1097/01.ta.0000058119.60074.25. [DOI] [PubMed] [Google Scholar]
- 23.Vergoni AV, Ottani A, Botticelli AR, et al. Neuroprotective effect of gamma-hydroxybutyrate in transient global cerebral ischemia in the rat. Eur J Pharmacol. 2000;397:75–84. doi: 10.1016/s0014-2999(00)00246-6. [DOI] [PubMed] [Google Scholar]
- 24.Taberner PV, Rick JT, Kerkut GA. The action of gamma-hydroxybutyric acid on cerebral glucose metabolism. J Neurochem. 1972;19:245–54. doi: 10.1111/j.1471-4159.1972.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 25.Schuler V, Luscher C, Blanchet C, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol. 2007;581:649–63. doi: 10.1113/jphysiol.2007.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinard V, Mikhail C, Pradervand S, et al. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci. 2012;32:12506–17. doi: 10.1523/JNEUROSCI.2306-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Struys EA, Verhoeven NM, Ten Brink HJ, Wickenhagen WV, Gibson KM, Jakobs C. Kinetic characterization of human hydroxyacid-oxoacid transhydrogenase: relevance to D-2-hydroxyglutaric and gamma- hydroxybutyric acidurias. J Inherit Metab Dis. 2005;28:921–30. doi: 10.1007/s10545-005-0114-x. [DOI] [PubMed] [Google Scholar]
- 29.Riachi M, Himms-Hagen J, Harper ME. Percent relative cumulative frequency analysis in indirect calorimetry: application to studies of transgenic mice. Can J Physiol Pharmacol. 2004;82:1075–83. doi: 10.1139/y04-117. [DOI] [PubMed] [Google Scholar]
- 30.McLean J.A, Tobin G. Cambridge England: Cambridge University Press; 1987. Animal and human calorimetry. [Google Scholar]
- 31.Donjacour CE, Aziz NA, Overeem S, Kalsbeek A, Pijl H, Lammers GJ. Glucose and fat metabolism in narcolepsy and the effect of sodium oxybate: a hyperinsulinemic-euglycemic clamp study. Sleep. 2014;37:795–801. doi: 10.5665/sleep.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blundell JE, Caudwell P, Gibbons C, et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Dis Model Mech. 2012;5:608–13. doi: 10.1242/dmm.009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahara J, Yunoki S, Yakushiji W, Yamauchi J, Yamane Y. Stimulatory effects of gamma-hydroxybutyric acid on growth hormone and prolactin release in humans. J Clin Endocrinol Metab. 1977;44:1014–7. doi: 10.1210/jcem-44-5-1014. [DOI] [PubMed] [Google Scholar]
- 34.Volpi R, Chiodera P, Caffarra P, et al. Muscarinic cholinergic mediation of the GH response to gamma-hydroxybutyric acid: neuroendocrine evidence in normal and parkinsonian subjects. Psychoneuroendocrinology. 2000;25:179–85. doi: 10.1016/s0306-4530(99)00048-7. [DOI] [PubMed] [Google Scholar]
- 35.Rigamonti AE, Muller EE. Gamma-hydroxybutyric acid and growth hormone secretion studies in rats and dogs. Alcohol. 2000;20:293–304. doi: 10.1016/s0741-8329(99)00094-4. [DOI] [PubMed] [Google Scholar]
- 36.Gerra G, Caccavari R, Fontanesi B, et al. Naloxone and metergoline effects on growth hormone response to gamma-hydroxybutyric acid. Int Clin Psychopharmacol. 1995;10:245–50. doi: 10.1097/00004850-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Gerra G, Caccavari R, Fontanesi B, et al. Flumazenil effects on growth hormone response to gamma-hydroxybutyric acid. Int Clin Psychopharmacol. 1994;9:211–5. doi: 10.1097/00004850-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Donjacour CE, Pardi D, Aziz NA, et al. Plasma total ghrelin and leptin levels in human narcolepsy and matched healthy controls: basal concentrations and response to sodium oxybate. J Clin Sleep Med. 2013;9:797–803. doi: 10.5664/jcsm.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Cauter E, Plat L, Scharf MB, et al. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young men. J Clin Invest. 1997;100:745–53. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meerlo P, Westerveld P, Turek FW, Koehl M. Effects of gamma-hydroxybutyrate (GHB) on vigilance states and EEG in mice. Sleep. 2004;27:899–904. doi: 10.1093/sleep/27.5.899. [DOI] [PubMed] [Google Scholar]
- 41.Rossetti AO, Heinzer RC, Tafti M, Buclin T. Rapid occurrence of depression following addition of sodium oxybate to modafinil. Sleep Med. 2010;11:500–1. doi: 10.1016/j.sleep.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Suhre K, Meisinger C, Döring A, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5:e13953. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierrefiche G, Topall G, Henriet I, Laborit H. Protective effects of gamma-hydroxybutyrate on alloxan induced diabetes in mice. Res Commun Chem Pathol Pharmacol. 1991;71:309–19. [PubMed] [Google Scholar]
- 44.Laborit G, Baron C, Topal G, Henriet I. [Value of sodium gamma hydroxybutyrate in cerebral protection during severe hypoxia in the rat] Agressologie. 1980;21:189–97. [PubMed] [Google Scholar]
- 45.Dana M, Baron C, Laborit H. [Radioprotective action of sodium gamma-hydroxybutyrate (preliminary notes)] Agressologie. 1962;3:497–506. [PubMed] [Google Scholar]
- 46.Mamelak M. Alzheimer' s disease, oxidative stress and gammahydroxybutyrate. Neurobiol Aging. 2007;28:1340–60. doi: 10.1016/j.neurobiolaging.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 47.van Nieuwenhuijzen PS, Kashem MA, Matsumoto I, Hunt GE, McGregor IS. A long hangover from party drugs: residual proteomic changes in the hippocampus of rats 8 weeks after gamma-hydroxybutyrate (GHB), 3,4-methylenedioxymethamphetamine (MDMA) or their combination. Neurochem Int. 2010;56:871–7. doi: 10.1016/j.neuint.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Kemmel V, Klein C, Dembele D, et al. A single acute pharmacological dose of gamma-hydroxybutyrate modifies multiple gene expression patterns in rat hippocampus and frontal cortex. Physiol Genomics. 2010;41:146–60. doi: 10.1152/physiolgenomics.00208.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main indirect calorimetric results of 4 days of GHB administration.