Abstract
Human theta (4−8 Hz) activity in the medial temporal lobe correlates with memory formation; however, the precise role that theta plays in the memory system remains elusive (Hanslmayr and Staudigl, 2013). Recently, prestimulus theta activity has been associated with successful memory formation, although its specific cognitive role remains unknown (e.g. Fell et al., 2011). In this report, we demonstrate that prestimulus theta in the hippocampus indexes encoding that supports old–new recognition memory but not recall. These findings suggest that human hippocampal prestimulus theta may preferentially participate in the encoding of item information, as opposed to associative information.
2 Introduction
Item recognition and free–recall, two common methods of testing human memory in the laboratory, differentially depend on a variety of encoding and retrieval operations (Kahana, 2012). Thus, it is of theoretical interest whether a neural signal that correlates with successful memory encoding comparatively indexes later recognition or recall. One of the fundamental differences between item recognition and free–recall is that these tasks rely to varying degrees on item and associative information stored in memory (Murdock, 1974). Item and associative information dissociate in many respects: forgetting rates (Hockley and Cristi, 1996), repetition effects (Greene, 1989), rates of information accumulation (Gronlund and Ratcliff, 1989; Nobel and Shiffrin, 2001) and rates of decline in human aging (Castel and Craik, 2003). Item recognition, in which participants study a list of unrelated items and later judge whether a specific item was present in the original list, preferentially depends on item information (Nosofsky, 1988; Kahana, 2012). In contrast, recall tasks such as cued– and free–recall rely more heavily on associative information as such associations enable cue–dependent retrieval (Sederberg et al., 2008; Polyn et al., 2009). In free–recall, each retrieved item serves as a cue for the next recall as seen in the tendency for successively recalled items to reflect both contiguity and similarity relations among items on the study list (Kahana, 1996; Miller et al., 2013). Thus, during encoding, neural activity that reflects item and associative processing should preferentially boost later recognition and recall, respectively.
A signal with a particularly puzzling function regarding memory is prestimulus 4−8 Hz theta activity. Several recent reports have shown theta activity before item presentation correlates with later successful memory retrieval (Guderian et al., 2009; Rutishauser et al., 2010; Fell et al., 2011; Gruber et al., 2013). However, the cognitive correlate of this stimulus–independent signal is poorly understood. Here we sought to clarify the role of prestimulus activity in the memory system by assessing whether it preferentially enhances subsequent recognition, recall, or both. Specifically, if prestimulus theta aids both later recall and recognition equally, then it is likely that such activity represents a non–specific memory signal (e.g., attention) that boosts encoding independent of the type of retrieval used to recover the memory. However, if prestimulus theta confers a relative benefit for recognition or recall, one could leverage the theoretical differences in these tasks to refine our understanding of prestimulus theta activity. For example, if it is linked more closely linked to recognition or free–recall, this may suggest that prestimulus theta preferentially aids the encoding of item or associative information, respectively.
We tested these competing hypotheses with intracranial electroencephalography (iEEG) recordings during a combined delayed free–recall, final–recognition task. We first assesed whether the theta power during the period prior to item presentation predicted successful encoding tested by later recall and recognition tasks. By directly recording from structures implicated in prestimulus theta generation, we also determined the spatial specificity of any memory–associated prestimulus theta activity. Finally, we analyzed the time–frequency characteristics in the hippocampus during both recognition and recall tasks to assess the timing of the theta subsequent memory effect (SME) in these two types of memory. We found that increased prestimulus theta occurred in the hippocampus but not lateral temporal or frontal areas, and that higher levels of this neural signal were associated with better recognition, but not recall, performance. These data suggest that prestimulus theta reflects a hippocampal memory signal rather than supporting a wide range of cognitive operations. Although there are several interpretations to these finding, we propose that hippocampal prestimulus theta enhances memory encoding by preferentially boosting item information processing, as opposed to associative information processing.
3 Materials and Methods
3.1 Subjects
Patients with medication-resistant epilepsy underwent surgical procedures in which grid, strip, or depth electrodes were implanted to localize epileptogenic regions. Data were collected over an eight year period as part of a multi-center collaboration. Our research protocol was approved by the institutional review board at each hospital and informed consent was obtained from the patients and their guardians. Our final subject pool consisted of 77 left-language dominant patients. A subset of these data have been reported previously (Burke et al., 2013). Unlike these previous reports which focused exclusively on free-recall, the novel analyses reported here focus on the comparison between free-recall and a final recognition memory task given following multiple free–recall lists. Further, we examine the time interval before an item appears on the screen rather than the post-presentation period. All of the analyses and results described here are novel.
3.2 Combined Free-Recall and Recognition Task
Each patient participated in an intentional combined delayed free-recall and final recognition task (Figures 1A & 1B). The task was developed using the python experiment-programming library (PyEPL; see Geller et al., 2007) and administered at the patients’s bedside using a laptop computer. A fixation cross presented in the center of the screen signaled the onset of each study list. Lists comprised fifteen words chosen randomly and without replacement from a pool of high-frequency nouns (http://memory.psych.upenn.edu/WordPools). During the encoding period, each word appeared individually for 1600 ms followed by a randomly jittered 800−1200 ms blank inter–stimulus interval (“Encoding Period” in figure 1A). Following presentation of the final list item patients were given a minimum 20 second mental arithmetic task (“Distractor” in figure 1A). The appearance of a row of asterisks along with an audible tone then signalled the start of a 45 second recall period during which patients were instructed to recall the just–presented list items in any order (“Recall Period” in figure 1A).
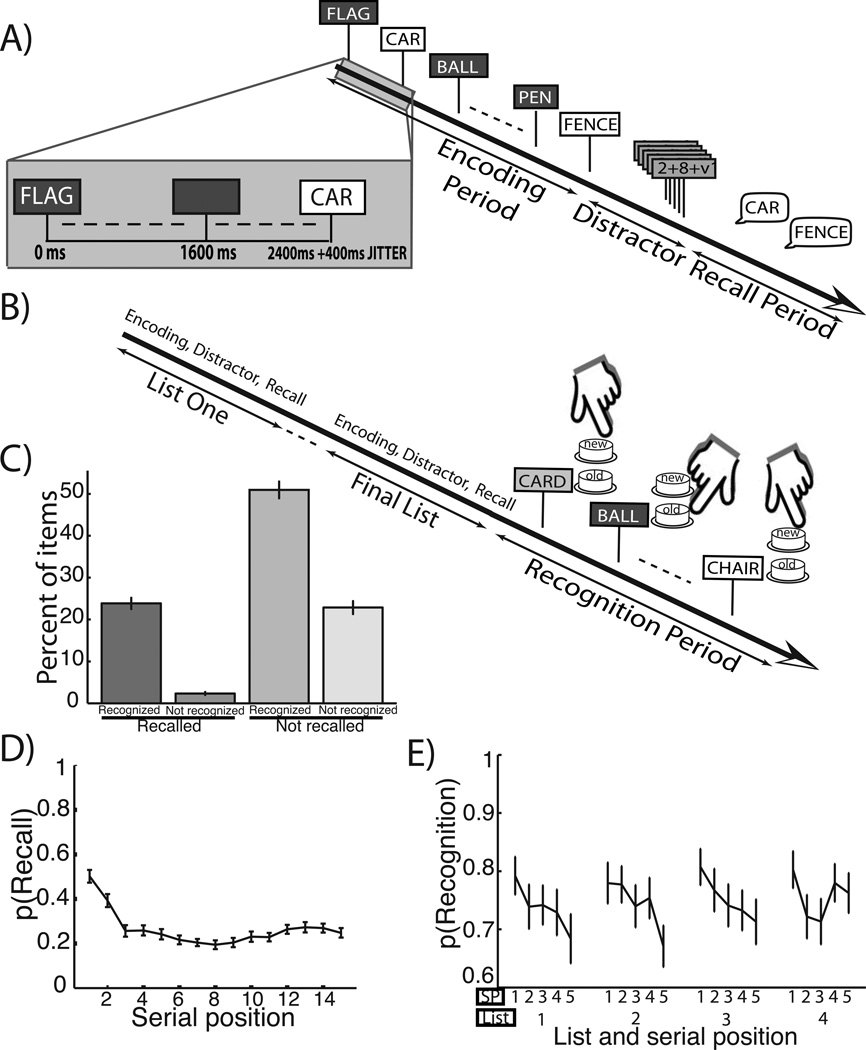
Figure 1.
A. Free–recall task. In this combined task, subjects were first shown a series of 15–word lists followed by a distractor and then asked to recall items from the most recent list. B. Recognition task following free–recall lists. After all recall lists were complete, the subjects were shown 60 targets from the studied items and 60 lures and asked to make a recognition judgment. C. Categorization of words by recall–recognition contingency. Across subject mean and ±1 SEM of the percentage of presented words in one of four categories based on later recall and recognition performance. D. Free–recall serial position curve. Across subject mean and ±1 SEM probability of recall as a function of serial position studied. E. Recognition performance by study list quartile and intra–list serial position quintile. Across subject mean and ±1 SEM probability of recognition as a function of study list quartile and serial position quintle within a list.
Following a series of between ten and sixteen free–recall lists, patients were given a final old–new recognition memory test (“Recognition Period” in figure 1B). The variation in the total list count reflected slight experimental modifications over the eight year period in which these data were collected. For the recognition test, 60 targets were randomly chosen from the studied items and intermixed with 60 lure items chosen from the same word pool. Each of these 120 test items was then presented individually and patients were asked to make old–new judgments by pressing one of two buttons on a computer keyboard with their right (“old”) or left (“new”) index finger. Patients were given a maximum of 5 s to respond to each probe item. Following a jittered inter–stimulus interval of 2400−2600 ms then next probe item was then presented.
3.3 Recordings and Spectral Power Computation
Intracranial EEG was recorded and converted to a bipolar montage by differencing the signals between each pair of immediately adjacent contacts on grid, strip, and depth electrodes (Burke et al., 2013). The sampling rates of initial recordings ranged from 256 – 1000 Hz depending on the clinical recording system. Signals were then re–sampled at 256 Hz. Contact localization was accomplished by co–registering the post–operative CTs with MRIs using FSL Brain Extraction Tool (BET) and FLIRT software packages. We convolved segments of iEEG signal (700 ms before the start of word presentation to the onset of word presentation, plus 3000 ms flanking buffers) with 10 complex–valued Morlet wavelets (wave number 6) with center frequencies linearly spaced from 4−8 Hz (Addison, 2002). We squared and log–transformed the wavelet convolutions, and then averaged the resulting log–power traces into one 700 ms epoch. Power was averaged across all frequencies yielding the 4−8 Hz theta frequency band. For each electrode, we then z-transformed power values separately for each session; further analyses were performed on these normalized power values. The power computation for our full time–frequency spectrogram analysis was similar to the theta–band specific power extraction. We convolved segments of iEEG signal (1000 ms before the start of word presentation to 1800 ms after the onset of word presentation, plus 3000ms flanking buffers) with 30 complex–valued Morlet wavelets (wave number 6) with center frequencies log–spaced from 2−100 Hz. In this case, we averaged the log–power traces into 100 ms epochs with a 20 ms sliding window and z-transformed power values within each frequency and session.
3.4 Regions of Interest
Based on subject electrode coverage and previous reports of prestimulus theta activity association with later memory performance we defined three regions of interest (ROI; hippocampus, temporal lobe, frontal lobe) (Guderian et al., 2009; Rutishauser et al., 2010; Fell et al., 2011; Gruber et al., 2013). Temporal and frontal lobe electrodes were classified using anatomic labels from the registration process (Burke et al., 2013). The temporal lobe ROI does not include regions in the medial temporal lobe, defined as brain tissue medial to the collateral sulcus. For the hippocampal ROI, a neuroradiologist experienced in neuroanatomical localization, but blinded to the electrophysiology data, manually reviewed post–operative CT and MRI images to accurately identify all depth contacts located within the hippocampus. Each electrode distance from the hippocampal head in the anterior–posterior plane of this structure was also calculated.
3.5 Statistical Procedures
For each subject’s sessions, a normalized power value was calculated for the −700 ms to 0 ms time epoch relative to the item–presentation period (henceforth “prestimulus period”). Based on the timing of our task, the previous word had been off the computer screen for at least 100 ms (maximum 500 ms) prior to the prestimulus period. Comparisons of two prestimulus SMEs were performed (Paller and Wagner, 2002) and these comparisons were dictated by the details of our memory task with the following goals in mind: 1. compare one type of memory (recognition, recall), while controlling for the other so that the difference in neural activity is associated with a the difference in the specific type of memory; 2. avoid the one memory contingency category (recalled but not recognized) with very few trials; 3. avoid the confounds of the study–effect, (words that were recalled had a second study before recognition, Kahana et al., 2005). To this end we formulated our SME comparisons as follows: among words that were not recalled, recognition hits were compared to recognition misses (recognition SME). Next, among words that were recognized, words that were recalled were compared to words that were not recalled (recall SME). Because of the dependencies between the two comparisons, i.e. the not-recalled, recognition hits formed part of both SMEs, we do not directly compare the recognition and recall SMEs. For all sessions and electrodes for each subject, we compared prestimulus theta power for successful versus unsuccessful encoding separately for each memory task (i.e. recognition and recall) using a parametric t–statistic. We then averaged t-statistics across sessions and across electrodes within a specified ROI, such that each subject contributed a t-statistic for both recall and recognition comparisons in each ROI that he/she had electrode coverage. One–factor (ROI), repeated–measures ANOVA tests were performed separately for each memory type. Post-hoc t-statistics were applied when significance was found in order to assess the strength of the SME in each ROI and compare the SME among ROIs.
We next performed a cluster–based permutation procedure to identify contiguous time–frequency bins which distinguished between later–remembered and later–forgotten words in both recognition and recall tasks (Maris and Oostenveld, 2007). We began by performing a series of parametric t–tests on the normalized power distributions following later–remembered and later–forgotten words at each time–frequency bin (29 frequencies, and 136 time windows surrounding word presentation, see Spectral Power Computation). This resulted in 3,944 t–statistics for each participant. To test the reliability of these t–statistics across participants, we performed a series of one–sample t–tests, one at each time–frequency bin, comparing the distribution of t–values to zero. To correct for multiple comparisons, we identified the largest clusters of spectrally and temporally adjacent windows that showed significantly different power between later–remembered and later–forgotten words (p <0.05 across participants) and computed the cluster statistic as the sum of t–statistics across these windows (true clus+). We also computed a cluster statistic for the largest contiguous decreases in power following forgotten compared to remembered words (true clus−). We then estimated the false–positive rate for each of these cluster statistics using a permutation–based shuffle procedure. For each iteration of the procedure, we randomly changed the sign of the t–statistics computed for each subject, and computed the cluster statistics associated with the largest contiguous significant increase and decrease observed in the shuffled data across subjects (null clus+ and null clus-, respectively). We repeated this procedure 1000 times and estimated a distribution of null clus+’s and null clus-‘s, which reflect cluster statistics that would be obtained if power values did not reliably differ between later–remembered and later–forgotten trials. Based on where the true clus+ and true clus- fell on these null distributions, we derived a p–value for each cluster statistic.
For all analyses α was set at 0.05 and a false discovery procedure (q = 0.05) (Benjamini and Hochberg, 1995) was applied when multiple statistical tests were used.
4 Results
77 subjects (24 women) undergoing intracranial EEG monitoring studied lists of 15 common nouns and then performed intentional, successive free–recall and a final item recognition tests on the studied items (see figures 1A, B). We limited our prestimulus theta analyses to sessions in which d', a common recognition performance metric, exceeded 0.66 in order to ensure patients were engaged in the memory task. Moreover, we excluded patients who did not accrue at least 5 trials in each category that composed our memory comparisons. Thus our final subject pool consisted of 58 patients (20 women), and all analyses below reflect this group. Mean/standard deviation/range of trial numbers for each condition composing our comparisons were as follows: recognized and recalled words: 28/24/5-136; recognized and not–recalled words: 52/33/19-165; not-recognized and not–recalled words: 25/18/7-55. The counts of each recognition–recall contingency as a percentage of all trials is illustrated in figure 1C. Subjects recalled a mean ±1 SEM of 26 ± 1% of the studied items on the delayed recall task. On the final old–new recognition task, subjects endorsed 75 ± 1% of targets and 36 ± 3% percent of lures as old–items, yielding an average d‘ of 1.16 ± .06. The probability of recall as a function of the serial position in the list it was studied is illustrated in figure 1D; these patients followed the law of primacy (earlier words tend to be more likely recalled) found in healthy controls (Tulving, 2007). Figure 1E shows the probability of recognition as a function of study list number quartile and intra–list serial position quintile. Because of the large number of words studied in a session (between 150 − 240) and the randomization of recognition test probe timing, nearly all words were associated with a substatial delay before recognition memory testing. A two–factor (list quartile, intra–list serial position quintile), repeated measures ANOVA was applied to these data to assess for differences in recognition performance as a function of study position. A main effect for intra–list serial position quintile was identified (F4,1140=3.71, MSE=0.227, p=0.005), but not for list number quartile (F3,1140=0, MSE=0.001, p=0.997) or the interaction between these factors (F12,1140=0, MSE=0.040, p=0.791). Post-hoc t-tests were applied to all combinations of intra–list serial position quintiles to assess for differences. The first intra–list serial position quintile (words 1−3) was more commonly recognized than the third and fifth serial position categories (words 7−9,13−15, respectively; t57=3.56, p=0.0008 and t57=2.73, p=0.008; false discovery rate correction, q=0.05). These data demonstrate a primacy effect, but no recency effect, based on the study serial position within a list; therefore, words that were more likely to be recalled were similarly more likely to be recognized.
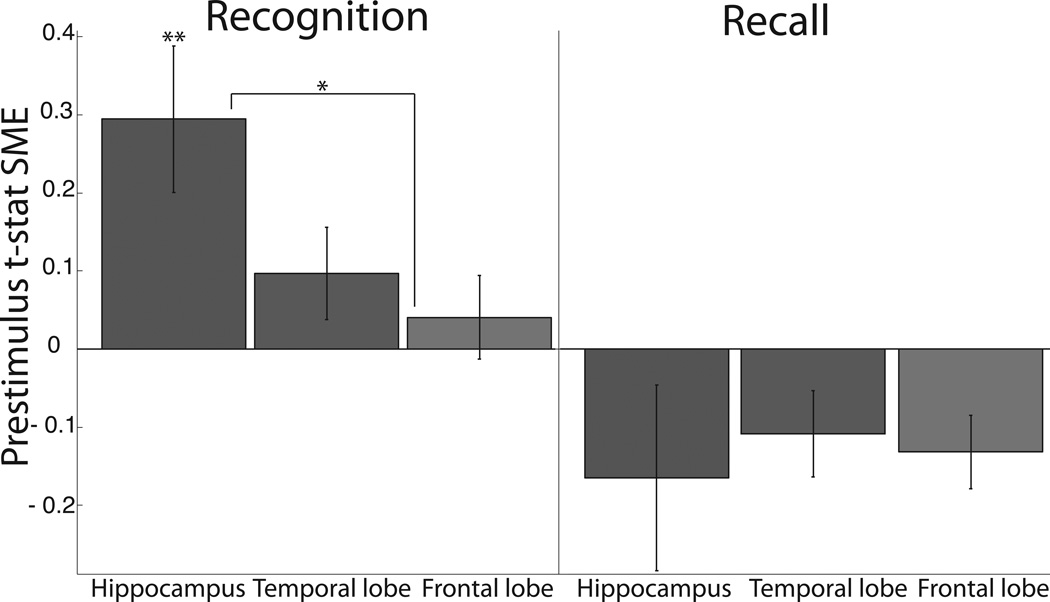
Recent human studies have identified prestimulus theta activity in several cortical locations - the hippocampus, temporal lobe, and frontal lobe - that predicts later episodic memory performance (Guderian et al., 2009; Rutishauser et al., 2010; Fell et al., 2011; Gruber et al., 2013). We sought to extend these findings in terms of memory type and anatomic specificity. Specifically, we sought to investigate whether prestimulus theta activity at encoding predicted later recognition, recall, or both. In order to best isolate these types of memories, we formulated two prestimulus SME comparisons (see Statistical Procedures) wherein the type of memory (recognition, recall) of interest differed but the other was held constant. One prestimulus theta recognition and one recall SME (unpaired t-statistic) was calculated for each subject in each ROI: the means and standard errors of these across–subject distributions are shown in figure 2. The number of patients in each ROI is as follows: hippocampal (28); temporal (50); frontal (48). To search for a regionally–localized prestimulus theta signal, a one-factor (ROI), repeated–measures ANOVA was applied to each type of SME. We did find differences amongst ROI for the mean recognition SME values (F2,125=6.91, MSE=0.645, p=0.002) but not the mean recall SME values (F2,125=0, MSE=0.001, p=0.997). Given our omnibus test identified regional differences amongst the recognition prestimulus theta SME, we further assessed these data with post-hoc t-tests. Specifically, we assessed if the recognition SME in each region was significantly greater than zero and if the recognition SMEs significantly differed from one another (six total post–hoc t-tests). Only the recognition SME in the hippocampus was significantly greater than zero (t27=3.14, p=0.004). The hippocampal recognition SME was greater than the frontal recognition SME (t76=2.54, p=0.013). Both of these remained significant following false discovery rate corrections (q = 0.05). The hippocampal recognition SME trended towards being greater than the temporal lobe SME (t74=1.87, p=0.066). Of note, recognition SME values for six subregions within the frontal and temporal ROIs (motor-, dorsolateral prefrontal, and orbitofrontal cortex; superior-, middle-, and inferior temporal gyrus, all n>35 subjects) were calculated to ensure a strong, local recognition SME was not obscured by heterogeneity with our pre-specified ROIs. No recognition SME value in these subregions reached significance (all uncorrected p>0.1). To summarize, we found that theta activity, only in the hippocampus, before learning an item was predictive of subsequent recognition but not subsequent recall.
Figure 2. Recognition and recall subsequent memory effects by region of interest.
Mean and ±1 SEM for the distribution of each subject’s t-statistic of prestimulus (−700 ms to 0 ms relative to word presentation) theta SME. Each bar represents the SME associated with a memory task (recognition or recall) and region of interest pair as labeled. Double and single asterisks denote levels of significance: p<0.01, p<0.05. n=28,50,48 respectively for hippocampal, temporal and frontal ROIs.
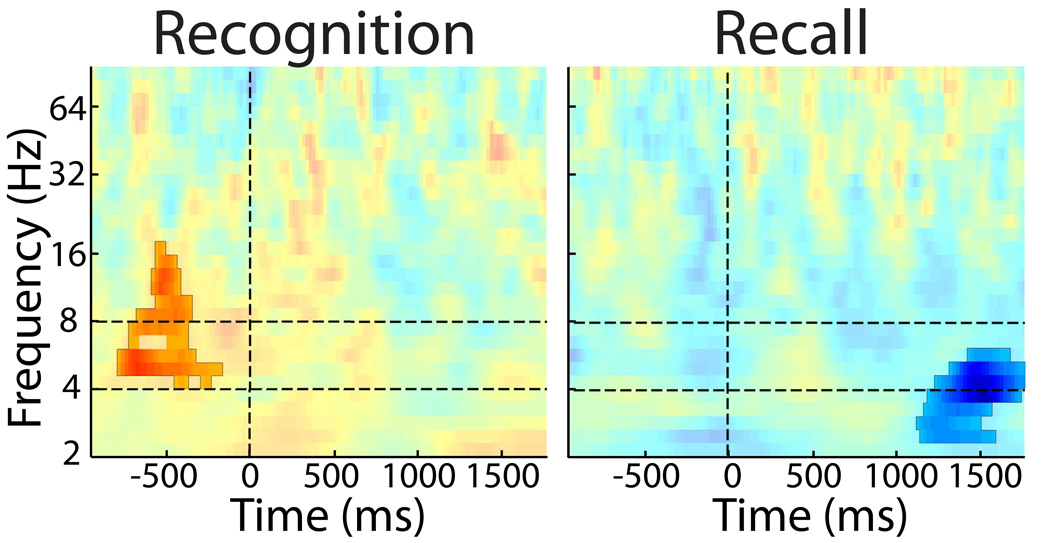
To investigate the temporal and spectral specificity of the prestimulus hippocampal recognition SME, we analyzed a broad time–frequency range of power values (figure 3). For the recognition SME, the only spectral cluster significantly associated with later memory began at 820 ms before word onset and ended 180 ms before the word onset (p=0.034), and was centered in the 4−8 Hz range with a brief extension into the alpha band. The recall SME spectral data appear quite different. There was no difference in time–frequency values during the prestimulus period. However, during the poststimulus period beginning 1120 ms after the word appeared and lasting until after the word came off the computer screen, activity peaking in the low theta range was significantly less for recalled words as compared to not-recalled words (p=0.023). The positive recognition and negative recall clusters remained significant following false discovery rate corrections (q = 0.05)
Figure 3. Hippocampus time–frequency cluster analysis.
A: Across subject t–statistics at each time–frequency bin for the recognition SME. Vertical dashed line represents time of word onset and horizontal lines mark the 4−8 Hz theta frequency band. All time–frequency clusters p<0.05 are highlighted. p=0.034 for the prestimulus positive (recognized > not–recognized power) cluster beginning at 820 ms before word onset. See methods for statistical details. B: Same plot as above for the recall SME. p=0.023, for the negative (not–recalled > recalled power) cluster beginning 1120 ms after word onset.
Lastly, given the differences in anatomic architecture and functional distinctions of the anterior and posterior hippocampus (Poppenk et al., 2013), we tested for a relationship between position along the long axis of the hippocampus and strength of the recognition SME. Our data did not reveal a correlation between location in the hippocampus and the predictive value of the prestimulus theta power for subsequent recognition performance (Pearson’s r27=−0.283, p=0.151).
5 Discussion
We set out to refine the understanding of prestimulus theta and its role in the memory system. Analysis of iEEG recordings in 58 patients performing a delayed free–recall, final–recognition memory task demonstrated that increased prestimulus hippocampal theta activity predicted subsequent recognition, but not subsequent free–recall. This effect was only found in the hippocampus and not in frontal or lateral temporal regions. A time–frequency analysis confirmed that increased encoding–related theta associated with later recognition was specific to the prestimulus period and centered in the theta band, whereas the recall analysis revealed a significant decreased theta SME in the post stimulus period (Sederberg et al., 2007; Burke et al., 2013). In our experimental design, studied items were tested successively, first by delayed recall and then by final recognition. This task has inherent strengths and weakness that we discuss below.
At the most basic level, our findings are consistent with the idea that different processes underlie recognition and free–recall, and that these processes are variably affected by the theta activity in the hippocampus before an item is learned. This study was motivated in part by the idea that neural activity supporting item or associative information processing will preferentially boost encoding for later recognition and recall, respectively. This assumption is consistent with a wealth of cognitive research documenting fundamental differences between these two forms of information. Laboratory studies of human memory have shown that item and associative information are encoded and retrieved via distinct processes (Gronlund and Ratcliff, 1989; Nobel and Shiffrin, 2001). Furthermore, recognition memory models posit that similarity of item features is the primary determinant of performance (Clark and Gronlund, 1996; Shiffrin and Steyvers, 1997; Nosofsky et al., 2011). In contrast, retrieval in free–recall relies on the interaction of associative information with self–generated cues to retrieve learned items (Sederberg et al., 2008; Polyn et al., 2009; Farrell, 2012). Thus our findings suggest that the prestimulus theta SME may aid in item encoding over associative encoding.
Our results are in many ways consistent with previous reports of prestimulus subsequent memory effects in the theta frequency range (Fell et al., 2011; Guderian et al., 2009) and build on these studies. We demonstrate that prestimulus theta predicts subsequent recognition; however, this relationship was not identified for free-recall. Moreover, we find the prestimulus recognition SME in the hippocampus but not in temporal or frontal regions. The hippocampus is a crucial anatomical region involved in the formation of contextually-defined memories. A substantial body of literature links hippocampal theta activity to these processes (Seager et al., 2002; Squire et al., 2004; Manns et al., 2007). As such, it is perhaps not surprising that we found declarative memory for items to be improved following periods of heightened prestimulus theta. That prestimulus theta was specific for item recognition but not free–recall suggests that hippocampal theta may index an endogenous neural mechanism that facilitate encoding of items but not associations. If so, this begs the question: What cognitive processes does hippocampal prestimulus theta represent?
One possibility that may relate prestimulus activity, if it indeed represents enhanced item information processing, to memory formation comes from the established literature linking prestimulus neural oscillations, including theta oscillations, with enhanced perception (Wyart and Tallon-Baudry, 2009; Busch et al., 2009; Hanslmayr et al., 2007). In particular, hippocampal prestimulus theta activity may represent a preparatory process that facilitates information flow from item perception into the memory system, thereby enhancing recognition memory but not recall memory. In contrast, the associative information, which forms after a longer latency relative to item presentation (Gronlund and Ratcliff, 1989; Nobel and Shiffrin, 2001), is less affected by the facilitation of item feature perception into the memory system, and thus does not correlate with the preparatory signal, i.e. the prestimulus theta oscillations. To be clear, we do not suggest that the hippocampus performs item and not associative encoding. But, one interpretation of our results is that prestimulus theta oscillations in the hippocampus mark enhancement of upcoming perception of item–level features thereby supporting memory for individual items but not associations.
Although we focus on the item vs. associative information distinction between recognition and free–recall, there are several alternative interpretations to our results that must be considered. In lieu of supporting item or associative information, one may hypothesize that the learning advantage conferred by prestimulus theta is masked by inhibitory retrieval factors, which differ between the two types of memory. For example, some items may not have been recalled because of output interference (Roediger, 1974), whereas recognition is less susceptible to such effects. Alternatively, prestimulus theta may differentially affect weak and strong memories (and thus recognition and recall performance). Future research should follow-up on this work with alternative testing approaches: experiments that systematically vary retrieval effects, e.g. output interference, or memory strength are needed to better understand the cognitive correlates of prestimulus theta. Moreover, concomitant electrographic recordings with experiments that dissociate item and associative information processing, for example with an associative recognition task or by manipulation of encoding strategy (e.g. Begg, 1978; McGee, 1980) would further assess the item–associative information distinction.
While our study benefited from a large dataset of intracranial recordings during this combined task, there are several limitations imposed by our experimental design. By testing the same words first by free–recall and subsequently by final recognition, memory performance may be differentially affected by factors inherent to the design rather than inherent to the the type of memory (i.e. recognition or free–recall). Recognition of recalled words was enhanced because the free–recall period acted as a second study (Kahana et al., 2005). We excluded recalled words from our recognition analysis for this reason. Moreover, the timing of the test periods may have affected the behavioral and neural effects we found. Free–recall occurred after a minimum 20 second delay but recognition occurred following all lists (approximately 2−35 minutes after learning a word). While our serial position behavioral analyses illustrate that primacy effects (present) and recency effects (absent) were similar between final recognition and delayed free–recall, future research with an interleaved trial design will provide a more straightforward approach to comparing recognition and recall. That is, by using separate words and alternating between testing item-recognition and free-recall, both limitations (second study, timing difference between memory tests) outlined here would be avoided.
Finally, it remains unclear whether the activity of the prestimulus hippocampal–generated theta signal we report varies stochastically or systematically. For instance, this signal may correlate with expectation of a stimulus: in this case theta power would rise before every item but the increase would be greater for to–be–recognized words. A better understanding of how the prestimulus mnemonic signal fluctuates across trials will be crucial in guiding experiments designed to harness hippocampal prestimulus theta as a means of enhancing human memory performance.
Acknowledgements
This work was supported by National Institutes of Health grants MH055687 and 5T32NS043126. We thank Dale H. Wyeth and Edmund Wyeth, for technical assistance at Thomas Jefferson Hospital and Ashwin G. Ramayya, Nicole M. Long, and Karl M. Healey for helpful discussion and input. We would also like to thank the members of the clinical teams where the data were collected: Ashwini Sharan, James Evans, Michael Sperling, Timothy Lucas and Gordon Baltuch. We are indebted to the patients who have selflessly volunteered their time to participate in our study.
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
References
- Addison PS. The illustrated wavelet transform handbook: introductory theory and applications in science, engineering, medicine and finance. Bristol: Institute of Physics Publishing; 2002. [Google Scholar]
- Begg I. Imagery and organization in memory: Instructional effects. Memory and Cognition. 1978;6(2):174–183. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. Journal of Royal Statistical Society, Series B. 1995;57:289–300. [Google Scholar]
- Burke JF, Zaghloul KA, Jacobs J, Williams RB, Sperling MR, Sharan AD, Kahana MJ. Synchronous and asynchronous theta and gamma activity during episodic memory formation. Journal of Neuroscience. 2013;33(1):292–304. doi: 10.1523/JNEUROSCI.2057-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. The Journal of Neuroscience. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Craik FI. The effects of aging and divided attention on memory for item and associative information. Psychology and aging. 2003;18(4):873–885. doi: 10.1037/0882-7974.18.4.873. [DOI] [PubMed] [Google Scholar]
- Clark SE, Gronlund SD. Global matching models of recognition memory: How the models match the data. Psychonomic Bulletin and Review. 1996;3:37–60. doi: 10.3758/BF03210740. [DOI] [PubMed] [Google Scholar]
- Farrell S. Temporal clustering and sequencing in short-term memory and episodic memory. Psychological Review. 2012;119(2):223–271. doi: 10.1037/a0027371. [DOI] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Staresina B, Wagner T, Kranz T, Elger CE, Axmacher N. Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial eeg. Journal of Neuroscience. 2011;31(14):5392–5397. doi: 10.1523/JNEUROSCI.3668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AS, Schleifer IK, Sederberg PB, Jacobs J, Kahana MJ. PyEPL: A cross-platform experiment-programming library. Behavior Research Methods. 2007;39(4):950–958. doi: 10.3758/bf03192990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene RL. Spacing effects in memory: Evidence for a two-process account. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1989;15:371–377. [Google Scholar]
- Gronlund SD, Ratcliff R. Time course of item and associative information: Implications for global memory models. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1989;15:846–858. doi: 10.1037//0278-7393.15.5.846. [DOI] [PubMed] [Google Scholar]
- Gruber MJ, Watrous AJ, Ekstrom AD, Ranganath C, Otten LJ. Expected reward modulates encoding-related theta activity before an event. NeuroImage. 2013;64:68–74. doi: 10.1016/j.neuroimage.2012.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guderian S, Schott B, Richardson-Klavehn A, Duzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proceedings of the National Academy of Sciences, USA. 2009;106(13):5365. doi: 10.1073/pnas.0900289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Klimesch W, Sauseng P, Gruber W, Doppelmayr M, Freunberger R, Pecherstorfer T, Birbaumer N. Alpha phase reset contributes to the generation of erps. Cerebral Cortex. 2007;17(1):1–8. doi: 10.1093/cercor/bhj129. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T. How brain oscillations form memories–a processing based perspective on oscillatory subsequent memory effects. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.05.121. [DOI] [PubMed] [Google Scholar]
- Hockley WE, Cristi C. Tests of encoding tradeoffs between item and associa- tive information. Memory & Cognition. 1996;24:202–216. doi: 10.3758/bf03200881. [DOI] [PubMed] [Google Scholar]
- Kahana MJ. Associative retrieval processes in free recall. Memory & Cognition. 1996;24:103–109. doi: 10.3758/bf03197276. [DOI] [PubMed] [Google Scholar]
- Kahana MJ. Foundations of Human Memory. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Kahana MJ, Dolan ED, Sauder CL, Wingfield A. Intrusions in episodic recall: Age differences in editing of overt responses. Journal of Gerontology: Psychological Sciences. 2005;60:92–97. doi: 10.1093/geronb/60.2.p92. [DOI] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56(3):530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG- data. Journal of Neuroscience Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- McGee R. Imagery and recognition memory: The effects of relational organization. Memory and Cognition. 1980;8(5):394–399. doi: 10.3758/bf03211135. [DOI] [PubMed] [Google Scholar]
- Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I, Hefft S, Merkow M, Polyn SM, Jacobs J, Kahana MJ, Schulze-Bonhage A. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science. 2013;342(6162):1111–1114. doi: 10.1126/science.1244056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock BB. Human memory: Theory and data. Potomac, MD: Lawrence Erlbaum and Associates; 1974. [Google Scholar]
- Nobel PA, Shiffrin RM. Retrieval processes in recognition and cued recall. Journal of Experimental Psychology: Learning Memory, and Cognition. 2001;27:384–413. doi: 10.1037/0278-7393.27.2.384. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM. Exemplar-based accounts of relations between classification, recognition, and typicality. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1988;14:700–708. [Google Scholar]
- Nosofsky RM, Little DR, Donkin C, Fific M. Short-term memory scanning viewed as exemplar-based categorization. Psychological Review. 2011;118(2):280–315. doi: 10.1037/a0022494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6(2):93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Norman KA, Kahana MJ. A context maintenance and retrieval model of organizational processes in free recall. Psychological Review. 2009;116(1):129–156. doi: 10.1037/a0014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis special- ization of the human hippocampus. Trends in Cognitive Science. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Roediger HL. Inhibiting effects of recall. Memory & Cognition. 1974;2(2):261–269. doi: 10.3758/BF03208993. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Ross I, Mamelak A, Schuman E. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464(7290):903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proceedings of the National Academy of Sciences, USA. 2002;99:1616–1620. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Howard MW, Kahana MJ. A context-based theory of recency and contiguity in free recall. Psychological Review. 2008;115(4):893–912. doi: 10.1037/a0013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cerebral Cortex. 2007;17(5):1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Steyvers M. A model for recognition memory: REM—retrieving effectively from memory. Psychonomic Bulletin and Review. 1997;4:145. doi: 10.3758/BF03209391. [DOI] [PubMed] [Google Scholar]
- Squire L, Stark C, Clark R. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and mind: A festschrift for Gordon H. Bower, chapter On the law of primacy. New Jersey: Lawrence 20 Erlbaum Associates; 2007. [Google Scholar]
- Wyart V, Tallon-Baudry C. How ongoing fluctuations in human visual cortex predict perceptual awareness: baseline shift versus decision bias. Journal of Neuroscience. 2009;29(27):8715–8725. doi: 10.1523/JNEUROSCI.0962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]