Abstract
Horizontal gene transfer drives the rapid evolution of bacterial populations. Classical processes that promote the lateral flow of genetic information are conserved throughout the prokaryotic world. However, some species have nonconserved transfer mechanisms that are not well known. This is the case for the ancient extreme thermophile Thermus thermophilus. In this work, we show that T. thermophilus strains are capable of exchanging large DNA fragments by a novel mechanism that requires cell-to-cell contacts and employs components of the natural transformation machinery. This process facilitates the bidirectional transfer of virtually any DNA locus but favors by 10-fold loci found in the megaplasmid over those in the chromosome. In contrast to naked DNA acquisition by transformation, the system does not activate the recently described DNA-DNA interference mechanism mediated by the prokaryotic Argonaute protein, thus allowing the organism to distinguish between DNA transferred from a mate and exogenous DNA acquired from unknown hosts. This Argonaute-mediated discrimination may be tentatively viewed as a strategy for safe sharing of potentially “useful” traits by the components of a given population of Thermus spp. without increasing the genome sizes of its individuals.
INTRODUCTION
Lateral gene flow is responsible for the enormous plasticity observed in prokaryotic genomes, which confers on these organisms the ability to adapt rapidly to environmental changes (1–5). Three conserved mechanisms are traditionally associated with lateral gene transfer (LGT) in prokaryotes: phage-mediated transduction, natural transformation, and conjugation. Besides, alternative DNA transfer systems based on nanotubes (6), membrane vesicles, and nanopods in Archaea (7–9), or on the so-called “gene transfer agents” (10), have been described as contributors to genetic exchange among prokaryotes. Among these processes, transformation and conjugation depend only on functions encoded by the bacterial cell. Transformation involves the uptake of naked DNA from the environment and relies entirely on the ability of a competent recipient cell to incorporate DNA. Conjugation, on the other hand, is traditionally seen as the unidirectional transfer of a plasmid DNA molecule from a donor to a recipient cell, where the proteins responsible for DNA transfer are provided exclusively by the donor cell (11).
Conjugation differs from transformation not only in the imperative need for a mate for the transfer of DNA but also in the nature of the transferred DNA. While transformation can be envisioned as a mechanism that might have originally evolved in ancestral bacteria, such as Thermus spp., for the acquisition of DNA of any sort and origin as a nutrient, conjugation is more frequently associated with a gain of new capabilities from related species and commonly involves plasmids or megaplasmids that encode all the functions required for conjugation. In fact, transfer of chromosomal genes is apparently less common and in many cases requires the integration of a conjugative plasmid to generate a high frequency of recombination (Hfr) strain capable of transferring extensive regions of chromosomal DNA into recipient cells in a polar fashion (12). Transfer of chromosomal DNA can also be mediated by integrative and conjugative elements (ICEs). ICEs have recently received growing attention due to their ubiquitous presence among prokaryotes (13–15).
Albeit with variations, the general mechanism of conjugation is shared by Archaea and Bacteria and involves cell-to-cell contacts through a type 4-like secretion system (T4SS) (16). In addition, unconventional conjugative transfers, such as those using the single polar transfer protein TraB from Streptomyces spp. (17), or the chromosomally encoded multiple cis-acting sequences in Mycobacterium smegmatis (18), have been described as devoid of T4SS elements.
The ancient Thermus-Deinococcus clade (19) involves a group of extremophilic bacteria, including extreme thermophiles and radiation-resistant isolates. In addition to their thermophilic character, one of the most remarkable properties of Thermus spp. is the ability to take up and incorporate foreign DNA through a highly efficient natural competence system (20–22). The Thermus thermophilus laboratory model strains HB8 and HB27 were the first to be sequenced. Both harbor a highly syntenic ∼1.8-Mbp chromosome and a more divergent and plastic megaplasmid (>200 kbp) that harbors most of the strain-specific genes (23). Actually, it has been suggested that this megaplasmid constitutes the main landing site for genes acquired laterally through natural competence (24), including loci that favor adaptation to a thermophilic lifestyle (22). Among the genes hypothetically acquired through LGT, several strains of T. thermophilus harbor a gene encoding a homologue of the Argonaute protein, which is involved in RNA silencing in eukaryotes (25). It has been reported recently that the Argonaute homologue of T. thermophilus (ttAgo) constitutes a barrier to DNA acquired by transformation through a mechanism involving DNA-DNA interference, resulting in a ∼10-fold decrease in the incorporation of genes acquired by natural competence. Also, the presence of ttAgo decreases the copy numbers of resident plasmids (26).
In addition to DNA acquisition by natural competence, T. thermophilus is able to transfer DNA by a mechanism that involves cell-to-cell contacts (21, 27). However, bioinformatic analyses conducted on the conjugation-proficient strains HB27 and HB8 have failed to detect the presence of conjugative T4SS homologues (21), supporting the existence of a distinct mechanism for conjugation in these isolates. In this work, we provide insights into the genetic basis of this mechanism of cell-to-cell DNA transfer. We show that this mechanism requires the presence of an active competence system in the recipient mate, that the transfer system has a strong preference for megaplasmid-associated genes over chromosomal genes, and that the DNA transferred by this mechanism does not elicit the DNA-DNA interference control mediated by the ttAgo protein. Taken together, these data suggest that this novel conjugation-like mechanism may play a major role in lateral gene transfer in this species, allowing the genus to distinguish between potentially malicious DNA acquired from the environment and reliable DNA transferred from a mate.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains used in this work are listed in Table 1. Escherichia coli strain DH5α, used for the construction of plasmids, was grown at 37°C in liquid or solid LB (28) selective medium. T. thermophilus strains were grown aerobically under rotational shaking (150 rpm) at 60°C in liquid or solid TB (Thermus broth [29]) medium, unless otherwise indicated. Kanamycin (Km; 30 μg/liter), ampicillin (Ap; 100 μg/liter), and/or hygromycin (Hyg; 100 μg/liter) was added when needed for selection.
TABLE 1.
Bacterial strains used in this work
| Strain | Genotype | Phenotype/use | Reference and/or source |
|---|---|---|---|
| E. coli DH5α | F− endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 Δ(argF-lacZYA)U169 ϕ80dlacΔM15 | Cloning | 47 |
| Thermus thermophilus | |||
| HB27 | ATCC BAA-163/DSM7039 | Wild type | Y. Koyama |
| HB27EC | HB27 ago::agoISTth7 | Enhanced competence | Lab strain; 26 |
| HB27Δago | Δago | Lacking Argonaute | 26 |
| NAR1 | Wild type | Wild type; partially denitrifying | 48 |
| PH1 | HB27EC(pTT27::ttp0146::hyg) | Hygr | This work |
| PH2 | HB27EC(pMHPnqosgfp) | Hygr | This work |
| CK1 | HB27 gdh::kat | Kmr | 48 |
| CK3 | NAR1 gdh::kat | Kmr; partially denitrifying | This work |
| CH1 | HB27EC::Δttc0313::hyg | Hygr | 49 |
| PK1 | HB27EC(pTT27::ttp0046::kat) | Kmr | This work |
| PK2 | HB27EC(pTT27::ttp0085::kat) | Kmr | This work |
| PK3 | HB27EC(pTT27::ttp0140::kat) | Kmr | This work |
| PK4 | HB27EC(pTT27::ttp0167::kat) | Kmr | This work |
| PK5 | HB27EC(pTT27::ttp0191::kat) | Kmr | This work |
| PK6 | HB27EC(pTT27::ttp0219::kat) | Kmr | This work |
| CK5 | HB27EC ttc0638::kat | Kmr | This work |
| CK6 | HB27EC ttc0893::kat | Kmr | This work |
| CK7 | HB27EC ttc1415::kat | Kmr | This work |
| CK8 | HB27EC ttc1844::kat | Kmr | This work |
| CK11 | HB27 ΔpilA4::kat Δago | Kmr; noncompetent | This work |
| CK12 | HB27 ΔpilQ::kat Δago | Kmr; noncompetent | This work |
| CK13 | HB27 pilF::kat | Kmr; noncompetent | 35 |
| CK14a | HB27 pilA1-3::kat | Kmr; noncompetent | 50 |
| CH2 | HB27 pilQ::hyg | Hygr; noncompetent | This work |
| CH3 | NAR1 pilQ::hyg | Hygr; noncompetent | This work |
| CH4 | HB27 ΔpilA4::hyg | Hygr; noncompetent | This work |
| CK15 | HB27 comEA::kat | Kmr; noncompetent | 35 |
| CK16 | HB27 pilT::kat | Kmr | 35 |
| CK17 | HB27 comZ::kat | Kmr; noncompetent | 50 |
| CK18 | HB27 pilD::kat | Kmr; noncompetent | 50 |
| CK19 | HB27 pilA3::kat | Kmr; noncompetent | 50 |
| CK20 | HB27 comEC::kat | Kmr; 0.001-fold competent | 35 |
| CK21 | HB27 pilT2::kat | Kmr | This work |
| CK22 | HB27 gdh::kat Δago | Kmr | This work |
| CK23 | HB27 gdh::kat | Kmr | This work |
| CH5 | HB27 Δttc0313::hyg Δago | Hygr | This work |
| CH6 | HB27 Δttc0313::hyg | Hygr | This work |
| CK24 | HB27 ttc0638::kat Δago | Kmr | This work |
| CK25 | HB27 ttc0638::kat | Kmr | This work |
| CK26 | HB27 ttc0858::kat | Kmr | This work |
| CK27 | HB27 ttc1621::kat Δago | Kmr | This work |
| CK28 | HB27 ttc1844::kat Δago | Kmr | This work |
| CK29 | HB27 ttc1844::kat | Kmr | This work |
Insertion mutant lacking the genes pilA1, pilA2, and pilA3.
Plasmids and construction of mutant strains.
The plasmids used in this work are listed in Table 2. The oligonucleotides used for PCR amplification are listed in Table S1 in the supplemental material. DNA manipulation and cloning were performed using standard laboratory techniques (30). All constructs were checked by restriction analysis and sequencing, and mutants were confirmed by PCR analysis.
TABLE 2.
Plasmids used in this work
| Plasmid | Description | Usea | Reference |
|---|---|---|---|
| pAB6 | pK18::ttp0191 | Insertion; Kmr; generation of PK5 mutant | This work |
| pAB8 | pK18::ttp0085 | Insertion; Kmr; generation of PK2 mutant | This work |
| pAB15 | pK18::ttp0140 | Insertion; Kmr; generation of PK3 mutant | This work |
| pAB22 | pUC19::ΔpilA4::kat | pilA4 deletion; Kmr; generation of CK11 and CK26 mutants | This work |
| pAB52 | pUC19::ΔpilA4::hyg | pilA4 deletion; Hygr; generation of CH4 mutant | This work |
| pAB36 | pUC19::ΔpilQ::kat | pilQ deletion; Kmr; generation of CK12 mutant | This work |
| pAB54 | pUC19::Δttc1844::kat | ttc1844 deletion; Kmr; generation of CK28 and CK29 mutants | This work |
| pCBG2 | pK18::ttp0046 | Insertion; Kmr; generation of PK1 mutant | This work |
| pCBG3 | pUC119::ttp0146::hyg | Insertion; Hygr; generation of PH1 mutant | This work |
| pCEC30 | pK18::ttp0167 | Insertion; Kmr; generation of PK4 mutant | This work |
| pCEC31 | pK18::ttp0219 | Insertion; Kmr; generation of PK6 mutant | This work |
| pCEC33 | pK18::ttc1415 | Insertion; Kmr; generation of CK7 and CK21 mutants | This work |
| pCEC34 | pK18::ttc1844 | Insertion; Kmr; generation of CK8 mutant | This work |
| pCEC35 | pK18::ttc0638 | Insertion; Kmr; generation of CK5, CK24, and CK25 mutants | This work |
| pCEC36 | pK18::ttc0893 | Insertion; Kmr; generation of CK6 mutant | This work |
| pCEC40 | pH118::pilQ | pilQ disruption; Hygr; generation of CH2 and CH3 mutants | This work |
| P#36 | porf333::kat | pilT disruption; Kmr; generation of CK16 and CK27 mutants | 35 |
| pH118 | Hyg resistant | Suicide vector | 49 |
| pK18 | Km resistant | Suicide vector | 51 |
| pMH::Pnqo::sgfp | Tth replicative | Hygr; episome transfer, generation of PH2 mutant | 52 |
| pMK184 | Tth replicative | Kmr; episome transfer | 48 |
| pUC119 | Ap resistant | Cloning vector | 31 |
| pUC119::Δ0313H | pUC119::Δttc0313::hyg | ttc0313 deletion; Hygr; generation of CH1 mutant | 49 |
Kmr or Hygr refers to a phenotype of resistance to kanamycin or hygromycin, respectively. Insertional plasmids contain a ∼1-kb sequence of the target gene in a suicide vector. Deletion plasmids include a ∼1-kb sequence upstream of the target gene and a ∼1-kb sequence downstream of the target gene, interrupted by the appropriate antibiotic marker.
T. thermophilus integration mutants containing appropriate selective markers were constructed by transformation with the suicide plasmid pK18 or pH118, which confers thermostable resistance to Km or Hyg, respectively. An internal fragment of the target gene was used for single recombination to obtain the corresponding insertion mutant. Deletion mutants were constructed by double recombination with a linearized DNA construct containing upstream and downstream flanking regions around the target gene separated by the hyg or kat gene cassette, encoding thermostable resistance to Hyg or Km, respectively (31). In all cases, the cassette was inserted in the same transcription sense as the target gene to allow the expression of downstream genes. Transformation and selection were performed as described previously (32).
Transformation and conjugation assays.
T. thermophilus strains were transformed by adding 10 to 200 ng of DNA to exponential-phase cultures of the strains as described previously (20, 32). Transformation frequencies were measured as the number of CFU on selective plates per viable cells of the transformed strain. For mating assays, T. thermophilus cultures, labeled with gene cassettes conferring thermostable resistance to Km or Hyg, were grown overnight to saturation. One hundred microliters of each mating pair was mixed in the presence of DNase I (5 units; Roche), and the mixture was centrifuged at 5,000 rpm for 4 min. Pellets were resuspended in 10 μl TB medium with DNase I and were laid on 0.22-μm sterile nitrocellulose filters (Protran BA85; Whatman) on prewarmed TB agar plates. After 5 h at 60°C, filters were introduced into Eppendorf tubes containing 1 ml liquid TB medium and were vortexed vigorously to wash off the cells. Appropriate serial dilutions were plated onto selective TB agar dishes. Conjugation frequencies were measured as the ratio of the number of CFU resistant to both antibiotics to the number of CFU corresponding to the chromosome-labeled mate.
For qualitative conjugation and transformation assays, 107 T. thermophilus cells were laid on top of selective agar medium and were topped either with 107 cells of the second member of the mating pair (1:1 ratio) or with 200 ng of naked DNA for transformation experiments.
Membrane pattern analyses.
Protein profiles of the cell envelope were obtained by scraping cells from spots of transconjugant cells grown on selective agar plates. The cells were resuspended in 500 μl of water and were broken by sonication, and the envelope fraction was concentrated by centrifugation (14,000 × g, 15 min). Proteins were solubilized by boiling in Laemmli sample buffer, separated by SDS-PAGE (33), and revealed by Coomassie blue staining.
Statistical methods.
Quantitative data were analyzed using SPSS Statistics, version 21.0 (2008; SPSS Inc., Chicago, IL). Results were considered significant if the P value was <0.05. To examine differences among conjugation frequencies (calculated as described above), Student's t test and the nonparametric Mann-Whitney U test and Kruskal-Wallis test were used. In studying differences in transfer frequencies among and within various loci, variance was addressed by one-way analysis of variance (ANOVA) and the Kruskal-Wallis test. Post hoc Tukey and Bonferroni tests were carried out when convenient.
RESULTS
Cell-to-cell DNA transfer among T. thermophilus strains shows preference for the pTT27 megaplasmid.
DNA transfer between T. thermophilus strains was monitored in mating experiments involving the transfer of genes conferring thermostable resistance to Km or Hyg, inserted at different positions in the chromosome (CK or CH clones, respectively) or the megaplasmid (PK or PH clones, respectively) (Table 1). Mating experiments were carried out with equal amounts of the two mates in the presence of thermostable DNase I in order to avoid transfer by natural competence after putative cell lysis or DNA secretion. Figure 1 shows that cell-to-cell transfer between strains CK1 and PH1 (referred to below as the CK1 × PH1 cross) or between strains CK1 and CH1 was insensitive to the presence of DNase I, whereas this enzyme prevented the transfer of plasmid pMH::Pnqo::sgfp by natural competence in the control experiment. Besides, experiments in which a nitrocellulose membrane was used to separate the mates did not produce recombinants harboring both resistances (not shown). Therefore, a DNA exchange system that, like classical conjugation processes, is not sensitive to DNase and requires direct cell-to-cell contacts for transfer exists in T. thermophilus. We will thus refer to the cells carrying both markers as transconjugants. However, in contrast to classical conjugation conditions, the strains used for these experiments are isogenic, which implies the absence of an active surface exclusion system and, at the same time, hinders discrimination between “donor” and “recipient” strains.
FIG 1.
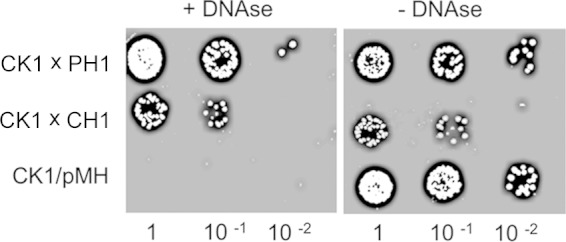
Cell-to-cell DNA transfer in T. thermophilus. Shown is the growth on Hyg-Km double-selective TB plates of dilutions of strain CK1 (Km resistant) mated in a 1:1 ratio with an isogenic Hyg-resistant strain labeled in the megaplasmid (PH1) or the chromosome (CH1). As a control, CK1 was transformed with 200 ng of plasmid pMH::Pnqo::sgfp, and the growth of serially diluted transformants was assessed. The media for matings and transformation experiments were either supplemented with DNase I before mixing (left) or left unsupplemented (right).
As can be observed in Fig. 1, a higher number of transconjugants was routinely detected when one of the mates was labeled in the megaplasmid (PH1) than when both mating strains were labeled in the chromosome. Thus, we quantified the frequency of transfer as the number of transconjugants (with both antibiotic resistances) per chromosomally labeled Km-resistant cell (CK1). For this experiment, either the chromosomally labeled strain CH1 or the megaplasmid-labeled strain PH1 or PH2 was mated in a 1:1 ratio with strain CK1. As shown in Fig. 2A, the numbers of transconjugants with megaplasmid-associated markers (PH1 and PH2) were around 15-fold higher (93.3 × 10−5 ± 6.7 × 10−5) than the number of transconjugants that had incorporated chromosomal genes (CH1) (6.1 × 10−5 ± 1.1 × 10−5). To determine if these differences were affected by the position of the transferred locus or the type of antibiotic marker used, we repeated a series of mating experiments with a common chromosomally labeled mating strain (CH1) and several kanamycin-tagged strains, labeled at different positions in the megaplasmid (PK1, PK2, PK3, PK4, PK5, and PK6) or in the chromosome (CK1, CK5, CK6, CK7, and CK8). As shown in Fig. 2B and in Fig. S2 in the supplemental material, the frequencies of transconjugants with megaplasmid-associated markers relative to strain CH1 were at least an order of magnitude higher than those involving only chromosome markers. Bearing in mind that the copy numbers of the chromosome and the megaplasmid are similar (4 to 5 according to reference 34), these figures support the existence of a strong bias toward genes located in the megaplasmid rather than the chromosome.
FIG 2.
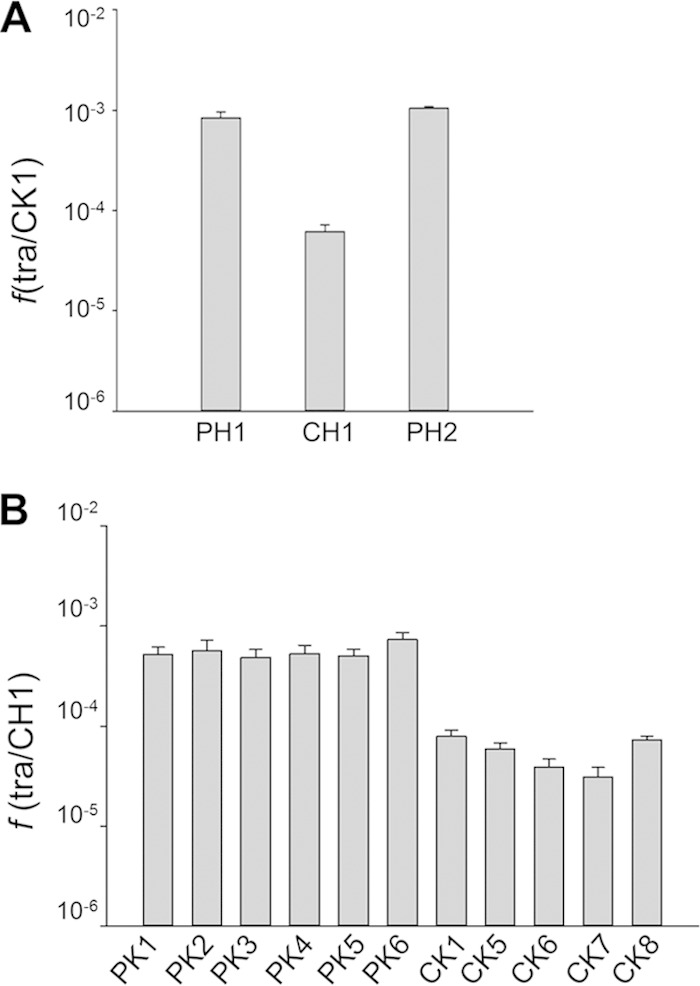
Preference for megaplasmid genes. (A) Transfer frequencies were obtained after equal amounts of a strain labeled with the Hyg cassette in the chromosome (CH1) or in the megaplasmid (PH1, PH2) and strain CK1, labeled with the Km marker in the chromosome, were mixed. Frequencies (f) are averages of ratios between colonies grown on selective medium containing Km and Hyg and colonies grown with Km (tra/CK1) in 9 independent experiments. Error bars correspond to the standard deviations of the means. Differences in transfer frequencies between chromosomal (CH1) and plasmid (PH1, PH2) markers were statistically significant, as assessed by t tests (n = 9) for the CK1 × PH1 (P, <0.001) and CK1 × PH2 (P, 0.001) mating pairs versus the CK1 × CH1 mating pair. No statistical difference could be observed between megaplasmid-linked frequencies (PH1 versus PH2 [P, 0.134]). (B) Transfer frequencies were obtained as described above for matings between the Hyg-resistant strain labeled in the chromosome (CH1) and Km-resistant strains labeled either in the chromosome (CK1, CK5 to CK8) or in the megaplasmid (PK1 to PK6). Frequencies are averages of ratios between colonies grown on selective medium containing Km and Hyg and colonies grown with Hyg (tra/CH1) in 9 independent experiments. Error bars correspond to the standard deviations of the means. Note that the transfer frequencies for megaplasmid-linked genes were approximately an order of magnitude higher than those for chromosomal genes.
In contrast, no significant differences were detected among the transfer frequencies of megaplasmid-labeled strains (Fig. 2B). Therefore, it was not possible to infer an order of transfer of loci in the megaplasmid. On the other hand, larger differences were found among the transfer frequencies of chromosomally labeled strains, but different tests failed to reveal enough significance to allow us to infer an order of transfer of the markers.
The natural competence system is involved in cell-to-cell DNA transfer.
In order to evaluate a putative relationship between cell-to-cell DNA transfer and natural competence, we employed a series of mutants (CK11 to CK21) known to be affected in natural competence (22) and carried out mating experiments with a transformation-proficient mate (CH1). We observed strong differences in the transfer frequencies among the mutants without any deducible pattern with regard to piliation (see Fig. S3 in the supplemental material). However, when we tried to mate these competence-deficient strains (CK11 to CK21) with another competence-deficient strain (CH4), no transconjugants were obtained (Fig. 3). Therefore, all the components of the natural competence system of T. thermophilus in at least one of the mates are required for cell-to-cell transfer.
FIG 3.
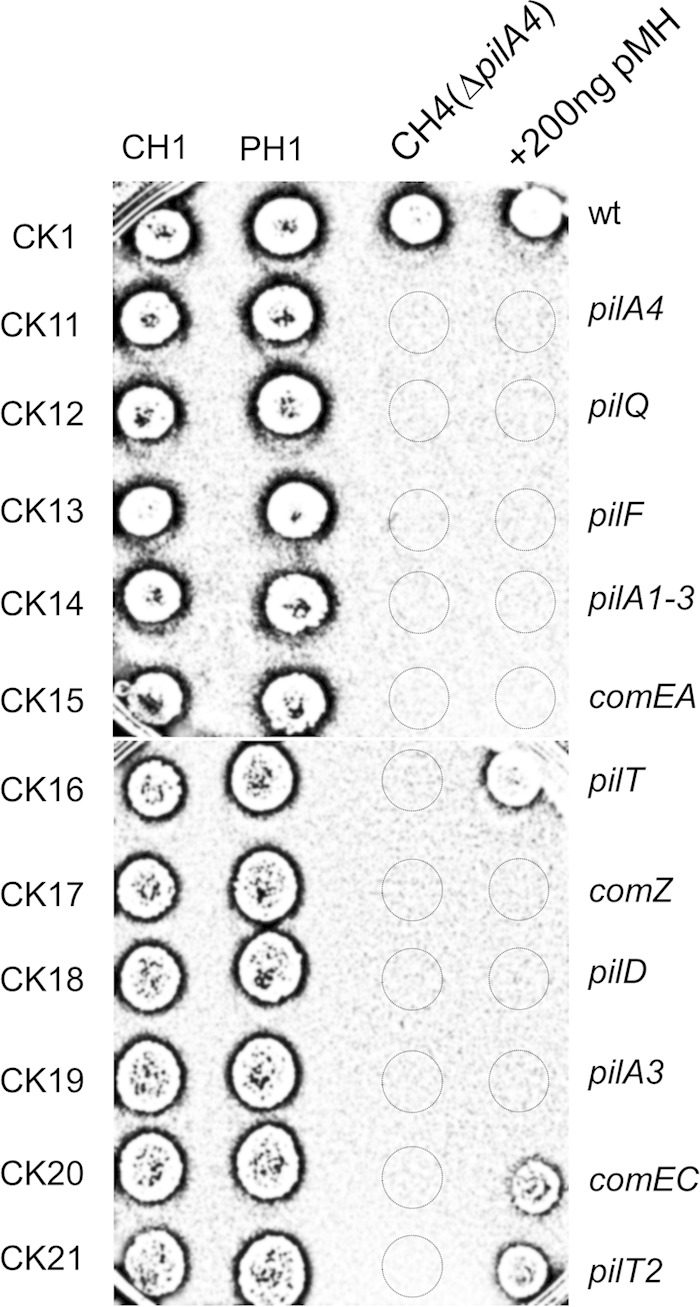
Competence genes are required for cell-to-cell DNA transfer. Selection plates with Hyg and Km were spotted in mating assays (1:1) between Km-resistant mutants in which the indicated components of the natural competence apparatus were affected (CK11 to CK21) and either of two competence-proficient strains, labeled in the chromosome (CH1) or the megaplasmid (PH1), or between CK11 to CK21 and another competence-deficient mutant (CH4). The results of transformation assays with a plasmid conferring Hyg resistance (pMH) are shown on the right. Note that most mutants are completely unable to take up DNA, whereas in others (e.g., CK16, CK20, and CK21), competence is still detectable. Other competence-defective mutants for which data are not shown here (pilO, pilW, pilM, pilN, and dprA mutants) yielded results similar to those for CK11.
To determine in which of the mates, donor or recipient, the competence system was required, we performed mating experiments between competence mutants of two different T. thermophilus strains, NAR1 and HB27, which can be phenotypically distinguished by differences in their respective patterns of membrane proteins (i.e., several traits encoded at different regions are followed simultaneously) (29). Thus, a competence-deficient HB27 (CH2) or NAR1 (CH3) strain was mated with an HB27 or NAR1 derivative proficient (CK1 or CK3, respectively) or deficient (CK11) in competence. As shown in Fig. 4A, plasmid transformation of strains CK11 (HB27 ΔpilA4::kat) (spot 4a), CH2 (HB27 ΔpilQ::hyg) (spot 3d), and CH3 (NAR1 ΔpilQ::hyg) (spot 2d) confirmed their deficiency in competence, whereas transformation was positive for strains CK3 (NAR1 gdh::kat) (spot 4b) and CK1 (HB27 gdh::kat) (spot 4c). When competence-deficient strain CH2 or CH3 was mated with the competence-proficient partner CK1 or CK3, we obtained transconjugants (Fig. 4A, spots 2b, 3b, 2c, and 3c). Thus, to analyze the parenthood of these transconjugant derivatives, we compared their whole membrane protein patterns. As shown in Fig. 4B, the membrane protein pattern of the transconjugants obtained after mating between a competent NAR1 strain (CK3) and a noncompetent HB27 mutant (CH2) is identical to the pattern obtained after mating between two NAR1 strains (the CK3 × CH3 cross). Conversely, the protein pattern of transconjugants after mating between a proficient HB27 strain (CK1) and a NAR1 competence mutant (CH3) is identical to the pattern observed for the mating between two HB27 derivatives (the CK1 × CH2 mating pair). Data for the complete experiment and controls are shown in Fig. S4 in the supplemental material. These data show that only competence-proficient strains can function as recipient strains in mating experiments.
FIG 4.
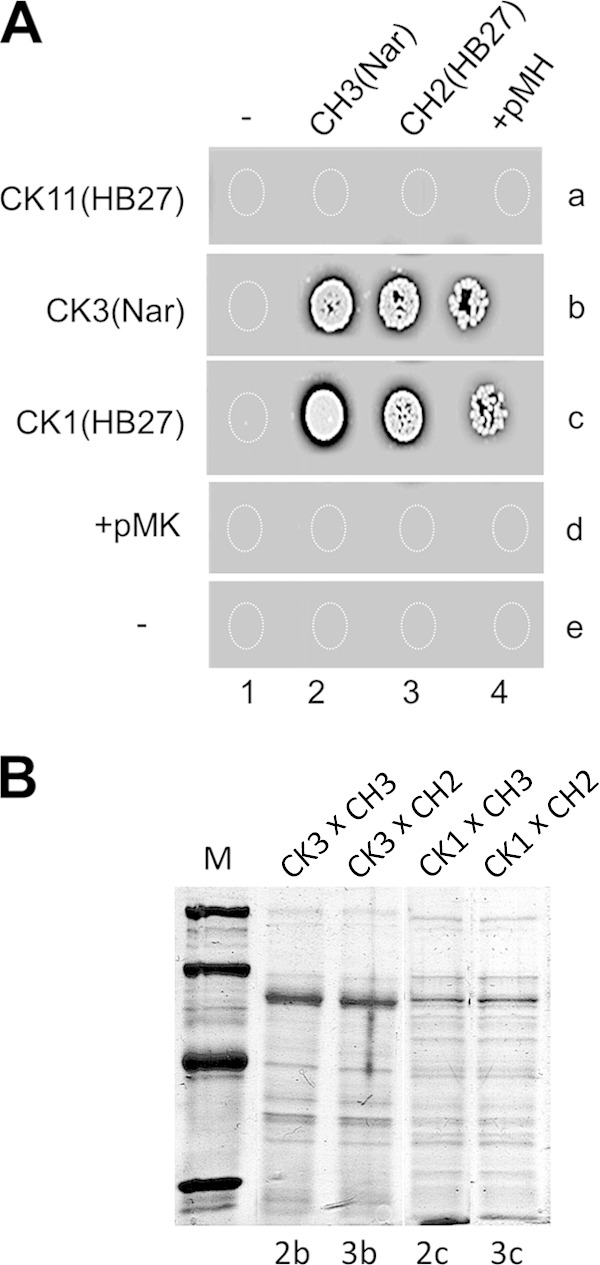
The competence system is required in the recipient but not in the donor cells. (A) A transformation-deficient derivative of strain HB27 (CK11, CH2) or NAR1 (CH3) was mated with the transformation-proficient derivative CK1 (HB27) or CK3 (NAR1). A total of 107 cells were spotted onto selective (Km and Hyg) plates either alone (−) or topped with the indicated mating counterpart or 200 ng of plasmid DNA conferring resistance to Km (+pMK) or Hyg (+pMH). (B) SDS-PAGE of whole membrane proteins from spots 2b (the CK3 × CH3 cross), 3b (the CK3 × CH2 cross), 2c (the CK1 × CH3 cross), and 3c (the CK1 × CH2 cross) of panel A. Note that the protein pattern always corresponds to the competence-proficient strain used in the mate: NAR1 for 2b and 3b, HB27 for 2c and 3c. Lane M, molecular size markers of 97.4, 66.2, 45, and 31 kDa.
The Argonaute protein distinguishes between DNA acquired by competence and DNA acquired by cell-to-cell transfer.
The presence of a homologue of the eukaryotic Argonaute protein in T. thermophilus (ttAgo) has been shown to protect the bacteria against plasmid DNA acquired by natural competence, leading to a decrease in the transformation efficiency by an order of magnitude (26). In order to know if ttAgo also protects the bacteria against DNA acquired by the cell-to-cell transfer process described here, we constructed a Δago (ΔTTP0026) mutant and labeled its chromosome at different positions with the Km (CK22) or Hyg (CH5) marker in two derivative strains that were used for mating. As shown in Fig. 5A, no significant differences in transfer efficiency were found in matings involving Δago or ago+ strains. In contrast, transformation of these strains with genomic DNA of the respective mating partners (high-G+C isogenic lineal DNA) revealed a decrease of an order of magnitude in transformation efficiency associated with the presence of the ttAgo protein (P, <0.001). Similar results were obtained when other pairs of ago+ and Δago counterpart mates were used (Fig. 5B). Therefore, despite the requirement for a functional competence system in the recipient cell, the way in which the DNA enters the cell by conjugation does not induce the ttAgo-mediated DNA-DNA interference mechanism that protects the cells from DNA acquired by natural competence.
FIG 5.
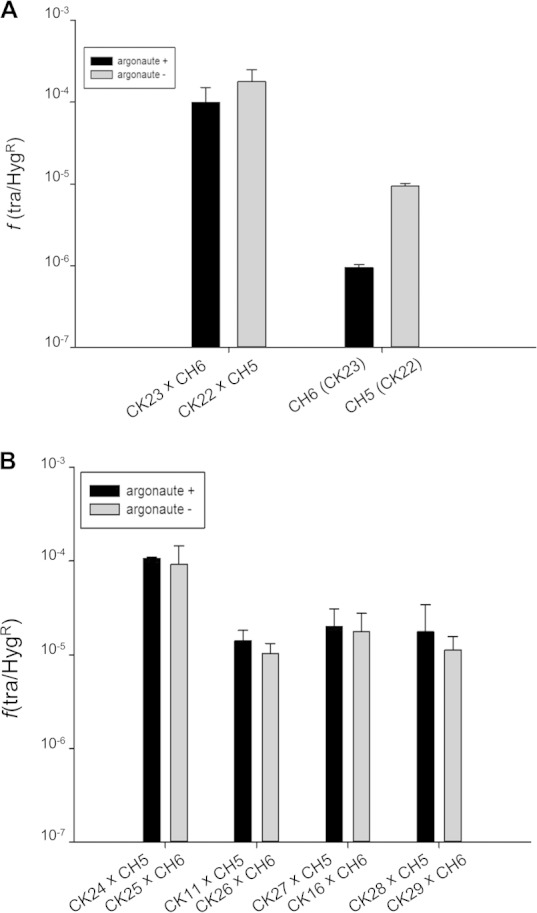
The ttAgo interference pathway is not activated by cell-to-cell DNA transfer. (A) Transfer frequencies were obtained after mating of equal cell amounts of Δago strains (the CK22 × CH5 mating pair) as well as ago+ strains labeled in the same genes (the CK23 × CH6 mating pair). Parallel transformation of strains CH5 and CH6 with 10 ng of genomic DNA isolated from the respective counterparts (CK22 and CK23) was carried out to show the Ago-mediated interference against high-G+C isogenic lineal DNA acquired by natural competence. Frequencies {ratios of Km- and Hyg-resistant transconjugants to the Hyg-resistant partner [f(tra/HygR)]} are averages of results from 9 independent experiments. Error bars correspond to the standard deviations of the means. Differences in transfer frequencies between Δago and ago+ matings were statistically nonsignificant (P, 0.501; n = 9). However, there were significant statistical differences between transformation frequencies (P, <0.001). (B) Transfer frequencies obtained after mating of equal cell amounts of CH5 (Δago) or CH6 (ago+) with Km-resistant mutants labeled in different chromosomal loci (CK11, CK16, CK24 to CK29) that were either Δago (shaded bars) or ago+ (filled bars). Frequencies are averages of results of 5 independent experiments. Error bars correspond to the standard deviations of the means. Differences in transfer frequencies between ago+ and Δago strains were not significant (P, 0.968). Likewise, no significant differences among locus groups or within each locus group could be detected (P, 0.339 and 0.105, respectively).
DISCUSSION
Most T. thermophilus strains contain a highly conserved chromosome (around 1.8 Mbp) and a very dynamic megaplasmid (230 to 400 kbp), which harbors most of the interstrain differences (23), including genes likely of archaeal or eukaryotic origin (24). It is believed that the extreme plasticity observed in these species is due to the presence of a highly efficient natural competence system (21, 22, 35) and that the concentration of such plasticity in the megaplasmid could be the result of counterselection of insertion in essential genes, which are mostly encoded in the chromosome. In contrast, our results suggest that concentration of foreign genes in the pTT27 megaplasmid could actually be associated with a preference for active cell-to-cell transfer of megaplasmid genes. In addition, we show that cell-to-cell DNA transfer does not elicit ttAgo-mediated DNA-DNA interference, which constitutes a major barrier against DNA acquired by natural competence.
Nature of the mating partners.
In several conjugation systems, DNA transfer takes place in an unidirectional way between a donor and a recipient mate (12, 15, 16). In this process, a mechanism of exclusion exists that prevents transfer between organisms that carry the same type of conjugative plasmids (36). However, we show here that in T. thermophilus, transfer takes place between completely isogenic strains. For this reason, the directionality of DNA transfer is difficult to assess, and simultaneous analysis of various phenotypes, such as membrane protein patterns, has to be used to confirm the parenthood of the transconjugants (Fig. 4B). This bidirectionality phenomenon is somehow similar to conjugative retrotransfer processes, where recipient cells can occasionally act as donor cells (37–40). However, in contrast to classical conjugative retrotransfer, where gene flow from recipient to donor is the consequence of two sequential rounds of DNA transfer in which the recipient becomes a secondary donor after obtaining DNA from the primary donor (39), the cell-to-cell DNA transfer system of T. thermophilus could a priori permit simultaneous bidirectional DNA transfer, resembling a hermaphroditism-like trait.
The cell-to-cell transfer described here is not restricted to isogenic strains. Actually, natural isolates of T. thermophilus that carry genes encoding the ability to respire nitrogen oxides anaerobically (PRQ25 or NAR1) can transfer such a property to the aerobic HB27 strain by cell-to-cell contacts (21, 41). In such cases, differences among membrane protein patterns provide the easiest way to identify donor and recipient strains, as shown in Fig. 4. In fact, a collection of T. thermophilus isolates actually behave as donors in mating experiments with strain HB27 (not shown), supporting the notion that this conjugative-like mechanism is widely distributed, at least within this species.
Requirement for competence genes.
The paradoxical aspect of the DNA transfer process described here is that while the DNA is transported from a donor to a recipient cell in a DNase I-resistant state, all the known components of the transformation machinery assayed are apparently required in the recipient cell (Fig. 3 and 4). A tentative explanation may be that the competence apparatus is required for pulling the DNA supplied by the donor cell, which, in turn, should have a DNA-pushing system apparently independent of competence. Therefore, we propose a two-step model for cell-to-cell conjugative transfer in T. thermophilus. The donor cell would actively transfer DNA to the recipient cell in a first, conjugation-like step, which does not require the transformation machinery. In the second, transformation-like step, the recipient cell would play an active role in pulling the transferred DNA to ease its passage into the cytoplasm, a process requiring the DNA transport machinery of the competence apparatus. This process is remarkably different from classical conjugation, where the recipient cell remains basically passive, waiting for DNA transfer (11).
An additional argument supporting the existence of a two-step push-pull mechanism is that it clearly favors the transfer of genes located in the megaplasmid over that of genes located in the chromosome (Fig. 2), despite their similar copy numbers. Actually, the genetic polymorphism observed in the megaplasmid could, to some extent, be related to this greater feasibility of transfer. The reasons underlying this preferential transfer of megaplasmid genes are not known, but it might be related to the presence of one or more yet unknown sequences acting as an origin of transfer, reminiscent of conjugative oriT sequences. In this scenario, the transfer of chromosomal genes could be the consequence of sporadic integration of the megaplasmid by recombination between insertion sequences (ISs), which are abundant in both elements (http://www-is.biotoul.fr/), as has been described for Hfr formation by the E. coli F factor (42). In this regard, previous data from our group suggest that some strains of T. thermophilus behave like Hfr strains (27), likely because such integration has become stable.
Similar generalized genomic transfer mechanisms have been described for Staphylococcus aureus, Mycobacterium smegmatis, and Streptococcus agalactiae (18, 43, 44). These transfer models differ from the classical Hfr model in that initiation of transfer is predicted to occur at multiple defined sites located throughout the genome. For S. aureus and S. agalactiae, conventional conjugation involves conjugative proteins (T4SS, a coupling protein, and a relaxase), which are responsible for the processive transfer of hundreds of kilobases of the bacterial chromosome (43, 44). In contrast, in M. smegmatis, DNA transfer is mediated by the virulence-associated locus esx-1 (45). In another example of unconventional conjugation models, Streptomyces conjugative transfer seems to depend exclusively on a single polar protein (17), which resembles the FtsK/SpoIIIE system (46). In the genus Thermus, the presence of a conspicuous conjugative T4SS is restricted to a few strains and species, including T. thermophilus strains SG0.5J (21) and JL-18 (NC_017590). However, neither strain HB27 nor any of its derivatives used in this work contain homologues of these classical conjugation-related genes. Likewise, no candidates for an oriT sequence, coupling protein (CP), or relaxase have been detected in their genomes by bioinformatic analysis. Hence, it is difficult to predict whether transfer initiation in the genus Thermus occurs at a single defined site or at multiple sites simultaneously. Nevertheless, the DNA to be transferred must first be secreted from the donor so that the recipient cell can pull it into the cytoplasm.
DNA discrimination by ttAgo.
As described in a previous article, the ttAgo protein is involved in a DNA-DNA interference mechanism that limits the entrance of DNA by natural competence (26). In that work, the ttAgo protein was shown in vitro to have a preference for A+T-rich regions in supercoiled plasmids. However, we show here that ttAgo is also able to protect the bacterium against the entrance of lineal isogenic DNA with a high G+C content (Fig. 5A). Such discrimination is not active when the same DNA is transferred by the cell-to-cell mechanism described here. Therefore, two hypotheses could explain this discrimination. Either the entry of DNA by competence stimulates specifically a putative ttAgo interference pathway, or the form in which DNA enters the cell makes the difference (i.e., double-stranded DNA versus single-stranded DNA, or the presence or absence of pilot proteins). In both cases, this discrimination system might have evolved to permit this very promiscuous bacterium to distinguish between potentially hazardous DNA of unknown origin and trustworthy DNA acquired from a reliable mate.
Supplementary Material
ACKNOWLEDGMENTS
This work has been supported by grant BIO2013-44963-R from the Spanish Ministry of Economy and Competence and FP7-PEOPLE-2012-IAPP grant 324439 from the European Union to J. Berenguer and by grant AV9/6-1 from the Deutsche Forschungsgemeinschaft to B. Averhoff. An institutional grant from the Fundación Ramón Areces to CBMSO and financial support to the Spanish National Network for Extremophilic Microorganisms (BIO2011-12879-E) are also acknowledged. An FPI fellowship for A. Blesa from the Ministry of Education is acknowledged. C. E. César thanks the Ministry of Science and Innovation for a Juan de la Cierva contract.
The contribution and advice of Carlos Bricio and Ralf Salzer are greatly appreciated.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02113-14.
REFERENCES
- 1.Koonin EV, Wolf YI. 2008. Genomics of bacteria and archaea: the emerging dynamic view of the prokaryotic world. Nucleic Acids Res 36:6688–6719. doi: 10.1093/nar/gkn668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain R, Rivera MC, Moore JE, Lake JA. 2003. Horizontal gene transfer accelerates genome innovation and evolution. Mol Biol Evol 20:1598–1602. doi: 10.1093/molbev/msg154. [DOI] [PubMed] [Google Scholar]
- 3.Lerat E, Daubin V, Ochman H, Moran NA. 2005. Evolutionary origins of genomic repertoires in bacteria. PLoS Biol 3:e130. doi: 10.1371/journal.pbio.0030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright NT, Raththagala M, Bruenger E, Schildbach J, Curtis JE, Krueger S. 2012. Towards the elucidation of antibiotic resistance transfer in bacteria: structural studies of the TraI protein, p 10–11. In Cappelletti RL. (ed), 2012 Accomplishments and opportunities. NIST special publication 1143. NIST Center for Neutron Research, Gaithersburg, MD. [Google Scholar]
- 5.Liu H, Fu Y, Li B, Yu X, Xie J, Cheng J, Ghabrial S, Li G, Yi X, Jiang D. 2011. Widespread horizontal gene transfer from circular single-stranded DNA viruses to eukaryotic genomes. BMC Evol Biol 11:276. doi: 10.1186/1471-2148-11-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubey GP, Ben-Yehuda S. 2011. Intercellular nanotubes mediate bacterial communication. Cell 144:590–600. doi: 10.1016/j.cell.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Marguet E, Gaudin M, Gauliard E, Fourquaux I, Du Plouy SLB, Matsui I, Forterre P. 2013. Membrane vesicles, nanopods and/or nanotubes produced by hyperthermophilic archaea of the genus Thermococcus. Biochem Soc Trans 41:436–442. doi: 10.1042/BST20120293. [DOI] [PubMed] [Google Scholar]
- 8.Juhas M, Van Der Meer JR, Gaillard M, Harding RM, Hood DW, Crook DW. 2009. Genomic islands: tools of bacterial horizontal gene transfer and evolution. FEMS Microbiol Rev 33:376–393. doi: 10.1111/j.1574-6976.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajon M, Fröls S, Van Wolferen M, Stoecker K, Teichmann D, Driessen AJ, Grogan DW, Albers SV, Schleper C. 2011. UV-inducible DNA exchange in hyperthermophilic archaea mediated by type IV pili. Mol Microbiol 82:807–817. doi: 10.1111/j.1365-2958.2011.07861.x. [DOI] [PubMed] [Google Scholar]
- 10.McDaniel LD, Young EC, Ritchie KB, Paul JH. 2012. Environmental factors influencing gene transfer agent (GTA) mediated transduction in the subtropical ocean. PLoS One 7:e43506. doi: 10.1371/journal.pone.0043506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Mendoza D, De La Cruz F. 2009. Escherichia coli genes affecting recipient ability in plasmid conjugation: are there any? BMC Genomics 10:71. doi: 10.1186/1471-2164-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wollman EL, Jacob F (ed). 1961. Sexuality and the genetics of bacteria. Academic Press Inc, London, United Kingdom. [Google Scholar]
- 13.Guglielmini J, Quintais L, Garcillan-Barcia MP, De La Cruz F, Rocha EP. 2011. The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. PLoS Genet 7:e1002222. doi: 10.1371/journal.pgen.1002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brochet M, Couvé E, Glaser P, Guédon G, Payot S. 2008. Integrative conjugative elements and related elements are major contributors to the genome diversity of Streptococcus agalactiae. J Bacteriol 190:6913–6917. doi: 10.1128/JB.00824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis BM, Waldor MK. 2013. Horizontal gene transfer: linking sex and cell fate. Curr Biol 23:R118–R119. doi: 10.1016/j.cub.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 16.de la Cruz F, Frost LS, Meyer RJ, Zechner EL. 2010. Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol Rev 34:18–40. doi: 10.1111/j.1574-6976.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- 17.Thoma L, Muth G. 2012. Conjugative DNA transfer in Streptomyces by TraB: is one protein enough? FEMS Microbiol Lett 337:81–88. doi: 10.1111/1574-6968.12031. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Karnati PK, Takacs CM, Kowalski JC, Derbyshire KM. 2005. Chromosomal DNA transfer in Mycobacterium smegmatis is mechanistically different from classical Hfr chromosomal DNA transfer. Mol Microbiol 58:280–288. doi: 10.1111/j.1365-2958.2005.04824.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu D, Hugenholtz P, Mavromatis K, Pukall R, Dalin E, Ivanova NN, Kunin V, Goodwin L, Wu M, Tindall BJ, Hooper SD, Pati A, Lykidis A, Spring S, Anderson IJ, D'haeseleer P, Zemla A, Singer M, Lapidus A, Nolan M, Copeland A, Han C, Chen F, Cheng JF, Lucas S, Kerfeld C, Lang E, Gronow S, Chain P, Bruce D, Rubin EM, Kyrpides NC, Klenk HP, Eisen JA. 2009. A phylogeny-driven genomic encyclopaedia of Bacteria and Archaea. Nature 462:1056–1060. doi: 10.1038/nature08656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyama Y, Hoshino T, Tomizuka N, Furukawa K. 1986. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J Bacteriol 166:338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.César CE, Alvarez L, Bricio C, Van Heerden E, Littauer D, Berenguer J. 2011. Unconventional lateral gene transfer in extreme thermophilic bacteria. Int Microbiol 14:187–199. doi: 10.2436/20.1501.01.148. [DOI] [PubMed] [Google Scholar]
- 22.Averhoff B. 2009. Shuffling genes around in hot environments: the unique DNA transporter of Thermus thermophilus. FEMS Microbiol Rev 33:611–626. doi: 10.1111/j.1574-6976.2008.00160.x. [DOI] [PubMed] [Google Scholar]
- 23.Brüggemann H, Chen C. 2006. Comparative genomics of Thermus thermophilus: plasticity of the megaplasmid and its contribution to a thermophilic lifestyle. J Biotechnol 124:654–661. doi: 10.1016/j.jbiotec.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 24.Omelchenko MV, Wolf YI, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Daly MJ, Koonin EV, Makarova KS. 2005. Comparative genomics of Thermus thermophilus and Deinococcus radiodurans: divergent routes of adaptation to thermophily and radiation resistance. BMC Evol Biol 5:57. doi: 10.1186/1471-2148-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarova KS, Wolf YI, Van Der Oost J, Koonin EV. 2009. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol Direct 4:29. doi: 10.1186/1745-6150-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swarts DC, Jore MM, Westra ER, Zhu Y, Janssen JH, Snijders AP, Wang Y, Patel DJ, Berenguer J, Brouns SJ, van der Oost J. 2014. DNA-guided DNA interference by a prokaryotic Arognaute. Nature 507:258–261. doi: 10.1038/nature12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramírez-Arcos S, Fernández-Herrero LA, Marin I, Berenguer J. 1998. Anaerobic growth, a property horizontally transferred by an Hfr-like mechanism among extreme thermophiles. J Bacteriol 180:3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennox EX. 1955. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Herrero LA, Olabarria G, Caston JR, Lasa I, Berenguer J. 1995. Horizontal transference of S-layer genes within Thermus thermophilus. J Bacteriol 177:5460–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 31.Vieira J, Messing J. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 32.de Grado M, Lasa I, Berenguer J. 1998. Characterization of a plasmid replicative origin from an extreme thermophile. FEMS Microbiol Lett 165:51–57. doi: 10.1111/j.1574-6968.1998.tb13126.x. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U, Favre M. 1973. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol 80:575–599. [DOI] [PubMed] [Google Scholar]
- 34.Ohtani N, Tomita M, Itaya M. 2010. An extreme thermophile, Thermus thermophilus, is a polyploid bacterium. J Bacteriol 192:5499–5505. doi: 10.1128/JB.00662-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedrich A, Hartsch T, Averhoff B. 2001. Natural transformation in mesophilic and thermophilic bacteria: identification and characterization of novel, closely related competence genes in Acinetobacter sp. strain BD413 and Thermus thermophilus HB27. Appl Environ Microbiol 67:3140–3148. doi: 10.1128/AEM.67.7.3140-3148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcillán-Barcia MP, De La Cruz F. 2008. Why is entry exclusion an essential feature of conjugative plasmids? Plasmid 60:1–18. doi: 10.1016/j.plasmid.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Heinemann JA, Ankenbauer RG. 1993. Retrotransfer in Escherichia coli conjugation: bidirectional exchange or de novo mating? J Bacteriol 175:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szpirer C, Top E, Couturier M, Mergeay M. 1999. Retrotransfer or gene capture: a feature of conjugative plasmids, with ecological and evolutionary significance. Microbiology 145:3321–3329. [DOI] [PubMed] [Google Scholar]
- 39.Sia EA, Kuehner DM, Figurski DH. 1996. Mechanism of retrotransfer in conjugation: prior transfer of the conjugative plasmid is required. J Bacteriol 178:1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ankenbauer RG. 1997. Reassessing forty years of genetic doctrine: retrotransfer and conjugation. Genetics 145:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez L, Bricio C, Gomez MJ, Berenguer J. 2011. Lateral transfer of the denitrification pathway genes among Thermus thermophilus strains. Appl Environ Microbiol 77:1352–1358. doi: 10.1128/AEM.02048-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guyer M, Reed R, Steitz J, Low K. 1981. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb Symp Quant Biol 45(Part 1):135–140. [DOI] [PubMed] [Google Scholar]
- 43.Brochet M, Rusniok C, Couve E, Dramsi S, Poyart C, Trieu-Cuot P, Kunst F, Glaser P. 2008. Shaping a bacterial genome by large chromosomal replacements, the evolutionary history of Streptococcus agalactiae. Proc Natl Acad Sci U S A 105:15961–15966. doi: 10.1073/pnas.0803654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson DA, Enright MC. 2004. Evolution of Staphylococcus aureus by large chromosomal replacements. J Bacteriol 186:1060–1064. doi: 10.1128/JB.186.4.1060-1064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coros A, Callahan B, Battaglioli E, Derbyshire KM. 2008. The specialized secretory apparatus ESX-1 is essential for DNA transfer in Mycobacterium smegmatis. Mol Microbiol 69:794–808. doi: 10.1111/j.1365-2958.2008.06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogelmann J, Ammelburg M, Finger C, Guezguez J, Linke D, Flotenmeyer M, Stierhof YD, Wohlleben W, Muth G. 2011. Conjugal plasmid transfer in Streptomyces resembles bacterial chromosome segregation by FtsK/SpoIIIE. EMBO J 30:2246–2254. doi: 10.1038/emboj.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grant SG, Jessee J, Bloom FR, Hanahan D. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A 87:4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cava F, Laptenko O, Borukhov S, Chahlafi Z, Blas-Galindo E, Gomez-Puertas P, Berenguer J. 2007. Control of the respiratory metabolism of Thermus thermophilus by the nitrate respiration conjugative element NCE. Mol Microbiol 64:630–646. doi: 10.1111/j.1365-2958.2007.05687.x. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez L, Bricio C, Hidalgo A, Berenguer J. 2014. Parallel pathways for nitrite reduction during anaerobic growth in Thermus thermophilus. J Bacteriol 196:1350–1358. doi: 10.1128/JB.01042-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedrich A, Rumszauer J, Henne A, Averhoff B. 2003. Pilin-like proteins in the extremely thermophilic bacterium Thermus thermophilus HB27: implication in competence for natural transformation and links to type IV pilus biogenesis. Appl Environ Microbiol 69:3695–3700. doi: 10.1128/AEM.69.7.3695-3700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cava F, De Pedro MA, Schwarz H, Henne A, Berenguer J. 2004. Binding to pyruvylated compounds as an ancestral mechanism to anchor the outer envelope in primitive bacteria. Mol Microbiol 52:677–690. doi: 10.1111/j.1365-2958.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 52.Cava F, De Pedro MA, Blas-Galindo E, Waldo GS, Westblade LF, Berenguer J. 2008. Expression and use of superfolder green fluorescent protein at high temperatures in vivo: a tool to study extreme thermophile biology. Environ Microbiol 10:605–613. doi: 10.1111/j.1462-2920.2007.01482.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


