Abstract
The practice of medicine is currently undergoing a transformation to become more efficient, cost-effective, and patient centered in its delivery of care. The aim of this article is to stimulate discussion within the sleep medicine community in addressing these needs by our approach as well as other approaches to sleep medicine care. The primary goals of the Sustainable Methods, Algorithms, and Research Tools for Delivering Optimal Care Study (SMART DOCS) are: (1) to introduce a new Patient-Centered Outcomes and Coordinated-Care Management (PCCM) approach for the future practice of sleep medicine, and (2) to test the PCCM approach against a Conventional Diagnostic and Treatment Outpatient Medical Care (CONV) approach in a randomized, two-arm, single-center, long-term, comparative effectiveness trial. The PCCM approach is integrated into a novel outpatient care delivery model for patients with sleep disorders that includes the latest technology, allowing providers to obtain more accurate and rapid diagnoses and to make evidence-based treatment recommendations, while simultaneously enabling patients to have access to personalized medical information and reports regarding their diagnosis and treatment so that they can make more informed health care decisions. Additionally, the PCCM approach facilitates better communication between patients, referring primary care physicians, sleep specialists, and allied health professionals so that providers can better assist patients in achieving their preferred outcomes. A total of 1,506 patients 18 y or older will be randomized to either the PCCM or CONV approach and will be followed for at least 1 y with endpoints of improved health care performance, better health, and cost control.
Clinical Trials Registration:
ClinicalTrials.gov Identifier: NCT02037438.
Citation:
Kushida CA, Nichols DA, Holmes TH, Miller R, Griffin K, Cardell CY, Hyde PR, Cohen E, Manber R, Walsh JK. SMART DOCS: a new patient-centered outcomes and coordinated-care management approach for the future practice of sleep medicine. SLEEP 2015;38(2):315–326.
Keywords: allied health professionals, cost-effective, outcomes, patient-centered care, sleep disorders, sleep medicine practice of the future, SMART DOCS
INTRODUCTION
The current national dialogue on the cost-effectiveness of medical care has forced experts to reexamine the traditional outpatient medical care model. This model dictates that within a time period ≤ 1 h for an initial evaluation, a patient is expected to convey symptoms and a medical history that are meaningful and relevant for diseases, including comorbidities that are frequently complex, so that the clinician can establish a differential diagnosis. The clinician, in turn, must effectively and appropriately diagnose and treat the patient's condition while simultaneously addressing patient questions and communicating detailed treatment plans.
Significant limitations have been identified with the traditional model. Frequently, the advantages and availability of new technologies for home-based diagnostic testing and electronic access to diagnostic and treatment results are not made available or effectively communicated to patients. Prescribed treatment plans are often not personalized, not communicated via clearly written instructions, and patients' questions are not adequately addressed. The provider and patient may not have complete access to subjective and/or objective records for assessing treatment outcomes. In addition, the diagnostic and treatment plan may not be fully communicated to, or discussed with, the patient's primary care physician (PCP).
The Patient-Centered Outcomes Research Institute (PCORI)-supported Sustainable Methods, Algorithms, and Research Tools for Delivering Optimal Care Study (SMART DOCS) that commenced on September 30, 2013 is designed to provide new solutions to limitations in the traditional outpatient medical care model. The overarching goal of SMART DOCS is to meet these challenges by introducing and testing a new approach for the future practice of sleep medicine, which is designed to provide better care and improve the health of patients while controlling costs, and could be practically implemented within academic institutions, hospitals, private practices, free-standing sleep centers, and rural communities. This future practice would employ a novel Patient-Centered Outcomes and Coordinated-Care Management (PCCM) approach that would serve as a new outpatient care delivery model for patients with sleep disorders. Cutting-edge tools are available to provide more accurate and rapid diagnoses; technology allows patients to have access to more information, resources, and data about their sleep disorders, comorbidities, risks associated with and without treatment, and management strategies so that they can make more informed health care decisions. Better communication between patients, referring clinicians, and sleep specialists (i.e., individuals who received specialized training in sleep medicine typically following postgraduate training in a medical specialty) can assist patients in achieving their preferred outcomes. “The Future of Sleep Medicine,”1 a 2011 article written by six authors (two of whom are SMART DOCS team members) as well as a recent editorial2 and American Academy of Sleep Medicine-sponsored conference (Sleep Medicine: Future Models of Care, November 16–17, 2013) highlight the need for this type of new model that will ensure better access to care and improved outcomes for patients. The new PCCM approach for sleep medicine will be tested against a Conventional Diagnostic and Treatment Outpatient Medical Care (CONV) approach with assessment of healthcare performance, health status, and cost control. Moreover, we believe that components of this approach are also applicable and extensible to other disciplines of medicine.
Our goal in describing this new approach is to encourage discussion within the sleep medicine community on this approach as well as other approaches to sleep medicine care. It is the authors' intent to stimulate dialogue among sleep medicine clinicians, allied health personnel, organizational leadership, and industry to ensure that the field of sleep medicine continues to evolve and advance toward becoming more efficient, outcomes-based, and patient oriented.
KEY COMPONENTS OF THE PATIENT-CENTERED OUTCOMES AND COORDINATED-CARE MANAGEMENT (PCCM) APPROACH
In the development of a new outpatient care delivery model for sleep medicine, we identified the following specific areas of focus for the PCCM approach that are expected to improve both clinical practice and patient experience of care.
Standardized Intake/Screening
There is a general lack of standardized intake/screening questionnaires, which assess the patient's sleep patterns and habits, medical conditions, medications that influence sleep quality/quantity, and level of daytime sleepiness that are used during an initial sleep medicine consultation. Each practice typically administers their own combination of different tools that vary in length and completion times; however, this approach makes the sharing of the results between practices more complicated due to dissimilar information gathered from the patients in each practice. We will administer the Alliance Sleep Questionnaire (ASQ) to our clinic patients in the PCCM arm, which is a novel electronic questionnaire developed through efforts led by Dr. Emmanuel Mignot and tested by the Academic Alliance for Sleep Research (a consortium composed of sleep centers at Stanford University, Harvard University, University of Pennsylvania, and University of Wisconsin-Madison). The ASQ is innovative because it: (1) is a standardized instrument, in which the content was selected by consensus among leaders in our field; (2) combines several relevant and important health-related domains to comprehensively assess the patient's overall health status including sleep problems; (3) is online and thus can be easily accessed by patients; (4) uses branching-logic algorithms, thus making more efficient use of patients' time; (5) can be used to assess longitudinal outcomes and incorporates disorder severity scales; and (6) generates a report for the sleep specialist that provides a list of predicted potential diagnoses for the patient in advance of the patient's initial visit. Use of the ASQ supports a more personalized patient approach beginning with the initial evaluation because the specialist can review the ASQ report prior to the patient visit, which organizes the pertinent patient information in a manner that enables the clinician to more efficiently diagnose the patient's condition. The ASQ integrates existing scales and questionnaires, many of which have been previously validated: Epworth Sleepiness Scale (ESS),3,4 Multivariate Apnea Risk Index (MAP Index),5 Fatigue Severity Scale (FSS),6 Functional Outcomes of Sleep Questionnaire (FOSQ),7 Insomnia Severity Index (ISI),8 Insomnia Symptom Questionnaire (ISQ),9 Reduced Hörne and Östberg Morningness-Eveningness Questionnaire (rMEQ),10 Generalized Anxiety Disorder 7-item (GAD-7) Scale,11 and Patient Health Questionnaire (PHQ-9).12 The narcolepsy module of the ASQ has been recently validated,13 and the restless legs and parasomnias module validations are currently in progress. Electronic questionnaires are gaining acceptance in clinical care; for example, use of a web-based questionnaire to generate data and questions about hormone therapy showed enhanced provider perception of patient engagement, relevance, and appropriateness of discussion.14
New Technology, Tests, and Tools
Novel medical technologies are constantly being developed; the challenges are to identify which of these new innovations provide the greatest benefit to clinical practice and patient engagement while being cost-effective, and thus are good candidates for rapid implementation in health care. For example, advancements in home-based technology allow us to use outof-center sleep and other testing in the patients' homes for many patients. Use of this technology has the advantages of patient testing in a nonlaboratory setting and lower health care costs. We will also employ unique treatment adherence monitoring, using new technology and devices to objectively measure adherence to therapy for common treatment modalities.15,16 This monitoring will enable clinicians to more accurately track their patients' treatment adherence, and modify therapy as needed. Additionally, the MATRx device (Zephyr Sleep Technologies, Inc., Calgary, Alberta, Canada)17–20 will be used during in-lab oratory polysomnography (PSG, sleep study) to assess whether an oral appliance can effectively treat a patient's obstructive sleep apnea and to initially titrate the oral appliance. This device is expected to identify treatment failures more rapidly because clinicians are provided information to better predict if a given patient will tolerate and benefit from an oral appliance, and to significantly shorten the adjustment duration (typically several months), because the oral appliance is optimized to control the patient's sleep disordered breathing during the in-laboratory PSG. Portable devices (actigraphs, a wristband-like device that measures motor activity to estimate sleep-wake patterns) will also be used to measure certain longitudinal outcomes, such as changes in sleep-wake patterns over time with treatment. These actigraphs provide an objective assessment of outcomes to complement subjective sleep measures, such as sleep diaries and other questionnaires. For actigraphy, we will be using and further validating a device (UP24, Jawbone, San Francisco, CA, USA) that has been used to self-track sleep, diet, and exercise for extended periods of time; data from a separate validation study on a version of this device compared to PSG on 30 participants at our institution has been collected and are now being analyzed. If the results of this validation study are negative for key outcome variables, we will revert to using conventional actigraphs for this study. Ambulatory blood pressure monitoring devices continuously measure blood pressure during sleep in patients who have borderline or definitive hypertension, and in particular will help to identify blood pressure surges associated with movement and other events. These blood pressure data assist the clinician in assessing the relationship between blood pressure changes with obstructive sleep apnea21,22 and periodic limb movement disorder,23–26 and in evaluating whether these blood pressure changes are improved with treatment over time. We will use dim-light melatonin onset (DLMO) assays to better characterize and diagnose complicated circadian rhythm sleep disorders.27–29 We will also collect blood samples from patients to identify known and future genetic markers as exploratory measures to more accurately diagnose sleep disorders in the future, and to assess risk factors of associated medical conditions and comorbidities, such as checking glucose, insulin, and lipid levels for diabetic risk assessment and C-reactive protein for cardiovascular disease risk assessment. Emerging patient-driven healthcare technology,30 such as electronic self-tracking, social networking methods, mobile technology, cloud services, and telemedicine will be explored, as well as automated methods for scoring sleep, respiratory, and periodic limb movements. We carefully selected these new technologies, tests, and tools, with the consideration of key factors such as patient benefit, provider acceptance, cost, practicality, utility, ease of use, and data quality; in particular, our goal was that they could be implemented in any type of sleep practice without much difficulty. Many of these new technologies, tests, and tools will require an initial investment of funds, time, personnel, and training resources. However, we believe that these expenditures will ultimately result in better, more patient-centered comprehensive care to patients as well as enhancement of communication between patients, sleep specialists, and referring physicians, ultimately leading to fewer clinical visits, both at the sleep center and at PCP offices. Further, as the principal role that the in-laboratory PSG performs in most sleep centers diminishes over time, it is important that the portfolio of services offered by the sleep center expands to more completely identify, diagnose, and manage sleep disorders and associated health-related issues and conditions for the patient.
Comanagement of Patients with Primary Care
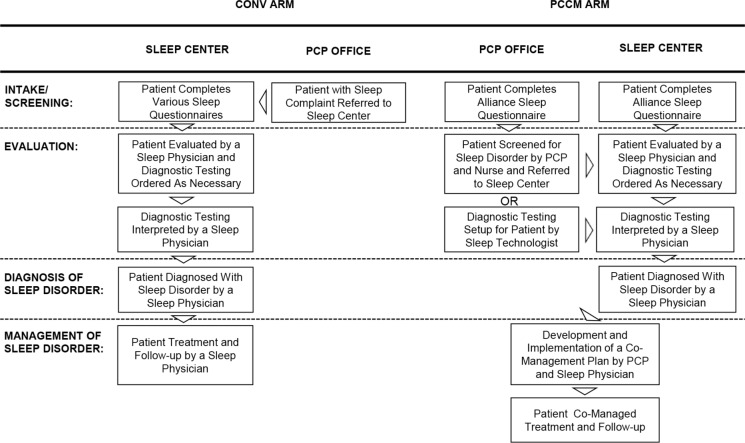
With up to 70 million individuals in the United States affected by sleep problems,31 the current sleep medicine model encounters difficulties in managing all patients with these conditions. Additionally, only 30–50% of patients mention their sleep difficulties during a primary care visit32 and providers neglect to ask their patients about sleep problems, so the prevalence of sleep disorders may be even higher than estimated. The PCCM approach relies on sleep specialists and allied health professionals (sleep medicine-trained nurses and sleep technologists) to assist local PCPs in screening their patients for sleep disorders (e.g., providing education in identifying the key symptoms and signs of these disorders and questions to routinely ask their patients about their sleep problems), to provide feedback when the patients are candidates to be tested and managed by both the PCP and sleep specialist, to conduct out-of-center sleep testing and other testing either through the PCP office or the sleep center, to assist PCPs in the long-term management of sleep disorders (including cognitive behavioral treatments), and to aid in the follow-up care (e.g., positive airway pressure mask fittings) of patients at the PCP office and/or sleep center, all without increasing PCP burden. The sleep specialists, sleep medicine-trained nurses, and sleep technologists serve as a resource for sleep related information to the PCPs, and through their interactions with the PCPs, provide improved access for the comanagement of their patients. We believe that this co-management approach enables greater identification of sleep disorders within the community, provides timelier and more efficient care than the current sleep medicine approach, and reserves specialty sleep medical care for complex patients. In addition, it will allow PCPs to play a greater role in the recognition and management of sleep disorders in their patients and allow patients to become more active participants in their health care.
Enhanced Patient-Provider Data and Information Sharing
Within the past decade there has been an increased emphasis on the importance of assessing patient satisfaction as a health-care outcome. Patient access to personalized health-related information facilitates more informed decision making, which leads to greater patient satisfaction. To deliver this type of access, we are creating a new secure, password-protected, online SMART DOCS web portal that will serve to meet personalized patient-centered needs and will be accessible to each patient 24 h a day, 7 days a week. This web portal contains integrated information about a patient's initial evaluation, tests, diagnoses, and treatments that are communicated with specific details, yet written in a clear and concise manner for a lay reader. This information will also be available as paper documents if the patient is without Internet access. The visit reports include targeted information about his/her sleep disorder and individual treatment plan, especially with respect to improving efficacy, describing adverse effects, and next steps. The patient, specialists, and PCPs will have web portal access to the results obtained from questionnaires, diagnostic testing, treatments, and adherence, so that the patient can recognize his/her successes or limitations with therapy and his/her role in improving the effectiveness of therapy through adherence. We expect that the enhanced record sharing of patient-specific information and results will provide substantial value to the patient, and we will be tracking the use of the web portal by each patient to assess its impact on outcomes. This web portal is built upon an existing reliable, secure, and extensible electronic informatics infrastructure developed during our Agency for Healthcare Research and Quality (AHRQ)-supported Comparative Outcomes Management with Electronic Data Technology (COMET) Study, which in turn used tools and methods derived from our National Heart, Lung and Blood Institute-supported Apnea Positive Pressure Long-term Efficacy Study (APPLES).33,34 Ul timately, components of the web portal will be integrated within our institution's electronic health record (EHR). We have discussed this integration with our medical center's information technology (IT) leadership, and our IT team has constructed the web portal to ensure that it is complementary and not redundant to Epic (Verona, WI, USA) software, which is one of the most common EHR software currently in use. Longitudinal outcomes data will be collected through a new patient registry that is a modified version of a patient registry proof-of-concept developed during the COMET Study. The registry data will be accessible through the web portal, which will enable providers and patients to better collaborate in improving these outcomes by adjustment of therapy.
Patient Education
In addition to personalized reports, the new web portal will also contain educational information and resources about an array of sleep topics, including the most prevalent of the approximately 90 different sleep disorders. This library of documents and videos about sleep and its disorders is currently being developed by our team of stakeholders to provide the interested patient with added information, enabling more informed decisions about their care. Further, we will hold free, small-group classes led by allied health professionals (sleep medicine-trained nurses and sleep technologists) that comprehensively cover the benefits and adverse effects of various treatment options. These classes will also encourage patients to ask questions and to serve as a forum for patients to relay successes or problems they have encountered with treatment. We have piloted this approach at our sleep center on a limited basis, and found it effectively supplements clinic visits with respect to patient understanding of management options and strengthening the patient-provider relationship. These classes will also be videotaped and provided online so that patients can view them when they desire.
DESCRIPTION OF THE PCCM APPROACH
Initial Evaluation
Conventionally, the sleep specialist captures medical history and physical examination data during an initial evaluation, with an emphasis on signs and symptoms of sleep disorders, as well as existing medical conditions. The patient is usually given one or more preliminary diagnoses belonging to the following six major diagnostic categories: sleep related breathing disorders, hypersomnias, insomnias, circadian rhythm sleep disorders, parasomnias, and/or sleep related movement disorders. In the PCCM approach, if the patient is suspected of having a sleep disorder, the patient will either be referred to a sleep specialist, or evaluated by the PCP with the assistance from the sleep specialist or sleep medicine-trained nurse. The ASQ will be completed either online or by electronic tablets in the waiting room prior to the sleep evaluation. Upon review of the ASQ report by the clinician, and taking into consideration all the information collected, the patient will be given one or more preliminary sleep disorder diagnoses.
Diagnosis and Treatment
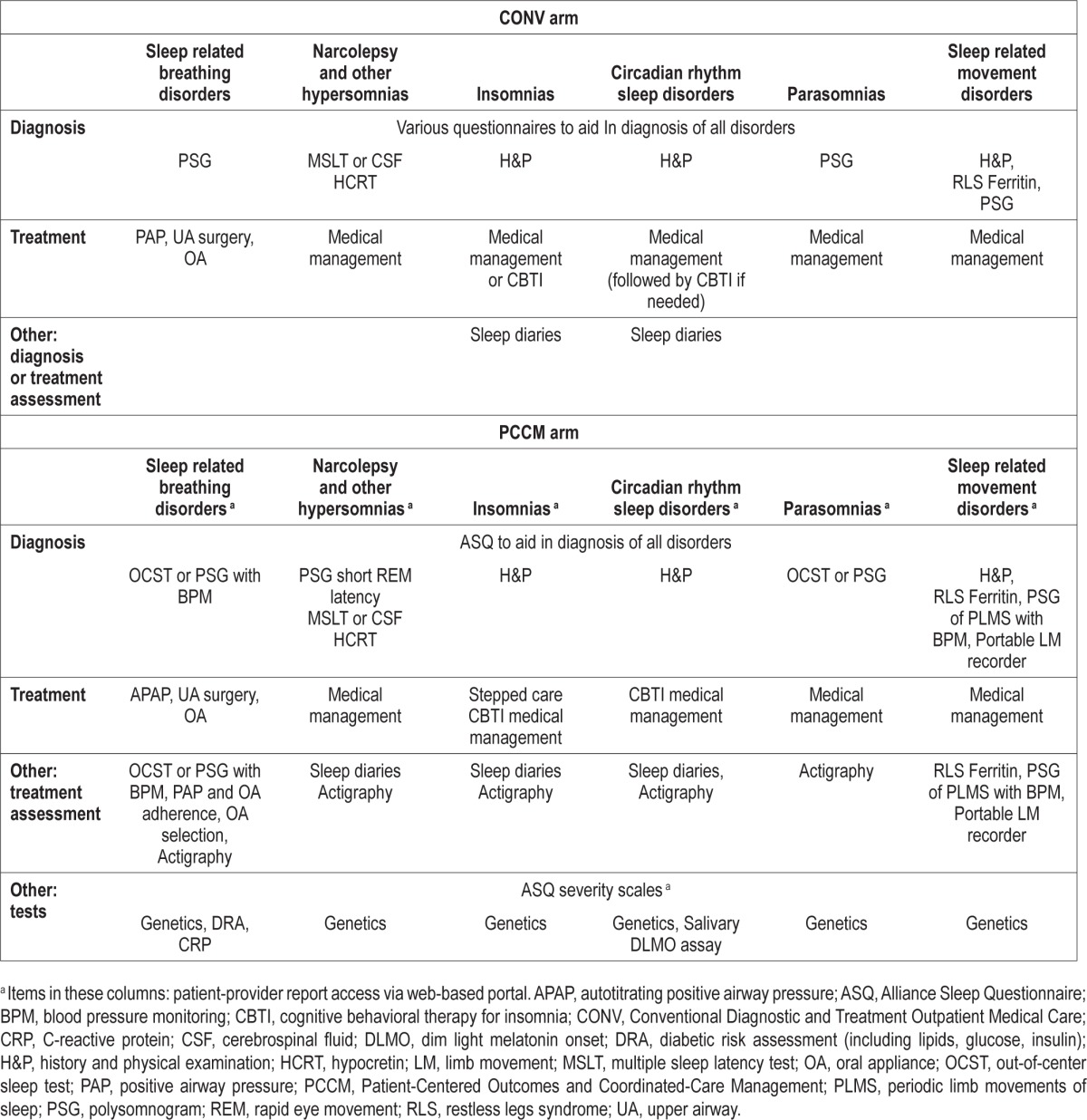
Sleep Related Breathing Disorders
In the conventional approach, the patient undergoes diagnostic testing that consists of an in-laboratory PSG or out-of-center sleep test (OCST) to confirm the diagnosis and to assess the severity of the disorder.35,36 At a follow-up visit, the PSG or OCST results are reviewed with the patient, and if the patient has a sleep related breathing disorder, treatment options are discussed, treatment is initiated, and the patient's progress is monitored through follow-up visits and PSGs or OCSTs. In the PCCM approach, a patient with a high pretest probability of a moderate to severe sleep related breathing disorder, but without suspicion of hypoventilation, central apnea, or serious cardiac, pulmonary, or neuromuscular disease, will have OCST37 using a Level III (unattended modified portable sleep apnea testing, minimum of four channels including ventilation, heart rate or electrocardiography, and oxygen saturation)38,39 device (SOMNOtouch RESP, SOMNOmedics GmbH, Randersacker, Germany; ApneaLink Plus, ResMed, San Diego, CA, USA; Nox T3 Sleep Monitor, Nox Medical, Reykjavík, Iceland) set up by the sleep technologist at either the sleep center or the PCP office. The patient will be provided the OCST device and instructed on its use by the sleep technologist; the patient will then use the device at home, and return the device the next day. The data will be downloaded, reviewed, and scored by the technologist, and a sleep specialist will examine the scored data and generate a report. If the OCST shows evidence of OSA, a management plan will be developed by the sleep specialist, and this plan and details from the report will be available to the PCP and patient through the SMART DOCS web portal.
If the patient does not meet the aforementioned criteria for OCST, the OCST is negative despite a high clinical suspicion for OSA, or there are patient- or technical-related issues regarding the OCST, an in-laboratory PSG will be conducted. At the follow-up visit, PSG results and treatment options are discussed with the patient by the sleep specialist; these results are also available to the PCP and patient through the web portal.
Additionally, the following diagnostic and therapeutic tools, methods, and algorithms will be employed as necessary in the PCCM approach:
Continuous overnight blood pressure assessment with a portable device (SOMNOtouch NIBP Blood Pressure Recorder,40–43 SOMNOmedics GmbH) will be conducted during both diagnostic and follow-up (e.g., positive airway pressure [PAP] titration, postsurgical) OCSTs and in-laboratory PSGs if the patient has borderline or definitive hypertension. Overnight blood pressure data will provide additional insight into each individual's OSA-related cardiovascular risk. We may also obtain C-reactive protein from a blood draw as another measure of cardiovascular risk.
Because of the increased risk of diabetes in patients with OSA, we may collect a blood sample from patients in whom diabetes has not been previously diagnosed to measure glucose, insulin, and lipid levels.
For patients who elect to try PAP therapy and who do not have hypoventilation or serious cardiopulmonary disease, an autoadjusting positive airway pressure (APAP) device will be prescribed to identify the optimal treatment pressure during home use.44 Reports regarding efficacy, air leak from the device or surrounding the mask, and adherence are available from the device manufacturers, and the relevant patient-provider outcome data will be uploaded to the secure web portal for access by the patient, his/her PCP, and sleep specialist. For patients who have complicated sleep related related breathing disorders or serious comorbidities, a PAP titration in-laboratory PSG will be conducted and an APAP device will be prescribed based on the results of the study. Treatment modifications (e.g., pressure adjustment) can be made remotely by the provider if necessary. A sleep medicine follow-up visit will occur within a few weeks of starting therapy, and the patient's APAP data will be reviewed on a regular basis (i.e., every few weeks) until the patient is stable.
If the patient declines PAP treatment and elects upper airway surgery, the patient will be referred to an otolaryngologist. Approximately 3 mo following surgery, the sleep specialist will order a postsurgical sleep study, either OCST or in-laboratory PSG, to assess surgical benefit. Key results from the OCST or PSG report will be exported to the secure web-based portal accessible to the patient and his/her clinicians. The need for additional treatment, if any, will be reviewed with the patient by the sleep specialist.
If the patient decides to try an oral appliance, the patient will be referred to a dentist for evaluation and determination of a range of mandibular protrusion that may be suitable to treat the patient's OSA. The patient will then be scheduled for an in-laboratory PSG using the MATRx device (Zephyr Sleep Technologies, Inc., Calgary, Alberta, Canada), which enables the dentist to target the patient's protrusive position for optimal treatment of OSA and also if the patient can be effectively treated with an oral appliance.17–20 If OA therapy is expected to be effective, a permanent appliance will be constructed with an innovative temperature-sensitive sensor (TheraMon, Handelsagentur Gschladt, Hargelsberg, Austria) embedded within the appliance by the dentist to objectively monitor adherence over time.15,16 These data can be downloaded from the sensor and a report will be uploaded to the secure web portal. If needed, the patient may later undergo either OCST or in-laboratory PSG while wearing the oral appliance to further assess efficacy and the potential need for further adjustments to the appliance. Results from the PSG, OCST, and adherence reports will be uploaded to the secure web portal for review by the patient, dentist, PCP, and sleep specialist for treatment modification.
Other sleep related breathing disorder treatment options such as Winx (ApniCure, Redwood City, CA, USA), Provent (Theravent, Inc., San Jose, CA, USA), genioglossus stimulation, bariatric surgery, oxygen therapy, or auto or adaptive servoventilation are also available to patients (as in the case of the conventional approach).
Blood samples will be collected to identify possible genetic markers for OSA.
Narcolepsy or Other Hypersomnias
Conventionally, a patient with an initial evaluation indicative of disorders of excessive sleep and sleepiness typically undergoes diagnostic testing for narcolepsy (e.g., PSG with a multiple sleep latency test [MSLT] or cerebrospinal fluid [CSF] hypocretin levels). The patient may be prescribed medications, and is assessed through regular follow-up visits. In the PCCM approach, a patient with symptoms of these disorders identified at either the sleep center or PCP office will undergo diagnostic testing at the sleep center. Instead of always proceeding with an MSLT or CSF hypocretin as in the conventional approach, we will use a short rapid eye movement (REM) latency during the in-laboratory PSG to assist in a diagnosis of narcolepsy. Diagnostic criteria typically mandate an MSLT or CSF hypocretin levels; however, recent evidence accommodated in the revised classification of sleep disorders shows that detecting a short REM latency (≤ 15 min) during a sleep study may alleviate the need for 2 MSLT sleep-onset REM periods (specificity = 99.2%, sensitivity 50.6%).45 CSF hypocretin may be needed in equivocal cases or with < 2 sleep-onset REM periods. Sleep diaries and actigraphy will be used to aid in the objective assessment of the quality and quantity of sleep as well as changes in sleep-wake cycles over time and following treatment. Blood samples will be collected to identify possible genetic markers for narcolepsy, and will include human leukocyte antigen (HLA) typing. The patient, sleep specialist, and PCP will have access to the reports through the secure web portal, and the patient will have follow-up visits at regular intervals.
Insomnia and/or Circadian Rhythm Sleep Disorders
In the conventional approach, if a patient has an initial evaluation consistent with insomnia or circadian rhythm sleep disorders, he or she is managed medically and/or with cognitive behavioral therapy for insomnia.46 The patient completes sleep diaries to document sleep-wake patterns at baseline and throughout treatment, and returns for regular follow-up visits. In the PCCM approach, we will employ a stepped care algorithm, in which a patient with symptoms consistent with an uncomplicated insomnia disorder (i.e., free of comorbid psychiatric disorders) will be provided the choice of undergoing mobile-based cognitive behavioral treatment for insomnia (CBTI) or brief CBTI47 by the sleep medicine-trained nurse at the PCP office. The mobile-based CBTI program (SleepRate, Palo Alto, CA, USA) provides a personalized sleep improvement plan using a CBTI protocol that is based on CBTI implemented at Stanford University. For the nurse-administered brief CBTI, the nurse, trained to competency by sleep center insomnia specialists, will deliver in-person individual treatment. Studies demonstrate that CBTI can be successfully administered in the primary care setting by nurses, physicians, or psychologists, with effect sizes that are roughly equivalent to those found in meta-analytic studies of CBTI in the general population.46,48–51 Complicated insomnia cases and patients requiring additional treatment after brief in-person or mobile-based CBTI will be referred to the sleep specialist or psychologist for continued care. A patient who has symptoms consistent with an uncomplicated circadian rhythm sleep disorder will be managed by a sleep specialist using medications, carefully timed light exposure, and behavioral techniques. For a patient with a complex circadian rhythm sleep disorder, DLMO assays (Salimetrics, Carlsbad, CA, USA) will be conducted on saliva samples obtained at 30-min intervals for 5 h prior to the usual bedtime during an in-laboratory PSG. Blood samples will be collected to identify possible genetic markers for insomnia and circadian rhythm sleep disorders. For both insomnia and circadian rhythm sleep disorders, longitudinal outcomes will be assessed by sleep diaries and actigraphy. The patient, sleep specialist, and PCP will have access to information from the longitudinal actigraphy reports on the secure web portal, and the patient will have regular follow-up visits. In cases where comorbid insomnia is present, the circadian rhythm sleep disorder will be managed prior to delivering CBTI.
Parasomnias
Conventionally, a patient with an initial evaluation consistent with a parasomnia diagnosis undergoes an in-laboratory PSG to identify and record the abnormal behavior and to rule out other possibilities (e.g., seizures), receives therapeutic and safety information, may be prescribed medications, and is monitored through regular follow-up visits. In the PCCM approach, a patient presenting with parasomnia symptoms will undergo actigraphy and an in-laboratory PSG or alternatively an OCST Level II (unattended comprehensive portable PSG)38,39 device with video monitoring to identify and record the abnormal behavior (e.g., sleepwalking, sleep terrors) and possibly the precipitant(s). Blood samples will be collected to identify possible genetic markers for parasomnias. The patient will receive information about his/her condition and precautions through the web portal, and may be prescribed medication by the sleep specialist to treat the condition. Via the web portal, the patient, sleep specialist, and PCP will also have access to findings from the sleep study reports and summaries of regular follow-up visits.
Sleep Related Movement Disorders
Using the conventional approach, when a patient has an initial evaluation consistent with a sleep related movement disorder, he/she may undergo an in-laboratory PSG to confirm the diagnosis, receive ancillary testing (e.g., ferritin levels), and be medically managed. In the PCCM approach, if a patient is suspected of having restless legs syndrome and/or periodic limb movement disorder, the patient may wear a portable limb movement recorder (SOMNOtouch NIBP with Periodic Limb Movement Recorder, SOMNOmedics GmbH), at home or in the laboratory to measure the frequency and duration of leg movements to confirm the diagnosis. A patient may also wear the limb movement recorder at regular intervals to objectively measure treatment response. The limb movement recorder also continuously measures and records blood pressure to assess potential periodic limb movement-related blood pressure spikes,23–26 which can provide further data for the patient, specialist, and PCP regarding possible cardiovascular associations with this condition. Patients with restless legs syndrome will also have ferritin levels checked to assess iron deficiency, as in the conventional approach. Blood samples will be collected to identify possible genetic markers for sleep related movement disorders. The patient, sleep specialist, and PCP will have access to longitudinal reports and visit summaries on the secure web portal, and the patient will have regular follow-up visits.
EVALUATING THE PCCM APPROACH
Study Design
The central question to be answered by SMART DOCS is whether a new PCCM approach for sleep medicine provides better care, from the patient perspective, and improves the health of patients while controlling costs as compared to a CONV approach. Thus, a randomized comparative effectiveness trial has been designed and will be conducted to inform health care decisions by providing evidence on patient-centered outcomes for these two different approaches of delivering outpatient sleep medical care. The CONV Arm is defined as the standard approach by which providers in a typical sleep medicine outpatient clinic manage their patients. Our study follows the standard methods and procedures for the management of the aforementioned sleep disorders, many of which were developed and published as practice parameters by the American Academy of Sleep Medicine (AASM) to guide the diagnosis and treatment of patients with sleep disorders. The PCCM Arm is defined as an approach that enables providers and patients access to specific and relevant information and resources, thereby allowing patients to make more informed health care decisions and providers to assist patients in achieving their preferred outcomes (described previously for each disorder). We will enroll 1,833 new patients to randomly assign 1,506 patients at a 50/50 ratio to each of these two management arms (Figure 1, Table 1), using a permuted block design52 (see sections on Sample Size Estimation, Statistical Methodology for Data Analyses, and Possible Limitations in the supplemental material).
Figure 1.
SMART DOCS Conventional Diagnostic and Treatment Outpatient Medical Care (CONV) Arm vs. Patient-Centered Outcomes and Coordinated-Care Management (PCCM) Arm. PCP, primary care physician.
Table 1.
Description of SMART DOCS sleep disorder management arms.
Population
The patients who are eligible for this study are consecutive new clinical outpatients ≥ 18 y of age who have a possible sleep disorder. In order to have a study population representative of a typical clinic population, there are no exclusion criteria. Each new patient consecutively seen at the Stanford Sleep Medicine Center or Stanford Primary Care will be informed about the study, and will also be apprised that he or she will be consenting to grant access to any and all clinical data collected during his or her evaluation and treatment to study personnel. A sleep medicine-trained nurse will assist in the screening and recruitment of patients at Stanford Primary Care. The patient will be notified that the study is a randomized trial and he or she could be assigned to either the CONV or PCCM arms, and will be followed for at least 1 y within the 3-y duration of the study. If he or she agrees to participate, informed consent will be obtained, he or she will be randomized to one of the study arms, detailed instruction about the study activities will be provided, and the patient will be asked to adhere to the study protocol, related to the diagnosis and treatment of his or her specific sleep disorder(s).
The patient catchment area of the Stanford Sleep Medicine Center and Stanford Primary Care is predominantly within the county of Santa Clara, CA, USA. Based on the 2010 United States Bureau of the Census data estimate, the population ≥ 18 y of age in this county (1,352,097) is 49.8% female and racially/ ethnically diverse with 35.7% minorities, which compares favorably with the national data (50.8% women and 27.6% minorities). The estimated proportion of sleep disorders diagnosed in our patient population are: sleep related breathing disorders (77%), insomnias (10%), sleep related movement disorders (5%), circadian rhythm sleep disorders (3%), parasomnias (3%), and narcolepsy and other hypersomnias (2%). We would expect that our patient population is demographically and diagnostically representative of other communities; however, we can adjust for selection bias through analyses53,54 that weight clinical and demographic subpopulations within our sample to match the distribution of patients across these subpopulations nationwide (see section on Selection Bias in the supplemental material).
Outcomes
The goals of this study are to provide better overall patient care and to improve the health of patients while controlling cost. In this light, there are two primary endpoints and a secondary endpoint associated with these goals (Table 2). The primary endpoint of improved health care performance or better care in SMART DOCS will use a survey developed within the Consumer Assessment of Healthcare Providers and Systems (CAHPS)55 program of the AHRQ, which asks patients to evaluate their experiences with health care, such as the communication skills of providers and ease of access to health care services. Specifically, the patients' global rating of the provider will be taken from the CAHPS Clinician and Group Survey (CGCAHPS) Adult 12-Month Questionnaire 2.0. The CAHPS Proportional Scoring Method to Clinician and Group Survey Composites will be used to score these results.55 Secondary CAHPS variables will include: (1) items from the CGCAHPS Adult 12-Month Questionnaire 2.0 on “How Well Providers (or Doctors) Communicate with Patients,” and (2) items from the CGCAHPS Health Information Technology Item Set.
Table 2.
SMART DOCS primary and secondary endpoints.

The primary endpoint to assess improved health will be the Vitality Component Summary Score on the Short Form (SF)-36 v.2 Health Survey.56 The SF-36 is a psychometrically validated measure used to assess perceived health in the prior 4 w, which will be administered after 12 mo of treatment to all participants. Data from the SF-36 can be used to generate a SF-6D score, a preference-based health utility index. Other secondary endpoints include disorder-specific severity measures as contained in the ASQ and the FOSQ-10 (Table 2).
For the secondary endpoint of cost containment, we will track the out-of-pocket costs of administering each pathway, including costs of the treatment, but excluding research data collection from survey instruments as well as related personnel time and other cost factors not related to patient care. In both approaches, we will track out-of-pocket healthcare costs for participants focusing particularly on costs associated with all outpatient visits, emergency department visits, and inpatient stays during the study period. With these data, out-of-pocket costs of treatment as well as the health care utilization between pathways can be compared. Other secondary endpoints are listed in Table 2, and the statistical methods are described in the section on Statistical Methodology for Data Analyses in the supplemental material.
EFFECT OF THE PCCM APPROACH ON HEALTHCARE PERFORMANCE
We hypothesize that the more efficient and convenient use of time, resources, and personnel in the PCCM approach will translate to improved delivery of care. In addition to the emphasis on new tests and technologies, expansion of the roles of allied health professionals and increased communication with PCP staff are major aspects of the PCCM approach. The care for many sleep disorders patients is a natural extension of traditional nursing care, requiring some additional knowledge, but utilizing traditional nursing methods and skills that generalize well to sleep medicine. The sleep medicine-trained nurse in this study will assist PCPs in the screening and diagnosis of sleep disorders in their patients, monitor outcomes and adverse events, provide cognitive behavioral treatment for their patients with insomnia, and assist PCPs and patients in using the patient-provider web portal especially with respect to assessing treatment efficacy, adherence, and next steps. Additionally, there are more than 15,000 registered sleep technologists in the United States and several thousand more who are employed but awaiting certification. These technologists are primarily responsible for conducting in-laboratory PSGs, but due to rapidly evolving technology in out-of-center sleep testing, it is anticipated that the number of these in-laboratory studies will significantly decrease within the next decade. This in turn will likely decrease the need for technologists to conduct in-laboratory procedures; however, these knowledgeable and skilled individuals can be rapidly reassigned to modified roles with minimal training. The PCCM approach will utilize sleep technologists to enhance care delivery and to serve as a resource for PCPs in the long-term comanagement of sleep disorders with sleep specialists. Specifically, sleep technologists will provide the setup and data collection for home testing in the diagnosis of sleep related breathing disorders, colead small-group classes with nurses for patients who have questions or issues with major treatment modalities, aid in patient treatment effectiveness and adherence issues, and assist in the collection and analysis of data from new diagnostic and treatment outcomes. For example, sleep technologists will contact patients with OSA shortly after they initiate PAP treatment to address any issues and concerns, and will recontact them at time intervals of 1 mo, 3 mo, and 6 mo, as necessary, until all problems are resolved. The enhanced teaching role of technologists is compatible with their frequent roles as educators in A-STEP (Accredited Sleep Technology Education Program), AWAKE (Alert, Well, and Keeping Energetic) groups, and clinical interactions with patients. It is our belief that the integration of sleep medicine-trained nurses and sleep technologists into the primary care setting will improve access to care and the management of patients with sleep disorders.
EFFECT OF THE PCCM APPROACH ON PATIENT EXPERIENCE
The PCCM approach for sleep medicine has been developed in direct response to our patients' needs and reflects the type of approach that our patients consider desirable. Our review of the Press Ganey quality of care data from the Stanford Sleep Medicine Center consistently reveals that the top three desires of our patients are better access to care, improved access to their records, and more information about their diseases. We believe that the PCCM approach will meet these patient needs and that our approach is patient centered in that it serves to address the following key patient-centered questions posed by PCORI.57
Given My Personal Characteristics, Conditions, and Preferences, What Should I Expect Will Happen to Me?
Through the use of technology and reallocation of healthcare personnel time, the PCCM approach provides a more tailored and customized method for patient care delivery that places more information and results related to the patient's care in the patient's hands. For example, a patient will be able to access personalized information about his/her sleep disorder and treatment, as well as longitudinal therapeutic effectiveness and adherence data. Educational materials regarding sleep disorders will also be available on the secure web portal. Access to this type of information will enable the patient to be more informed about his/her condition, and these data plus the new web portal will permit collaboration between the patient, PCP, and sleep specialists to assist the patient in determining the ongoing success or limitations of the current treatment.
What Are My Options and What Are the Potential Benefits and Harms of Those Options?
The PCCM approach will allow the patient to evaluate various treatment options through small group classes with other sleep medicine patients and by visits with the PCP, sleep specialist, and allied health professionals as well as through review of educational content on the web portal. These avenues of information will allow thorough discussions of the potential benefits and limitations of the various treatment options so that the optimal management plan is selected.
What Can I Do to Improve the Outcomes That are Most Important to Me?
Our team firmly believes that one of the primary ways to improve outcomes is through extended and readily accessible knowledge about the disorder and current/new treatments. The sharing of information between the patient and his/her providers as well as the comprehensiveness of the results and reports available to the patient through the PCCM approach will offer the patient the best chance in improving his/her outcomes.
How Can Clinicians and the Care Delivery Systems in Which They Work in Help Me Make the Best Decisions About My Health and Health Care?
SMART DOCS is expected to demonstrate several methods of providing results and information to patients, including from home-based devices, a secure web portal, and discussions with their PCPs, specialists, and allied health professionals through visits and group settings. The sharing of these results between the patient and his/her providers ensures that they are up to date regarding the patient's health status and thus are in the best position to make a collaborative decision about the patient's health and healthcare needs.
SUMMARY
It is our team's hope that SMART DOCS will lead to meaningful improvements in patient health and quality of care by transforming the manner in which patients with sleep problems are currently diagnosed and managed, via a new PCCM approach that is designed to be sustainable, effective, and exportable to other academic institutions, hospitals, private practices, free-standing sleep centers, and rural communities. We realize that there is a delicate balance in the management approach of patients with sleep disorders in avoiding pathways that are either too narrow or “one size fits all.” Rather, the goal is to develop pathways that might aid the clinician in managing the majority of his or her patients, and to use feedback from stakeholders and the larger sleep medicine community to further enhance these pathways. The selection of the methods, algorithms, and tools to be tested in the PCCM arm were considered by our Core Team as having the highest likelihood of improving patient health and quality of care by providing patients greater access to personalized results and relevant outcomes, and ultimately placing the patient more in control of his or her choice of treatment. These processes, in turn, have been subject to ongoing review and revision by our 22-member SMART DOCS Stakeholder Team, composed of patients, patient advocacy/support group leadership, providers from various disciplines and practice settings, leadership from professional organizations relevant to sleep medicine, and industry (medical device and pharmaceutical manufacturers/ suppliers) leadership (see section on Stakeholder Team in the supplemental material). It is anticipated that the PCCM approach and other new approaches for sleep medicine will help remedy the current inefficiencies and gaps in effective patient care delivery by enabling providers and patients access to specific and relevant information and educational resources, thereby enabling patients to make more informed health care decisions and allowing providers to assist patients in achieving their preferred outcomes.
DISCLOSURE STATEMENT
This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Pilot Project Program Award (CE-12-11-4137) Sustainable Methods, Algorithms, and Research Tools for Delivering Optimal Care Study (PI: Kushida). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. Investigational Use: SOMNOtouch NIBP Blood Pressure Recorder, (SOMNOmedics GmbH, Randersacker, Germany). Dr. Kushida has received grant/research support from Aerial BioPharma, Apnex Medical, Cephalon, Impax Laboratories, Inc., Jawbone, Pacific Medico Co, Ltd, and Resmed; has served as a consultant for Apnex Medical, Noven Pharmaceuticals, Philips Respironics, Seven Dreamers laboratories, UCB, and Zephyr Sleep Technologies, Inc; and has received royalties from Philips Respironics. Mr. Miller is a part owner and salaried employee of Microflow DBMS, Inc. Ms. Griffin has received grant/research support from Apnex Medical, Merck, Novo Nordisk, and Vanda Pharmaceuticals. Ms. Cardell has received grant/research support from Apnex Medical, Jawbone, Pacific Medico Co., Ltd, and Resmed. Dr. Manber has served as a consultant for General Sleep and has received royalties from New Harbinger. Dr. Walsh has received grant/research support from Apnex Medical, Merck, Novo Nordisk, Philips Respironics, and Vanda Pharmaceuticals; and has served as a consultant for Aptalis, Cereve, Merck, Somnus, Ventus, and Vivus. The other authors have indicated no financial conflicts of interest. The work was performed at Stanford University, Stanford, CA.
ACKNOWLEDGMENTS
The Sustainable Methods, Algorithms, and Research Tools for Delivering Optimal Care Study (SMART DOCS) is funded by contract CE-12-11-4137 from the Patient-Centered Outcomes Research Institute (PCORI).
Administrative Core
Clete A. Kushida, MD, PhD (Stanford University, Stanford, CA), James K. Walsh, PhD (St. Luke's Hospital, St. Louis, MO), Kara Griffin, MA (St. Luke's Hospital, St. Louis, MO), Pamela R. Hyde, MA (Stanford University, Stanford, CA)
Patient-Centered Outcomes Research Institutue (PCORI)
Diane Bild, MD, MPH (Senior Program Officer), David Salinas (Contract Administrator), Jana-Lynn Louis, MPH (Program Associate)
Data and Safety Monitoring Board (DSMB)
Stuart F. Quan, MD (Chair; Harvard University, Boston, MA), Sonia Ancoli-Israel, PhD (University of California, San Diego, CA), Nathaniel F. Watson, MD, MSc (University of Washington, Seattle, WA)
Data Coordinating Center
Deborah A. Nichols, MS (Stanford University, Stanford, CA), Ric Miller, MA (Sausalito, CA), Oscar Carrillo, RPSGT (Stanford University, Stanford, CA), Charles Chen (Stanford University, Stanford, CA), Tyson H. Holmes, PhD (Stanford University, Stanford, CA)
Clinical Center
Emmanuel Mignot, MD, PhD (Stanford University, Stanford, CA), Rachel Manber, PhD (Stanford University, Stanford, CA), Chia-Yu Cardell, RPSGT (Stanford University, Stanford, CA), Elyse Cohen, RN (Stanford University, Stanford, CA), Kayla Griffith, RN (Stanford University, Stanford, CA), Jan Anderson (Stanford University, Stanford, CA), Linda Kresge (Stanford University, Stanford, CA)
Consultants
Uli Gal-Oz (SleepRate, Palo Alto, CA, USA), Steve Granger, PhD (Salimetrics, Carlsbad, CA), Gerhard Gschladt (TheraMon, Handelsagentur Gschladt, Hargelsberg, Austria), Randall Inouye, DDS (Stanford University, Stanford, CA), Gert Küchler, MD (SOMNOmedics GmbH, Randersacker, Germany), Eileen B. Leary, MS, RPSGT (Stanford University, Stanford, CA), Alice Liu, MD (Stanford University, Stanford, CA), Hyatt Moore, PhD (Stanford University, Stanford, CA), Gerald Reaven, MD (Stanford University, Stanford, CA), John Remmers, MD (University of Calgary, Alberta, Canada), Max Utter (Jawbone, San Francisco, CA), Jaime Zeitzer, PhD (Stanford University, Stanford, CA)
Stakeholder Team
Patient Representatives
John F. Dee, Cambridge, MA; Joe David Ramirez, PhD, San Francisco, CA; Janice Wait, Portola Valley, CA
Patient Support/Advocacy Group Leadership
Georgianna Bell (Executive Director, Willis-Ekbom Disease Foundation, Rochester, MN), Julie Flygare, JD (Narcolepsy spokesperson, Boston, MA), Ed Grandi (Executive Director, American Sleep Apnea Association, Washington, DC), Robert Tognoli, RPSGT (Director, Alert, Well, and Keeping Energetic [AWAKE] Patient Support Group, Stanford University Chapter, Stanford, CA)
Providers/Professional Organization Leadership
Nancy Collop, MD (Pulmonary/Sleep Medicine, Director, Emory Sleep Center, Emory University, Atlanta, GA; Past-President, American Academy of Sleep Medicine), Jack Edinger, PhD (Psychology/Sleep Medicine, National Jewish Health, Denver, CO), Joyce Lee, MD, MPH (Endocrinology, Co-Director, Program on Mobile Technology in Child Health, University of Michigan, Ann Arbor, MI), Teofilo Lee-Chiong, MD (Pulmonary/Sleep Medicine, National Jewish Hospital, Denver, CO), Nancy Morioka-Douglas, MD (Family Practice, Medical Director, Stanford Family Practice, Stanford University, Stanford, CA), Anil Rama, MD (Neurology/Sleep Medicine, Medical Director, Division of Sleep Medicine, Kaiser Permanente, San Jose, CA), Richard Simon, MD (Internal Medicine/Sleep Medicine, St Marys Medical Center, Walla Walla, WA), Baldeep Singh, MD (Internal Medicine, Medical Director, Stanford Internal Medicine, Stanford University, Stanford, CA), Patrick Strollo, MD (Pulmonary/Sleep Medicine, Medical Director, UPMC Sleep Medicine Center, University of Pittsburgh, Pittsburgh, PA, Past-President, American Academy of Sleep Medicine), Terri Weaver, PhD (Nursing/Sleep Medicine, Dean, School of Nursing, University of Illinois, Chicago, IL; Board of Directors, American Academy of Sleep Medicine), David P. White, MD (Pulmonary/Sleep Medicine, Clinical Professor of Medicine, Harvard University, Boston, MA; Past-President, American Academy of Sleep Medicine)
Industry - Medical Device and Pharmaceutical Manufacturers/ Suppliers Leadership
Mark Aloia, PhD (Senior Director of Global Clinical Research, Philips Healthcare, Denver, CO), Ron Barrett, PhD (CEO, XenoPort, Santa Clara, CA), Adam Benjafield (Vice President, Medical Affairs, ResMed Ltd, San Diego, CA), Kelly Garber (National Director, Clinical and Respiratory Services, Apria Healthcare, Lake Forest, CA).
SUPPLEMENTAL MATERIAL
Sample Size Estimation
We will enroll 1,833 patients and randomize 1,506 to obtain a final sample of 1,054 randomized participants each followed for at least 1 y in SMART DOCS. Estimates for prerandomization (20%) and postrandomization (30%) participant losses are based upon our National Heart, Lung,and Blood Institute (NHLBI)-supported Apnea Positive Pressure Long-term Efficacy Study (APPLES). A sample size of 1,054 is feasible for this pilot trial given our current estimated annual new patient volume of 4,920 at Stanford. Effect size was defined as d = (m1 – m2) / sd, where m1 and m2 are the respective means of the two groups and sd is their common within-group standard deviation. A small effect size was defined as d = 0.2, per standards provided by Cohen.1 We will assume that this effect size holds for our primary outcomes of improved health care performance and health and that these two outcomes are moderately correlated (ρ = 0.5). For each outcome, means will be compared using a two-tailed, two-sample t-test,2 with correction for unequal variances, if necessary. Type I error rate will be controlled at 5% across the two primary hypotheses using the method of Holm.3 Based on ten-thousand simulations performed in R,4 a sample size of 527 per group (1,054 total completers) is required to obtain 88% power.
Statistical Methodology For Data Analyses
“Study arm” refers to the Conventional Diagnostic and Treatment Outpatient Medical Care (CONV) approach versus the Patient-Centered Outcomes and Coordinated-Care Management (PCCM) approach. Randomization will be 1:1 to study arms using a permuted block design, with 251 patients per block; randomization will not be stratified. Simulations estimate that randomization of the planned 1,506 recruited participants along with the anticipated 30% post-randomization random dropout will achieve approximately the randomization goal of 1,054 completers (Figure S1) and result in good balance between study arms (Figure S2).
Distribution of quantity of completers across 2,500 simulated permuted-block randomizations (block size = 251) of 1,506 patients with 30% postrandomization random dropout.
Distribution of proportion assigned to PCCM approach across 2,500 simulated permuted-block randomizations (block size = 251) of 1,506 patients with 30% postrandomization random drop-out.
Baseline Analyses
Demographic and other baseline features will be compared between study arms. These two-group comparisons will employ t-tests with correction for unequal variances as needed. Categorical variables, including dichotomous factors, will be summarized as percentages that will be compared between arms using chi-square tests, two-sample t-tests,2 or Boschloo exact unconditional tests,5 depending on the smallest expected cell sizes. Categorical variables, including dichotomous factors, will be summarized as percentages that will be compared between arms using chi-square tests or Boschloo exact unconditional tests,5 depending on the smallest expected cell sizes.
Primary Analyses
The primary endpoint of improved health care performance will be assessed by the proportion of patients answering with a “9” or “10” on an 11-point scale (where 0 is the worst provider possible and 10 is the best provider possible) for the patients' global rating of the provider from the CAHPS Clinician and Group Survey (CGCAHPS) Adult 12-Month Questionnaire 2.0. This dichotomized outcome will be logistically regressed on study arm. Hypothesis testing will employ a Wald statistic (t-test). In secondary analyses, random intercepts will be employed for providers to account for possible nesting of patients' responses within providers; and the CGCAHPS global rating score without dichotomization, will be regressed on study arm using a finite mixture6 of binomial distributions with random intercepts employed for providers. A finite mixture is recommended because the CGCAHPS global rating score is likely to have a non-standard distribution. Binomial distributions will be employed because the CGCAHPS global rating score is discrete and bounded above by a positive integer and below by zero. Means of the component binomial distributions will be formulated in terms of the same regression coefficient for study arm (but different intercepts), a parsimonious model structure that will facilitate interpretation. Hypothesis testing for secondary analyses will also employ a Wald statistic (t-test).
For the primary endpoint of improved health, the vitality scale score for improved health from the SF-36 will be collected at baseline and end-of-study. Analysis will parallel that for the CGCAHPS global rating score with the addition of baseline score as a covariate. Baseline value will be centered and scaled7 via subtracting the sample mean and dividing that difference by the sample standard deviation prior to analysis in order to improve numerical stability of the fitting algorithm. A finite mixture of binomial distributions is recommended here as well because the distribution of vitality scale scores may be complex with some floor and ceiling effects (Table 7 in Gandek et al., 1998).8
Secondary Analyses
All secondary outcomes are longitudinal, with measurement planned at more than two visits per person. Regression analyses will be performed using generalized linear mixed models (GLMM),9 with regression of longitudinal outcome values on study arm, baseline value, and visit. Baseline value will be centered and scaled7 as previously described. GLMMs are useful because they accommodate a wide variety of parametric outcome distributions. To account for the repeated-measures structure, each GLMM model will include a random intercept for patient. A random intercept will be employed for provider to account for nesting of patients' responses within providers.
For the secondary endpoint of out-of-pocket cost, because within either arm, some individuals may have out-of-pocket costs that are much larger than most other participants (e.g., emergency surgical intervention), we will compare mean cost between arms using finite mixture models.6 To be thorough, as a secondary analysis, we will also compare arms in a more generalized fashion by comparing out-of-pocket cost deciles between arms using quantile regression,10 as this may identify other cost disparities not revealed by the comparison of means. If the PCCM treatment arm proves associated with reductions in out-of-pocket health care costs, we will also perform a more in-depth assessment of the nature of this reduction in relationship to changes in health care utilization.
Special Analyses
The effect of CONV versus PCCM care on the primary outcomes may vary depending on diagnostic category, which will have direct bearing on the exportability and expandability of this study's findings. Some of these diagnostic categories are more prevalent than others. As a result, sample sizes will vary, causing corresponding variation in the reliability of diagnostic category-specific estimates of the effect of CONV versus PCCM. A special statistical study will be performed and separately published that is aimed at improving the reliability of diagnostic-category specific estimates, particularly through exploration of statistical shrinkage techniques, such as empirical Bayes.11 In addition, time permitting, special statistical studies may be conducted to permit a more fine-grained assessment of health as an outcome and collectively across diagnoses. For each major diagnostic category, a mathematical model will be derived, in consultation with the study's clinical team, of the biological process of disease progression and remission. These models will be stochastic12 to allow for the inherent variability in disease status. These models will be fit using normalized measures of disease status, where normalization will be accomplished using either published norms or norms derived from those who entered the study but were not of sufficient severity to receive a diagnosis.13 The intent of normalization is to permit assessment of longitudinal trends in disease status across all diagnoses.
Because levels of web portal use may vary, in secondary analyses randomization assignment will be employed as an instrument14 for adherence to assess the causal relationship between level of web portal use and outcome.
Retention and Missing Data
Retention fractions and reasons for loss to follow-up may differ between arms. Losses may be caused by voluntary withdrawal, medical disqualification, or death. Cumulative incidences of each of these three competing risks will be compared between study arms via modeling of their subdistribution hazards.15
For primary analyses, data will be treated as missing completely at random. Time permitting, secondary analyses will explore use of multiple imputation under the assumption that data are missing at random and, separately, using selection modeling16 for cross-sectional analyses and correlated random effects for longitudinal analyses17 under the assumption that data are missing not at random.
General Considerations
For all the aforementioned regression analyses, to minimize loss in statistical power due to collinearity and to avoid numerical computational problems, study arm and randomization factors will be orthogonally coded7 prior to fitting the regression model. Random effects will be estimated via adaptive gaussian quadrature and replaced by either conditioning18 or fixed effects if indicated by a Hausman-Wu test.19
Results of hypothesis testing will be declared nominally significant for attained significance levels of P ≤ 1/20. For reference, multiple-comparison adjusted P values will also be calculated across all primary and, separately, across all secondary outcomes using the sequential adjustment method of Holm,3 which is preserving of statistical power and applicable to a set of nonindependent tests.
Possible Limitations
Selection Bias
The recruitment of patients from one of the largest tertiary referral academic sleep centers that receives patients referred from diverse locales, practice settings, and medical specialties will provide a representative sample of patients with sleep disorders. The enrollment of consecutive patients seen by sleep specialists and primary care physicians, use of broad inclusion criteria with no exclusion criteria, and random assignment to the CONV or PCCM arms will minimize potential patient selection bias. However, some self-selection bias may occur because patients can choose not to sign informed consent and thus not participate in the study. We can adjust for selection bias through analyses that weight clinical and demographic subpopulations within our sample to match the distribution of patients across these subpopulations nationwide.20,21
Attrition Bias
Close monitoring of the patients while they are enrolled in the study will minimize dropouts. Differential dropout is possible (e.g., fewer participants exiting the study in the PCCM versus CONV arm because of the new tools, enhanced patient resources, etc. in the PCCM arm) (see section on Retention and Missing Data), but unlikely because the CONV arm is the standard-of-care approach in our center. Additionally, we will explore the impacts of any differential dropout using the sensitivity analyses described in Todem et al.22 The Data Coordinating Center (DCC) (see section on Study and Data Governance) will implement live online enrollment and randomization reports that were developed for the Agency for Healthcare Research and Quality (AHRQ)-supported Comparative Outcomes Management with Data Technology (COMET) Study, and the DCC will be responsible for ensuring that recruitment, enrollment, and randomization are on track. Additionally, the use of new technology such as the electronic measures of patient adherence to positive airway pressure and oral appliances to be used in this study will enable quick and accurate assessment of adherence to therapy, allowing the providers to rapidly identify and remedy the source of any potential reduction in adherence on the part of the subject. Based on our prior NHLBI-supported APPLES, we estimate prerandomization and postrandomization dropout rates of 20% and 30%, respectively, for the study (see section on Sample Size Estimation).
Missing Data
We have an experienced DCC that was established 15 y ago, which has developed methods and procedures for minimizing data loss and handling missing data (see section on Retention and Missing).
Outcome Data Comparisons
The primary outcomes selected for this study have normative and other population datasets that can be used for comparisons of the data collected from our CONV and PCCM populations.
Study and Data Governance
The organization of the study and the management of the trial are depicted in Figure S3.
SMART DOCS Organizational Chart.
The roles and responsibilities of the individuals (see Acknowledgments for names and affiliations) participating in the proposed study are outlined below.
Administrative Core
The primary function of the Core is to serve as the administrative and communications hub for the study. This includes responsibilities such as: (1) organizing conference calls, meetings, and training sessions; (2) ensuring subject safety and compliance with the institutional review board; (3) developing mechanisms to ensure a smooth transfer of data between the site and the DCC; (4) ensuring compliance with deadlines, protocols, and procedures (e.g., subject recruitment, randomization); and (5) monitoring federal regulatory compliance, fiscal and personnel management, and development of conflict-of-interest policies. Most importantly, the Core is responsible in maintaining the scientific integrity, cooperation, and morale among the staff and stakeholders of the study.
Patient-Centered Outcomes Research Institute (PCORI)
The PCORI program officer is responsible for the oversight of the conduct of the study, and will monitor study progress by regular communication with the Principal Investigator and the Data and Safety Monitoring Board.
Data and Safety Monitoring Board (DSMB)
The roles of the DSMB are to ensure the safety of the patients and the scientific integrity of the study by monitoring the study participants, supervising the safety and quality control activities of the DCC, and recommending modifications of the clinical trial protocol. The DSMB will work closely with the Core Team and the DCC.
Data Coordinating Center (DCC)
The DCC serves as an independent unit within our study organization. The main function of this center is to serve as the central organizing site for all data collected and reviewed for the proposed study. The DCC was established 15 y ago and has participated in multiple studies, including the NHLBI-supported APPLES and the AHRQ-supported COMET Study.
Clinical Center (CC)
The CC is responsible for conducting the clinical trial and the proper execution of the study protocol. The CC personnel are under the direct supervision of the SMART DOCS Core Team, who ensure that the staff complies with the study schedule, treats the patients in a respectful and courteous manner, collects the data in an efficient and careful manner, performs meticulous and reliable data entry, and maintains communication of data with the DCC.
Consultants (CC)
These other investigators, clinicians, and industry leadership provide specialized expertise in the diverse areas of circadian rhythms, endocrinology, informatics, product development, electronic questionnaires, and web design.
Stakeholder Team
In order to accomplish its goals, SMART DOCS will establish partnerships with multiple stakeholders, who will be directly engaged as part of the study and who perform an integral role in assessing the methods, algorithms, and tools in this project. Active participation of stakeholders is necessary to review and provide feedback on the PCCM approach during the progress of the study, to determine the best structure and communication pathways to ensure success and sustainability of this multistakeholder involvement model, to guide the exploration of use of newer electronic self-tracking and social networking methods, and to establish plans to disseminate, expand, and export the PCCM approach to other medical disciplines and practice settings. The SMART DOCS Stakeholder Team is composed of 22 members: (1) Patients and Patient Representatives: Patients from our current patient population and representatives from local and national patient advocacy and support groups ensure that the patient always has access to his/ her data and control of care with our approach. (2) Providers: Providers from various disciplines and practice settings allow the successful repurposing of these approaches to other areas of medicine, and will be engaged for applicability of our approach to various practice settings (e.g., academic institutions, large hospital and physician networks, private practices). (3) Professional Organization Leadership: The leadership from organizations relevant to sleep medicine will assist in the rapid dissemination of the findings to their respective organizations. (4) Industry - Medical Device and Pharmaceutical Manufacturers/Suppliers Leadership: The leadership from medical manufacturers of diagnostic/therapeutic devices, a durable medical equipment provider, and a pharmaceutical company will provide an industry perspective on our approach.
Dissemination Plan
We plan to disseminate descriptions of this approach and the results of the study through upcoming APSS meetings as well as other national and international meetings associated with sleep medicine and research. We will also rely on the three members of our Core Team and several consultants on the 22-member SMART DOCS Stakeholder Team who hold or have held leadership positions within the two main professional organizations of sleep medicine and research, the American Academy of Sleep Medicine and the Sleep Research Society, to assist in the dissemination of this approach. In addition, we plan to utilize our entire Stakeholder Team to further disseminate our approach to their respective specialties and organizations. In particular, the collective background and knowledge of the Stakeholder Team not only within the field of sleep medicine, but also in the patient experience, industry relationships, leadership, and other areas of medicine will help the Core Team best decide which components of the approach are most applicable to a given setting or medical discipline. The Core Team will also ensure that PCORI and the EDM (Evidence, Data, and Methods to Build Learning Health Systems of the Future) Forum, of which the SMART DOCS PI is a Steering Committee Member, will also provide a means of dissemination. Last, many members of the Core and Stakeholder Teams participate in the Sleep Research Network (SRN). The SRN conferences have attracted representatives from many of the current Clinical and Translational Science Award (CTSA) institutions, and, as it provides a forum for the leadership of sleep centers to become aware and in turn disseminate and transform new research findings into clinical practice, it will be an important vehicle for disseminating our approach and findings.
REFERENCES
- 1.Cohen J. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 2.D'Agostino RB, Chase W, Belanger A. The appropriateness of some common procedures for testing the equality of two independent binomial proportions. Am Stat. 1988;42:198–202. [Google Scholar]
- 3.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 4.R Development Core Team. R: A Language and Environment for Statistical Computing. 2009 Available at: http://www.R-project.org. [Google Scholar]
- 5.Mehrotra DV, Chan ISF, Berger RL. A cautionary note on exact unconditional inference for a difference between two independent binomial proportions. Biometrics. 2003;59:441–50. doi: 10.1111/1541-0420.00051. [DOI] [PubMed] [Google Scholar]
- 6.McLachlan G, Peel D. Finite Mixture Models. New York: John Wiley & Sons; 2000. [Google Scholar]
- 7.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. 4th ed. Boston, MA: WCB McGraw-Hill; 1996. [Google Scholar]
- 8.Gandek B, Ware JE, Jr, Aaronson NK, et al. Tests of data quality, scaling assumptions, and reliability of the SF-36 in eleven countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1149–58. doi: 10.1016/s0895-4356(98)00106-1. [DOI] [PubMed] [Google Scholar]
- 9.McCulloch CE, Searle SR. New York, NY: John Wiley & Sons, Inc; 2001. Generalized, Linear and Mixed Models. [Google Scholar]
- 10.Koenker R, Hallock KF. Quantile regression. J Econ Perspect. 2001;15:143–56. [Google Scholar]
- 11.Casella G. Illustrating empirical Bayes methods. Chemometrics and Intelligent Laboratory Syst. 1992;16:107–25. [Google Scholar]
- 12.Taylor H, Karlin S. 3rd ed. San Diego CA: Academic Press; 1998. An Introduction to Stochastic Modeling. [Google Scholar]
- 13.Holmes TH, Anderson AL, Li SH, Elkashef AM. Advantages of joint modeling of component HIV risk behaviors and non-response: application to randomized trials in cocaine-dependent and methamphetamine-dependent populations. Front Psychiatry. 2011;2:41. doi: 10.3389/fpsyt.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bond SJ, White IR, Sarah Walker A. Instrumental variables and interactions in the causal analysis of a complex clinical trial. Stat Med. 2007;26:1473–96. doi: 10.1002/sim.2644. [DOI] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 16.Little R, Rubin D. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2002. Statistical Analysis with Missing Data. [Google Scholar]
- 17.McCulloch C. Joint modelling of mixed outcome types using latent variables. Stat Methods Med Res. 2008;17:53–73. doi: 10.1177/0962280207081240. [DOI] [PubMed] [Google Scholar]
- 18.Neuhaus JM, Kalbfleisch JD. Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics. 1998;54:638–45. [PubMed] [Google Scholar]
- 19.Wu DM. Alternative tests of independence between stochastic regressors and disturbances. Econometrica. 1973;41:733–50. [Google Scholar]
- 20.Haapea M, Veijola J, Tanskanen P, Jaaskelainen E, Isohanni M, Miettunen J. Use of inverse probability weighting to adjust for non-participation in estimating brain volumes in schizophrenia patients. Psychiatry Res. 2011;194:326–32. doi: 10.1016/j.pscychresns.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Shen C, Li X, Robins JM. On weighting approaches for missing data. Stat Methods Med Res. 2013;22:14–30. doi: 10.1177/0962280211403597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todem D, Kim K, Fine J, Peng L. Semiparametric regression models and sensitivity analysis of longitudinal data with nonrandom dropouts. Statistica Neerlandica. 2010;64:133–56. doi: 10.1111/j.1467-9574.2009.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Strollo PJ, Jr, Badr MS, Coppola MP, Fleishman SA, Jacobowitz O, Kushida CA. The future of sleep medicine. Sleep. 2011;34:1613–9. doi: 10.5665/sleep.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badr MS. The future is here. J Clin Sleep Med. 2013;9:841–3. doi: 10.5664/jcsm.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 4.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 5.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 6.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 7.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 8.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 9.Okun ML, Kravitz HM, Sowers MF, Moul DE, Buysse DJ, Hall M. Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J Clin Sleep Med. 2009;5:41–51. [PMC free article] [PubMed] [Google Scholar]
- 10.Adan A, Almirall H. Horne and Ostberg Morningness Eveningness Questionnaire - a Reduced Scale. Pers Indiv Differ. 1991;12:241–53. [Google Scholar]
- 11.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leary EB, Einen M, Malunjkar S, Ruoff CM, Walsh JK, Mignot E. Validation of the Alliance Sleep Questionnaire (ASQ) narcolepsy module in sleep disordered patients. Sleep. 2014;37:A360. (Abstract Suppl) [Google Scholar]
- 14.Barnabei VM, O'Connor JJ, Nimphius NM, Vierkant RA, Eaker ED. The effects of a web-based tool on patient-provider communication and satisfaction with hormone therapy: a randomized evaluation. J Womens Health (Larchmt) 2008;17:147–58. doi: 10.1089/jwh.2007.0369. [DOI] [PubMed] [Google Scholar]
- 15.Schott TC, Goz G. Applicative characteristics of new microelectronic sensors Smart Retainer(R) and TheraMon(R) for measuring wear time. J Orofac Orthop. 2010;71:339–47. doi: 10.1007/s00056-010-1019-3. [DOI] [PubMed] [Google Scholar]
- 16.Schott TC, Goz G. Young patients' attitudes toward removable appliance wear times, wear-time instructions and electronic wear-time measurements--results of a questionnaire study. J Orofac Orthop. 2010;71:108–16. doi: 10.1007/s00056-010-9925-y. [DOI] [PubMed] [Google Scholar]
- 17.Remmers J, Charkhandeh S, Grosse J, et al. Remotely controlled mandibular protrusion during sleep predicts therapeutic success with oral appliances in patients with obstructive sleep apnea. Sleep. 2013;36:1517–5. 1525A. doi: 10.5665/sleep.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dort LC, Hadjuk E, Remmers JE. Mandibular advancement and obstructive sleep apnoea: a method for determining effective mandibular protrusion. Eur Respir J. 2006;27:1003–9. doi: 10.1183/09031936.06.00077804. [DOI] [PubMed] [Google Scholar]
- 19.Petelle B, Vincent G, Gagnadoux F, Rakotonanahary D, Meyer B, Fleury B. One-night mandibular advancement titration for obstructive sleep apnea syndrome: a pilot study. Am J Respir Crit Care Med. 2002;165:1150–3. doi: 10.1164/ajrccm.165.8.2108056. [DOI] [PubMed] [Google Scholar]
- 20.Tsai WH, Vazquez JC, Oshima T, et al. Remotely controlled mandibular positioner predicts efficacy of oral appliances in sleep apnea. Am J Respir Crit Care Med. 2004;170:366–70. doi: 10.1164/rccm.200310-1446OC. [DOI] [PubMed] [Google Scholar]
- 21.Wolf J, Hering D, Narkiewicz K. Non-dipping pattern of hypertension and obstructive sleep apnea syndrome. Hypertens Res. 2010;33:867–71. doi: 10.1038/hr.2010.153. [DOI] [PubMed] [Google Scholar]
- 22.Baguet JP, Barone-Rochette G, Pepin JL. Hypertension and obstructive sleep apnoea syndrome: current perspectives. J Hum Hypertens. 2009;23:431–43. doi: 10.1038/jhh.2008.147. [DOI] [PubMed] [Google Scholar]
- 23.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14:163–5. [PubMed] [Google Scholar]
- 24.Cuellar NG. The effects of periodic limb movements in sleep (PLMS) on cardiovascular disease. Heart Lung. 2013;42:353–60. doi: 10.1016/j.hrtlng.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–80. doi: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 27.Lewy AJ. The dim light melatonin onset, melatonin assays and biological rhythm research in humans. Biol Signals Recept. 1999;8:79–83. doi: 10.1159/000014573. [DOI] [PubMed] [Google Scholar]
- 28.Rahman SA, Kayumov L, Tchmoutina EA, Shapiro CM. Clinical efficacy of dim light melatonin onset testing in diagnosing delayed sleep phase syndrome. Sleep Med. 2009;10:549–55. doi: 10.1016/j.sleep.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 30.Swan M. Emerging patient-driven health care models: an examination of health social networks, consumer personalized medicine and quantified self-tracking. Int J Environ Res Public Health. 2009;6:492–525. doi: 10.3390/ijerph6020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colten HR, Altevogt BM. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington DC: National Academies Press; 2006. Institute of Medicine (US) Committee on Sleep Medicine and Research. [PubMed] [Google Scholar]
- 32.Bartlett DJ, Marshall NS, Williams A, Grunstein RR. Predictors of primary medical care consultation for sleep disorders. Sleep Med. 2008;9:857–64. doi: 10.1016/j.sleep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35:1593–602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kushida CA, Nichols DA, Quan SF, et al. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med. 2006;2:288–300. [PubMed] [Google Scholar]
- 35.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 36.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 37.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med. 2007;3:1–16. [PMC free article] [PubMed] [Google Scholar]
- 38.Standards of Practice Committee of the American Sleep Disorders Association. Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Sleep. 1994;17:372–7. [PubMed] [Google Scholar]
- 39.Ferber R, Millman R, Coppola M, et al. Portable recording in the assessment of obstructive sleep apnea. ASDA standards of practice. Sleep. 1994;17:378–92. doi: 10.1093/sleep/17.4.378. [DOI] [PubMed] [Google Scholar]
- 40.Bilo G, Zorzi C, Ochoa Munera E, Torlasco C, Giuli V, Parati G. Validation according to ESH International Protocol of SOMNOtouch NIBP, a device for noninvasive continuous blood pressure monitoring. Paper presented at: Joint Meeting of the European Society of Hypertension (ESH) and International Society of Hypertension (ISH); 2014; Athens, Greece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hennig A, Patzak A. Continuous blood pressure measurement using pulse transit time. Somnologie. 2013;17:104–10. [Google Scholar]
- 42.Gesche H, Grosskurth D, Kuchler G, Patzak A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol. 2012;112:309–15. doi: 10.1007/s00421-011-1983-3. [DOI] [PubMed] [Google Scholar]
- 43.Bartsch S, Ostojic D, Schmalgemeier H, et al. [Validation of continuous blood pressure measurements by pulse transit time: a comparison with invasive measurements in a cardiac intensive care unit] Deutsche Medizinische Wochenschrift. 2010;135:2406–12. doi: 10.1055/s-0030-1269408. [DOI] [PubMed] [Google Scholar]
- 44.Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31:141–7. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA. 2013;70:891–902. doi: 10.1001/jamaneurol.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morin C, Espie C. New York, NY: Kluwer Academic/Plenum Publishers; 2003. Insomnia: A Clinician's Guide to Assessment and Treatment. [Google Scholar]
- 48.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39:45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 49.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26:177–82. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 50.Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30:574–84. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 51.Goodie JL, Isler WC, Hunter C, Peterson AL. Using behavioral health consultants to treat insomnia in primary care: a clinical case series. J Clin Psychol. 2009;65:294–304. doi: 10.1002/jclp.20548. [DOI] [PubMed] [Google Scholar]
- 52.Matthews JNS. 2nd ed. Boca Raton, FL: Chapman and Hall/CRC; 2006. Introduction to Randomized Controlled Clinical Trials. [Google Scholar]
- 53.Haapea M, Veijola J, Tanskanen P, Jaaskelainen E, Isohanni M, Miettunen J. Use of inverse probability weighting to adjust for non-participation in estimating brain volumes in schizophrenia patients. Psychiatry Res. 2011;194:326–32. doi: 10.1016/j.pscychresns.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Shen C, Li X, Robins JM. On weighting approaches for missing data. Stat Methods Med Res. 2013;22:14–30. doi: 10.1177/0962280211403597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agency for Healthcare Research and Quality. [Accessed January 24, 2012]. http://www.cahps.ahrq.gov/Surveys-Guidance/CG.aspx.
- 56.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Patient-Centered Outcomes Research Institute. [Accessed December 26, 2013]. http://www.pcori.org/research-we-support/pcor/establishing-a-definition/
- 58.Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11:934–40. doi: 10.1016/j.sleep.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siccoli MM, Pepperell JC, Kohler M, Craig SE, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31:1551–8. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weaver TE. Outcome measurement in sleep medicine practice and research. Part 1: assessment of symptoms, subjective and objective daytime sleepiness, health-related quality of life and functional status. Sleep Med Rev. 2001;5:103–28. doi: 10.1053/smrv.2001.0152. [DOI] [PubMed] [Google Scholar]
- 61.Chakravorty I, Cayton RM, Szczepura A. Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2002;20:1233–8. doi: 10.1183/09031936.00.00014401. [DOI] [PubMed] [Google Scholar]
- 62.Snedecor SJ, Botteman MF, Bojke C, Schaefer K, Barry N, Pickard AS. Cost-effectiveness of eszopiclone for the treatment of adults with primary chronic insomnia. Sleep. 2009;32:817–24. doi: 10.1093/sleep/32.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Institute for Health and Clinical Excellence (NICE) Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. NICE technology appraisal guidance 139. 2008. Mar, Available at: http://guidance.nice.org.uk/ta139.
- 64.Tousignant P, Cosio MG, Levy RD, Groome PA. Quality adjusted life years added by treatment of obstructive sleep apnea. Sleep. 1994;17:52–60. [PubMed] [Google Scholar]
- 65.Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep. 2011;34:695–709. doi: 10.5665/SLEEP.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkelman JW, Redline S, Baldwin CM, Resnick HE, Newman AB, Gottlieb DJ. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep. 2009;32:772–8. doi: 10.1093/sleep/32.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32:915–9. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golicki D, Bala MM, Niewada M, Wierzbicka A. Modafinil for narcolepsy: systematic review and meta-analysis. Med Sci Monit. 2010;16:RA177–86. [PubMed] [Google Scholar]
- 69.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abetz L, Arbuckle R, Allen RP, et al. The reliability, validity and responsiveness of the International Restless Legs Syndrome Study Group rating scale and subscales in a clinical-trial setting. Sleep Med. 2006;7:340–9. doi: 10.1016/j.sleep.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Borreguero D, Grunstein R, Sridhar G, et al. A 52-week open-label study of the long-term safety of ropinirole in patients with restless legs syndrome. Sleep Med. 2007;8:742–52. doi: 10.1016/j.sleep.2006.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of quantity of completers across 2,500 simulated permuted-block randomizations (block size = 251) of 1,506 patients with 30% postrandomization random dropout.
Distribution of proportion assigned to PCCM approach across 2,500 simulated permuted-block randomizations (block size = 251) of 1,506 patients with 30% postrandomization random drop-out.
SMART DOCS Organizational Chart.