Abstract
The bacterial flagellum is assembled from over 20 structural components, and flagellar gene regulation is morphogenetically coupled to the assembly state by control of the anti-sigma factor FlgM. In the Gram-negative bacterium Salmonella enterica, FlgM inhibits late-class flagellar gene expression until the hook-basal body structural intermediate is completed and FlgM is inhibited by secretion from the cytoplasm. Here we demonstrate that FlgM is also secreted in the Gram-positive bacterium Bacillus subtilis and is degraded extracellularly by the proteases Epr and WprA. We further demonstrate that, like in S. enterica, the structural genes required for the flagellar hook-basal body are required for robust activation of σD-dependent gene expression and efficient secretion of FlgM. Finally, we determine that FlgM secretion is strongly enhanced by, but does not strictly require, hook-basal body completion and instead demands a minimal subset of flagellar proteins that includes the FliF/FliG basal body proteins, the flagellar type III export apparatus components FliO, FliP, FliQ, FliR, FlhA, and FlhB, and the substrate specificity switch regulator FliK.
INTRODUCTION
Bacterial transcription is initiated when RNA polymerase is directed to specific promoter sequences by the action of the sigma subunit (1). All bacteria encode a highly conserved ubiquitous σA/σ70 class sigma factor for generalized, vegetative, and housekeeping gene expression. For specialized gene expression, however, many bacteria also encode alternative sigma factors that differentially control regulons of genes in response to physiological or environmental conditions such as nutrient starvation, heat shock, envelope stress, and motility (2). One way to conditionally restrict alternative sigma factor activity is by production of an anti-sigma factor that binds to, and directly antagonizes, its cognate sigma factor (3). One of the better-understood examples of alternative sigma factor regulon control is that of an alternative sigma factor governing flagellar assembly, σ28, by its anti-sigma factor, FlgM (4).
Flagella are constructed from over 20 different proteins that must be assembled in the correct order and in the correct stoichiometry (5). To ensure proper assembly, flagellar gene expression is organized in at least two hierarchical levels defined here as “early-class” genes, recognized by σ70, and “late-class” genes, recognized by the alternative sigma factor σ28 (6). Early-class flagellar genes encode the flagellar type III export apparatus, the structural components of the hook-basal body, and the alternative sigma factor σ28, which is held inactive through direct protein interaction with its cognate anti-sigma factor, FlgM (Fig. 1A) (7–9). Flagella are assembled in part by type III secretion, and when the hook-basal body is complete, the regulator FliK instructs the secretion apparatus to change specificity to recognize and secrete late-class flagellar proteins (10–12), including the anti-sigma factor FlgM (Fig. 1B) (13, 14). FlgM secretion liberates its cognate, σ28, to direct expression of the late-class flagellar genes, such as the gene that encodes the flagellar filament structural protein, flagellin (Fig. 1C) (8, 15). Thus, completion of an assembly intermediate (the hook-basal body) permits the expression of late-class flagellar genes by controlling secretion of an anti-sigma factor. The FlgM secretion model of morphogenetic coupling of flagellar structure and regulation was established in the Gram-negative bacterium Salmonella enterica but has not been supported outside the gammaproteobacteria (16).
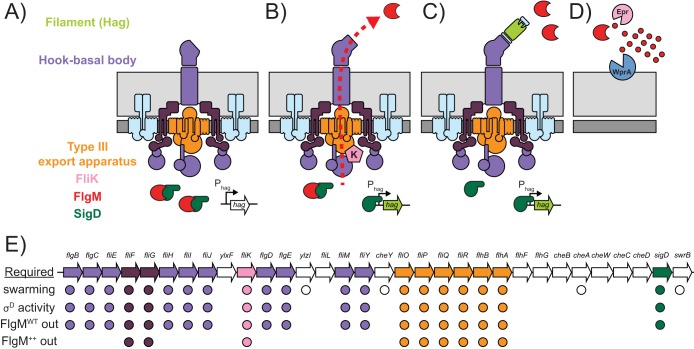
FIG 1.
Model for FlgM regulation. (A to C) Extrapolation of the S. enterica model for FlgM inhibition as it might function for a Gram-positive organism. (A) Prior to hook-basal body completion (purple), FlgM (red) binds to and inhibits σD (dark green) intracellularly. (B) Upon hook-basal body completion, FliK (pink) changes the secretion specificity of the flagellar type III export apparatus (orange) to recognize and secrete FlgM. (C) Liberated σD directs transcription of the σD regulon, including the hag gene (light green), encoding the flagellar filament protein Hag. (D) In B. subtilis, extracellular FlgM is proteolyzed by the activity of two proteases, Epr and WprA. Light gray represents peptidoglycan, and dark gray represents cytoplasmic membrane. (E) Cartoon diagram of the fla-che operon. Genes predicted to encode flagellar structural proteins are colored purple, and genes predicted to encode type III export apparatus components are colored orange. For each phenotype examined in the study, i.e., swarming motility (migration over 0.7% LB agar), σD activity (expression of Phag-GFP), FlgMWT out (secretion of FlgM expressed from its native promoter), and FlgM++ out (secretion of FlgM expressed from an IPTG-inducible promoter), a circle is indicated below the gene if that gene product was found to be essential for the respective phenotype. Thus, purple circles indicate hook-basal body structural proteins, white circles indicate nonstructural proteins, and orange circles indicate type III export apparatus homologs required for the indicated phenotype. Green circles indicate sigD, and pink circles indicate fliK.
The Gram-positive bacterium Bacillus subtilis synthesizes flagella and encodes both a homolog of the σ28 alternative sigma factor, σD, and FlgM (17–19). The early-class genes are organized in a long, 32-gene, 27-kb operon called the fla-che operon, which is primarily expressed from a σA-dependent promoter (20–23). The late-class flagellar genes are σD dependent and part of a large regulon that also includes cell-separating peptidoglycan hydrolases (24–28). Mutation of certain genes in the fla-che operon required for hook-basal body assembly has been shown to abolish σD-dependent gene expression and cause cells both to lose motility and to grow in long chains (20, 21, 29). Furthermore, mutation of FlgM has been shown to restore σD-dependent gene expression in these mutants (18, 30–33) suggesting that, like in S. enterica, an incomplete hook-basal body antagonizes FlgM function. Unlike what has been reported for S. enterica, however, secretion of B. subtilis FlgM has never been shown, and thus the mechanism of FlgM inhibition has remained unknown.
Here we show that B. subtilis FlgM is secreted by the flagellar export apparatus, consistent with the model of morphogenetic coupling proposed in S. enterica (Fig. 1B). Once secreted, FlgM is degraded by extracellular proteases, and we propose that FlgM proteolysis likely prohibited detection of FlgM secretion previously (Fig. 1D). By mutating every gene in the fla-che operon, we found that FlgM secretion and σD activation were tightly correlated (Fig. 1E). Finally, by overriding native regulation, we identify a minimal core subset of flagellar proteins required for FlgM secretion, including six genes for the flagellar type III export apparatus, the FliF and FliG basal body components, and the substrate specificity switch protein FliK.
MATERIALS AND METHODS
Strains and growth conditions.
B. subtilis strains were grown in Luria-Bertani (LB) (10 g tryptone, 5 g yeast extract, 5 g NaCl per liter) broth or on LB plates fortified with 1.5% Bacto agar at 37°C. When appropriate, antibiotics were included at the following concentrations: 10 μg/ml tetracycline (Tet), 100 μg/ml spectinomycin (Spc), 5 μg/ml chloramphenicol (Cat), 5 μg/ml kanamycin (Kan), and 1 μg/ml erythromycin plus 25 μg/ml lincomycin (Mls). IPTG (isopropyl β-d-thiogalactopyranoside; Sigma) was added to the medium at the indicated concentration when appropriate.
Strain construction.
Constructs were introduced into the PY79 or DS2569 strain background using DNA transformation and were then transferred to wild-type (WT) strain 3610 using SPP1-mediated generalized phage transduction (34). All strains are in the 3610 background unless otherwise noted. Strains used in this study are listed in Table 1. Plasmids used in this study are listed in Table S1 in the supplemental material. Primers used in this study are listed in Table S2 in the supplemental material.
TABLE 1.
Strains used in this study
| B. subtilis strain | Relevant genotype or description |
|---|---|
| Parental strains | |
| 3610 | Wild type (ancestral strain) |
| DS2569 | Cured strain (3610 lacking pBS32) |
| DS6329 | ΔaprE ΔnprE Δbpr Δepr Δvpr ΔwprA Δmpr (Δ7 protease or Δ7) |
| PY79 | swrAPY79 sfp0 (laboratory strain) |
| Derivative strains | |
| DK214 | ΔaprE ΔnprE Δbpr Δepr wprA::cat amyE::Physpank-flgM Spcr |
| DK215 | ΔaprE ΔnprE Δbpr wprA::cat amyE::Physpank-flgM Spcr |
| DK216 | ΔaprE ΔnprE wprA::cat amyE::Physpank-flgM Spcr |
| DK217 | ΔaprE wprA::cat amyE::Physpank-flgM Spcr |
| DK382 | wprA::cat |
| DK383 | epr::kan |
| DK462 | wprA::cat epr::kan |
| DK717 | Δ7 ΔflgB |
| DK1142 | Δ7 ΔflhA amyE::Physpank-flgM Spcr |
| DK1143 | Δ7 ΔflgC amyE::Physpank-flgM Spcr |
| DK1144 | Δ7 ΔflgE amyE::Physpank-flgM Spcr |
| DK1856 | ΔflgB flgM::tet amyE::Phag-GFP Catrr |
| DK1857 | ΔflgC flgM::tet amyE::Phag-GFP Catrr |
| DK1858 | ΔfliE flgM::tet amyE::Phag-GFP Catrr |
| DK1859 | ΔfliG flgM::tet amyE::Phag-GFP Catrr |
| DK1860 | ΔfliH flgM::tet amyE::Phag-GFP Catrr |
| DK1861 | ΔfliI flgM::tet amyE::Phag-GFP Catrr |
| DK1862 | ΔfliJ flgM::tet amyE::Phag-GFP Catrr |
| DK1863 | ΔylxF flgM::tet amyE::Phag-GFP Catrr |
| DK1864 | ΔfliK flgM::tet amyE::Phag-GFP Catrr |
| DK1865 | ΔflgD flgM::tet amyE::Phag-GFP Catrr |
| DK1866 | ΔflgE flgM::tet amyE::Phag-GFP Catrr |
| DK1867 | ΔylzI flgM::tet amyE::Phag-GFP Catr |
| DK1868 | ΔfliL flgM::tet amyE::Phag-GFP Catr |
| DK1869 | ΔfliM flgM::tet amyE::Phag-GFP Catr |
| DK1870 | ΔfliY flgM::tet amyE::Phag-GFP Catr |
| DK1871 | ΔcheY flgM::tet amyE::Phag-GFP Catr |
| DK1872 | ΔfliO flgM::tet amyE::Phag-GFP Catr |
| DK1873 | ΔfliP flgM::tet amyE::Phag-GFP Catr |
| DK1874 | ΔfliQ flgM::tet amyE::Phag-GFP Catr |
| DK1875 | ΔfliR flgM::tet amyE::Phag-GFP Catr |
| DK1876 | ΔflhB flgM::tet amyE::Phag-GFP Catr |
| DK1877 | ΔflhA flgM::tet amyE::Phag-GFP Catr |
| DK1878 | ΔflhF flgM::tet amyE::Phag-GFP Catr |
| DK1879 | ΔflhG flgM::tet amyE::Phag-GFP Catr |
| DK1880 | ΔcheB flgM::tet amyE::Phag-GFP Catr |
| DK1881 | ΔcheA flgM::tet amyE::Phag-GFP Catr |
| DK1882 | ΔcheW flgM::tet amyE::Phag-GFP Catr |
| DK1883 | ΔcheC flgM::tet amyE::Phag-GFP Catr |
| DK1884 | ΔcheD flgM::tet amyE::Phag-GFP Catr |
| DK1885 | ΔsigD flgM::tet amyE::Phag-GFP Catr |
| DK1886 | ΔswrB flgM::tet amyE::Phag-GFP Catr |
| DK1902 | Δ7 ΔfliG |
| DK1903 | Δ7 ΔfliQ |
| DK1904 | Δ7 ΔfliY |
| DK1935 | Δ7 ΔcomI |
| DK1951 | Δ7 ΔcomI ΔfliH |
| DK1952 | Δ7 ΔcomI ΔfliJ |
| DK1953 | Δ7 ΔcomI ΔfliM |
| DK1968 | Δ7 ΔcomI ΔflhB |
| DK1969 | Δ7 ΔcomI ΔfliO |
| DK1970 | Δ7 ΔcomI ΔflgD |
| DK1971 | Δ7 ΔcomI ΔfliK |
| DK2012 | Δ7 ΔcomI ΔfliE |
| DK2013 | Δ7 ΔcomI ΔcheA |
| DK2014 | Δ7 ΔcomI ΔylzI |
| DK2026 | Δ7 ΔcomI ΔylxF |
| DK2027 | Δ7 ΔcomI ΔcheC |
| DK2028 | Δ7 ΔcomI ΔfliL |
| DK2029 | Δ7 ΔcomI ΔcheD |
| DK2030 | Δ7 ΔcomI ΔcheW |
| DK2031 | Δ7 ΔcomI ΔcheY |
| DK2058 | Δ7 ΔcomI ΔfliI |
| DK2059 | Δ7 ΔcomI ΔfliP |
| DK2060 | Δ7 ΔcomI ΔcheB |
| DK2071 | Δ7 ΔcomI ΔflhG |
| DK2230 | Δ7 ΔcomI ΔsigD |
| DK2301 | Δ7 ΔcomI ΔsigD amyE::Physpank-flgM Spcr |
| DK3051 | Δ7 ΔflgB amyE::Physpank-flgM Spcr |
| DK3052 | Δ7 ΔcomI ΔfliH amyE::Physpank-flgM Spcr |
| DK3053 | Δ7 ΔcomI ΔfliE amyE::Physpank-flgM Spcr |
| DK3054 | Δ7 ΔfliG amyE::Physpank-flgM Spcr |
| DK3055 | Δ7 ΔcomI ΔfliI amyE::Physpank-flgM Spcr |
| DK3056 | Δ7 ΔcomI ΔfliJ amyE::Physpank-flgM Spcr |
| DK3057 | Δ7 ΔcomI ΔylxF amyE::Physpank-flgM Spcr |
| DK3058 | Δ7 ΔcomI ΔfliK amyE::Physpank-flgM Spcr |
| DK3059 | Δ7 ΔcomI ΔflgD amyE::Physpank-flgM Spcr |
| DK3060 | Δ7 ΔcomI ΔylzI amyE::Physpank-flgM Spcr |
| DK3061 | Δ7 ΔcomI ΔfliL amyE::Physpank-flgM Spcr |
| DK3062 | Δ7 ΔcomI ΔfliM amyE::Physpank-flgM Spcr |
| DK3063 | Δ7 ΔfliY amyE::Physpank-flgM Spcr |
| DK3064 | Δ7 ΔcomI ΔcheY amyE::Physpank-flgM Spcr |
| DK3065 | Δ7 ΔcomI ΔfliO amyE::Physpank-flgM Spcr |
| DK3066 | Δ7 ΔcomI ΔfliP amyE::Physpank-flgM Spcr |
| DK3067 | Δ7 ΔfliQ amyE::Physpank-flgM Spcr |
| DK3068 | Δ7 ΔcomI ΔflhB amyE::Physpank-flgM Spcr |
| DK3069 | Δ7 ΔcomI ΔflhG amyE::Physpank-flgM Spcr |
| DK3070 | Δ7 ΔcomI ΔcheB amyE::Physpank-flgM Spcr |
| DK3071 | Δ7 ΔcomI ΔcheA amyE::Physpank-flgM Spcr |
| DK3072 | Δ7 ΔcomI ΔcheW amyE::Physpank-flgM Spcr |
| DK3073 | Δ7 ΔcomI ΔcheC amyE::Physpank-flgM Spcr |
| DK3074 | Δ7 ΔcomI ΔcheD amyE::Physpank-flgM Spcr |
| DK3075 | Δ7 ΔcomI ΔswrB |
| DK3076 | Δ7 ΔcomI ΔfliR |
| DK3077 | Δ7 ΔcomI ΔflhF |
| DK3078 | Δ7 ΔcomI ΔswrB amyE::Physpank-flgM Spcr |
| DK3079 | Δ7 ΔcomI ΔfliR amyE::Physpank-flgM Spcr |
| DK3080 | Δ7 ΔcomI ΔflhF amyE::Physpank-flgM Spcr |
| DS908 | amyE::Phag-GFP Catr |
| DS2509 | ΔswrB |
| DS3772 | amyE::Physpank-flgM Spcr |
| DS4029 | ΔflgM |
| DS4264 | flgM::tet amyE::Phag-GFP Catr |
| DS4536 | ΔfliK |
| DS4680 | ΔflgB |
| DS4681 | ΔflgE |
| DS5384 | ΔfliY |
| DS5648 | ΔaprE |
| DS5700 | ΔaprE ΔnprE |
| DS5771 | ΔaprE ΔnprE Δbpr |
| DS5810 | ΔaprE ΔnprE Δbpr Δepr |
| DS5893 | ΔaprE ΔnprE Δbpr Δepr Δvpr |
| DS5913 | ΔflhA |
| DS6105 | ΔaprE ΔnprE Δbpr Δepr Δvpr ΔwprA |
| DS6420 | ΔsigD |
| DS6468 | ΔfliO |
| DS6540 | ΔfliL |
| DS6554 | ΔylxF |
| DS6555 | ΔflgD |
| DS6657 | ΔylzI |
| DS6658 | ΔflhF |
| DS6728 | ΔfliI |
| DS6729 | ΔfliH |
| DS6775 | ΔfliM |
| DS6806 | Δ7 amyE::Physpank-flgM Spcr |
| DS6867 | ΔcheC |
| DS6868 | ΔcheD |
| DS6869 | ΔcheW |
| DS6870 | ΔcheY |
| DS6871 | Δ7 ΔfliF |
| DS6887 | ΔcheA |
| DS6919 | Δ7 ΔfliF amyE::Physpank-flgM Spcr |
| DS7080 | ΔfliF |
| DS7118 | ΔfliQ |
| DS7119 | ΔfliP |
| DS7120 | ΔflhB |
| DS7161 | Δ7 ΔflgM |
| DS7303 | ΔflgC |
| DS7306 | ΔcheB |
| DS7308 | ΔfliE |
| DS7317 | ΔfliR |
| DS7357 | ΔfliG |
| DS7358 | ΔflhG |
| DS7359 | ΔfliJ |
| DS7457 | ΔfliF flgM::tet amyE::Phag-GFP Catr |
| DS7684 | ΔflgB amyE::Phag-GFP Catr |
| DS7685 | ΔflgC amyE::Phag-GFP Catr |
| DS7686 | ΔfliE amyE::Phag-GFP Catr |
| DS7687 | ΔfliF amyE::Phag-GFP Catr |
| DS7688 | ΔfliG amyE::Phag-GFP Catr |
| DS7689 | ΔfliH amyE::Phag-GFP Catr |
| DS7690 | ΔfliI amyE::Phag-GFP Catr |
| DS7691 | ΔfliJ amyE::Phag-GFP Catr |
| DS7692 | ΔylxF amyE::Phag-GFP Catr |
| DS7693 | ΔfliK amyE::Phag-GFP Catr |
| DS7694 | ΔflgD amyE::Phag-GFP Catr |
| DS7695 | ΔflgE amyE::Phag-GFP Catr |
| DS7696 | ΔylzI amyE::Phag-GFP Catr |
| DS7697 | ΔfliL amyE::Phag-GFP Catr |
| DS7698 | ΔfliM amyE::Phag-GFP Catr |
| DS7699 | ΔfliY amyE::Phag-GFP Catr |
| DS7700 | ΔcheY amyE::Phag-GFP Catr |
| DS7701 | ΔfliO amyE::Phag-GFP Catr |
| DS7702 | ΔfliP amyE::Phag-GFP Catr |
| DS7703 | ΔfliQ amyE::Phag-GFP Catr |
| DS7704 | ΔfliR amyE::Phag-GFP Catr |
| DS7705 | ΔflhB amyE::Phag-GFP Catr |
| DS7706 | ΔflhA amyE::Phag-GFP Catr |
| DS7707 | ΔflhF amyE::Phag-GFP Catr |
| DS7708 | ΔflhG amyE::Phag-GFP Catr |
| DS7709 | ΔcheB amyE::Phag-GFP Catr |
| DS7710 | ΔcheA amyE::Phag-GFP Catr |
| DS7711 | ΔcheW amyE::Phag-GFP Catr |
| DS7712 | ΔcheC amyE::Phag-GFP Catr |
| DS7713 | ΔcheD amyE::Phag-GFP Catr |
| DS7714 | ΔsigD amyE::Phag-GFP Catr |
| DS7715 | ΔswrB amyE::Phag-GFP Catr |
| DS8080 | ΔaprE ΔnprE Δbpr Δepr Δvpr ΔwprA amyE::Physpank-flgM Spcr |
| DS8081 | ΔaprE ΔnprE Δbpr Δepr Δvpr amyE::Physpank-flgM Spcr |
| DS8082 | ΔaprE ΔnprE Δbpr Δepr amyE::Physpank-flgM Spcr |
| DS8083 | ΔaprE ΔnprE Δbpr amyE::Physpank-flgM Spcr |
| DS8084 | ΔaprE ΔnprE amyE::Physpank-flgM Spcr |
| DS8085 | ΔaprE amyE::Physpank-flgM Spcr |
| DS8117 | Δ7 ΔflhA |
| DS8365 | Δ7 ΔflgE |
| DS8404 | Δ7 ΔflgC |
(i) In-frame deletions.
To build the integrative in-frame deletion plasmids, one ∼500-bp fragment upstream and one ∼500-bp fragment downstream of the gene of interest were PCR amplified, digested with the appropriate restriction endonucleases, and cloned into pMiniMad (35). DNA amplicons were designed such that they removed a portion of the coding sequence of the gene of interest without disrupting the reading frame.
Plasmid DNA purified from Escherichia coli TG1was introduced into strain DS2569 by single-crossover integration using transformation at the restrictive temperature for plasmid replication (37°C) and Mls resistance as a selection. The integrated plasmid was then transduced into 3610. To evict the plasmid, the strain was grown overnight (∼14 h) in 3 ml LB broth at a permissive temperature for plasmid replication (22°C) and then serially diluted and plated on LB agar plates at 37°C. Individual colonies were patched onto LB plates and LB plates containing Mls to identify Mls-sensitive colonies that had evicted the plasmid. Chromosomal DNA from colonies that had excised the plasmid was purified and screened by PCR using appropriate primers to determine which isolate retained the in-frame deletion allele.
(a) Δ7 protease mutant.
The aprE deletion plasmid pDP314 was built using primer pairs 1751/1752 and 1753/1754. The aprE deletion was resolved in DS2569 to generate strain DS5648. The nprE deletion plasmid pDP313 was built using primer pairs 1747/1748 and 1749/1750. The nprE deletion was resolved in DS5648 to generate strain DS5700. The bpr deletion plasmid pDP311 was built using primer pairs 1739/1740 and 1741/1742. The bpr deletion was resolved in DS5700 to generate strain DS5771. The epr deletion plasmid pDP315 was built using primer pairs 1755/1756 and 1757/1758. The epr deletion was resolved in DS5771 to generate strain DS5810. The vpr deletion plasmid pDP312 was built using primer 1743/1744 and 1745/1746. The vpr deletion was resolved in DS5810 to generate strain DS5893. The wprA deletion plasmid pDP317 was built using primer pairs 1763/1764 and 1765/1766. The wprA deletion was resolved in DS5893 to generate strain DS6105. The mpr deletion plasmid pDP320 was built using primer pairs 1759/1760 and 1761/1762. The mpr deletion was resolved in DS6105 to generate strain DS6329, the Δ7 protease mutant strain.
(b) fla-che operon gene deletions.
The flgB deletion plasmid pDP305 was built using primer pairs 1479/1480 and 1481/1482. The flgC deletion plasmid pDP349 was built using primer pairs 2317/2318 and 2319/2320. The fliE deletion plasmid pDP350 was built using primer pairs 2321/2322 and 2323/2324. The fliF deletion plasmid pLC16 was built using primer pairs 1900/1901 and 1898/1899. The fliG deletion plasmid pKB40 was built using primer pairs 770/771 and 772/773. The fliH deletion plasmid pDP335 was built using primer pairs 2122/2123 and 2124/2125. The fliI deletion plasmid pDP336 was built using primer pairs 2126/2127 and 2128/2129. The fliJ deletion plasmid pLC25 was built using primer pairs 857/858 and 859/860. The ylxF deletion plasmid pDP327 was built using primer pairs 2029/2030 and 2031/2032. The fliK deletion plasmid pKB93 was built using primer pairs 1387/1388 and 1389/1390. The flgD deletion plasmid pDP328 was built using primer pairs 2033/2034 and 2035/2036. The flgE deletion plasmid pDP306 was built using primer pairs 1483/1484 and 1485/1486. The ylzI deletion plasmid pDP329 was built using primer pairs 2037/2038 and 2039/2040. The fliL deletion plasmid pDP330 was built using primer pairs 2041/2042 and 2043/2044. The fliM deletion plasmid pSG32 was built using primer pairs 1569/1570 and 1571/1572. The fliY deletion plasmid pSG6 was built using primer pairs 1574/1575 and 1576/1577. The cheY deletion plasmid pDP343 was built using primer pairs 2197/2198 and 2199/2200. The fliO deletion plasmid pDP332 was built using primer pairs 1692/1693 and 1694/1695. The fliP deletion plasmid pDP346 was built using primer pairs 2290/2291 and 2292/2293. The fliQ deletion plasmid pDP345 was built using primer pairs 2286/2287 and 2288/2289. The fliR deletion plasmid pDP351 was built using primer pairs 2325/2326 and 2327/2328. The flhB deletion plasmid pDP347 was built using primer pairs 2294/2295 and 2296/2297. The flhA deletion plasmid pLC47 was built using primer pairs 976/977 and 978/979. The flhF deletion plasmid pDP333 was built using primer pairs 2103/2104 and 2105/2106. The flhG deletion plasmid pLC22 was built using primer pairs 826/827 and 828/829. The cheB deletion plasmid pDP344 was built using primer pairs 2282/2283 and 2284/2285. The cheA deletion plasmid pDP338 was built using primer pairs 2177/2178 and 2179/2180. The cheW deletion plasmid pDP342 was built using primer pairs 2193/2194 and 2195/2196. The cheC deletion plasmid pDP340 was built using primer pairs 2185/2186 and 2187/2188. The cheD deletion plasmid pDP341 was built using primer pairs 2189/2190 and 2191/2192. The sigD deletion plasmid pDP326 was built using primer pairs 2019/2020 and 2021/2022. The swrB deletion plasmid pDP242 was built using primer pairs 740/741 and 839/840. The swrB deletion plasmid pRC62 was built using primer pairs 4190/4191 and 4192/4193.
(c) fla-che operon gene deletions in Δ7 protease mutant background.
To generate the Δ7 protease ΔcomI mutant, the in-frame markerless deletion construct pMP50 was introduced into the Δ7 protease mutant background by SPP1-mediated generalized transduction. The comI gene was deleted as previously described to generate DK1935. Each fla-che gene deletion construct purified from E. coli TG1 was transformed into DK1935 and deleted as previously described. For certain fla-che gene deletions, SPP1-mediated generalized transduction was successfully used to introduce the deletion constructs directly into the Δ7 protease mutant background (Table 1).
(ii) ΔflgM::tet.
The ΔflgM::tet insertion-deletion allele was generated by long flanking homology PCR (using primer pairs 140/141 and 142/143), and DNA containing a tetracycline drug resistance gene (pDG1515) was used as a template for marker replacement (36).
(iii) ΔwprA::cat.
The ΔwprA::cat insertion-deletion allele was generated by long flanking homology PCR (using primer pairs 3260/3261 and 3262/3263), and DNA containing a chloramphenicol resistance gene (pAC225) was used as a template for marker replacement (pAC225 was a generous gift from Amy Camp, Mount Holyoke College).
(iv) Δepr::kan.
The Δepr::kan insertion-deletion allele was generated by long flanking homology PCR (using primer pairs 721/722 and 723/724), and DNA containing a kanamycin resistance gene (pDG780) was used as a template for marker replacement (36).
(v) Inducible constructs.
To generate the inducible amyE::Physpank-flgM Spc overexpression construct pRC21, a fragment containing the flgM gene was PCR amplified using primer pair 3330/1135 and 3610 genomic DNA as a template. The resulting PCR product was digested with NheI and SphI and ligated into the NheI and SphI sites of pDR111 containing a spectinomycin resistance cassette, a polylinker downstream of the Physpank promoter, and the gene encoding the LacI repressor between the arms of the amyE gene (37).
(vi) 6His-SUMO-FlgM protein expression constructs.
To generate the translational fusion of FlgM to the 6His-SUMO tag, a fragment containing flgM was PCR amplified using strain 3610 DNA as a template and primer pair 933/934 and was digested with SapI and XhoI. The fragment was ligated into the SapI and XhoI sites of plasmid pTB146 containing an ampicillin resistance cassette to create pDP266 (38).
SPP1 phage transduction.
To 0.2 ml of dense culture grown in TY broth (LB broth supplemented after autoclaving with 10 mM MgSO4 and 100 μM MnSO4), serial dilutions of SPP1 phage stock were added and statically incubated for 15 min at 37°C. To each mixture, 3 ml TYSA (molten TY supplemented with 0.5% agar) was added, poured atop fresh TY plates, and incubated at 30°C overnight. Top agar from the plate containing near-confluent plaques was harvested by scraping into a 15-ml conical tube, vortexed, and centrifuged at 5,000 × g for 10 min. The supernatant was treated with 25 μg/ml DNase (final concentration) before being passed through a 0.45-μm syringe filter and stored at 4°C.
Recipient cells were grown to stationary phase in 2 ml TY broth at 37°C. One milliliter of cells was mixed with 25 μl of SPP1 donor phage stock. Nine milliliters of TY broth was added to the mixture and allowed to mix by gentle rocking at 25°C for 30 min. The transduction mixture was then centrifuged at 5,000 × g for 10 min, the supernatant was discarded, and the pellet was resuspended in the residual volume. One hundred microliters of cell suspension was then plated on LB fortified with 1.5% agar, the appropriate antibiotic, and 10 mM sodium citrate.
Swarm expansion assay.
Cells were grown to mid-log phase at 37°C in LB broth and resuspended to an optical density at 600 nm (OD600) of 10 in pH 8.0 phosphate-buffered saline (PBS) buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4) containing 0.5% India ink (Higgins). Freshly prepared LB containing 0.7% Bacto agar (25 ml/plate) was dried for 20 min in a laminar flow hood, centrally inoculated with 10 μl of the cell suspension, dried for another 10 min, and incubated at 37°C. After 5 h, the radius of swarm expansion was measured and recorded for each strain.
Microscopy.
Fluorescence microscopy was performed with a Nikon 80i microscope with a phase-contrast objective Nikon Plan Apo 100X and an Excite 120 metal halide lamp. FM4-64 was visualized with a C-FL HYQ Texas Red filter cube (excitation filter, 532 to 587 nm; barrier filter, >590 nm). Green fluorescent protein (GFP) was visualized using a C-FL HYQ fluorescein isothiocyanate (FITC) filter cube (for FITC, excitation filter, 460 to 500 nm, and barrier filter, 515 to 550 nm). Images were captured with a Photometrics Coolsnap HQ2 camera in black and white, false colored, and superimposed using Metamorph image software.
For GFP fluorescence microscopy, cells were grown to mid-log phase (OD600, 0.4 to 0.9) in 2 ml of LB broth at 37°C, and 1 ml was pelleted and washed with 1 ml PBS. Membranes were stained by resuspending the cell pellet in 50 μl of PBS containing 5 μg/ml FM4-64 and incubated for 5 min at room temperature in the dark. Samples were observed by spotting 3 μl of the suspension on a glass microscope slide and were immobilized with a poly-l-lysine-treated coverslip.
FlgM protein purification.
The 6His-SUMO-FlgM fusion protein expression vector pDP266 was transformed into Rosetta-gami II E. coli, and the resulting strain was grown to an OD600 of ∼0.8 at 37°C with shaking in 1 liter of Terrific Broth supplemented with 100 μg/ml ampicillin (12 g tryptone, 25 g yeast extract, 0.4% glycerol per 900 ml, with addition of 100 ml sterile potassium phosphate solution [2.31 g KH2PO4, 12.54 g K2HPO4] after autoclaving). Protein expression was induced with the addition of 0.1 mM IPTG, and growth was continued overnight at 16°C. Cells were pelleted, resuspended in lysis/binding buffer (50 mM Na2HPO4, 500 mM NaCl, 20 mM imidazole, 0.1 mM phenylmethylsulfonyl fluoride [PMSF]; pH 8.0), and lysed by sonication. Lysed cells were centrifuged at 8,000 × g for 30 min at 4°C. Cleared supernatant was combined with 1 ml of nickel-nitrilotriacetic acid (Ni-NTA) His Bind resin (Novagen) equilibrated in lysis/binding buffer and incubated overnight with gentle rotation at 4°C. The resin-lysate mixture was poured into a 1-cm separation column (Bio-Rad), the resin was allowed to pack, and the lysate was allowed to flow through the column. The resin was washed with wash buffer (50 mM Na2HPO4, 500 mM NaCl, 40 mM imidazole, 0.1 mM PMSF; pH 8.0). The 6His-SUMO-FlgM fusion protein bound to the resin was eluted using elution buffer (50 mM Na2HPO4, 500 mM NaCl, 500 mM imidazole, 0.1 mM PMSF; pH 8.0). Elution products were separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue stained to verify purification of the 6His-SUMO-FlgM fusion protein and pure fractions.
FlgM antibody production.
One milligram of purified 6His-SUMO-FlgM protein was sent to Cocalico Biologicals Inc. for serial injection into a rabbit host for antibody generation. Anti-FlgM serum was mixed with FlgM-conjugated Affigel-10 beads and incubated overnight at 4°C. The beads were packed onto a 1-cm column (Bio-Rad) and then washed with 100 mM glycine (pH 2.5) to release the antibody and immediately neutralized with 2 M Tris base. The purification of the antibody was verified by SDS-PAGE. Purified anti-FlgM antibody was dialyzed into 1× PBS–50% glycerol and stored at −20°C.
FlgM secretion assay.
For the pellet fraction (cytoplasmic and cell-associated proteins), B. subtilis strains were grown in 25 ml LB broth to an OD600 of ∼1.0, and 1-ml and 10-ml samples of broth culture were harvested by centrifugation, resuspended to 10 OD600 units in lysis buffer (20 mM Tris [pH 7.0], 10 mM EDTA, 1 mg/ml lysozyme, 10 μg/ml DNase I, 100 μg/ml RNase I, 1 mM PMSF), and incubated 30 min at 37°C. For the supernatant fraction (secreted extracellular proteins), 10 ml of supernatant was collected from the same cultures as those used to generate the pellet fractions. The supernatant was clarified by centrifugation at 5,000 × g for 30 min and treated with 1 ml of freshly prepared 0.015% sodium deoxycholate for 10 min at room temperature. Proteins from the supernatant were precipitated by adding 500 μl chilled trichloroacetic acid (TCA) and incubating the mixture for >2 h on ice at 4°C. Precipitated proteins were pelleted at 9,447 × g for 10 min at 4°C, washed twice with 1 ml ice-cold acetone, and resuspended to 10 OD600 units in 0.1 N sodium hydroxide. Ten microliters of cell pellet or supernatant sample was mixed with 2 μl 6× SDS loading buffer. Samples were separated in parallel by 15% SDS-PAGE. Proteins were electroblotted onto nitrocellulose for 50 min at 400 mA and probed with a 1:10,000 dilution of anti-FlgM primary antibody, with a 1:40,000 dilution of anti-SigA primary antibody (a generous gift from Masaya Fujita, University of Huston), and with a 1:10,000 dilution of secondary antibody (horseradish peroxidase [HRP]-conjugated goat anti-rabbit immunoglobulin G). Immunoblots were developed using the Pierce ECL Western blotting substrate kit (Thermo Scientific).
RESULTS
FlgM is secreted and degraded extracellularly.
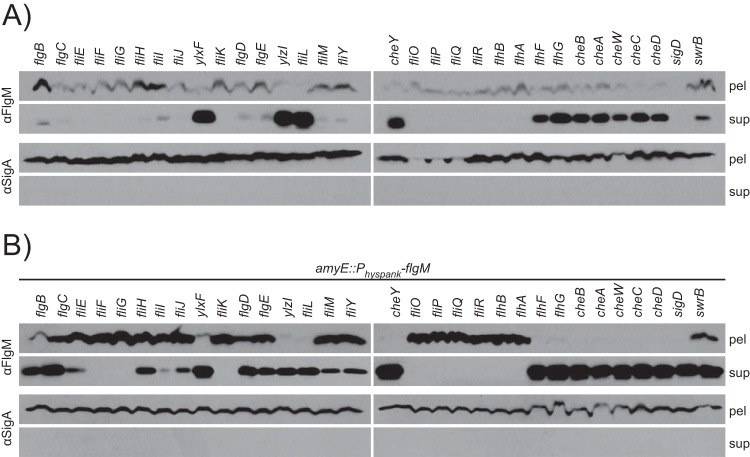
FlgM is the anti-sigma factor that inhibits the activity of the motility sigma factor σD (19). In S. enterica, FlgM activity is inhibited when FlgM is secreted through the flagellar hook-basal body and becomes spatially separated from its cognate sigma factor (13, 14). To test whether FlgM is secreted in B. subtilis, Western blot analysis was performed using anti-FlgM primary antibody on TCA-precipitated supernatant and cell pellet lysate fractions of the wild type, an flgM mutant, and a strain that expressed flgM from the artificial IPTG-inducible Physpank promoter integrated at the ectopic amyE locus (amyE::Physpank-flgM). In parallel, Western blot assays were conducted using primary antibody against SigA, the constitutive cytoplasmic vegetative sigma factor. SigA serves both as a loading control for cell pellet samples and as a control for cytoplasmic contamination of supernatant samples caused by cell lysis, as SigA is cytoplasmic and is not secreted. FlgM protein was barely detectable in the cell pellet lysates of the wild type, absent from the flgM mutant, and enhanced in the Physpank-flgM strain when induced with 1 mM IPTG (Fig. 2). Neither FlgM nor SigA was detected in the any of the TCA-precipitated supernatant fractions. We conclude that either FlgM is not secreted in B. subtilis or FlgM is secreted and degraded extracellularly.
FIG 2.
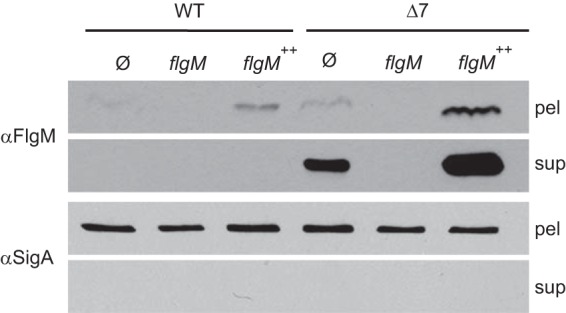
FlgM is secreted and proteolyzed extracellularly. (A) FlgM secretion assay. Cell pellets (pel) were lysed, and supernatants (sup) were concentrated by TCA precipitation of dissolved proteins, resolved by SDS-PAGE, electroblotted, and probed in Western analysis. Anti-FlgM (αFlgM) primary antibody was used to detect FlgM, and anti-SigA (αSigA) served as a loading control and as a control for cytoplasmic contamination of supernatant samples caused by cell lysis. The horizontal bars group strains with the indicated common genetic background. “Ø” indicates no additional change to the genetic background. The following strains were used to generate the figure: 3610 (WT strain), DS4029 (flgM), DS3772 (flgM++), DS6329 (Δ7), DS7161 (Δ7 flgM), and DS6806 (Δ7 flgM++).
FlgM could be extracellularly degraded by one or more of the seven extracellular proteases secreted by B. subtilis: AprE, NprE, Bpr, Epr, Vpr, WprA, and Mpr (39–45). To test whether extracellular proteases degrade FlgM, a secretion assay was conducted using a mutant containing multiple in-frame markerless deletions disrupting each of the exoprotease-encoding genes (“Δ7 mutant”). In the Δ7 mutant background, FlgM was detected in both the cell pellet lysates and the corresponding TCA-precipitated supernatant fractions from the wild-type and IPTG-induced Physpank-flgM strains (Fig. 2). SigA was detected in the cell lysate fraction but was not detected in the TCA-precipitated supernatant fraction, suggesting that SigA was neither secreted nor released by cell lysis. We conclude that FlgM is secreted in B. subtilis and that one or more of the seven secreted proteases degraded FlgM in the extracellular environment.
A series of mutants was constructed to reductively deduce which exoprotease(s) contributed to the degradation of FlgM (Fig. 3A). Each strain tested in order lacked a single protease encoded by the preceding strain. Thus, when FlgM was detected in the supernatant, we could infer that the most recently mutated protease contributed to FlgM degradation. FlgM was not detected by Western blotting of TCA-precipitated supernatant fractions when strains encoded the exoprotease WprA (Fig. 3A, lane 6) but was detected when WprA was mutated (Fig. 3A, lane 7), suggesting that WprA was involved in FlgM degradation. Mutation of WprA alone, however, was not sufficient to allow for the detection of FlgM in the supernatant fraction, suggesting that one or more of the remaining six exoproteases was also involved (Fig. 3B).
FIG 3.

FlgM is specifically proteolyzed by WprA and Epr. (A) FlgM secretion assay. All strains contain the Physpank-flgM construct (flgM++) and were grown in the presence of 1 mM IPTG. Blots were probed with both anti-FlgM (αFlgM) and anti-SigA (αSigA) to serve as a loading and lysis control. The following strains were used to generate the figure: DS6806 (lane 1), DS8085 (lane 2), DS8084 (lane 3), DS8083 (lane 4), DS8082 (lane 5), DS8081 (lane 6), DS8080 (lane 7), DK217 (lane 8), DK216 (lane 9), DK215 (lane 10), and DK214 (lane 11). We note that some SigA was detected in the supernatant of lane 6, presumably due to a low level of spontaneous cell lysis prior to harvesting the supernatant. We further note that the cell lysis that occurred did not seem to contribute significantly to the amount of FlgM in the supernatant. (B) FlgM secretion assay. All strains contain the Physpank-flgM construct (flgM++) and were grown in the presence of 1 mM IPTG. The following strains were used to generate the figure: DS6806 (WT strain), DK382 (wprA), DK383 (epr), DK462 (wprA epr), and DS6329 (Δ7).
To find additional exoproteases that contributed to FlgM degradation, we reanalyzed a reductive series of exoprotease mutants in which WprA was also mutated. FlgM was not detected in TCA-precipitated supernatants in WprA-mutated strains that encoded the protease Epr (Fig. 3A, lane 10) but was detected when Epr was also mutated, suggesting that Epr contributed to FlgM degradation (Fig. 3A, lane 11). Mutation of Epr alone was also not sufficient to allow for the detection of FlgM in the supernatant fraction, likely due to the presence of WprA (Fig. 3B). Mutation of both WprA and Epr, however, was sufficient to allow for the detection of FlgM in the TCA-precipitated supernatant fraction (Fig. 3B). We conclude that FlgM is proteolytically degraded in the extracellular environment by the combined activity of WprA and Epr. Henceforth, all FlgM secretion assays were conducted in the Δ7 protease mutant background.
FlgM inhibits σD activity in flagellar basal body mutants.
In S. enterica, cells defective in synthesis of the flagellar hook-basal body inhibit σ28 activity by an enhanced accumulation of the anti-sigma factor FlgM (7). Thirty-two genes within the B. subtilis fla-che operon are predicted to be involved in either flagellar assembly or flagellar function based on their homology to flagellar proteins in S. enterica (20). To determine which genes in the fla-che operon were required for flagellar assembly and/or function, swarm expansion motility assays were conducted in strains mutated for each of the fla-che operon genes (Fig. 4). Mutants able to expand from the point of inoculation after 5 h of incubation were deemed swarming proficient, and based on this criterion, the following genes were considered unlikely candidates essential for the assembly of the hook-basal body: ylxF, fliL, flhF, flhG, cheB, cheW, cheC, and cheD (Fig. 1E).
FIG 4.
Mutations in the fla-che operon abolish swarming motility. Swarm expansion assays were conducted on the indicated strains, and the swarm radius was measured after 5 h of incubation at 37°C. Bars represent averages of three replicates. The dashed line indicates the swarming motility of the wild type (strain 3610) at 5 h. The following strains (mutated genes in parentheses) were used to generate the figure: DS4680 (flgB), DS7303 (flgC), DS7308 (fliE), DS7080 (fliF), DS7357 (fliG), DS6729 (fliH), DS6728 (fliI), DS7359 (fliJ), DS6554 (ylxF), DS4536 (fliK), DS6555 (flgD), DS4681 (flgE), DS6657 (ylzI), DS6540 (fliL), DS6775 (fliM), DS5384 (fliY), DS6870 (cheY), DS6468 (fliO), DS7119 (fliP), DS7118 (fliQ), DS7317 (fliR), DS7120 (flhB), DS5913 (flhA), DS6658 (flhF), DS7358 (flhG), DS7306 (cheB), DS6887 (cheA), DS6869 (cheW), DS6867 (cheC), DS6868 (cheD), DS6420 (sigD), and DS2509 (swrB).
In S. enterica, defects in flagellar hook-basal body assembly result in the inhibition of σ28 activity (7). To determine which mutants in the fla-che operon were defective in σD activity, a reporter for σD-dependent gene expression in which the Phag promoter for the hag gene encoding the flagellar filament Hag was transcriptionally fused to green fluorescent protein was integrated at the ectopic amyE locus (amyE::Phag-GFP) in the wild type and each mutant background (Fig. 5, top left images). Expression of σD-dependent genes is heterogenous in the population, and wild-type cells displayed bistable expression of Phag-GFP such that short motile cells were brightly fluorescent and nonmotile chains were dark (28, 46). Mutants that robustly expressed the Phag-GFP reporter did not have a strong defect in σD-dependent gene expression, and based on this criterion, the following genes were also considered unlikely candidates essential for the assembly of the hook-basal body: ylzI, cheY, cheA, and swrB (Fig. 1E). As further support that ylzI, cheY, cheA, and swrB are not essential for hook-basal body assembly, liquid cultures of each mutant exhibited swimming motility when observed in phase-contrast microscopy of wet mounts. In sum, we infer that the following fla-che-encoded proteins are required for hook-basal body completion in B. subtilis: FlgB, FlgC, FliE, FliF, FliG, FliH, FliI, FliJ, FliK, FlgD, FlgE, FliM, FliY, FliO, FliP, FliQ, FliR, FlhB, and FlhA.
FIG 5.
Mutations in the fla-che operon impair σD-dependent gene expression. Fluorescence microscopy of the indicated strains that contain the amyE::Phag-GFP reporter construct. The top left triangle of each square is an image of the strain containing an in-frame deletion of the indicated gene; the bottom right triangle is an image of the corresponding deletion mutant strain containing an additional flgM::tet mutation (see the legend in the top left panel). Cell membranes were stained with FM4-64 and are false-colored red. GFP fluorescence is false-colored green. Scale bar, 4 μm. The following strains (mutated genes in parentheses) were used to generate the figure: DS908 (WT strain), DS4264 (flgM), DS7684 (flgB), DK1856 (flgB flgM), DS7685 (flgC), DK1857 (flgC flgM), DS7686 (fliE), DK1858 (fliE flgM), DS7687 (fliF), DS7457 (fliF flgM), DS7688 (fliG), DK1859 (fliG flgM), DS7689 (fliH), DK1860 (fliH flgM), DS7690 (fliI), DK1861 (fliI flgM), DS7691 (fliJ), DK1862 (fliJ flgM), DS7692 (ylxF), DK1863 (ylxF flgM), DS7693 (fliK), DK1864 (fliK flgM), DS7694 (flgD), DK1865 (flgD flgM), DS7695 (flgE), DK1866 (flgE flgM), DS7696 (ylzI), DK1867 (ylzI flgM), DS7697 (fliL), DK1868 (fliL flgM), DS7698 (fliM), DK1869 (fliM flgM), DS7699 (fliY), DK1870 (fliY flgM), DS7700 (cheY), DK1871 (cheY flgM), DS7701 (fliO), DK1872 (fliO flgM), DS7702 (fliP), DK1873 (fliP flgM), DS7703 (fliQ), DK1874 (fliQ flgM), DS7704 (fliR), DK1875 (fliR flgM), DS7705 (flhB), DK1876 (flhB flgM), DS7706 (flhA), DK1877 (flhA flgM), DS7707 (flhF), DK1878 (flhF flgM), DS7708 (flhG), DK1879 (flhG flgM), DS7709 (cheB), DK1880 (cheB flgM), DS7710 (cheA), DK1881 (cheA flgM), DS7711 (cheW), DK1882 (cheW flgM), DS7712 (cheC), DK1883 (cheC flgM), DS7713 (cheD), DK1884 (cheD flgM), DS7714 (sigD), DK1885 (sigD flgM), DS7715 (swrB), and DK1886 (swrB flgM).
In S. enterica, defects in flagellar hook-basal body assembly result in the inhibition of σ28 activity due to a failure to inhibit the FlgM anti-sigma factor (7). To determine whether the low fluorescence from the Phag-GFP reporter in the fla-che operon mutants was due to enhanced FlgM activity, FlgM was mutated in each fla-che operon mutant background (Fig. 5, bottom right images). Mutation of flgM increased Phag-GFP fluorescence magnitude in the wild type and was epistatic to all fla-che operon mutants irrespective of basal Phag-GFP fluorescence level. We conclude that each of the fla-che operon mutants expressing low levels of Phag-GFP experience enhanced inhibition of σD-dependent gene expression due to an inability to antagonize FlgM.
FlgM is secreted through the hook-basal body by the flagellar export apparatus.
In S. enterica, FlgM is inhibited by secretion through a flagellar type III export apparatus that becomes proficient for FlgM secretion only upon completion of the hook-basal body structural intermediate (15). To test whether FlgM secretion depends on a functional hook-basal body in B. subtilis, in-frame markerless deletions of each gene in the fla-che operon were constructed in the Δ7 protease mutant background. Low levels of FlgM were detected in the cell pellet lysate fraction in each mutant save sigD, as flgM gene expression is σD dependent (Fig. 6A). Importantly, FlgM secretion was reduced in each mutant background that also exhibited low σD-dependent gene expression (Fig. 1E). We conclude that the ability to activate σD is correlated with the ability to secrete FlgM into the extracellular environment.
FIG 6.
Mutations in the fla-che operon abolish FlgM secretion. (A) FlgM secretion assay in which control of FlgM is under the native promoter. All mutations were constructed in the Δ7 protease mutant background. The two panels were generated using the same samples. Blots were probed with both anti-FlgM (αFlgM) and anti-SigA (αSigA) to serve as a loading and lysis control. The strains with mutations for flgB (DK717), flgC (DS8404), fliE (DK2012), fliF (DS6871), fliG (DK1902), fliH (DK1951), fliI (DK2058), fliJ (DK1952), ylxF (DK2026), fliK (DK1971), flgD (DK1970), flgE (DS8365), ylzI (DK2014), fliL (DK2028), fliM (DK1953), fliY (DK1904), cheY (DK2031), fliO (DK1969), fliP (DK2059), fliQ (DK1903), fliR (DK3076), flhB (DK1968), flhA (DS8117), flhF (DK3077), flhG (DK2071), cheB (DK2060), cheA (DK2013), cheW (DK2030), cheC (DK2027), cheD (DK2029), sigD (DK2230), and swrB (DK3075) were used to generate the figure. (B) FlgM secretion assay in which FlgM is artificially expressed from an IPTG-inducible promoter. Strains contain the amyE::Physpank-flgM construct (flgM++) and were grown in the presence of 1 mM IPTG. The strains with mutations flgB flgM++ (DK3051), flgC flgM++ (DK1143), fliE flgM++ (DK3053), fliF flgM++ (DS6919), fliG flgM++ (DK3054), fliH flgM++ (DK3052), fliI flgM++ (DK3055), fliJ flgM++ (DK3056), ylxF flgM++ (DK3057), fliK flgM++ (DK3058), flgD flgM++ (DK3059), flgE flgM++ (DK1144), ylzI flgM++ (DK3060), fliL flgM++ (DK3061), fliM flgM++ (DK3062), fliY flgM++ (DK3063), cheY flgM++ (DK3064), fliO flgM++ (DK3065), fliP flgM++ (DK3066), fliQ flgM++ (DK3067), fliR flgM++ (DK3079), flhB flgM++ (DK3068), flhA flgM++ (DK1142), flhF flgM++ (DK3080), flhG flgM++ (DK3069), cheB flgM++ (DK3070), cheA flgM++ (DK3071), cheW flgM++ (DK3072), cheC flgM++ (DK3073), cheD flgM++ (DK3074), sigD flgM++ (DK2301), and swrB flgM++ (DK3078) were used to generate the figure.
In B. subtilis, the expression of FlgM is under explicit control of σD, and thus the reduced ability of some mutants to inhibit FlgM could limit FlgM expression and thus lower FlgM levels, both intracellularly and extracellularly, below the limit of detection (18, 47). To override native transcriptional regulation of FlgM, the flgM gene was artificially expressed from the artificial, IPTG-inducible Physpank promoter (Physpank-flgM) in each of the fla-che operon mutants in the Δ7 protease mutant background. FlgM secretion was abolished in cells mutated for the FliF and FliG basal body components, the FliO, FliP, FliQ, FliR, FlhA, and FlhB type III export apparatus components, and the FliK substrate specificity switch regulator (Fig 6B). By contrast, the remaining proteins required for hook-basal body completion were not essential for FlgM secretion when FlgM was artificially expressed (Fig 6B). We conclude that completion of the hook-basal body structural assembly intermediate enhances but is not absolutely required for FlgM secretion (Fig. 1E).
DISCUSSION
Flagellar biosynthesis is complex, and FlgM secretion is a paradigm for the morphogenetic coupling of flagellar structure and gene transcription (4). Prior to completion of the hook-basal body structural intermediate, FlgM inhibits the expression of the σD regulon such that the gene encoding the abundant flagellar filament protein Hag is not expressed prematurely (18, 30–33). In S. enterica, the flagellar type III export apparatus changes substrate specificity upon hook-basal body completion to recognize, export, and inhibit FlgM (13, 14). While the FlgM paradigm is widely accepted, it has rarely been tested outside a subset of Gram-negative gammaproteobacteria (16). Here we show that the Gram-positive bacterium B. subtilis conforms to the established paradigm of flagellar morphogenetic coupling, as the activity of σD was tightly correlated with the ability to efficiently secrete FlgM from the cytoplasm.
FlgM secretion was not originally detected in the wild type due to the fact that FlgM was degraded by Epr and WprA, two relatively understudied representatives of the seven extracellular proteases secreted by B. subtilis. The mechanisms that govern extracellular protease specificity are unclear, so why FlgM is degraded by those two enzymes in particular and not by the general specificity protease AprE (subtilisin) is unknown (39). While the involvement of Epr and WprA may be arbitrary, it is also possible that protease-specific degradation of FlgM is biologically relevant. For example, Epr is the only protease expressed under the control of σD (48), and WprA (wall protease A) is anchored to the peptidoglycan and thus is in close proximity to the flagellar basal body (44). We note that the same two proteases, Epr and WprA, were reported to proteolytically restrict localization of the autolysin LytF, which is required for separating daughter cells after division, and LytF is coincidentally under strict σD control (46, 49). Proteolysis of FlgM and LytF suggests that at least some members of the σD regulon may be coordinately processed at the posttranslational level, but if so, the coprocessing seems unrelated to both motility and cell separation, as here we show that strains with deletion of all seven proteases produce single motile cells, comparable to what occurs in the wild type (see Fig. S1 in the supplemental material). The relevance of FlgM and/or LytF extracellular proteolysis is unknown.
It has been shown that mutation of a subset of fla-che operon genes abolishes σD-dependent gene expression in an FlgM-dependent manner (31–33). To further explore this correlation, we tested mutations of every gene in the fla-che operon and measured both motility and σD-dependent gene expression. Mutation of 24 of the 32 fla-che operon genes resulted in severe swarming motility defects. Four of the mutants defective for swarming motility expressed σD-dependent genes and were proficient for swimming in liquid (swrB, cheA, cheY, and ylzI), suggesting that the corresponding gene products were not essential for hook-basal body assembly. The remaining 20 genes were defective for both swarming and σD-dependent gene expression, and with the exceptions of sigD and fliK, all were predicted to encode a conserved structural component of the flagellum (20). Furthermore, each mutant defective for σD-dependent gene expression also failed to secrete FlgM. Thus, as in S. enterica, the ability to secrete FlgM depended on hook-basal body completion and FlgM secretion was tightly correlated with σD activity.
FlgM is autoinhibitory, as the flgM gene is expressed by σD (18). Thus, reduced FlgM secretion could be misinterpreted due to reduced FlgM expression. Indeed, artificial IPTG induction of FlgM revealed that many genes required for hook-basal body assembly improved but were not in fact absolutely essential for FlgM secretion. Ultimately, nine gene products, including the flagellar baseplate FliF, the flagellar rotor FliG, the flagellar type III export apparatus components FliO, FliP, FliQ, FliR, FlhB, and FlhA, and the substrate specificity switch mediator FliK constituted the minimal set of proteins strictly required to secrete FlgM. In contrast, the completion of the hook-basal body, including the rod (FlgB and FlgC), the hook (FlgD and FlgE), the C-ring (FliM and FliY), and the C-rod (FliH, FliI, and FliJ), strongly enhances, but is not strictly essential, for FliK to switch the specificity of the type III export apparatus and permit FlgM secretion. We note that this may be the first report of robust FlgM secretion in the absence of a complete hook-basal body, perhaps due to the technical limitations of the Gram-negative model systems. In contrast, Gram-positive model organisms such as B. subtilis permit the study of early events in type III export as the substrates are secreted directly into the extracellular environment even in flagellar mutants with severe structural defects.
In sum, the control of FlgM in B. subtilis largely adheres to the paradigm of morphogenetic coupling of structural assembly and gene regulation established in S. enterica. In S. enterica, however, FlgM is believed to be the primary form of regulation on the expression of the flagellar filament protein; in B. subtilis, the flagellar filament protein Hag is regulated posttranscriptionally by CsrA, an RNA-binding protein that inhibits Hag translation (31, 50). Importantly, like FlgM, CsrA is controlled (albeit indirectly) by the activity of the flagellar type III export apparatus and completion of the hook-basal body (31, 50). Thus, B. subtilis engages both FlgM and CsrA during the assembly of the flagellum, but unlike CsrA, which is specific for one protein, FlgM controls an entire regulon of genes. Perhaps FlgM inhibition by secretion through the completed hook-basal body has less to do with regulating motility per se and more to do with coordinating motility gene expression with other aspects of B. subtilis biology, peptidoglycan hydrolases in particular.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH Training Grant T32 GM007757 to R.A.C. and NIH GM093030 to D.B.K.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02324-14.
REFERENCES
- 1.Wösten MMSM. 1998. Eubacterial sigma-factors. FEMS Microbiol Rev 22:127–150. doi: 10.1016/S0168-6445(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 2.Haldenwang WG. 1995. The sigma factors of Bacillus subtilis. Microbiol Rev 59:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes KT, Mathee K. 1998. The anti-sigma factors. Annu Rev Microbiol 52:231–286. doi: 10.1146/annurev.micro.52.1.231. [DOI] [PubMed] [Google Scholar]
- 4.Chilcott GS, Hughes KT. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol Mol Biol Rev 64:694–708. doi: 10.1128/MMBR.64.4.694-708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macnab RM. 1992. Genetics and biogenesis of bacterial flagella. Annu Rev Genet 26:131–158. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- 6.Chevance FFV, Hughes KT. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillen KL, Hughes KT. 1991. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J Bacteriol 173:2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohnishi K, Kutsukake K, Suzuki H, Iino T. 1992. A novel transcriptional regulation mechanism in the flagellar regulon of Salmonella typhimurium: an anti-sigma factor inhibits the activity of the flagellum-specific sigma factor, σF. Mol Microbiol 6:3149–3157. doi: 10.1111/j.1365-2958.1992.tb01771.x. [DOI] [PubMed] [Google Scholar]
- 9.Sorenson MK, Ray SS, Darst SA. 2004. Crystal structure of the flagellar σ/anti-σ complex σ28/FlgM reveals an intact σ factor in an inactive conformation. Mol Cell 14:127–138. doi: 10.1016/S1097-2765(04)00150-9. [DOI] [PubMed] [Google Scholar]
- 10.Moriya N, Minamino T, Hughes KT, Macnab RM, Namba K. 2006. The type III flagellar export specificity switch is dependent on FliK ruler and a molecular clock. J Mol Biol 359:466–477. doi: 10.1016/j.jmb.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Minamino T, Moriya N, Hirano T, Hughes KT, Namba K. 2009. Interaction of FliK with the bacterial flagellar hook is required for efficient export specificity switching. Mol Microbiol 74:239–251. doi: 10.1111/j.1365-2958.2009.06871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erhardt M, Singer HM, Wee DH, Keener JP, Hughes KT. 2011. An infrequent molecular ruler controls flagellar hook length in Salmonella enterica. EMBO J 30:2948–2961. doi: 10.1038/emboj.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes KT, Gillen KL, Semon MJ, Karlinsey JE. 1993. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science 262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 14.Kutsukake K. 1994. Excretion of the anti-sigma factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol Gen Genet 243:605–612. [DOI] [PubMed] [Google Scholar]
- 15.Karlinsey JE, Tanaka S, Bettenworth V, Yamaguchi S, Boos W, Aizawa S, Hughes KT. 2000. Completion of the hook-basal body complex of the Salmonella typhimurium flagellum is coupled to FlgM secretion and fliC transcription. Mol Microbiol 37:1220–1231. doi: 10.1046/j.1365-2958.2000.02081.x. [DOI] [PubMed] [Google Scholar]
- 16.Rust M, Borchert S, Niehus E, Kuehne SA, Gripp E, Bajceta A, McMurry JL, Suerbaum S, Hughes KT, Josenhans C. 2009. The Helicobacter pylori anti-sigma factor FlgM is predominantly cytoplasmic and cooperates with the flagellar basal body protein FlhA. J Bacteriol 191:4824–4834. doi: 10.1128/JB.00018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helmann JD, Márquez LM, Chamberlin MJ. 1988. Cloning, sequencing, and disruption of the Bacillus subtilis σ28 gene. J Bacteriol 170:1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirel DB, Lauer P, Chamberlin MJ. 1994. Identification of flagellar synthesis regulatory and structural genes in a σD-dependent operon of Bacillus subtilis. J Bacteriol 176:4492–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertero MG, Gonzales B, Tarricone C, Ceciliani F, Galizzi A. 1999. Overproduction and characterization of the Bacillus subtilis anti-sigma factor FlgM. J Biol Chem 274:12103–12107. doi: 10.1074/jbc.274.17.12103. [DOI] [PubMed] [Google Scholar]
- 20.Albertini AM, Caramori T, Crabb WD, Scoffone F, Galizzi A. 1991. The flaA locus of Bacillus subtilis is part of a large operon coding for flagellar structures, motility functions, and an ATPase-like polypeptide. J Bacteriol 173:3573–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Márquez-Magaña LM, Chamberlin MJ. 1994. Characterization of the sigD transcription unit of Bacillus subtilis. J Bacteriol 176:2427–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estacio W, Santa Anna-Arriola S, Adedipe M, Márquez-Magaña LM. 1998. Dual promoters are responsible for transcription initiation of the fla/che operon in Bacillus subtilis. J Bacteriol 180:3548–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West JT, Estacio W, Márquez-Magaña LM. 2000. Relative roles of the fla/che PA, PD-3, and PsigD promoters in regulating motility and sigD expression in Bacillus subtilis. J Bacteriol 182:4841–4848. doi: 10.1128/JB.182.17.4841-4848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirel DB, Chamberlin MJ. 1989. The Bacillus subtilis flagellin gene (hag) is transcribed by the σ28 form of RNA polymerase. J Bacteriol 171:3095–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirel DB, Lustre VM, Chamberlin MJ. 1992. An operon of Bacillus subtilis motility genes transcribed by the σD form of RNA polymerase. J Bacteriol 174:4197–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda A, Sekiguchi J. 1993. High-level transcription of the major Bacillus subtilis autolysin operon depends on expression of the sigma D gene and is affected by a sin (flaD) mutation. J Bacteriol 175:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serizawa M, Yamamoto H, Yamaguchi H, Fujita Y, Kobayashi K, Ogasawara N, Sekiguchi J. 2004. Systematic analysis of SigD-regulated genes in Bacillus subtilis by DNA microarray and Northern blotting analyses. Gene 329:125–136. doi: 10.1016/j.gene.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Kearns DB, Losick R. 2005. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev 19:3083–3094. doi: 10.1101/gad.1373905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Márquez LM, Helmann JD, Ferrari E, Parker HM, Ordal GW, Chamberlin MJ. 1990. Studies of σD-dependent functions in Bacillus subtilis. J Bacteriol 172:3435–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caramori T, Barilla D, Nessi C, Sacchi L, Galizzi A. 1996. Role of FlgM in σD-dependent gene expression in Bacillus subtilis. J Bacteriol 178:3113–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee S, Yakhnin H, Kysela D, Sokoloski J, Babitzke P, Kearns DB. 2011. CsrA-FliW interaction governs flagellin homeostasis and a checkpoint on flagellar morphogenesis in Bacillus subtilis. Mol Microbiol 82:447–461. doi: 10.1111/j.1365-2958.2011.07822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtney CR, Cozy LM, Kearns DB. 2012. Molecular characterization of the flagellar hook in Bacillus subtilis. J Bacteriol 194:4619–4629. doi: 10.1128/JB.00444-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cozy LM, Kearns DB. 2010. Gene position in a long operon governs motility development in Bacillus subtilis. Mol Microbiol 76:273–285. doi: 10.1111/j.1365-2958.2010.07112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasbin RE, Young FE. 1974. Transduction in Bacillus subtilis by bacteriophage SPP1. J Virol 14:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrick JE, Kearns DB. 2008. MinJ (YvjD) is a topological determinant of cell division in Bacillus subtilis. Mol Microbiol 70:1166–1179. doi: 10.1111/j.1365-2958.2008.06469.x. [DOI] [PubMed] [Google Scholar]
- 36.Guérout-Fleury AM, Shazand K, Frandsen N, Stragier P. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Yehuda S, Rudner DZ, Losick R. 2003. RacA, a bacterial protein that anchors chromosomes to the cell poles. Science 299:532–536. doi: 10.1126/science.1079914. [DOI] [PubMed] [Google Scholar]
- 38.Bendezú FO, Hale CA, Bernhardt TG, de Boer PA. 2009. RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli. EMBO J 28:193–204. doi: 10.1038/emboj.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl ML, Ferrari E. 1984. Replacement of the Bacillus subtilis subtilisin structural gene with an in vitro-derived deletion mutation. J Bacteriol 158:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang MY, Ferrari E, Henner DJ. 1984. Cloning of the neutral protease gene of Bacillus subtilis and the use of the cloned gene to create an in vitro-derived deletion mutation. J Bacteriol 160:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu XC, Nathoo S, Pang AS, Carne T, Wong SL. 1990. Cloning, genetic organization, and characterization of a structural gene encoding bacillopeptidase F from Bacillus subtilis. J Biol Chem 265:6845–6850. [PubMed] [Google Scholar]
- 42.Brückner R, Shoseyov O, Doi RH. 1990. Multiple active forms of a novel serine protease from Bacillus subtilis. Mol Gen Genet 221:486–490. [DOI] [PubMed] [Google Scholar]
- 43.Sloma A, Rufo GA, Theriault KA, Dwyer M, Wilson SW, Pero J. 1991. Cloning and characterization of the gene for an additional extracellular serine protease of Bacillus subtilis. J Bacteriol 173:6889–6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margot P, Karamata D. 1996. The wprA gene of Bacillus subtilis 168, expressed during exponential growth, encodes a cell-wall associated protease. Microbiology 142:3437–3444. doi: 10.1099/13500872-142-12-3437. [DOI] [PubMed] [Google Scholar]
- 45.Rufo GA, Sullivan BJ, Sloma A, Pero J. 1990. Isolation and characterization of a novel extracellular metalloprotease from Bacillus subtilis. J Bacteriol 172:1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen RC, Guttenplan SB, Blair KM, Kearns DB. 2009. Role of the σD-dependent autolysins in Bacillus subtilis population heterogeneity. J Bacteriol 191:5775–5784. doi: 10.1128/JB.00521-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsueh Y-H, Cozy LM, Sham L-T, Calvo RA, Gutu AD, Winkler ME, Kearns DB. 2011. DegU-phosphate activates expression of the anti-sigma factor FlgM in Bacillus subtilis. Mol Microbiol 81:1092–1108. doi: 10.1111/j.1365-2958.2011.07755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixit M, Murudkar CS, Rao KK. 2002. epr is transcribed from a σD promoter and is involved in swarming of Bacillus subtilis. J Bacteriol 184:596–599. doi: 10.1128/JB.184.2.596-599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto H, Kurosawa S, Sekiguchi J. 2003. Localization of the vegetative cell wall hydrolases LytC, LytE, and LytF on the Bacillus subtilis cell surface and the stability of these enzymes to cell wall-bound or extracellular proteases. J Bacteriol 185:6666–6677. doi: 10.1128/JB.185.22.6666-6677.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yakhnin H, Pandit P, Petty TJ, Baker CS, Romeo T, Babitzke P. 2007. CsrA of Bacillus subtilis regulates translation initiation of the gene encoding the flagellin protein (hag) by blocking ribosome binding. Mol Microbiol 64:1605–1620. doi: 10.1111/j.1365-2958.2007.05765.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.