Abstract
For over 70 years, researchers have debated whether the ability to use day length as a cue for the timing of seasonal events (photoperiodism) is related to the endogenous circadian clock that regulates the timing of daily events. Models of photoperiodism include two components: (1) a photoperiodic timer that measures the length of the day, and (2) a photoperiodic counter that elicits the downstream photoperiodic response after a threshold number of days has been counted. Herein, we show that there is no geographical pattern of genetic association between the expression of the circadian clock and the photoperiodic timer or counter. We conclude that the photoperiodic timer and counter have evolved independently of the circadian clock in the pitcher-plant mosquito Wyeomyia smithii and hence, the evolutionary modification of photoperiodism throughout the range of W. smithii has not been causally mediated by a corresponding evolution of the circadian clock.
Keywords: Geographic variation, Biological clocks, Seasonality, Diapause, Photoperiodism
Introduction
There are two great rhythms of light and temperature in the biosphere: the daily rhythm caused by the rotation of the earth about its axis and the yearly seasonal rhythm caused by the rotation of the earth about the sun. All eukaryotes (Edmunds 1988) and some prokaryotes (Johnson et al. 1996) possess an endogenous circadian (daily) clock that has been shown to be adaptive1 in 24-h environments (Yan et al. 1998; Sharma 2003; Emerson et al. 2008a). A wide diversity of plants, annelids, arthropods, echinoderms, fish, birds and mammals use daylength (photoperiodism) to anticipate and prepare for the changing seasons (Withrow 1959; Anonymous 1960; Bünning 1964; Menaker 1971; Bradshaw and Holzapfel 2007a), and this photoperiodic response has been shown to be adaptive in year-long seasonal environments (Bradshaw et al. 2004).
Photoperiodism consists of two separate, though related, components. The photoperiodic timer measures the length of day (or night), and the photoperiodic counter accumulates information from the timer and elicits the downstream photoperiodic response after a threshold number of inductive days has been counted (reviewed in Saunders 2002, Ch. 12). Over 70 years ago, Bunning (1936), hypothesized that the circadian clock formed the basis (Grundlage) of photoperiodic response and since then, the main focus of studies of the mechanistic basis of photoperiodism has been on this relationship (Saunders 2002; Danks 2005). Bunning’s (1936) model posits that there is a rhythmic, circadian sensitivity to light that persists in constant darkness. If light interacts with the rhythm when it is in its sensitive phase, induction of the photoperiodic response follows, i.e., Bunning’s (1936) model assumed a causal connection between the circadian clock and photoperiodic response.
The main experimental support for Bunning’s hypothesis of the causal relationship between the circadian clock and photoperiodism are results from Nanda–Hamner experiments (NH) in which organisms are exposed for the duration of the experiment to light for a fixed number of hours (usually a short-day ~ 10–12 h) followed by long nights of varying duration in separate experiments with separate individuals, creating cycle lengths (T = light + dark) of typically 24–72 h total duration (Blaney and Hamner 1957; Nanda and Hamner 1958; Bradshaw et al. 2003). For instance, some individuals are exposed to L:D (light: dark) = 10:14 (T = 24) for the duration of their life, while others are exposed to L:D = 10:26 (T = 36), or L:D = 10:62 (T = 72). Historically, the idea was that if, during the long dark phase, there were a circadian-based sensitivity to light, then the animals should exhibit a rhythmic long-day response to increasing duration of the T cycle (e.g., Saunders 1968, 1974; Pittendrigh 1981; Bradshaw et al. 2003).
Recently, however, it has been argued that rhythmic responses to NH experiments in and of themselves do not support Bunning’s hypothesis of a causal circadian basis of photoperiodism (Veerman 2001; Danks 2005; Bradshaw et al. 2006). When all experiments are run under the same environmental conditions using populations that have been reared through at least two laboratory generations to minimize maternal (field) effects, then persistent phenotypic variation among populations represents evolved (genetic) differences among them. If the evolutionary modification of two physiological processes is causally (genetically) connected through common genes (pleiotropy), then there should be a strong correlation between the two processes among evolutionary lineages: If the evolution of photoperiodic response is due to evolution of the circadian clock, then the formal properties of both processes should be correlated among populations within a single species. Among populations of the pitcher-plant mosquito, Wyeomyia smithii, critical photoperiod (an overt expression of the photoperiodic timer) is closely correlated with latitude and altitude of population origin (R2 repeatedly>0.92) but is not correlated with either the period or amplitude of response to NH with a short day and variable night lengths (Bradshaw and Holzapfel 2001; Bradshaw et al. 2003, 2006). Bradshaw et al. (2006) therefore concluded that W. smithii provides an example “that Nanda–Hamner periodicity is an expression of basic circadian rhythmicity, but PPTM (photoperiodic time measurement) is a separate mechanism” (one of three alternate proposals by Saunders 2002, p 481; emphasis Saunders’). Consequently, we use NH response as a direct assay of circadian rhythmicity, i.e., a circadian phenotype analogous to eclosion rhythms (Bradshaw et al. 2006).
Herein, we determine whether there is an evolutionary association of critical photoperiod (an overt expression of the photoperiodic timer) and depth of diapause (an overt expression of the photoperiodic counter) with the circadian clock (as measured by a rhythmic response of development time to a long-day NH). A long-day NH consists of a diapause-terminating long day (18 h) followed by night lengths of 6–54 h to create T-cycles from 24 to 72 h using different animals in the different L:D = 18:D regimens. For the termination of diapause, W. smithii counts an L:D = 18:D cycle as a single long day, regardless of night length (Emerson et al. 2008b). The question then remains whether there is a rhythmic expression in development time of diapausing larvae exposed to T cycles from 24 to 72 h and whether the degree of rhythmicity covaries with critical photoperiod or depth of diapause over the geographic, evolutionary trajectory of W. smithii.
Methods
The pitcher-plant mosquito, Wyeomyia smithii (Coq), completes its pre-adult development within the water-filled leaves of the purple pitcher-plant, Sarracenia purpurea, and its range closely follows that of is host plant in North America. Throughout its range, W. smithii undergoes an hibernal larval diapause whose onset, maintenance and termination are regulated by photoperiod (Bradshaw and Lounibos 1977). Ancestral, southern populations enter diapause in the fourth instar; derived northern (and southern mountain) populations enter diapause in the third instar (Bradshaw and Lounibos 1977; Armbruster et al. 1998). The critical photoperiod for the onset, maintenance and termination of diapause increases linearly with both altitude and latitude (Bradshaw and Lounibos 1977). Critical photoperiod and depth of diapause form part of a diapause syndrome (Campbell and Bradshaw 1992) where southern, diapause-averse populations enter a shallower diapause at a later instar under shorter days later in the fall; northern, diapause-prone populations enter a deeper diapause at an earlier instar under longer days earlier in the fall (Table 1).
Table 1.
Geographic origin, stage of diapause, critical photoperiod, depth of diapause, and both short-day (L = 10 h) and long-day (L = 18 h) Nanda–Hamner (NH) responses of the eight populations (two from each region) used in this study
| Cladea | Regionb | Latitude | Altitude (m) | Stage of diapausec |
Critical photoperiodd |
Depth of diapausee |
Short-day NH Responsef |
Long-day NH Responseg |
|---|---|---|---|---|---|---|---|---|
| South | Southern | 30–31°TN | <100 | IV | 12.3 ± 0.1 | 4.0 ± 0.4 | Strong rhythm | Linear |
| Lowland | 34–35°N | <110 | IV | 12.9 ± 0.2 | 6.3 ± 0.9 | Strong rhythm | Rhythmic | |
| North | Mountain | 35°N | ≥900 | III | 13.9 ± 0.0 | 7.5 ± 2.3 | Arrhythmic | Rhythmic |
| Northern | 46°N | 295–365 | III | 15.2 ± 0.2 | 8.2 ± 1.5 | Weak Rhythm | Linear |
Taxonomic clade (Bradshaw and Lounibos 1977; Armbruster et al. 1998)
General geographic region of origin: Southern Gulf Coast of FL, Lowland coastal and piedmont NC, Mountain southern Appalachian Mountains in NC, Northern ME and WI
Stage of diapause, III or IV instar (Bradshaw and Lounibos 1977)
Mean ± SE (hours) critical photoperiod, a measure of the photoperiodic timer, of populations within the region (Bradshaw et al. 2003). Note that in W. smithii, critical photoperiod is the same for both the initiation and termination of diapause in unchilled animals (Bradshaw and Lounibos 1972)
Mean ± SE (days) depth of diapause, a measure of the photoperiodic counter, within each region using the same populations as in this study. Data are from Emerson et al. 2008b, Table 1, LDC5 018:06
NH response with a light period of 10 h (Bradshaw et al. 2003)
NH response with a light period of 18 h (Fig. 1)
Eight populations of W. smithii were collected from four geographic regions, with two populations at least 80 km apart within each region, representing extremes in latitude from 30 to 46N and altitude from 20 to 1,000 m (Table 1). All eight populations used in this study are the same as those reported in (Emerson et al. 2008b): two from Florida (Southern), two from the coastal plain and piedmont of North Carolina (Lowland), two from the mountains of North Carolina (Mountain), one from northern Maine and one from northern Wisconsin (Northern) (populations WI, CR, GS, SH, DB, HS, KC and RY, respectively from Emerson et al. 2008b). After factoring out maternal effects by rearing the populations in the laboratory as detailed in (Emerson et al. 2008b), experiments were carried out as a single block to ensure constant temperature, light and humidity conditions across all treatments. Experimental larvae were reared in short days (L:D = 8:16) at 21 ± 0.C for at least 30 days to ensure that all animals were synchronized in diapause before the experiment began. Diapausing larvae from each population were exposed to L:D cycles with 18 h of light and, in separate experiments with separate individuals, from 6 to 54 h of darkness in 2-h increments. The experiment involved 25 treatments X 8 populations X 105 larvae treatment−1 population−1, totaling 21,000 larvae in a single block. Experiments were carried out until all larvae developed (<70 days for the longest L:D cycles). All experiments were run in light-tight experimental chambers with an aircooled 4 W cool-white fluorescent lamp at 21 ± 0.C.
Development time was measured as the time from the start of the experiment with diapausing larvae until pupation. Development time is therefore a composite index including both the time required for larvae to terminate diapause using the photoperiodic counter and the time required for larvae to complete post-diapause development culminating in pupation. Experimental animals were cleaned and fed and all pupae were counted and removed every 2–4 days during the light phase of the L:D cycles. We tested whether there is a significant rhythm in the response to a long day T-experiment using a likelihood-based statistical approach. We independently fit two classes of models of the relationship between development time (DT) and cycle length (T) to the data from the four separate geographic regions: (1) a linear model of the form DT = a + bT, and (2) a rhythmic model of the form DT = a + bT + c Cos(2pi * (T-d)/e), where a–e, are parameters fit by maximum likelihood: a intercept, b slope of the linear function, c amplitude of the rhythmic function, d lag between the internal rhythm and the external L:D cycle, e the period of the internal rhythm. Within each geographic region, Akiake’s information criterion (AIC), a measure of the relative correctness-of-fit for a given model, was used to determine whether a linear or a rhythmic model was better supported by the data (Burnham and Anderson 2004). This measure gives an estimate of the “information lost” when using the model rather than the data itself and can be thought of as a measure of distance between the model and the data. Hence, smaller values for AIC indicate a better fit of the model to the observed data. We determine whether the relationship between development time and T is better explained by a linear or rhythmic function by choosing the model with the smallest AIC value.
We use log-likelihood ratios as a measure of the strength of our test between the two models. The log-likelihood ratio is calculated as Log(L) = log(likelihood of non-linear model/likelihood of linear model). Large positive values of Log(L) > 5 indicate confidence in the non-linear model being a better fit than the linear model; whereas large negative values of Log(L) < −5 indicate confidence in linear models being a better fit than the non-linear model. All analyses use the statistical computing program R (R Development Core Team 2007).
Results
Percent development was greater than 95% in all treatments used in this study, showing that all the treatments were interpreted as long (diapause-terminating) days by the mosquitoes, confirming previous results that showed that W. smithii measures the length of day (Emerson et al. 2008b) rather than the length of the dark period.
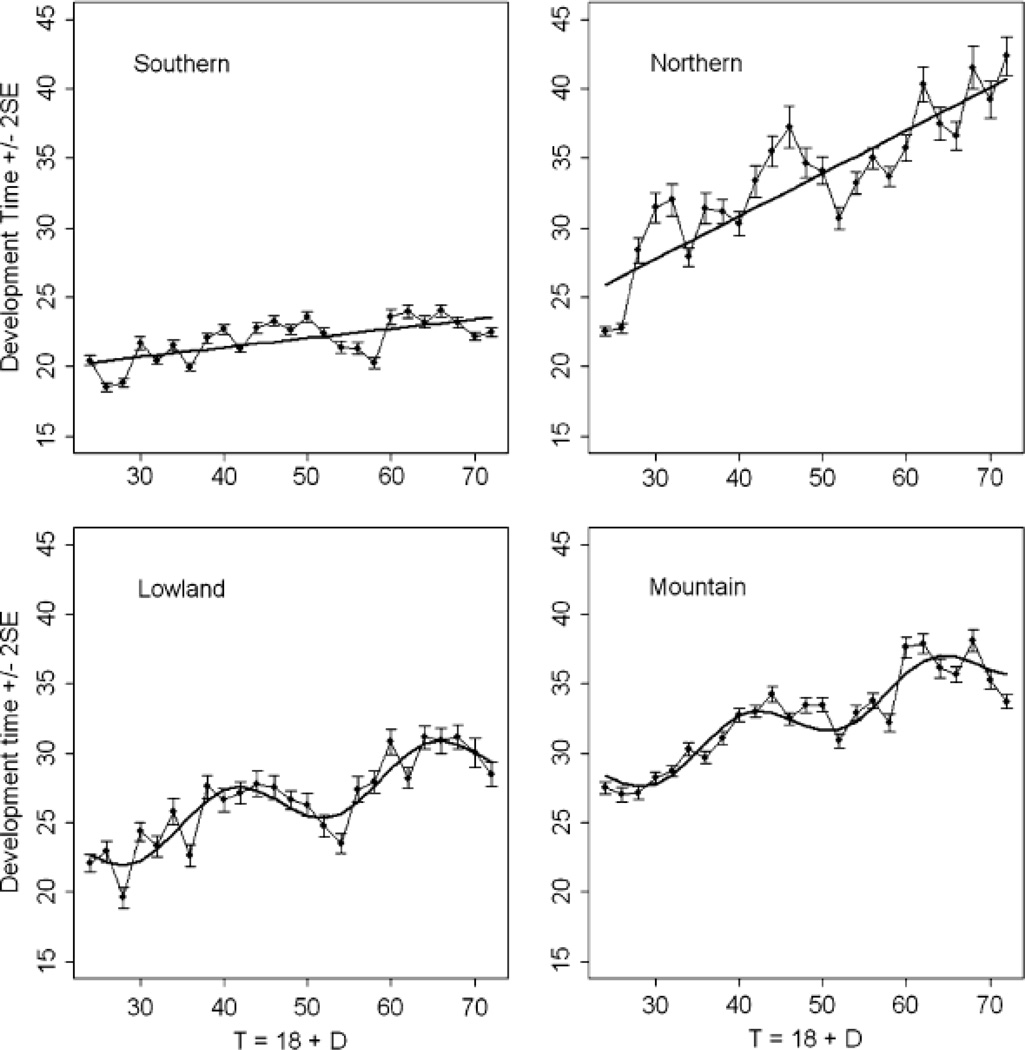
Development time in all four regions increased with increasing duration of the L:D cycle (Fig. 1). There was a rhythmic response in development time as a function of cycle length in the lowland and mountain regions, whereas the relationship had no significant rhythm in southern and northern regions (Fig. 1; Table 2). The magnitude of the log-likelihood ratios, a quantitative measure of support for one model over the other, ranged from 9 to 62, suggesting that, at a minimum, the better supported model was more than 109 times more likely to have produced the observed data. The period of oscillations for the rhythmic regions were slightly less than 24 h (Lowland 23.90 ± 0.78 SE, Mountain 22.29 ± 0.56; t = 1.68, P = 0.342), and fell within the range of 19–26 h reported for insect circadian rhythms (Saunders 2002; Lankinen and Forsman 2006).
Fig 1.
Developmental time (mean number of days ± 2 SE) as a response to long days (18 h) followed by 6–54 h of darkness in 25 separate experiments for eight populations of W. smithii from four geographic regions; lines plot the best fitting model (Table 2)
Table 2.
The support for the linear and rhythmic models fit to development time for each geographic region
| Geographic region | Log(L) |
Log(L) ratiob | AIC |
Better fitting model | ||
|---|---|---|---|---|---|---|
| Lineara | Rhythmica | Linearc | Rhythmicc | |||
| Southern | −161.78 | −199.15 | −37.37 | 749.03 | 927.14 | Linear |
| Lowland | −169.67 | −160.65 | 9.02 | 785.36 | 749.84 | Rhythmic |
| Mountain | −380.47 | −332.09 | 48.38 | 1756.11 | 1539.31 | Rhythmic |
| Northern | −321.59 | −383.75 | −62.16 | 1484.98 | 1777.24 | Linear |
Log-likelihood of the given model
Estimate of the strength of support for the better fitting model: large negative values < −5 support the linear model over the rhythmic model; large positive values > 5 support the rhythmic model over the linear model
Akiake’s Information Criterion, a measure of the relative correctness-of-fit to the linear and to the rhythmic models. Boldface indicates the better fitting model for each geographic region
Discussion
Critical photoperiod in W. smithii is closely correlated with latitude and altitude (R2 repeatedly > 0.92, Bradshaw and Holzapfel 2001). To be rhythmic a function must have both a period and an amplitude of oscillation. The period of the rhythmic response to short-day NH is not correlated with latitude, altitude or critical photoperiod (Bradshaw et al. 2003). The amplitude of the rhythmic response to short-day NH is correlated only with altitude and is not significantly correlated with critical photoperiod over W. smithii’s range (Bradshaw et al. 2006). In the present study using long-day NH experiments, a linear model best describes development times in regions representing the extremes in both critical photoperiod and depth of diapause (Fig. 1; Table 2, southern and northern); a rhythmic model best describes development times in regions representing intermediate critical photoperiods and depths of diapause (Fig. 1; Table 2, lowland and mountain). Hence, there is no consistent geographic association of critical photoperiod or depth of diapause with the rhythmic response to long-day NH.
Wyeomyia smithii is the single temperate species of an otherwise neotropical genus (Lane 1953; Stone et al. 1959). Wyeomyia smithii’s geographic distribution, natural history, morphology, reproductive biology, physiology and allozyme variation all support an evolutionary history that originates with the invasion of North America from the tropics along the Gulf of Mexico and was subsequently followed by dispersal to higher latitudes and altitudes (Bradshaw and Lounibos 1977; Armbruster et al. 1998). There is a strong association (Table 1) of evolutionary history with both critical photoperiod and depth of diapause. This association is not true for the rhythmic response to long-day NH, which is more similar among regions between than within evolutionary groups. Hence, there is no consistent evolutionary association between critical photoperiod (a measure of the photoperiodic timer) or depth of diapause (a measure of the photoperiodic counter) with the circadian-based, rhythmic response to long-day NH.
Saunders et al. (2004) proposed that the presence of a robust photoperiodic response curve despite the absence of a response to Nanda–Hamner experiments may be due to rapid damping of the circadian oscillator during long dark periods. This proposition might have applied to the mountain populations that show a flat, arrhythmic developmental response to short-day NH (Table 1). Our present results provide an unintended test of Saunders’ proposal. The same mountain populations that are arrhythmic in response to short-day NH show a clear rhythmic response to long-day NH that persists for at least 54 h in darkness (Fig. 1). Hence, there is a robust, non-damping circadian oscillation in the mountain populations and the lack of a rhythmic response to the short-day NH is not due to damping of the circadian oscillator.
All populations of W. smithii exhibit a rhythmic response to short-day NH, to long-day NH or to both, but neither the period nor the amplitude of this rhythmic response is associated with the photoperiodic timer or counter. We therefore conclude that the circadian rhythm expressed through NH experiments is not a causal factor in the evolution of photoperiodic response over the eco-climatic gradient of North America by W. smithii.
The hypothesis of a causal connection between circadian rhythms and photoperiodism has been a tantalizing concept for over 70 years. Such a relationship would mean that one or more key genes are mediating both processes, i.e., their functional connection is due to pleiotropy. Pleiotropy is bi-directional: selection on one trait generates a correlated response in the other trait, leading to potential tradeoffs between fitness-related traits (Rose 1991; Roff 1992). If the circadian clock were related to photoperiodic time measurement through pleiotropy, the evolutionary modification of one trait would have a modifying effect on the other. The circadian clock orchestrates the daily temporal coordination of hundreds of genes in Drosophila (Claridge-Chang et al. 2001; McDonald and Rosbash 2001); and, by one estimate, nearly all genes in mouse adipose tissue have a circadian component to their expression (Ptitsyn et al. 2007). Proper entrainment of the circadian clock to the external L:D cycle has been shown to be critical for the maintenance of fitness in natural populations of W. smithii and, in lab populations of diverse organisms, fitness or its correlates are sensitive to mutations that interfere with circadian clock function (Yan et al. 1998; Sharma 2003; Emerson et al. 2008a). By contrast, photoperiodic time measurement enables organisms to anticipate and prepare in advance for seasonal changes in their environment, and the correct, climate-specific photoperiodic response is essential for maintaining fitness in temperate seasonal environments (Bradshaw al. 2004). If circadian rhythmicity and the evolutionary modification of photoperiodic time measurement were causally connected through pleiotropy, then rapid evolution of photoperiodic time measurement by invading species (Hoy 1978) or in response to rapid climate change (Bradshaw and Holzapfel 2001) would involve significant tradeoffs between the evolution of photoperiodic time measurement and the maintenance of internal circadian organization.
The daily circadian clock and the seasonal photoperiodic timer serve two, separate adaptive functions and are affected by different suites of environmental inputs (Danks 2005). This is not to say that a particular gene involved in the circadian clock mechanism may not also be involved in photoperiodic time measurement, independently of its role in the circadian clock (Mathias et al. 2005; Bradshaw and Holzapfel 2007b; Tauber et al. 2007; Stehlík et al. 2008); rather, the evolutionary modification of photoperiodic response is independent of the evolution of the circadian clock as a system. This independent evolution should be expected over geographic gradients that vary in the amplitude of seasonal day length, in the mean and amplitude of daily temperature, in the length of the growing season, in the duration and severity of winter and in ecological contexts that are not the same between times of day and between times of year.
Acknowledgments
We thank A. Letaw for discussion, A. Letaw and two anonymous reviewers for their comments on previous versions of this paper, and B. Kolaczkowski for valuable discussions on likelihood methods. All work presented here complied with the “Principles of animal care,” publication No. 86-23 of the National Institute of Health, and also with current laws of the United States, where these experiment were performed. This work was made possible by generous support from the National Science Foundation through grants DEB-0412573, IOB-0445710 and IOB-0520799 (REU supplement for SJD) to WEB, and the National Science Foundation and National Institutes of Health through training grants DGE- 0504727 and 5-T32-GMO7413 to KJE
Abbreviations
- NH
Response to Nanda–Hamner protocols
- L:D
Number of hours of light (L) and dark (D) in a given environmental cycle
- T
Total numbers of hours in a given environmental cycle (T = L ? D)
- DT
Development time
- AIC
Akiake’s Information Criterion
- Log(L)
Log-likelihood of a given model
Appendix: Glossary of terms highlighted in the text
- Adaptive
A trait is adaptive if it is genetically determined and the possession of that trait improves fitness. We do not use adaptive or adaptation to mean phenotypically plastic, accommodative or acclimative responses of individuals to the environment
- Akiake’s information criterion (AIC)
A measure of the goodness of fit of a model to a given set of data. AIC estimates the information lost by using the model rather than the data itself and, hence, lower values of AIC indicate better support of a given model.
- Amplitude
One half of the difference between the maximum and minimum magnitude of a rhythm or oscillation. If the amplitude is zero, then there is no rhythm.
- Critical photoperiod
The length of day that induces or maintains 50% diapause and stimulates 50% development in a sample cohort. Critical photoperiod is an overt expression of the photoperiodic timer.
- Depth of diapause
Herein, the number of long-days required to terminate diapause in 50% of a sample cohort (Bradshaw and Lounibos 1977; Emerson et al. 2008b). Depth of diapause is an overt expression of the photoperiodic counter. Depth of diapause is also referred to as the intensity of diapause (Danks 1987, p. 17)
- NH response
Response to Nanda–Hamner experiments in which organisms are exposed to a fixed day length and, in separate experiments with separate animals, varying night length. The phenotypic response may be either rhythmic or non-rhythmic (linear).
- Period
Peak-to-peak or valley-to-valley interval of a rhythm or oscillation. If there is no significant period of oscillation, then there is no rhythm.
- Pleiotropy
The influence of a locus on more than one trait. Pleiotropic effects can be assessed either by molecular genetic techniques showing the effect of a single gene on more than one phenotype, or by quantitative genetic techniques showing a correlated response to selection in the absence of linkage disequilibrium (Roff 1997).
- T
Total period of light plus dark = L + D of an L:D = light:dark cycle.
Footnotes
All words and terms first appearing in bold are explicitly defined in the glossary found in appendix 1
References
- Anonymous. Biological clocks. Cold Spring Harbor, New York: The Biological Laboratory; 1960. [Google Scholar]
- Armbruster PA, Bradshaw WE, Holzapfel CM. Effects of postglacial range expansion on allozyme and quantitative genetic variation in the pitcher-plant mosquito, Wyeomyia smithii. Evolution. 1998;52:1697–1704. doi: 10.1111/j.1558-5646.1998.tb02249.x. [DOI] [PubMed] [Google Scholar]
- Blaney LT, Hamner KC. Inter-relations among the effects of temperature, photoperiod, and dark period on floral initiation of Biloxi soybean. Bot Gaz. 1957;119:10–24. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Genetic shift in photoperiodic response correlated with global warming. Proc Natl Acad Sci USA. 2001;98:14509–14511. doi: 10.1073/pnas.241391498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Evolution of animal photoperiodism. Annu Rev Ecol Evol Syst. 2007a;38:1–25. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Tantalizing timeless. Science. 2007b;316:1851–1852. doi: 10.1126/science.1145053. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Lounibos LP. Photoperiodic control of development in the pitcher-plant mosquito, Wyeomyia smithii. Can J Zool. 1972;50:713–719. [Google Scholar]
- Bradshaw WE, Lounibos LP. Evolution of dormancy and its photoperiodic control in pitcher-plant mosquitoes. Evolution. 1977;31:546–567. doi: 10.1111/j.1558-5646.1977.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Quebodeaux MC, Holzapfel CM. Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: adaptive response to the photic environment or correlated response to the seasonal environment? Am Nat. 2003;161:735–748. doi: 10.1086/374344. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Zani PA, Holzapfel CM. Adaptation to temperate climates. Evolution. 2004;58:1748–1762. doi: 10.1111/j.0014-3820.2004.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM, Mathias D. Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: can the seasonal timer evolve independently of the circadian clock? Am Nat. 2006;167:601–605. doi: 10.1086/501032. [DOI] [PubMed] [Google Scholar]
- Bünning E. Die endogene Tagesrhythmik als Grundlage der photoperiodischen Reaktion. Ber Dtsch Bot Ges. 1936;54:590–607. [Google Scholar]
- Bünning E. The physiological clock. Berlin: Springer; 1964. [Google Scholar]
- Burnham KP, Anderson DR. Multimodal inference: understanding AIC and BIC in model selection. Soc Meth Res. 2004;33:261–304. [Google Scholar]
- Campbell MD, Bradshaw WE. Genetic coordination of diapause in the pitcher-plant mosquito, Wyeomyia smithii (Diptera, Culicidae) Ann Entomol Soc Am. 1992;85:445–451. [Google Scholar]
- Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW. Circadian regulation of gene expression systems in the Drosophila head. Neuron. 2001;32:657–671. doi: 10.1016/s0896-6273(01)00515-3. [DOI] [PubMed] [Google Scholar]
- Danks HV. Insect dormancy: an ecological perspective. Ottawa: Biological Survey of Canada (terrestrial arthropods); 1987. [Google Scholar]
- Danks HV. How similar are daily and seasonal biological clocks? J Insect Physiol. 2005;51:609–619. doi: 10.1016/j.jinsphys.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Edmunds LN. Cellular and molecular bases of biological clocks: models and mechanisms for circadian timekeeping. New York: Springer; 1988. [Google Scholar]
- Emerson KJ, Bradshaw WE, Holzapfel CM. Concordance of the circadian clock with the environment is necessary to maximize fitness in natural populations. Evolution. 2008a;62:979–983. doi: 10.1111/j.1558-5646.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson KJ, Letaw AD, Bradshaw WE, Holzapfel CM. Extrinsic light:dark cycles, rather than endogenous circadian cycles, affect the photoperiodic timer in the pitcher-plant mosquito, Wyeomyia smithii. J Comp Phys A. 2008b;194:611–615. doi: 10.1007/s00359-008-0334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy MA. Variability in diapause attributes of insects and mites: some evolutionary and practical implications. In: Dingle H, editor. Evolution of insect migration and diapause. New York: Springer; 1978. pp. 101–126. [Google Scholar]
- Johnson CH, Golden SS, Ishiura M, Kondo T. Circadian clocks in prokaryotes. Mol Microbiol. 1996;21:5–11. doi: 10.1046/j.1365-2958.1996.00613.x. [DOI] [PubMed] [Google Scholar]
- Lane J. Neotropical Culicidae. São Paulo: University of São Paulo; 1953. [Google Scholar]
- Lankinen P, Forsman P. Independence of genetic geographical variation between photoperiodic diapause, circadian eclosion rhythm, and Thr-Gly repeat region of the period gene in Drosophila littoralis. J Biol Rhythms. 2006;21:3–12. doi: 10.1177/0748730405283418. [DOI] [PubMed] [Google Scholar]
- Mathias D, Jacky L, Bradshaw WE, Holzapfel CM. Geographic and developmental variation in expression of the circadian rhythm gene, timeless, in the pitcher-plant mosquito, Wyeomyia smithii. J Insect Physiol. 2005;51:661–667. doi: 10.1016/j.jinsphys.2005.03.011. [DOI] [PubMed] [Google Scholar]
- McDonald MJ, Rosbash M. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 2001;107:567–578. doi: 10.1016/s0092-8674(01)00545-1. [DOI] [PubMed] [Google Scholar]
- Menaker M. Biochronometry. Washington, DC: National Academy of Sciences; 1971. [Google Scholar]
- Nanda KK, Hamner KC. Studies on the nature of the endogenous rhythm affecting photoperiodic response of Biloxi soybean. Bot Gaz. 1958;120:14–25. [Google Scholar]
- Pittendrigh CS. Circadian organization and the photoperiodic phenomena. In: Follett BK, Follett DE, editors. Biological clocks in seasonal reproductive cycles. Bristol: Wright; 1981. pp. 1–35. [Google Scholar]
- Ptitsyn AA, Zvonic S, Gimble JM. Digital signal processing reveals circadian baseline oscillation in majority of mammalian genes. PLoS Comp Biol. 2007;3:e120. doi: 10.1371/journal.pcbi.0030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007. [Google Scholar]
- Roff DA. The evolution of life-histories: theory and analysis. New York: Chapman and Hall; 1992. [Google Scholar]
- Roff DA. Evolutionary quantitative genetics. New York: Chapman and Hall; 1997. [Google Scholar]
- Rose MR. Evolutionary biology of aging. New York: Chapman & Hall; 1991. [Google Scholar]
- Saunders DS. Photoperiodism and time measurement in the parasitic wasp, Nasonia vitripennis. J Insect Physiol. 1968;14:433–450. [Google Scholar]
- Saunders DS. Evidence for ‘dawn’ and ‘dusk’ oscillators in the Nasonia photoperiodic clock. J Insect Physiol. 1974;20:77–88. [Google Scholar]
- Saunders DS. Insect clocks. Amsterdam: Elsevier Science; 2002. [Google Scholar]
- Saunders DS, Lewis RD, Warman GR. Photoperiodic induction of diapause: opening the black box. Physiol Entomol. 2004;29:1–15. [Google Scholar]
- Sharma VK. Adaptive significance of circadian clocks. Chronobiol Int. 2003;20:901–919. doi: 10.1081/cbi-120026099. [DOI] [PubMed] [Google Scholar]
- Stehlík J, Závodská R, Shimada K, Šauman I, Koštál V. Photoperiodic induction of diapause requires regulated transcription of timeless in the larval brain of Chymomyza costata. J Biol Rhythms. 2008;23:129–139. doi: 10.1177/0748730407313364. [DOI] [PubMed] [Google Scholar]
- Stone A, Knight KL, Starke H. A synoptic catalog of the mosquitoes of the world (Diptera: Culicidae) Washington, DC: Entomological Society of America; 1959. [Google Scholar]
- Tauber E, Zordan M, Sandrelli F, Pegoraro M, Osterwalder N, Breda C, Daga A, Selmin A, Monger K, Benna C, Rosato E, Kyriacou CP, Costa R. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science. 2007;316:1895–1898. doi: 10.1126/science.1138412. [DOI] [PubMed] [Google Scholar]
- Veerman A. Photoperiodic time measurement in insects and mites: a critical evaluation of the oscillator-clock hypothesis. J Insect Physiol. 2001;47:1097–1109. doi: 10.1016/s0022-1910(01)00106-8. [DOI] [PubMed] [Google Scholar]
- Withrow RB. Photoperiodism and related phenomena in plants and animals. Washington, DC: American Association for the Advancement of Science; 1959. [Google Scholar]
- Yan OY, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci USA. 1998;95:8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]