Abstract
The ubiquity of endogenous, circadian (daily) clocks among eukaryotes has long been held as evidence that they serve an adaptive function, usually cited as the ability to properly time biological events in concordance with the daily cycling of the environment. Herein we test directly whether fitness is a function of the matching of the period of an organism’s circadian clock with that of its environment. We find that fitness, measured as the per capita expectation of future offspring, a composite measure of fitness incorporating both survivorship and reproduction, is maximized in environments that are integral multiples of the period of the organism’s circadian clock. Hence, we show that organisms require temporal concordance between their internal circadian clocks and their external environment to maximize fitness and thus the long-held assumption is true that, having evolved in a 24-h world, circadian clocks are adaptive.
Keywords: Diapause, life history trade-offs, Nanda-Hamner protocol, photoperiodism, resonance
Circadian clocks with a period of about a day are ubiquitous among eukaryotes and also occur in Cyanobacteria (Edmunds 1988; Johnson et al. 1996). The pervasiveness of circadian clocks across a diverse spectrum of organisms and the requirement of a functional circadian clock for the temporal coordination of both overt behavior and internal organization of cellular biochemistry is often used as evidence that circadian rhythmicity serves an adaptive function (Bunning 1960; Pittendrigh 1961; Aschoff 1964; Hastings et al. 1991; Pittendrigh 1993; Yan et al. 1998; Sharma 2003). Indeed, mutant strains of the cyanobacterium Synechococcus are most competitive in light:dark (L:D) environments whose period (light plus dark) approximates that of their circadian free-running period (Yan et al. 1998). In the drosophilid Drosophila melanogaster (Pittendrigh and Minis 1972; Klarsfeld and Rouyer 1998), the blowfly Phormia terranovae, (von Saint Paul and Aschoff 1978), and the golden hamster Mesocricetus auratus (Hurd and Ralph 1998), adult longevity is enhanced in environmental cycles that are of the same length as the endogenous circadian period. Relative to wild-type, null mutants of genes involved in the circadian clock (clock genes) show reduced carbon fixation, vegetative growth and survivorship in Arabidopsis (Dodd et al. 2005), reduced adult longevity in Drosophila (Hendricks et al. 2003), and reduced reproductive success in both male and female Drosophila (Beaver et al. 2002, 2003).
In these cases, although, it is not clear what effects the mutations are having on reproductive physiology independent of their effects on circadian organization. Also, in studying the effect of a genetic mutant on fitness, one must maintain a standardized genetic background for both the experimental and control lines, making it hard to generalize the effects of those mutations in natural populations where dominance and epistasis play such large roles (Wolf et al. 2000). Thus it is important to test the effects of phenotypes on fitness using populations of animals segregating naturally occurring levels of genetic variation. With this in mind, we study the effects of concordance of the circadian clock with the environment in natural populations of the pitcher-plant mosquito.
Circadian clocks can be rendered dysfunctional either through genetic means (Hall 1999; Sehgal 2004) or by imposing external light:dark (L:D) cycles whose period (T = L + D) varies substantially from the period of oscillation (τ) of an organism’s internal circadian clock (Pittendrigh 1965, 1966). The circadian clock resonates with the external L:D cycle when T = 24 + nτ, but resonance fails when T = 24 +τ (n +0.5), where n is an integer. Periods of endogenous circadian rhythms in insects generally range from τ ~19 to 26 h (Saunders 2002; Lankinen and Forsman 2006). The endogenous circadian rhythm of the flesh fly, Sarcophaga argyrostoma, is about 24 h (Saunders 1976). When S. argyrostoma are exposed to resonant T cycles of 24 or 48 h, there is a strong pupal eclosion rhythm, but when they are exposed to nonresonant T cycles of 36 or 60 h the rhythm is very weak (Saunders 1978), reflecting disorganization of the circadian clock much as if the flies were subjected to perpetual jet lag.
The pitcher-plant mosquito, Wyeomyia smithii, lays its eggs and completes its preadult development entirely within the water-filled leaves of the purple pitcher plant in eastern North America. Throughout their range, they enter a larval dormancy (diapause) that is initiated, maintained, and terminated by day length (Bradshaw and Lounibos 1977). Under a 24 h L:D cycle, short days initiate and maintain diapause whereas long days avert or terminate diapause. Under longer L:D cycles with a fixed short day and increasing night lengths, W. smithii exhibit a rhythmic response, alternating between peaks of development with valleys of diapause (Fig. 1A). In D. melanogaster (Saunders 1990), D. auraria (Pittendrigh et al. 1991; Pittendrigh and Takamura 1993), Calliphora vicina (Saunders 1997), and S. argyrostoma (Saunders 1973, 1978), the peak-to-peak or valley-to-valley interval equals the period of adult eclosion or locomotor rhythms and represents the period of the underlying circadian rhythm (Pittendrigh 1981; vaz Nunes and Saunders 1999). In W. smithii (Fig. 1A), these experiments repeatedly show resonant short-day responses when T equals 24, 46, or 68 h and nonresonant long-day responses when T equals 35 or 56 h, indicating a circadian period) of ~ 21 h in W. smithii.
Figure 1.
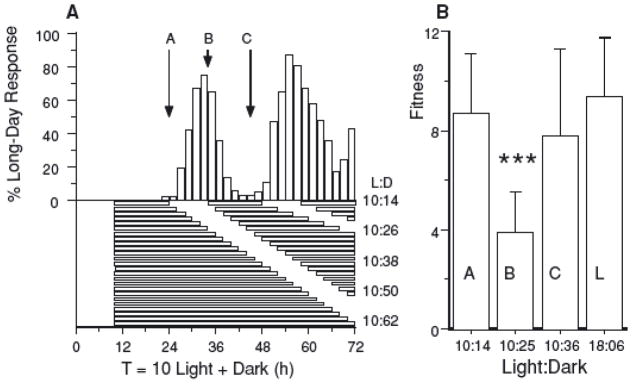
Resonance experiments. (A) Rhythmic response of Wyeomyia smithii from the Gulf Coast of North America (30–31°N) to light:dark (L:D) cycles ranging from L:D = 10:14–10:62 in separate experiments (Bradshaw et al. 2003). The data are pooled from the two populations used in this study. Note that all of these regimens consist of diapause-maintaining short days and long nights so that the rhythmic long-day response represents rhythmic transitions from resonant cycles producing short-day response “valleys” to nonresonant long-day response “peaks.” The arrows indicate three of the four experimental regimens: (A) L:D = 10:14; (B) L:D = 10:25; (C) L:D = 10:36. (B) Fitness (per-capita expectation of future offspring) in response to the L:D cycles indicated in Figure 1A and to a long-day L:D = 18:6 cycle (L). Error bars represent two standard errors. ***P < 0.001 when comparing L:D = 10:25 with the other three cycles, which did not differ from each other.
Herein, we test whether a composite measure of fitness, the net, per-capita expectation of future offspring, depends on resonance of T with the circadian clock in natural populations of W. smithii. We break down fitness into its components: pupal survivorship, fecundity, and embryonic viability and, because of its frequent use as a surrogate for fitness, we also consider adult longevity. We test the specific a priori hypothesis that mosquitoes exposed to a nonresonant cycle (peak B in Fig. 1A) achieve lower fitness than mosquitoes exposed to resonant cycles (valleys A and C in Fig. 1A). A comparison of fitness between two different resonant cycles (valleys A and C in Fig. 1A) controls for the possibility that a decline in fitness in nonresonant cycles might be due to mosquitoes experiencing extended nights. In addition, we determine fitness in response to long days in a resonant cycle (L:D = 18:6). A comparison of fitness between the 24 h short-day resonant cycle (valley A in Fig. 1A) and L:D = 18:6 controls for the possibility that a decline in fitness in the nonresonant cycle might be due to a response to day length, per se.
Materials and Methods
Animals from two populations from northern Florida, USA (30N and 31N, populations WI and CR from previous studies) were used in this experiment. Both populations were maintained as large, outbred populations with N > 1000 in the laboratory for 2 (WI) or > 10 (CR) generations to reduce field and maternal effects while maintaining the naturally occurring genetic diversity of populations. Assuming genetic drift, after 10 generations of size of Ne = 200, heterozygosity of these populations would be reduced by less than 4%, and thus the populations represent naturally occurring genetic variation.
Before starting the experiment larvae were reared under short-day conditions to synchronize all individuals into diapause. Diapausing larvae from each population were then reared (Bradshaw et al. 2003) on a L:D = 18:6 cycle to promote continuous development without diapause. On the day of pupation, 6–10 replicate cohorts were either maintained on L:D=18:6 (long days) or transferred to L:D = 10:14 (valley A in Fig. 1A), L:D = 10:25 (peak B in Fig. 1A), or L:D = 10:36 (valley C in Fig. 1A) at 23±0.5°C and 80%RHin light-tight experimental chambers held within a climate-controlled room. Cohort size was determined by the number of larvae actually pupating on a single day and ranged from 50 to 85 pupae in each cohort. The pupae, resulting adults, and their embryonating eggs were maintained under these same conditions until the eggs hatched. Fitness was then equated with the per-capita expectation of future offspring = (number of first instar larvae eventually hatching from a cohort) ÷ (number of pupae in the original cohort).
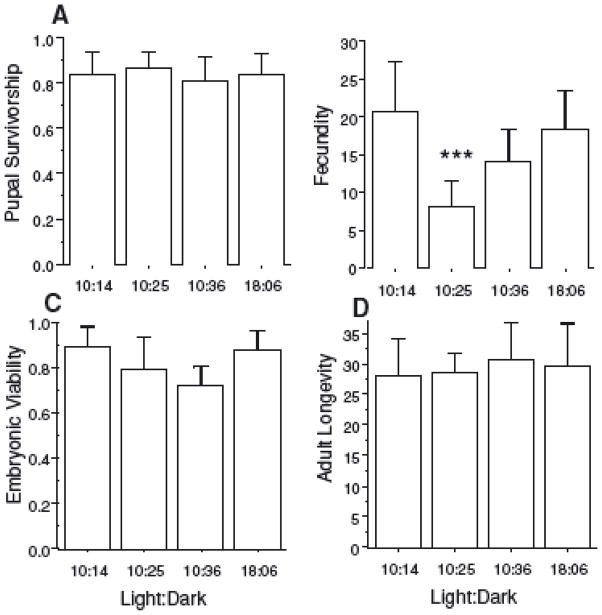
Pupal survivorship was measured as the number of pupae initiating the cohort that survived to adult eclosion; fecundity was calculated as the mean number of eggs per eclosing female; embryonic viability was measured as the percentage of eggs laid that successfully hatched; and adult longevity was measured as the time between median adult eclosion and median adult death of each cohort.
Differences among light regimens in both fitness, its components, and adult longevity were tested with a one-way ANOVA; if there was a significant effect of light treatment, orthogonal contrasts with 1 degree of freedom each were used to test the specific a priori hypotheses (Sokal and Rohlf 1995). Prior to analysis of the data, all assumptions of ANOVA were tested and the appropriateness of ANOVA was confirmed (Sokal and Rohlf 1995). Data from both populations were pooled to increase power in the final analysis as the two separate populations showed the same qualitative results.
Results
Fitness varied among L:D treatments (Fig. 1B) (F3,24 =5.12, P< 0.001). Fitness achieved under L:D = 10:25 was 55% lower than the three other L:D regimens (F1,24 = 14.62, P < 0.001), whereas fitness under the latter three regimens did not differ from each other (P > 0.40). Fitness under the longer L:D = 10:36 cycle did not differ from the two 24-h cycles (F1,24 = 0.63, P = 0.43), showing that the reduction in fitness in the L:D = 10:25 cycle is not due to a longer exotic L:D cycle, per se. Finally, in 24-h regimens, fitness did not differ (F1,24 = 0.13, P = 0.72) between long days (L:D = 10:14) and short days (L:D = 18:06), showing that fitness was not due to variation in day length.
Pupal survivorship (F3,24 = 0.33, P = 0.80), embryonic viability (F3,24 = 1.43, P = 0.26), and adult longevity (F3,24 = 0.25, P=0.85) did not vary among L:D treatments (Fig. 2). By contrast, per capita female fecundity varied among L:D treatments (F3,24 = 5.12, P <0.001). Fecundity under L:D = 10:25 was 58% lower than the three other L:D regimens (F1,24 = 15.36, P < 0.001), whereas fecundity under the latter three regimens did not differ from each other (P > 0.09). In a similar pattern to that of our composite measure of fitness, fecundity under the longer L:D = 10:36 cycle did not differ from the two 24-h cycles (F1,24 = 3.11, P = 0.09) nor did it differ (F1,24 = 0.47, P = 0.49) between short days (L:D = 10:14) and long days (L:D = 18:06).
Figure 2.
Components of fitness: (A) pupal survivorship; (B) average fecundity per female; (C) embryonic viability, and (D) adult longevity, in the four different light treatments (Light:Dark) used in this study. Error bars represent two standard errors. ***P<0.001 when comparing L:D = 10:25 with the other three cycles, which did not differ from each other.
Discussion
We show for the first time in natural populations of animals that concordance of the circadian clock with the cycling environment is necessary to maximize fitness. We show that the fitness reduction in nonresonating L:D environments is mainly due to a reduction in fecundity, whereas pupal survivorship, embryonic viability, and adult
Much of the recent data on the fitness consequences of circadian organization in animals have only been in reference to a single component or correlate of fitness, in most cases longevity (Pittendrigh and Minis 1972; Hurd and Ralph 1998; Klarsfeld and Rouyer 1998; Kumar et al. 2005). Studying the effect of circadian disorganization on longevity alone may be misleading in that longevity is often not positively correlated with composite measures of fitness (Bell 1984; Partridge and Harvey 1985; Reznick 1992; Roff 1992; Stearns 1992; Zwaan 1999; Sheeba et al. 2000). For instance, Sheeba et al. (2000) compared adult life span and fecundity among several lines of D. melanogaster under constant light (LL), 12L: 12D and constant darkness (DD), and found that a reduction in longevity in adult flies in LL is at least partly a function of an increase in reproductive output early in life. In W. smithii, we found that adult longevity did not vary among treatments, despite significant differences in fitness. This result underscores the unreliability of using a single fitness correlate as a surrogate for an appropriate composite index of fitness.
Using loss-of-function mutants in central circadian clock genes, period, timeless, cycle, and clock (per°, tim°, cyc°, and Clkjrk) in D. melanogaster, Beaver et al. (2002) showed that single matings using mutant males produced ~40% fewer progeny, and those progeny had low survivorship to adulthood. This reduction in fitness was associated with the reduction in the amount of sperm released from the testes to the seminal vesicles in null mutant males (Beaver et al. 2002). Mutations in per and tim may also have clock-independent effects on the production of mature oocytes and viable progeny in females of D. melanogaster (Beaver et al. 2003). The mutant lines from these studies come from different genetic backgrounds and are compared to Canton-S flies as a control, making generalizations from these comparisons difficult. In addition, using mutant, inbred lines, allows for pleiotropic and epistatic effects to be fixed within the line, potentially affecting the resulting phenotype. We overcome this problem by using natural populations of mosquitoes that are segregating naturally occurring alleles among large numbers of individuals.
Several studies on the fitness effects of having an environment with the same periodicity as the circadian clock involve the use of short- and long-period mutant lines (Yan et al. 1998; Dodd et al. 2005). These studies use L:D treatments that have the same total period length as the mutant lines and showthat fitness is optimized under the environment that matches the organism’s free-running period. Here we avoid having to induce mutations by using two classes of extended night environments, one which is resonant with the underlying circadian rhythm and one that is nonresonant as shown in experiments with natural populations of W. smithii (Fig. 1A). We have controlled for the novelty of the experimental treatments by using resonant cycles with integral (1 and 2) multiples of the circadian period. We also control for the effect of genetic background by averaging across many individuals from populations with naturally segregating genetic variation; and, the error term in our analyses incorporates variation between as well as within populations. We have shown that the loss of fitness in the nonresonant cycle is not due either to the extended nights of that cycle or to an effect of day length. The loss of fitness in W. smithii in environmental cycles that are not integral multiples of the circadian clock’s period is primarily due to reduced female fecundity (Fig. 2). We observed no effect of cycle length on pupal survivorship, indicating that the switch itself from a resonant (L:D = 18:06) to a nonresonant (L:D = 10:25) was not the cause of reduced fitness. Finally, we observed no effect of cycle length on adult longevity and, had we measured longevity alone, we would not have observed the effect of cycle length on fitness. We conclude that concordance of the period of the circadian clock with the environment is necessary to maximize fitness and confirm the long-held proposition that circadian clocks are adaptive in natural populations.
Acknowledgments
We thank D. Mathias, A. Johnson, A. Letaw, M. Sparks, D. Wright, and K. Neall for technical assistance and for comments on previous versions of the manuscript, two anonymous reviewers for insightful comments, the National Science Foundation through grant DEB-0412573 to WEB, and the National Science Foundation and National Institutes of Health through training grants DGE-0504727 and 5-T32-GMO7413, respectively, to KJE.
LITERATURE CITED
- Aschoff J. Survival value of diurnal rhythms. In: Edholm O, editor. Biology of survival; Symposium of the Zoological Society of London; London. 1964. pp. 79–98. [Google Scholar]
- Beaver LM, Gvakharia BO, Vollintine TS, Hege DM, Stanewsky R, Giebultowicz JM. Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc Natl Acad Sci USA. 2002;99:2134–2139. doi: 10.1073/pnas.032426699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver LM, Rush BL, Gvakharia BO, Giebultowicz JM. Noncircadian regulation and function of clock genes period and timeless in oogenesis of Drosophila melanogaster. J Biol Rhythms. 2003;18:463–472. doi: 10.1177/0748730403259108. [DOI] [PubMed] [Google Scholar]
- Bell G. Measuring the cost of reproduction. 1. The correlation structure of the life table of a plankton rotifer. Evolution. 1984;38:300–313. doi: 10.1111/j.1558-5646.1984.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Lounibos LP. Evolution of dormancy and its photoperiodic control in pitcher-plant mosquitoes. Evolution. 1977;31:546–567. doi: 10.1111/j.1558-5646.1977.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Quebodeaux MC, Holzapfel CM. Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: adaptive response to the photic environment or correlated response to the seasonal environment? Am Nat. 2003;161:735–748. doi: 10.1086/374344. [DOI] [PubMed] [Google Scholar]
- Bünning E. Circadian rhythms and the time measurement in photoperiodism. Cold Spring Harbor Symp Quant Biol. 1960;25:249–256. doi: 10.1101/sqb.1960.025.01.029. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJAA, Webb R. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- Edmunds LN. Cellular and molecular bases of biological clocks: models and mechanisms for circadian timekeeping. Springer-Verlag; New York, NY: 1988. [Google Scholar]
- Hall JC. Genetics and molecular biology of rhythms in Drosophila and other insects. Academic Press; Amsterdam: 1999. [DOI] [PubMed] [Google Scholar]
- Hastings JW, Rusak B, Boulos Z. Circadian rhythms: the physiology of biological timekeeping. In: Prosser C, editor. Comparative animal physiology. Neural and integrative animal physiology. Wiley-Liss; New York, NY: 1991. pp. 435–546. [Google Scholar]
- Hendricks J, Lu S, Kume K, Yin J, Yang Z, Sehgal A. Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster. J Biol Rhythms. 2003;18:12–25. doi: 10.1177/0748730402239673. [DOI] [PubMed] [Google Scholar]
- Hurd MW, Ralph M. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Golden SS, Ishiura M, Kondo T. Circadian clocks in prokaryotes. Mol Microbiol. 1996;21:5–11. doi: 10.1046/j.1365-2958.1996.00613.x. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A, Rouyer F. Effects of circadian mutations and LD periodicity on the life span of Drosophila melanogaster. J Biol Rhythms. 1998;13:471–478. doi: 10.1177/074873098129000309. [DOI] [PubMed] [Google Scholar]
- Kumar S, Mohan A, Sharma VK. Circadian dysfunction reduces lifespan in Drosophila melanogaster. Chronobiol Int. 2005;22:641–653. doi: 10.1080/07420520500179423. [DOI] [PubMed] [Google Scholar]
- Lankinen P, Forsman P. Independence of genetic geographical variation between photoperiodic diapause, circadian eclosion rhythm, and Thr-Gly repeat region of the period gene in Drosophila littoralis. J Biol Rhythms. 2006;21:3–12. doi: 10.1177/0748730405283418. [DOI] [PubMed] [Google Scholar]
- Partridge L, Harvey PH. Evolutionary biology—costs of reproduction. Nature. 1985;316:20–20. [Google Scholar]
- Pittendrigh CS. On temporal organization in living systems. Harvey Lectures. 1961;56:93–125. [PubMed] [Google Scholar]
- Pittendrigh CS. On the mechanism of the entrainment of a circadian rhythm by light cycles. In: Aschoff J, editor. Circadian clocks. North-Holland Publishing Co; Amsterdam, NL: 1965. pp. 277–297. [Google Scholar]
- Pittendrigh CS. The circadian oscillation in Drosophila pseudoobscura pupae: A model for the photoperiodic clock. Z Pflanzenphysiol. 1966;54:275–307. [Google Scholar]
- Pittendrigh CS. Circadian organization and the photoperiodic phenomena. In: Follett BK, Follett DE, editors. Biological clocks in seasonal reproductive cycles. Wright; Bristol: 1981. pp. 1–35. [Google Scholar]
- Pittendrigh CS. Temporal organization—reflections of a Darwinian clockwatcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Minis DH. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc Natl Acad Sci USA. 1972;69:1537–1539. doi: 10.1073/pnas.69.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Takamura T. Homage to Sinzo Masaki: circadian components in the photoperiodic response of Drosophila auraria. In: Takeda M, Tanaka S, editors. Seasonal adaptation and diapause in insects. Bun-ichi Sôgô Shuppan; Tokyo, Japan: 1993. pp. 288–305. (in Japanese) [Google Scholar]
- Pittendrigh CS, Kyner WT, Takamura T. The amplitude of circadian oscillations—temperature-dependence, lattitudinal clines, and the photoperiodic time measurement. J Biol Rhythms. 1991;6:299–313. doi: 10.1177/074873049100600402. [DOI] [PubMed] [Google Scholar]
- Reznick D. Measuring the costs of reproduction. Trends Ecol Evol. 1992;7:42–45. doi: 10.1016/0169-5347(92)90104-J. [DOI] [PubMed] [Google Scholar]
- Roff . The evolution of life-histories: theory and analysis. Chapman and Hall; New York, NY: 1992. [Google Scholar]
- Saunders DS. The photoperiodic clock in the flesh-fly, Sarcophaga argyrostoma. J Insect Physiol. 1973;19:1941–1954. doi: 10.1016/0022-1910(73)90188-1. [DOI] [PubMed] [Google Scholar]
- Saunders DS. Circadian eclosion rhythm in Sarcophaga-argyrostoma—some comparisons with photoperiodic clock. J Comp Physiol. 1976;110:111–133. [Google Scholar]
- Saunders DS. An experimental and theoretical analysis of photoperiodic induction in the flesh-fly Sarcophaga argyrostoma. J Comp Physiol. 1978;124:75–95. [Google Scholar]
- Saunders DS. The circadian basis of ovarian diapause regulation in Drosophila melanogaster: is the period gene causally involved in photoperiodic time measurement? J Biol Rhythms. 1990;5:315–331. doi: 10.1177/074873049000500404. [DOI] [PubMed] [Google Scholar]
- Saunders DS. Insect circadian rhythms and photoperiodism. Invertebr Neurosci. 1997;3:155–164. doi: 10.1007/BF02480370. [DOI] [PubMed] [Google Scholar]
- Saunders DS. Insect clocks. Elsevier Science; Amsterdam: 2002. [Google Scholar]
- Sehgal A, editor. Molecular biology of circadian rhythms. John Wiley & Sons; Hoboken, NJ: 2004. [Google Scholar]
- Sharma VK. Adaptive significance of circadian clocks. Chronobiol Int. 2003;20:901–919. doi: 10.1081/cbi-120026099. [DOI] [PubMed] [Google Scholar]
- Sheeba V, Sharma VK, Shubha K, Chandrashekaran MK, Joshi A. The effect of different light regimes on adult life span in Drosophila melanogaster is partly mediated through reproductive output. J Biol Rhythms. 2000;15:380–392. doi: 10.1177/074873000129001477. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. W.H. Freeman and Company; New York, NY: 1995. [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford Univ. Press; New York, NY: 1992. [Google Scholar]
- vaz Nunes M, Saunders D. Photoperiodic time measurement in insects: a review of clock models. J Biol Rhythms. 1999;14:84–104. doi: 10.1177/074873049901400202. [DOI] [PubMed] [Google Scholar]
- von Saint Paul U, Aschoff J. Longevity among blowflies Phormia terranovae (RD) kept in non-24-hour light-dark cycles. J Comp Physiol. 1978;127:191–195. [Google Scholar]
- Wolf JB, Brodie ED III, Wade MJ, editors. Epistasis and the evolutionary process. Oxford Univ. Press; New York, NY: 2000. [Google Scholar]
- Yan OY, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci USA. 1998;95:8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan BJ. The evolutionary genetics of ageing and longevity. Heredity. 1999;82:589–597. doi: 10.1046/j.1365-2540.1999.00544.x. [DOI] [PubMed] [Google Scholar]