Abstract
Background
Laiwu District is recognized as a hyper-endemic region for scrub typhus in Shandong Province, but the seriousness of this problem has been neglected in public health circles.
Methodology/Principal Findings
A disability-adjusted life years (DALYs) approach was adopted to measure the burden of scrub typhus in Laiwu, China during the period 2006 to 2012. A multiple seasonal autoregressive integrated moving average model (SARIMA) was used to identify the most suitable forecasting model for scrub typhus in Laiwu. Results showed that the disease burden of scrub typhus is increasing yearly in Laiwu, and which is higher in females than males. For both females and males, DALY rates were highest for the 60–69 age group. Of all the SARIMA models tested, the SARIMA(2,1,0)(0,1,0)12 model was the best fit for scrub typhus cases in Laiwu. Human infections occurred mainly in autumn with peaks in October.
Conclusions/Significance
Females, especially those of 60 to 69 years of age, were at highest risk of developing scrub typhus in Laiwu, China. The SARIMA (2,1,0)(0,1,0)12 model was the best fit forecasting model for scrub typhus in Laiwu, China. These data are useful for developing public health education and intervention programs to reduce disease.
Author Summary
Scrub typhus, also known as tsutsugamushi disease, is a zoonosis transmitted by chigger bites (larval trombiculid mites) and the pathogen Orientia tsutsugamushi (O. tsutsugamushi), a Gram-negative obligate intracellular bacterium. It is distributed widely in the Pacific regions of Asia, and the islands of the western Pacific and Indian Oceans. People with outdoor activities that involve contact with grasses or shrubs are at highest risk. Scrub typhus has existed in Southern China for thousands of years, but it has been noted to spread from the South to the North of China in recent decades. Though this research we studied the disease burden of scrub typhus with disability-adjusted life years (DALYs), and developed a forecasting time series model for human clinical disease in Laiwu, China. Results demonstrated that the disease burden of scrub typhus was increasing year by year in Laiwu, and it was higher in females than males. Moreover, DALY rates in females and males were highest for persons in the 60–69 years age group. Of all the seasonal autoregressive integrated moving average (SARIMA) models tested, the SARIMA(2,1,0)(0,1,0)12 model was the best fit for scrub typhus cases in Laiwu. The disease occurred mainly in autumn, with a peak in October.
Introduction
Scrub typhus, also known as tsutsugamushi disease, is a zoonosis transmitted by chigger bites (larval trombiculid mites) and infection with Orientia tsutsugamushi (O. tsutsugamushi), a Gram-negative obligate intracellular bacterium. Globally, scrub typhus is distributed widely in the Pacific region of Asia. It is prevalent in a triangle from Northern Japan and far-eastern Russia in the north, to Northern Australia in the south, to Pakistan and Afghanistan in the west, and also involves islands of the western Pacific and Indian Oceans [1]–[3].
Scrub typhus has existed in China for thousands of years. Before 1986, the disease was found only in Southern China, with epidemics occurring mainly in summer [4]. Beginning in 1986, scrub typhus was also reported in areas of Northern China, such as Shandong Province, Tianjin City, Heilongjiang Province, Shanxi Province and Hebei Province [5]. Clinical infections in Northern China seemed to occur mainly in autumn and winter [4].
Shandong Province is most noted to the one of the most serious foci for scrub typhus in Northern China [5]. In 1986, the first outbreak of scrub typhus occurred in Linyi District, followed other outbreaks in Jinan in 1988, in Jining in 1996, in Yantai in 1997, in Weifang and in Tai′an in 2000 [6]. From 2006 to 2012, a total of 2337 scrub typhus cases were notified in the Diseases Reporting Information System of Shandong Center for Disease Control and Prevention. At present, 13 of 17 districts have reported scrub typhus cases in Shandong Province where Laiwu is the district of the highest scrub typhus incidence.
In 1999, the earliest cases of scrub typhus were documented in Laiwu District [7]. Since 2006, scrub typhus cases have been included in the Notifiable Infectious Diseases System managed by Shandong Center for Disease Control and Prevention. From 2006 to 2012, 308 cases were detected in Laiwu (134 males and 174 females). The average annual incidence of scrub typhus in Laiwu (3.59/100,000) was approximately ten times of that in entire Shandong Province (0.35/100,000). However, researchers and health authorities have more often focused their attentions to Linyi, the initial focus of scrub typhus in Shandong, where the annual incidence was 0.89/100,000. As a new focus of scrub typhus in Northern China, Laiwu (117°19′-117°58′E,36°02′-36°33′N) lies in the center of Shandong Province, an important geographical position in Shandong. The population of Laiwu was 1,226,393.
Incidence, prevalence, duration and mortality indicators were most frequently used to estimate the burden of disease [8]. Of available indexes, we highlight disability-adjusted life years (DALYs) to measure the disease burden of scrub typhus in Laiwu, China. The DALYs metric was jointly developed by the World Bank, Harvard School of Public Health and the World Health Organization (WHO) for the Global Burden of Disease and Injury Study(GBD) [9]. The European Centre for Disease Prevention and Control (ECDC) had adopted an incidence- and pathogen-based DALYs approach to measure the Burden of Communicable Diseases in Europe Project (BCoDE) across European Member States [10]–[12].
DALY, a summary metric of population health, is often used to identify health gaps by measuring the state of a population's health compared to a normative goal which is for individuals to live the standard life expectancy in full health. One DALY means one-year loss of ‘healthy’ life. It integrates disease-specific mortality, morbidity and severity together, and quantifies morbidity associated with different clinical outcomes by assigning disability weights on a scale between 0 and 1, where by 0 means no morbidity and 1 means death [13]. DALYs and DALY rates (DALYs per 100,000 population or DALYs per 1000 population) [8], [14], are often used to compare disease burden of the same disease among regions, areas, countries, districts [15], or disease burden of different diseases in the same place [16]. Moreover, DALYs and DALY rate could be used to identify high risk population for targeted interventions or intervention prioritization. In this research, DALYs and DALY rate were adopted to evaluate the disease burden of scrub typhus in Laiwu, China.
Epidemic modeling and forecasting is more recognized as an essential tool in preventing and controlling infectious disease. Cases of scrub typhus in Laiwu, China seem to have considerable variation during the period 2006 to 2012. A seasonal time series autoregressive integrated moving average (SARIMA) modeling introduced by Box and Jenkins [17] is most useful in examining data for seasonal or periodic fluctuations that recur with about the same intensity each year. SARIMA models have been successfully applied for forecasting economic, marketing, and social problems. While this model has the advantage of accurate forecasting over short periods, it has a limitation that at least 50 observations are needed [17]. In this study, we sought to identify the most suitable SARIMA model we could use to predict scrub typhus cases in Laiwu over time.
By combined the results of disease burden measured by DALYs and DALY rate with a SARIMA forecasting model of scrub typhus, we hoped to identify high-risk populations and interventions or intervention prioritization of scrub typhus. The research could help health authorities to prevent and control of scrub typhus efficiently.
Materials and Methods
Data of disease and population
The dataset of the human scrub typhus cases in Laiwu, China from 2006 to 2012 was obtained from the Diseases Reporting Information System of Shandong Center for Disease Control and Prevention. The notification system recorded the detailed information for the scrub typhus cases, including gender, age, dates of symptom onset and diagnosis, and recovery or death. The symptom onset date was used in this study, and it was thought to be more useful than the date of diagnosis or the date of notification. The cases recorded were the anonymized in this study. Population data for Laiwu was obtained from the Laiwu Statistical Bureau and stratified by age group and gender [18].
Calculation of DALYs and DALY rate
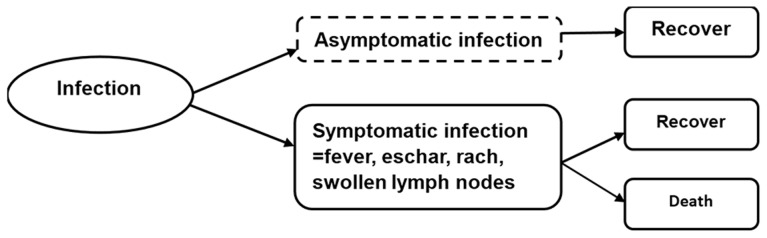
In this research, we adopted the incidence- and pathogen-based DALYs approach [10]–[13] to estimate the disease burden of scrub typhus in Laiwu, China. This approach has been previously used in the BcoDE project by ECDC. Fig. 1 shows a disease outcome tree for Orientia tsutsugamushi infection. All cases of scrub typhus in this study recovered for their illness.
Figure 1. The Outcome tree for Orientia tsutsugamushi.
DALYs were the sum of years of life lost due to premature death (YLLs) and years lived with disability (YLDs) [19]. According to the outcome tree, YLDs were calculated for each health outcome (l) by multiplying the number of incident cases (n) with the disability weight (w) for a specific health outcome (l), and the duration of the disabling condition (t) [see equation (1)]. All input parameters in both YLDs and YLLs formula were chosen to be age (a) and sex (s) dependent when such information was available, where a stands for age at infection and ã for age at onset of a condition or death [11], [12].
| (1) |
To estimate the YLLs for those health outcomes (l) that can lead to death, the number of fatal cases (d) for a specific health outcome (i) for an infection acquired at age (a) is multiplied by the remaining life expectancy (e) at age ã [see equation (2)].
| (2) |
In this research, the Coale and Demeny West Level 26 Life Table adopted in many disease burden studies was used in the calculation of YLLs to assure comparability to other disease burden assessments [20]. Life expectancy for males and females at birth were set at 80 and 82.5 years, respectively. The average durations of scrub typhus in different age groups and gender were achieved by DisMod II software developed for the calculation of GBD [21]. DisMod II is powered by two basic inputs: the make-up of a region's population by gender and age, and the overall mortality rate for each demographic group. This model stratifies the relationships between a set of indicators relevant by age and gender: incidence, prevalence, remission, mortality, duration, case fatality, and RR mortality (the relative risk on total mortality) [22]. It requires powering with a minimum of three of these variables (by age groups and gender) and these three variables allow the prediction of the others [22]. Comparing the internal epidemiological consistency of estimates of incidences, prevalence, duration and mortality, we found that when inputting the variables were incidence, remission (100%), and case fatality of scrub typhus clarified by age and gender, the outputs fitted well. Thus, the average durations for scrub typhus in gender and different age groups were obtained (Table 1).
Table 1. The duration (year) of scrub typhus in different age groups and gender in Laiwu, China.
| Gender | Age (years old) | |||||||
| 0–4 | 5–14 | 15–29 | 30–44 | 45–59 | 60–69 | 70–79 | 80+ | |
| Male | 0.9990 | 0.9996 | 0.9992 | 0.9975? | 0.9926 | 0.9769 | 0.9367 | 0.8042 |
| Female | 0.9990 | 0.9998 | 0.9994 | 0.9988 | 0.9959 | 0.9832 | 0.9557 | 0.9061 |
No paper has previously reported the disability weight of scrub typhus, including “Global Burden of Disease 2004 Update: Disability Weights for Diseases and Conditions,” the widely adopted document about disability weights of disease [13], [16], [23]. Scrub typhus and dengue fever have so many similarities in duration, high-risk population, signs and symptoms, pathogenesis and prognosis [1], [24]–[26] that disability weight of dengue fever (0.197) [13] was referred in the research of scrub typhus.
According to BCoDE-project, these raw incidence data should be corrected for underestimation by pathogen-specific multiplication factors (MF), representing either correction for underestimation in one step, or separate correction for under-ascertainment and underreporting in two steps [12], [27]. The overall extent of underestimation can be explained by two major effects represented by under-ascertainment and under-reporting [27]. Since one person infected with Orientia tsutsugamushi will demonstrate acute symptoms, such as fever, rash, eschar and swollen lymph nodes, the person is likely to visit a doctor, and due to physical awareness, scrub typhus is relatively easy to diagnosis in Laiwu, China. Moreover, as a notifiable disease in Laiwu, scrub typhus must be reported to the Diseases Reporting Information System of Shandong Center for Disease Control and Prevention. So, under-ascertainment and under-reporting of scrub typhus in Laiwu was thought to be minimal and not included in the research. We used “1” as the MF value of scrub typhus in Laiwu, China in the research.
SARIMA model
Many time series data contain seasonal periodic components. To deal with seasonality, a general multiplicative SARIMA model was extended from the ARIMA model (Box et al) [28]. This building process of the SARIMA (p,d,q)(P,D,Q)s model was designed to take advantage of associations in the sequentially lagged relationships which usually exist in periodically collected data. Seven main parameters are selected when fitting a SARIMA model: p, the order of process autoregression; d, the order of difference; q, the order of process moving average; P, D and Q, the corresponding seasonal orders; s, the length of seasonal period. If d is nonzero, a general differencing can be used to remove trend. If D is not zero, seasonal differencing can be used to remove seasonality. In order to construct and validate the model, the database of scrub typhus was verified by dividing the data file into two data sets, i.e. the data between January and December 2006–2011 and data between January and December 2012.
The original series of scrub typhus cases was not a stable time series, so it was necessary to make it stable by differential. After general difference of 1 order, followed by seasonal difference of 1 order and length of seasonal period was 12, the series was satisfied with stability. Thus d = 1, D = 1, S = 12. Then the order of autoregression and moving average were identified using autocorrelation function (ACF) and partial autocorrelation function (PACF) of the differenced series. The most suitable model was selected on the basis of Normalized Bayesian Information Criteria (BIC) [29] and Ljung-Box test. Lower values of Normalized BIC and Ljung-Box test (higher significant) were preferable. Furthermore, Ljung-Box test was performed to test if ACF of the residuals at different lag times were significantly different from zero, where no different from zero was expected [30]. Compare the predicted values in 2012 using the most suitable SARIMA model with the number of scrub typhus cases notified in 2012 to validate the forecasting ability of the model. The analyses were carried out with STATA version 12.0 (Stata Corporation, College Station, USA).
Results
DALYs, DALY rate and incidence of scrub typhus in Laiwu, China
No cases of death were reported in Laiwu from 2006 to 2012, so DALYs of scrub typhus in Laiwu was equal to YLDs. From 2006 to 2012, DALYs of scrub typhus were 5, 10, 10, 5, 8,10 and 13, respectively. The average annual DALYs of scrub typhus was 9, and DALYs were higher in females (5) than males (4) (Table 2).
Table 2. DALYs of scrub typhus in different age groups and gender in Laiwu, China (2006–2012).
| Year | Gender | Age(years) | Total | |||||||
| 0–4 | 5–14 | 15–29 | 30–44 | 45–59 | 60–69 | 70–79 | 80+ | |||
| 2006 | M* | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| F* | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 3 | |
| B* | 0 | 0 | 0 | 1 | 3 | 1 | 0 | 0 | 4 | |
| 2007 | M* | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 4 |
| F* | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 0 | 6 | |
| B* | 1 | 1 | 0 | 1 | 4 | 2 | 1 | 0 | 10 | |
| 2008 | M* | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 5 |
| F* | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 5 | |
| B* | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 0 | 10 | |
| 2009 | M* | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 3 |
| F* | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | |
| B* | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 5 | |
| 2010 | M* | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 4 |
| F* | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 4 | |
| B* | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 0 | 8 | |
| 2011 | M* | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 4 |
| F* | 0 | 0 | 1 | 1 | 1 | 2 | 1 | 0 | 6 | |
| B* | 0 | 1 | 1 | 2 | 2 | 3 | 1 | 0 | 10 | |
| 2012 | M* | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 0 | 6 |
| F* | 0 | 0 | 1 | 1 | 3 | 1 | 1 | 0 | 7 | |
| B* | 0 | 0 | 1 | 2 | 5 | 3 | 2 | 0 | 13 | |
| Mean | M* | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 4 |
| F* | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 | |
| B* | 1 | 1 | 0 | 1 | 3 | 2 | 1 | 0 | 9 | |
* M, males; F, females; B, both males and females.
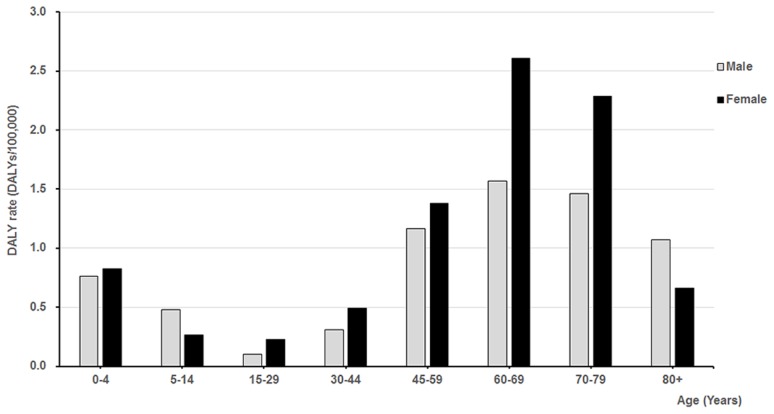
DALY rates (DALYs/100,000) of scrub typhus were 0.3663, 0.7933, 0.8106, 0.4437, 0.6179, 0.7883 and 1.0618 from 2006 to 2012, respectively. The average annual DALY rate was 0.6974 DALYs/100,000. The DALY rate was higher among females (0.8019 DALYs/1 00,000) than among males (0.5961DALYs/100,000). Moreover, DALY rates in females and males were both highest for the 60–69 years age group (Table 3). Fig. 2 shows the breakdown of average DALY rates of scrub typhus in different age groups and gender in Laiwu. Since all cases of scrub typhus were cured in Laiwu, no sequelae were recorded.
Table 3. DALY rate (DALYs/100,000) of scrub typhus in different age groups and gender in Laiwu, China (2006–2012).
| Year | Gender | Age(years) | Total | |||||||
| 0–4 | 5–14 | 15–29 | 30–44 | 45–59 | 60–69 | 70–79 | 80+ | |||
| 2006 | M* | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.8696 | 0.9135 | 0.9418 | 0.0000 | 0.2486 |
| F* | 0.0000 | 0.0000 | 0.1464 | 0.5581 | 1.4550 | 0.4793 | 0.0000 | 0.0000 | 0.4877 | |
| B* | 0.0000 | 0.0000 | 0.0722 | 0.2737 | 1.1562 | 0.7011 | 0.4194 | 0.0000 | 0.3663 | |
| 2007 | M* | 0.4863 | 1.1923 | 0.0000 | 0.1073 | 1.5655 | 0.9135 | 0.9033 | 0.0000 | 0.5943 |
| F* | 1.0486 | 0.2637 | 0.1464 | 0.5581 | 1.8182 | 3.8496 | 2.2917 | 2.3098 | 0.9987 | |
| B* | 0.7569 | 0.7513 | 0.0722 | 0.3284 | 1.6892 | 2.3501 | 1.6734 | 1.4937 | 0.7933 | |
| 2008 | M* | 1.4588 | 0.7154 | 0.1426 | 0.5366 | 1.3910 | 0.4593 | 1.8836 | 4.0058 | 0.7482 |
| F* | 2.0950 | 0.2637 | 0.2930 | 0.7812 | 1.2733 | 2.4003 | 0.7510 | 0.0000 | 0.8750 | |
| B* | 1.7650 | 0.5009 | 0.2167 | 0.6565 | 1.3334 | 1.4090 | 1.2554 | 1.4153 | 0.8106 | |
| 2009 | M* | 1.4588 | 0.7154 | 0.0000 | 0.1072 | 0.8703 | 0.9186 | 0.9033 | 0.0000 | 0.4690 |
| F* | 1.0486 | 0.2637 | 0.0000 | 0.2232 | 0.3642 | 0.9624 | 3.0620 | 0.0000 | 0.4176 | |
| B* | 1.2613 | 0.5009 | 0.0000 | 0.1641 | 0.6225 | 0.9400 | 2.1006 | 0.0000 | 0.4437 | |
| 2010 | M* | 0.9725 | 0.4769 | 0.1425 | 0.3217 | 0.6963 | 2.7406 | 0.0000 | 0.0000 | 0.5634 |
| F* | 1.5728 | 0.0000 | 0.0000 | 0.2232 | 1.2727 | 2.3967 | 3.0233 | 0.0000 | 0.6742 | |
| B* | 1.2614 | 0.2504 | 0.0722 | 0.2734 | 0.9785 | 2.5723 | 1.6769 | 0.0000 | 0.6179 | |
| 2011 | M* | 0.4863 | 0.2385 | 0.1426 | 0.5362 | 0.6944 | 1.3728 | 2.7869 | 3.4995 | 0.5830 |
| F* | 0.0000 | 0.5274 | 0.5858 | 0.5582 | 0.9098 | 5.2840 | 3.0814 | 0.0000 | 1.0001 | |
| B* | 0.2522 | 0.3757 | 0.3612 | 0.5470 | 0.7999 | 3.2865 | 2.9503 | 1.2364 | 0.7883 | |
| 2012 | M* | 0.4863 | 0.0000 | 0.2851 | 0.5363 | 2.0869 | 3.6642 | 2.8254 | 0.0000 | 0.9666 |
| F* | 0.0000 | 0.5274 | 0.4393 | 0.5582 | 2.5470 | 2.8948 | 3.8131 | 2.3098 | 1.1602 | |
| B* | 0.2522 | 0.2505 | 0.3612 | 0.5470 | 2.3122 | 3.2877 | 3.3732 | 1.4937 | 1.0618 | |
| Mean | M* | 0.7641 | 0.4769 | 0.1018 | 0.3065 | 1.1677 | 1.5689 | 1.4634 | 1.0721 | 0.5961 |
| F* | 0.8236 | 0.2637 | 0.2301 | 0.4943 | 1.3772 | 2.6095 | 2.2888 | 0.6599 | 0.8019 | |
| B* | 0.7928 | 0.3756 | 0.1651 | 0.3986 | 1.2703 | 2.0781 | 1.9212 | 0.8056 | 0.6974 | |
*M, males; F, females; B, both males and females.
Figure 2. The breakdown of average DALY rate of scrub typhus in different age groups and gender in Laiwu, China (2006–2012).
Scrub typhus was an acute illness. YLLs were zero. No sequelae.
The annual incidence (/100,000) of scrub typhus was 1.88, 4.08, 4.16, 2.28, 3.18, 4.08, and 5.46 from 2006 to 2012, respectively. The average annual incidence was 3.59/100,000. There was no statistical difference between incidence of scrub typhus in males and females each year (2006–2012)(P>0.05). Related x 2 and P values were showed in Table 4. But, the average annual incidence in females (4.11/100,000)was higher than males (3.07/100,000) (x 2 = 5.850, P = 0.016). The average annual incidence was the highestin 60–69 age group (Table 4).
Table 4. Incidence (/100,000) of scrub typhus in different age groups and gender in Laiwu, China (2006–2012).
| Year | Gender | Age(years) | Total | x 2 (P) | |||||||
| 0–4 | 5–14 | 15–29 | 30–44 | 45–59 | 60–69 | 70–79 | 80+ | ||||
| 2006 | M* | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 4.4469 | 4.7556 | 5.0467 | 0.0000 | 1.2844 | 2.357(0.125) |
| F* | 0.0000 | 0.0000 | 0.7438 | 2.8362 | 7.4170 | 2.4818 | 0.0000 | 0.0000 | 2.4854 | ||
| B* | 0.0000 | 0.0000 | 0.3669 | 1.3909 | 5.9011 | 3.6430 | 2.2476 | 0.0000 | 1.8754 | ||
| 2007 | M* | 2.4708 | 6.0545 | 0.0000 | 0.5459 | 8.0044 | 4.7556 | 5.0467 | 0.0000 | 3.0504 | 3.272(0.070) |
| F* | 5.3264 | 1.3388 | 0.7438 | 2.8362 | 9.2712 | 19.8546 | 12.1568 | 12.9400 | 5.1364 | ||
| B* | 3.8450 | 3.8150 | 0.3669 | 1.6691 | 8.6247 | 12.1434 | 8.9903 | 8.3682 | 4.0770 | ||
| 2008 | M* | 7.4123 | 3.6327 | 0.7242 | 2.7294 | 7.1151 | 2.3778 | 10.0934 | 23.6855 | 3.8532 | 0.284(0.594) |
| F* | 10.6527 | 1.3388 | 1.4877 | 3.9707 | 6.4899 | 12.4091 | 4.0523 | 0.0000 | 4.4737 | ||
| B* | 8.9718 | 2.5433 | 1.1008 | 3.3381 | 6.8090 | 7.2861 | 6.7427 | 8.3682 | 4.1585 | ||
| 2009 | M* | 7.4123 | 3.6327 | 0.0000 | 0.5459 | 4.4469 | 4.7556 | 5.0467 | 0.0000 | 2.4082 | 0.087(0.768) |
| F* | 5.3264 | 1.3388 | 0.0000 | 1.1345 | 1.8542 | 4.9636 | 16.2091 | 0.0000 | 2.1540 | ||
| B* | 6.4084 | 2.5433 | 0.0000 | 0.8345 | 3.1775 | 4.8574 | 11.2378 | 0.0000 | 2.2831 | ||
| 2010 | M* | 4.9415 | 2.4218 | 0.7242 | 1.6376 | 3.5575 | 14.2667 | 0.0000 | 0.0000 | 2.8899 | 0.335(0.563) |
| F* | 7.9896 | 0.0000 | 0.0000 | 1.1345 | 6.4899 | 12.4091 | 16.2091 | 0.0000 | 3.4795 | ||
| B* | 6.4084 | 1.2717 | 0.3669 | 1.3909 | 4.9932 | 13.3578 | 8.9903 | 0.0000 | 3.1801 | ||
| 2011 | M* | 2.4708 | 1.2109 | 0.7242 | 2.7294 | 3.5575 | 7.1333 | 15.1400 | 23.6855 | 3.0504 | 3.272(0.070) |
| F* | 0.0000 | 2.6777 | 2.9754 | 2.8362 | 4.6356 | 27.3000 | 16.2091 | 0.0000 | 5.1364 | ||
| B* | 1.2817 | 1.9075 | 1.8347 | 2.7818 | 4.0854 | 17.0008 | 15.7330 | 8.3682 | 4.0770 | ||
| 2012 | M* | 2.4708 | 0.0000 | 1.4484 | 2.7294 | 10.6726 | 19.0223 | 15.1400 | 0.0000 | 4.9770 | 0.548(0.459) |
| F* | 0.0000 | 2.6777 | 2.2315 | 2.8362 | 12.9797 | 14.8909 | 20.2614 | 12.9400 | 5.9649 | ||
| B* | 1.2817 | 1.2717 | 1.8347 | 2.7818 | 11.8022 | 17.0008 | 17.9806 | 8.3682 | 5.4632 | ||
| Mean | M* | 3.8826 | 2.4218 | 0.5173 | 1.5597 | 5.9716 | 8.1524 | 7.9305 | 6.7673 | 3.0734 | 5.850(0.016) |
| F* | 4.1850 | 1.3388 | 1.1689 | 2.5120 | 7.0196 | 13.4727 | 12.1568 | 3.6971 | 4.1186 | ||
| B* | 4.0281 | 1.9075 | 0.8387 | 2.0267 | 6.4847 | 10.7556 | 10.2746 | 4.7818 | 3.5878 | ||
*M, males; F, females; B, both males and females.
The trend of DALY rates of scrub typhus was consistent with incidences from 2006 to 2012 in Laiwu, China. Since 2009, DALY rates and the incidences were both increasing year by year (Table 3, 4). Fig. 2 showed that in 60–69 age group, the DALY rate in females was sharply higher than males.
SARIMA model
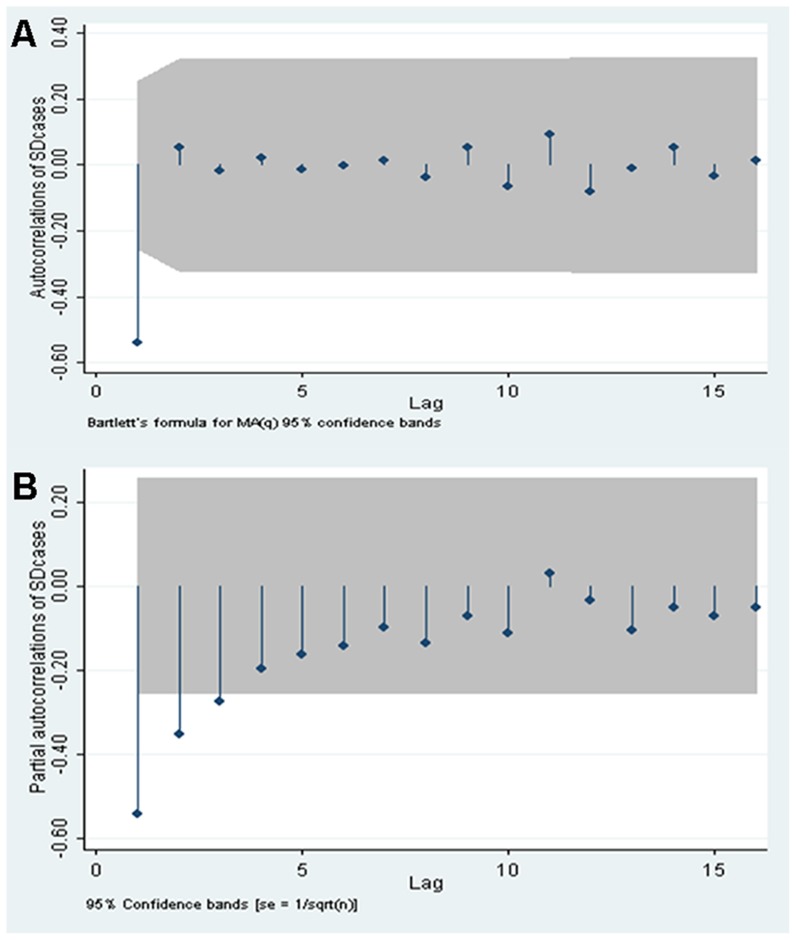
The series of notified cases was a non-stationary series. Therefore, by taking 1 order general difference, followed by 1 order seasonal difference and length of seasonal period was 12, the time series of scrub typhus cases was corrected into stationary series. Fig. 3 shows the autocorrelation function (ACF) and partial autocorrelation function (PACF) of scrub typhus cases in Laiwu, China after differencing. Based on the distribution characteristic, we conducted several models, SARIMA(2,1,0)(0,1,1)12, SARIMA(1,1,0)(1,1,1)12, SARIMA(0,1,0)(2,1,1)12, SARIMA(2,1,0)(0,1,0)12, SARIMA(1,1,0)(1,1,0)12, SARIMA(0,1,0)(2,1,0)12, SARIMA(2,1,1)(0,1,0)12 and SARIMA(2,1,1)(0,1,0)12. Of all the models tested, the SARIMA(2,1,0)(0,1,0)12 model was the best fit for the data (Table 5). Moreover, the Ljung-Box test suggested that the ACF of residuals for the model at different lag times was not significantly different from zero, i.e. the residuals of the SARIMA(2,1,0)(0,1,0)12 model was satisfied with white noise. The stationary residuals provided the evidence that the SARIMA(2,1,0)(0,1,0)12 model was adequate. All the coefficients of the SARIMA(2,1,0)(0,1,0)12model were significant (Table 5, 6). The equation of the SARIMA was 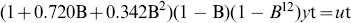 .
.
Figure 3. The autocorrelation function and partial autocorrelation function of differenced time series of scrub typhus cases in Laiwu, China.
A, Autocorrelation function; B, Partial autocorrelation function.
Table 5. Comparisons of the tested SARIMA models for scrub typhus cases in Laiwu, China.
| Model | Ljing-Box Q Statistics(P) | BIC* | Parameters(P) |
| SARIMA(2,1,0)(0,1,1)12 | 9.219(0.866) | 3.723 | AR, lag1(<0.001) |
| MA, lag 2(0.013) | |||
| MA Seasonal, lag 1(0.383) | |||
| SARIMA(1,1,0)(1,1,1)12 | 15.656(0.405) | 3.912 | AR, lag1(<0.001) |
| AR Seasonal, lag1(0.283) | |||
| MA Seasonal, lag 1(0.912) | |||
| SARIMA(0,1,0)(2,1,1)12 | 28.743(0.017) | 4.083 | AR Seasonal, lag1(<0.001) |
| AR Seasonal, lag2(<0.001) | |||
| MA Seasonal, lag 1(0.897) | |||
| SARIMA(2,1,0)(0,1,0)12 | 7.325(0.966) | 3.764 | AR, lag1(<0.001) |
| AR, lag2(<0.001) | |||
| SARIMA(1,1,0)(1,1,0)12 | 9.676(0.883) | 3.888 | AR, lag1(<0.001) |
| AR Seasonal, lag1(0.226) | |||
| SARIMA(0,1,0)(2,1,0)12 | 23.342(0.105) | 3.990 | AR Seasonal, lag1(0.001) |
| AR Seasonal, lag2(<0.001) | |||
| SARIMA(2,1,1)(0,1,0)12 | 1.056(1.000) | 3.638 | AR, lag1(0.766) |
| AR, lag2(0.823) | |||
| MA,lag1(0.009) | |||
| SARIMA(2,1,1)(0,1,0)12 | 0.679(1.000) | 3.634 | AR, lag1(0.771) |
| MA,lag1(0.945) | |||
| AR Seasonal, lag1(0.348) | |||
| SARIMA(0,1,1)(2,1,0)12 | 4.918(0.993) | 3.319 | MA,lag1(0.981) |
| AR Seasonal, lag1(<0.001) | |||
| AR Seasonal, lag2(<0.001) |
*BIC, Bayesian Information Criteria.
Table 6. Parameters estimated by a SARIMA(2,1,0)(0,1,0)12 model for scrub typhus cases in Laiwu, China.
| Coefficient | Std. err. | P | [95% Conf. interval] | |
| Constant | 0.023 | 0.411 | 0.060 | [−0.784,0.829] |
| AR1 | −0.720 | 0.072 | <0.001 | [−0.862,−0.578] |
| AR2 | −0.342 | 0.087 | <0.001 | [−0.513,−0.172] |
All the number of notified cases from Jan 2012 to Dec 2012 were in the 95% confidence interval of the forecasting values by the SARIMA(2,1,0)(0,1,0)12 model. The model was used to predict values from January to December 2012 for validation. The notified cases and fitted cases by the best fitted SARIMA model from 2006 to 2011, and the actual cases and predicted cases from January to December 2012, were illustrated in Fig. 4. It showed that the predicted values could follow the upturn and downturn of the observed series reasonably well. In addition, the fitted values appeared some negative values, which was a common case with the series with too many zeros as observed values in the series of scrub typhus cases. The SARIMA(2,1,0)(0,1,0)12 model showed that the prevailing disease occurred mainly in autumn and peaked in October.
Figure 4. Notified cases, model fit cases (2006–2011), predictive cases (2012) of scrub typhus by SARIMA(2,1,0)(0,1,0)12 model.
Discussion
Incidence is often used as one measure index of disease frequency, and since its calculation only concerns the cases number and basic population data, it must be standardized by some standard population when compared between different areas or different times. However, DALYs consider both survival time and life quality caused by a certain disease and its calculation requires age and sex dependent incident cases, fatal cases, severity of disease and life expectancy. Therefore, DALYs can demonstrate the threat of some disease to the whole population, and it is more objective and comprehensive than incidence [23]. In addition, standardization issues have been covered inherently in the calculation of DALYs [8], [14]–[16], [31], [32], such as different age groups and gender are assigned with different life expectancies and different diseases are assigned with different disability weights. So, DALYs and DALY rate could be compared among areas or diseases directly. From 2006 to 2012, the average annual DALYs of scrub typhus was 9, and DALY rate was 0.6974 DALYs/100,000 in Laiwu District. The average annual DALYs and DALY rate of scrub typhus were 15 and 0.1504 DALYs/100,000 in Linyi District, the initial foci of scrub typhus in Shandong Province. Populations of Linyi and Laiwu were 10,039,435 and 1,226,393, respectively. So, even though DALYs of scrub typhus was higher in Linyi than Laiwu, DALY rate was apparently higher in Laiwu than Linyin. Therefore, considering the limited health resources, more attention should be paid to Laiwu, a new focus of scrub typhus.
No cases of death were reported in Laiwu, China from 2006 to 2012, so the YLLs were 0 and the DALYs were equal to YLDs. There were several reasons why DALYs were adopted rather than solely YLDs. Worldwide acceptance of DALYs and DALY rate enabled international comparisons or ranking of areas by disease burden [8], [15], [16], [33]–[43]. DALYs represent unified summary estimate of disease burden that takes mortality as well as morbidity into consideration, i.e., DALYs and DALY rate would be more useful in comparing the disease burden among different diseases and different areas than other indexes. In addition, the levels of diagnosis and treatment of scrub typhus in Laiwu, China were high enough to cure all the patients, but there were still death cases in some other areas in China [44], [45]. For example, there were death cases of scrub typhus reported in Guangzhou, China in 2012. When comparing the disease burden of scrub typhus between these two districts, as a single metric DALYs is better than YLDs [44]. The DALYs of scrub typhus were equal to YLDs in Laiwu demonstrated that more attention should be paid to the unhealthy conditions of scrub typhus.
Since 2009, DALY rates and incidences of scrub typhus have both been increasing year by year (Table 3 and Table 4). With global warming, the predicted scenarios of increased temperature and rainfall were also causing concern for increases in vector-borne diseases, particularly, endemic arboviruses [46]. In 2010, Kim SH and Jang JY [47] reported that the incidence of scrub typhus in hyper-endemic region during the outbreak period was positively correlated with temperature and humidity during the summer. In addition, scrub typhus was transmitted to humans by the bites of chiggers which are mainly active in forest clearings, riverbanks, and grassy regions [47], [48]. With the increased urbanization and higher green coverage rate, people have more opportunity to contact chiggers. In 2012, there were several death cases of scrub typhus reported in Guangzhou, China and these patients had lied, stood, or sat on lawn in gardens before onset of the disease [44]. Green coverage rate achieved 42.19% and park green space per capita was 18.49 square meters in Laiwu, China in 2012 [49]. More suitable environment for chiggers' multiplication, more cases might appear in Laiwu.
From 15 to 80 years old, the average annual DALY rate and incidence of scrub typhus in females were both higher than males (Table 3, Table 4). Moreover, in the 60–69 years age group, females had a sharply higher DALY rate (2.6095 DALYs/100,000) than males (1.5689 DALYs/100,000) (Fig. 2). Thus, females, especially those from 60 to 69 years old, were the highest risk population for scrub typhus in Laiwu, China. As a zoonosis, scrub typhus was infected with O. tsutsugamushi, and rodents were the main host of O. tsutsugamushi. With increasing touch chance with O. tsutsugamushi, people in fields were easier to suffer from the disease [50]. Being a modern industrial area, Laiwu had become one home of numerous iron and steel businesses in China. Therefore, with more and more young and males moving from rural to urban areas, more and more elderly and females now engage in agriculture activity [18]. Additionally, with sparse awareness of the disease, the elderly have a greater chance of contact with chiggers [18]. Hence, health education efforts regards scrub typhus should be focused upon high risk groups like females who are 60–69 years old, especially those who live in countryside.
SARIMA modeling was useful for interpreting and applying surveillance data in disease control and prevention [51], [52]. As with many infectious diseases, the time series data of scrub typhus in Laiwu, China showed the components of trend and seasonal pattern. One of the most recognized disadvantages of this approach is the necessity of a large amount of data (i.e., a minimum of 50 observations) to build a reasonable SARIMA model [53]. In this research, monthly number of cases from 2006 to 2011 (72 observations) was used to build the SARIMA model, and monthly cases during the period of January to December in 2012 were used to validate the corresponding SARIMA model. The chosen SARIMA(2,1,0)(0,1,0)12 model fit the observations well and the residual series were satisfied with white noises. Therefore, the SARIMA(2,1,0)(0,1,0)12 model could be used to forecast the monthly cases of scrub typhus in Laiwu, China. The prevailing disease occurred in autumn and peaked in October, which suggests that education and other protective measures should occur just before October.
In the research, we adopted the incidence- and pathogen-based DALYs approach used by BcoDE Project to estimate the disease burden of scrub typhus rather than prevalence-based DALYs method presented in the GBD 2010. In case of an infectious pathogen, and in particular for priority settings of intervention to prevent primary infection, incidence is a more appropriate input for the DALYs metric than prevalence with the reason that only with the initial start of the infection is it possible to include all the disease squeals that result from the infection [12]. In addition, the incident cases of scrub typhus in Laiwu could be obtained from surveillance systems accurately. Considering above mentioned, the incidence- and pathogen-based DALYs were adopted in this research.
Limitations of this research should be acknowledged. The occurrence of scrub typhus was influenced by many factors such as pathogen prevalence, mites, rodents, human being's activities, and social or environmental factors. While building the SARIMA model, factors mentioned above were not considered. In addition, our research regarding disease burden and time series analysis of scrub typhus only focused on Laiwu, China. The results may not generalize to the most of China's population.
The disease burden of scrub typhus has increased year-by-year in Laiwu, China. Public health authorities should make concerted efforts to control and prevent the disease. The results of disease burden can also assist authorities in identifying the high-risk population. The SARIMA(2,1,0)(0,1,0)12 model developed in the research could offer prediction of scrub typhus monthly cases in Laiwu. Combined with disease burden measurement and SARIMA forecasting, these data should help official as a decision support tool in a scrub typhus risk management program and in planning various prevention efforts.
Acknowledgments
We thank Shandong Center for Disease Control and Prevention for providing the disease data. We thank Prof. Xue-Jie Yu, School of Public Health, Shandong University, for his helpful comments and suggestions. We thank Prof. Gregory C. Gray, Duke Infectious Diseases & Duke Global Health Institute, Duke University, for his review and helpful suggestions.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. The disease data are available from Shandong Center for Disease Control and Prevention Data Access for researchers who meet the criteria for access to confidential data. (tel: 86-531-82679681).
Funding Statement
The study was designed by the National Basic Research Program of China (973 Program http://124.127.202.241/Share/index.jsp, No. 2012CB955502) received by WM. Data collection and analysis, preparation of the manuscript were supported by the funds of Independent Innovation Foundation of Shandong University (No. 2012TS087) and Youth Innovation Foundation of School of Public health, Shandong University (No. 201101) which were received by LPY.
References
- 1. Jensenius M, Davis X, von Sonnenburg F, Schwartz E, Keystone JS, et al. (2009) Multicenter GeoSentinel analysis of rickettsial diseases in international travelers, 1996–2008. Emerg Infect Dis 15: 1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jensenius M, Montelius R, Berild D, Vene S (2006) Scrub typhus imported to Scandinavia. Scand J Infect Dis 38: 200–202. [DOI] [PubMed] [Google Scholar]
- 3. Razak A, Sathyanarayanan V, Prabhu M, Sangar M, Balasubramanian R (2010) Scrub typhus in Southern India: are we doing enough? Trop Doct 40: 149–151. [DOI] [PubMed] [Google Scholar]
- 4. Yu ES (1997) The epidemic characteristics of scrub typhus in China. Chinese Journal of Epidemiology 18: 56. [Google Scholar]
- 5. Zhang M, Wang XJ, Zhao ZT (2011) Current epidemic status and issues on prevention and control of scrub typhus. Chinese Journal of Disease Control and Prevention 32: 419–423. [PubMed] [Google Scholar]
- 6. Yang LP, Zhao ZT, Li Z, Wang XJ, Liu YX, et al. (2008) Comparative analysis of nucleotide sequences of Orientia tsutsugamushi in different epidemic areas of scrub typhus in Shandong, China. Am J Trop Med Hyg 78: 968–972. [PubMed] [Google Scholar]
- 7. Si PX, Qi JB (2002) Treatment of 43 cases of scrub typhus with qingying decoction subtraction. Shanxi Journal of Traditional Chinese Medicine 18: 27. [Google Scholar]
- 8. Borges M, Gouveia M, Costa J, Dos SPL, Paulo S, et al. (2009) The burden of disease attributable to smoking in Portugal. Rev Port Pneumol 15: 951–1004. [PubMed] [Google Scholar]
- 9. Murray CJ (1994) Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ 72: 429–445. [PMC free article] [PubMed] [Google Scholar]
- 10. Kretzschmar M, Mangen MJ, Pinheiro P, Jahn B, Fèvre EM, et al. (2012) New methodology for estimating the burden of infectious diseases in Europe. PLoS Med 9 (4) e1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plass D, Mangen MJ, Kraemer A, Pinheiro P, Gilsdorf A, et al.. (2014) The disease burden of hepatitis B, influenza, measles and salmonellosis in Germany: first results of the Burden of Communicable Diseases in Europe Study. Epidemiol Infect 2: ePub:1–12. [DOI] [PMC free article] [PubMed]
- 12. Mangen MJ, Plass D, Havelaar AH, Gibbons CL, Cassini A, et al. (2013) The Pathogen- and Incidence-Based DALY Approach: An Appropriated Methodology for Estimating the Burden of Infectious Diseases. PLoS One 8: e79740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. The global burden of disease: 2004 update [cited May 20, 2014]. http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/.
- 14. Ding D, Hong Z, Wang WZ, Wu JZ, de Boer HM, et al. (2006) Assessing the disease burden due to epilepsy by disability adjusted life year in rural China. Epilepsia 47: 2032–2037. [DOI] [PubMed] [Google Scholar]
- 15. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 16. Jankovic S, Vlajinac H, Bjegovic V, Marinkovic J, Sipetic-Grujicic S, et al. (2007) The burden of disease and injury in Serbia. Eur J Public Health 17: 80–85. [DOI] [PubMed] [Google Scholar]
- 17.Box GP, Jenkins GM (1976) Time series analysis: forecasting and control. San Francisco: Holden-Day. 784 p. [Google Scholar]
- 18.Laiwu Statistical Bureau. The Fifth Population Cencus in Laiwu District.[In Chinese, cited October 20, 2014].http://www.lwtjj.gov.cn/.
- 19. Murray CJ, Lopez AD (1994) Quantifying disability: data, methods and results. Bull World Health Organ 72: 481–494. [PMC free article] [PubMed] [Google Scholar]
- 20. Coale A, G Guo (1989) Revised regional model life tables at very low levels of mortality. Popul Index 55: 613–643. [PubMed] [Google Scholar]
- 21.Barendregt JJ (2001) DisMod II version 1.01. Global programme on evidence for health policy, World Health Organization.
- 22.(2001) Disease modeling using Dismod. In: Mathers CD, Vos T, Lopez AD, Salomon J, EzzatiM, editors. National Burden of Disease Studies: A Practical Guide. Edition 2.0. Global Program on Evidence for Health Policy. Geneva: World Health Organization. Pp. 64–83. [Google Scholar]
- 23.Lopez AD, Mathers CD, Ezzati M, Jamison DT, et al.. (2006) Global burden of disease and risk factors. Washington: Oxford University Press and the World Bank. [Google Scholar]
- 24. Watt G, Jongsakul K, Chouriyagune C, Paris R (2003) Differentiating dengue virus infection from scrub typhus in Thai adults with fever. Am J Trop Med Hyg 68: 536–538. [DOI] [PubMed] [Google Scholar]
- 25. Rajapakse S, Rodrigo C, Fernando D (2012) Scrub typhus: pathophysiology, clinical manifestations and prognosis. Asian Pac J Trop Med 5: 261–264. [DOI] [PubMed] [Google Scholar]
- 26. Ferreira GL (2012) Global dengue epidemiology trends. Rev Inst Med Trop Sao Paulo 54 Suppl 18S5–6. [DOI] [PubMed] [Google Scholar]
- 27. Gibbons CL, Mangen MJ, Plass D, Havelaar AH, Brooke RJ, et al. (2014) Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health 14: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alkasasbeh M, Raqab MZ (2009) Estimation of the generalized distribution parameters: comparative study. Statistical Methodology 6: 262–279. [Google Scholar]
- 29. Schwarz G (1978) Estimating the dimension of a model. Ann Statist 6: 461–464. [Google Scholar]
- 30.Brockwell P, Davis R (2002) Introduction to time series and forecasting. New York: Springer. 437 p. [Google Scholar]
- 31. Gaunt ER, Harvala H, McIntyre C, Templeton KE, Simmonds P (2011) Disease burden of the most commonly detected respiratory viruses in hospitalized patients calculated using the disability adjusted life year (DALY) model. J Clin Virol 52: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathers CD, Vos ET, Stevenson CE, Begg SJ (2001) The burden of disease and injury in Australia. Bull World Health Organ 79: 1076–1084. [PMC free article] [PubMed] [Google Scholar]
- 33. Costilla R, Tobias M, Blakely T (2013) The burden of cancer in New Zealand: a comparison of incidence and DALY metrics and its relevance for ethnic disparities. Aust N Z J Public Health 37: 218–225. [DOI] [PubMed] [Google Scholar]
- 34. Matsuda A, Katanoda K (2013) Estimated disability-adjusted life year (DALY) in all cancers in GLOBOCAN 2008, in Asia by the county. Jpn J Clin Onco l43: 943–944. [DOI] [PubMed] [Google Scholar]
- 35. Machii R, Saika K (2013) Estimated Disability-adjusted Life Year (DALY) in Asia in GLOBOCAN 2008. Jpn J Clin Oncol 43: 846–847. [DOI] [PubMed] [Google Scholar]
- 36. Saika K, Matsuda T (2013) Estimated disability-adjusted life year (DALY) in Japan in GLOBOCAN 2008. Jpn J Clin Oncol 43: 768–769. [DOI] [PubMed] [Google Scholar]
- 37. Hu G, Mamady K (2013) Alcohol-related road traffic injury and Global Burden of Disease 2010. Lancet 382: 1092–1093. [DOI] [PubMed] [Google Scholar]
- 38. Byass P, de Courten M, Graham WJ, Laflamme L, McCaw-Binns A, et al. (2013) Reflections on the global burden of disease 2010 estimates. PLoS Med 10: e1001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, et al. (2013) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382: 1575–1586. [DOI] [PubMed] [Google Scholar]
- 40. Murray CJ, Richards MA, Newton JN, Fenton KA, Anderson HR, et al. (2013) UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 381: 997–1020. [DOI] [PubMed] [Google Scholar]
- 41. Ortblad KF, Lozano R, Murray CJ (2013) The burden of HIV: insights from the Global Burden of Disease Study 2010. Aids 27: 2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, et al. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Struijk EA, May AM, Beulens JW, de Wit GA, Boer JM, et al. (2013) Development of methodology for disability-adjusted life years (DALYs) calculation based on real-life data. PLoS One 8: e74294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liao YZ, Ye XG, Zhuo SH (2013) Clinical treatment of 200 cases of scrub typhus. Guangdong Medical Journal 34: 735–737. [Google Scholar]
- 45. He S, Xie ZH, Chen Y, Chen L, Deng YQ, et al. (2011) Epidemiological characteristics of scrub typhus from 2006 to 2009 in Fujian Province. Chinese Journal of Disease Control and Prevention 15: 123–125. [Google Scholar]
- 46. Russell RC (1998) Vectors vs. humans in Australia–who is on top down under? An update on vector-borne disease and research on vectors in Australia. J Vector Ecol 23: 1–46. [PubMed] [Google Scholar]
- 47. Kim SH, Jang JY (2010) Correlations between climate change-related infectious diseases and meteorological factors in Korea. J Prev Med Public Health 43: 436–444. [DOI] [PubMed] [Google Scholar]
- 48. Kelly DJ, Fuerst PA, Ching WM, Richards AL (2009) Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis 48 Suppl 3S203–230. [DOI] [PubMed] [Google Scholar]
- 49.Laiwu Statistical Bureau. 2012 Laiwu Economic and Social Development Statistics Bulletin [in Chinese, cited May 20, 2014]. http://www.lwtjj.gov.cn/
- 50. Vliegenthart-Jongbloed K, de Mendonca MM, Slobbe L, Beersma MF, van Genderen PJ (2013) Imported scrub typhus in The Netherlands. Travel Med Infect Dis 11: 197–199. [DOI] [PubMed] [Google Scholar]
- 51. Abeku TA, de Vlas SJ, Borsboom G, Teklehaimanot A, Kebede A, et al. (2002) Forecasting malaria incidence from historical morbidity patterns in epidemic-prone areas of Ethiopia: a simple seasonal adjustment method performs best. Trop Med Int Health 7: 851–857. [DOI] [PubMed] [Google Scholar]
- 52. Hu W, Nicholls N, Lindsay M, Dale P, McMichael AJ, et al. (2004) Development of a predictive model for ross river virus disease in Brisbane, Australia. Am J Trop Med Hyg 71: 129–137. [PubMed] [Google Scholar]
- 53.Wei W (1990) Time series analysis. New York: Addison-Wesley. 642 p. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The disease data are available from Shandong Center for Disease Control and Prevention Data Access for researchers who meet the criteria for access to confidential data. (tel: 86-531-82679681).