Abstract
Morphology of the corpus callosum is a useful biomarker of neuronal loss, as different patterns of cortical atrophy help to distinguish between dementias such as Alzheimer’s disease (AD) and frontotemporal lobar degeneration (FTLD).
We used a sophisticated morphometric analysis of the corpus callosum in FTLD subtypes including frontotemporal dementia (FTD) semantic dementia (SD), and progressive non-fluent aphasia (PNFA), and compared them to AD patients and 27 matched controls.
FTLD patient subgroups diverged in their callosal morphology profiles, with: FTD patients showing marked widespread differences, PNFA patients with differences largely in the anterior half of the callosum, and SD patients differences in a small segment of the genu. AD patients showed differences in predominantly posterior callosal regions.
This study is consistent with our previous findings showing significant cortical and subcortical regional atrophy across FTLD subtypes, and suggests that callosal atrophy patterns differentiate AD from FTLD, and FTLD subtypes.
Keywords: Frontotemporal dementia, Alzheimer’s disease, neuroimaging, morphometry, corpus callosum, white matter, atrophy, magnetic resonance imaging
BACKGROUND
Frontotemporal lobar degeneration (FTLD), a group of dementias affecting predominantly the frontal and temporal lobes, is the third most common cortical dementia type after Alzheimer’s disease (AD) and Lewy body dementia. FTLD poses a diagnostic challenge due to its clinical and pathological heterogeneity, and the phenotypic characterization of patients with this disorder can be augmented by using neuroimaging to map the brain changes in FTLD and its subtypes. As grey matter volume decreases due to loss of projecting pyramidal cells in layer III of the neocortex, Wallerian degeneration of the corresponding axons results in a loss of volume in white matter tracts [1]; this is prominent in the corpus callosum, the brain’s largest white matter bundle, where regional atrophy of the callosum reflects the pattern of cortical atrophy in cortical dementias [2]. Most extensively studied in AD, callosal morphometry is a useful marker of neuronal loss and disease severity [3–5].
In contrast to AD, where the temporal and parietal neocortex is particularly involved, in patients with FTLD the frontal and anterior temporal cortex show the greatest regional atrophy [6,7]. This suggests that the pattern of callosal atrophy may be able to distinguish these two cortical dementias. The first study to examine the role of callosal morphometry in distinguishing AD and FTLD was undertaken by Kaufer et al., who parcellated the callosum into four segments based on 10 equal radial divisions of the callosum in FTLD and AD patients compared to matched controls. Whereas the AD subjects did not show detectable differences from controls, but FTLD subjects had significantly smaller total and anterior CC regions [8]. Yamauchi et al. examined similar groups, but added a progressive supranuclear palsy (PSP) group, and measured antero-posterior quartile areas of the callosum; all patient groups showed lower total callosal area, and anterior regions were most reduced in FTLD, posterior in AD, and middle-anterior in PSP [9]. Hensel et al. examined anterior-posterior quintile areas of the callosum in a smaller group FTLD and AD patients with milder illness than the two previous studies, and found no difference, albeit in groups unmatched in age [10]. Wiltshire et al. examined antero-posterior quartile areas in AD patients, but also in Parkinson’s disease (PD) patients and controls, showing lower total callosal area in AD patients, and a significant positive correlation between Mini-Mental State Examination (MMSE) score and the size of the most anterior and posterior callosal quartiles [11]. However, these four studies used unaligned images and an operator-chosen “best” mid-sagittal slice, possibly introducing bias, and arbitrary parcellation methods that may be insensitive to localised thickness changes. More recently, Di Paola and colleagues examined callosal morphometry using a more robust method that measured callosal thickness at 100 points across an aligned callosum in patients with mild cognitive impairment (MCI), mild and severe AD, and controls [12]. They demonstrated reductions in anterior and posterior callosal regions. The white matter intensity in these regions strongly correlated with atrophy in interconnected grey matter regions as detected using voxel-based morphometric analysis, thus supporting the relationship between callosal morphometric change and loss of grey matter in the cortical regions that these fibres interconnect [12].
However, while studies examining callosal morphometry in AD have examined patients both mild and severe illness and individuals diagnosed with MCI, no study has looked at the subtypes of FTLD patients: behavioural variant frontotemporal dementia (FTD), primary non-fluent aphasia (PNFA) and semantic dementia (SD). We have previously shown that different subtypes of FTLD show different shape or morphometric “signatures” in a number of grey matter structures, including the caudate, putamen and hippocampus [13,14] that correspond to regional neocortical reductions that affect fronto-striatal and fronto-limbic networks, and that regional shape reductions may serve as a map of structural change in cortical afferent pathways across FTLD subtypes [15,16]. Given the well-defined regional callosal reductions seen in the callosum in Alzheimer’s disease, we might expect FTLD subtypes to show a regional pattern of callosal atrophy that may aid in the neuroradiological, and hence phenotypic, differentiation of these subtypes.
The aim of this study was to apply a sophisticated callosal morphometric analysis in a well-characterised group of FTLD and AD patients and matched controls to further quantify the differences in callosal morphometry between AD and FTLD, and to also determine if the pattern of atrophy in the callosum differed across FTLD subtypes.
METHODS
Subjects
Participants were recruited retrospectively from the Memory Clinic at the Karolinska University Hospital, Huddinge, Stockholm, Sweden and have been previously described [13,14,17–20]. The study was approved by the local ethics committee.
Eighty subjects participated in the study: 34 FTLD patients [12 behavioural variant frontotemporal dementia (FTD), 13 semantic dementia (SD), 9 progressive non fluent aphasia (PNFA)], 19 with Alzheimer’s disease (AD) and 27 in a control group (See Table 1). All subjects in the studies underwent the standard investigation procedure for memory clinic patients. The clinical diagnosis was determined at a multidisciplinary consensus conference with physicians, neuropsychologists, speech-language pathologists and nurses. The medical examination included history from a close informant, as well as assessment of physical, neurological, and psychiatric status. Laboratory investigation of blood, CSF and urine (including vitamin B12, folic acid levels and thyroid function) was performed. Neuroradiologic examination consisted of magnetic resonance imaging (MRI) of the brain and single-photon emission computed tomography (SPECT) imaging of cerebral blood flow. Detailed EEG, neuropsychological and speech-language examinations were performed [18].
Table 1.
Sample demographic data
| Group | Age at scan | Illness duration | MMSE | n | M/F | Intracranial volume |
|---|---|---|---|---|---|---|
| Controls | 62.39 (±6.51) | 28.90 (±1.22) | 21 | 7/14 | 1424 (±124) | |
| AD | 63.88 (±7.22) | 2.49 (±1.25) | 22.30 (±4.89)* | 19 | 8/12 | 1379 (±137) |
| PNFA | 64.93 (±7.21) | 3.56 (±2.69) | 16.88 (±11.37)* | 9 | 3/6 | 1373 (±77) |
| SD | 63.77 (±7.13) | 3.90 (±1.81) | 22.92 (±6.63)* | 13 | 5/8 | 1440 (±156) |
| FTD | 61.25 (±4.18) | 1.85 (±0.92) | 20.27 (±6.51)* | 12 | 4/8 | 1419 (±150) |
Mean (min-max) of: age at scan, illness duration, MMSE, and intracranial volume.
significantly different from controls in Kruskal-Wallis ANOVA with a Mann-Whitney U-test post-hoc.
AD, Alzheimer’s disease, FTD, Frontotemporal dementia; M/F: the number of males/females included in the study; MMSE, Mini-Mental State Examination,; PNFA, progressive nonfluent aphasia; SD, semantic dementia.
Clinical criteria for the subtypes of FTLD were based on international consensus criteria [21]. The subtypes included were: frontotemporal dementia (FTD), semantic dementia (SD) and progressive non-fluent aphasia (PNFA). Only patients suffering from a primary degenerative cerebral process were selected, excluding patients with signs of cerebrovascular or systemic disorders. FTLD patients at different stages of the disease were included. Alzheimer’s disease was diagnosed using DSM-IV and ICD-10 criteria [22,23]. Participants with Alzheimer’s disease (AD) displayed the development of multiple cognitive deficits including memory impairment and one or more of aphasia, apraxia, or agnosia, plus disturbance in executive functioning. This presented as an illness of gradual onset, with continuing decline from previous levels of functioning. These symptoms were not due to another dementing process or psychiatric disorder. The control group comprised individuals who were found, after careful assessment, not to fulfil criteria for FTLD, AD, or any other cognitive disorder, but to sometimes feel forgetful in everyday life. Objective impairment was ruled out through comprehensive neuropsychological assessment; impairment was defined as performance ≥ 1.5 standard deviation units below the mean on any cognitive test. Accordingly, controls had no objective cognitive impairment by definition. To further minimize the risk of including participants with neurodegenerative disease in very early stages, only those participants that did not deteriorate over a minimum of two years follow-up were included.
MRI Scanning
T1-weighted MRI scans were acquired on a 1.5 T Siemens Magnetom Vision Plus scanner (Siemens Medical Systems, Erlangen, Germany). A three-dimensional magnetization-prepared rapid gradient echo (3D-MPRAGE) pulse sequence (TR 11.4 ms; TE 4.4 ms; TI 300 ms; FA 10°; NEX 1) was used to obtain 72 contiguous coronal 2.5 mm-slices with 512 × 144 matrix and 230-mm FOV.
Callosal Thickness Analysis
Images were linearly registered to the Montreal Neurological Institute (MNI) 305 template using 9-parameter transformations to adjust for brain orientation, translation, and size. The corpus callosum was then outlined automatically based on the Chan-Vese model for active contours [24]. This resulted in two midsagittal callosal segments (i.e., the upper and lower callosal boundary) for each subject. Subsequently, each callosal segment was overlaid onto the MR image from which it had been extracted and visually inspected to ensure that automatically generated callosal outlines precisely followed the natural course and boundaries of the corpus callosum. Using the upper and lower callosal boundaries, callosal thickness was calculated in millimetres at 100 locations distributed evenly over the callosal surface, as detailed elsewhere [25,26]. Point-wise callosal measurements were compared between controls and the patient groups, while co-varying for sex and age. When comparing the patient groups to each other, we additionally removed the variance associated with disease duration. Significance maps for the associations were corrected for multiple comparisons using the standard false discovery rate (FDR) method, at the conventionally accepted level of Q=0.05, whereby only 5% of the voxels shown in the thresholded statistical maps are expected to be false positives. The critical P value is reported, which represents the highest P-value threshold for which only 5% of the surviving voxels are expected to be false positives. The critical P-value threshold for this map is generally larger when standardized effect sizes are larger. Significance maps for each comparison, showing both uncorrected and corrected P values, were then generated. In the primary analysis, patient groups (AD, and the three FTLD subtypes: FTD, PNFA and SD) were compared to controls; in a secondary analysis, patient groups were compared to each other.
RESULTS
Dementia Groups Compared to Control Group
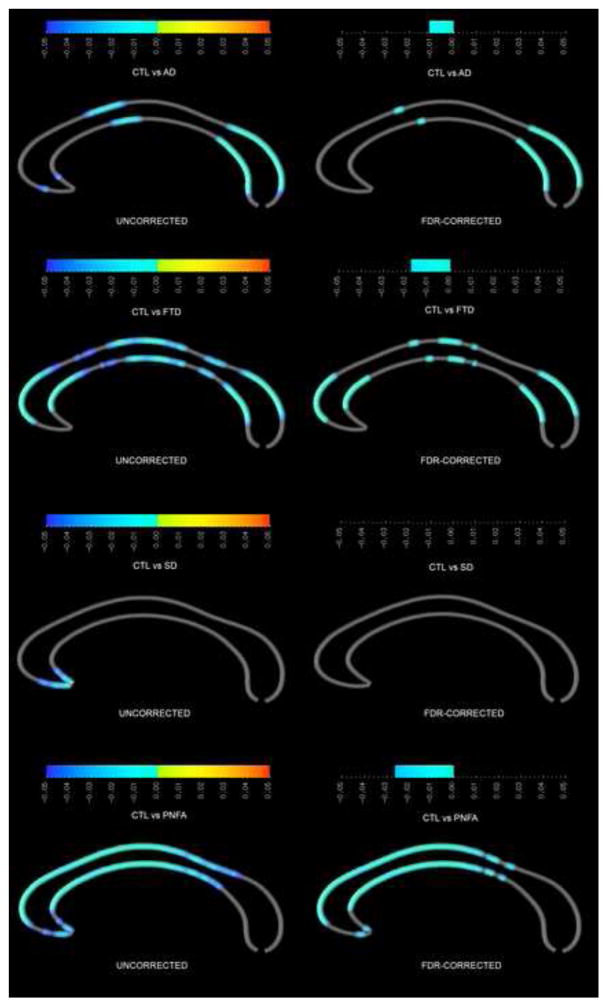
When patients with AD were compared to healthy controls, the splenium, anterior body and the most anterior segment of the genu were significantly thinner in AD; all findings but those in the genu survived FDR correction for multiple comparisons (figure 1). FTD patients also had thinner corpora callosa than controls, where effects were even more pronounced and widespread than in AD patients with significant differences occurring within large segments of the splenium, mid-body, and across the genu; these findings largely survived FDR correction. SD patients had a significantly thinner genu compared to controls, which did not survive FDR correction. The PNFA group showed differences across the anterior half of the callosum, surviving FDR.
Figure 1.
Callosal comparison between controls and the four patient groups. On the left, uncorrected p-value maps, with significance for group 2 being thinner than group 1 on the left (blue colour bar), and thicker on the right (yellow-red colour bar). On the right, FDR-corrected p-value maps.
Dementia Groups Compared to Each Other
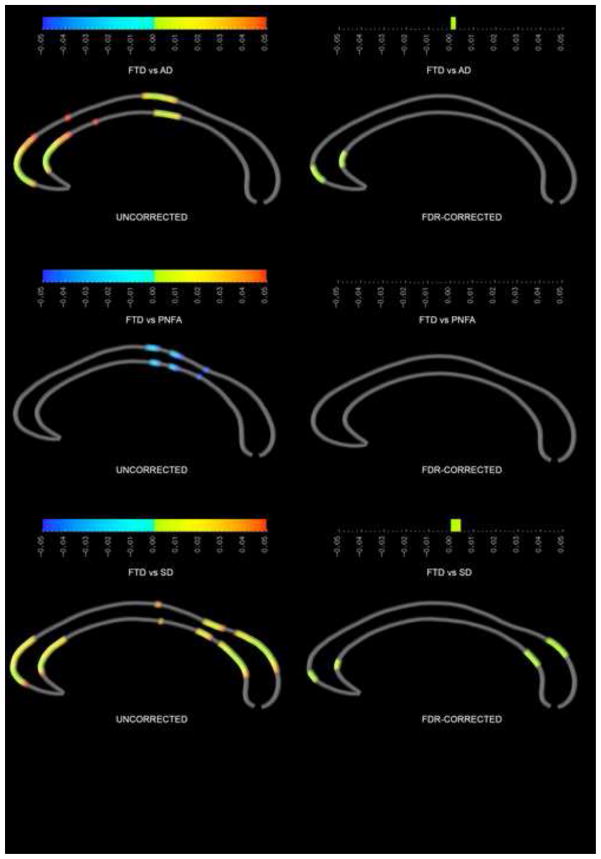
When the FTD group was compared to the AD group, AD subjects had thicker corpora callosa in the genu and midbody (figure 2), with genu findings surviving FDR correction. The PNFA group had a thinner posterior body and isthmus compared to the FTD group, but these findings did not survive FDR correction. Compared to the SD group, the FTD patients had a thinner genu and splenium, which did survive FDR correction. The PNFA and SD groups did not differ significantly from each other.
Figure 2.
Callosal comparison between FTD patient group and the three other dementia groups. On the left, uncorrected p-value maps; on the right, FDR-corrected p-value maps.
DISCUSSION
Here we used advanced morphometry techniques to extend previous reports that different cortical dementias, AD and FTLD, show characteristic shape signatures in the corpus callosum. We also set out to determine if well-characterised FTLD subtypes also present with characteristic shape profiles, which to our knowledge has not been done before.
In the AD group, our findings strongly corroborate other data demonstrating significant reduction in the posterior callosum in AD patients across illness stages [2,4,12,27–29]; this posterior atrophy is thought to relate to Wallerian degeneration in the commissural fibres that arise from the projecting cells in layer III of the temporal and parietal cortices, regions of the neocortex affected most significantly in AD [5]. Parietal and temporal commissural fibres course through the posterior body/isthmus and anterior splenium respectively, with the most posterior regions of the splenium containing fibres from occipital regions [30]. Hence, the regions that show the most significant reductions in our AD patients contain fibres from the regions of neocortex most affected in AD. Not all studies have shown splenial reductions in AD, although a wide variety of methods have been used to quantify the callosum in this disorder and not all have been robust [5]. Unlike many previous studies, we used a 9-parameter normalisation step for all scans before generating callosal contours. This ensures robust selection of the mid-sagittal slice by minimising the effect of head orientation/rotation on apparent mid-sagittal area. It also adjusts, linearly, for the relationship between skull size and callosal area [31]. Furthermore, studies using parcellated regions of the callosum largely do not take into account the non-independence of adjacent callosal measures in their statistical model, and frequently do not correct for multiple comparisons; FDR ensures that non-independence and multiple comparisons are accounted for in our statistical analysis [32]. As a result, our FDR-corrected findings for the AD group compared to controls are likely to be robust.
Our findings in FTLD subtypes are broadly consistent with findings from studies analysing grey matter change in these groups: FTD with (often medial) frontal and temporal atrophy; PNFA with left inferior frontal and insula atrophy; and SD with asymmetrical atrophy of the temporal poles [33,34]. These patterns of atrophy across cortical regions strongly differentiate these subtypes of FTLD [17]. In our callosal morphometry analysis, the FTD group showed corresponding changes in regions associated with medial and orbitofrontal anterior cortex (anterior changes), superior and inferior temporal regions (posterior changes) and the insula (mid-callosal changes) [30]. The PNFA group showed striking changes across frontal regions of the callosum, extending through zones that connect the entire prefrontal cortex, premotor and motor regions, and anterior and posterior cingulate [30]. These findings are largely consistent with previous work involving FTD and PNFA subjects.
The group demonstrating the least significant difference compared to controls was the SD group, who only showed differences in the inferior genu of the callosum, which connects contralateral ventromedial frontal lobe regions [30]. Ventromedial changes have been described in SD [35,36], which may account for these regional changes. Also, much of the regional change in temporal pole grey matter may not be reflected in anterior callosal change, as many of these fibres traverse the anterior commissure, which conveys fibres for the anterior one third of the temporal cortex [37,38]. In support of this, the anterior commissure shows significant microstructural change and regional thinning in SD [39], and the larger FTLD group [40].
When dementia groups were compared to each other, we found difference that were expected given the different pattern of differences between each dementia group and controls. AD and FTD groups both demonstrated posterior change, but only the FTD group showed significant genu change; i.e., the FTD group showed genu shape reductions compared to AD patients. However, not all comparisons showed significant effects; for example, the SD and PNFA groups did not show significant differences from each other, but each group showed a different profile from controls. Additionally, modest uncorrected changes in some other comparisons failed to survive FDR. These inter-group comparisons were less well-powered than the patient group-control analyses (Table 1). It is harder to draw definitive conclusions from the patient group comparisons, which warrant replication in larger datasets.
One additional consideration when interpreting these results is the role that white matter pathology plays in FTLD, and whether white matter changes are viewed as secondary to grey matter changes, or as reflecting at least some primary white matter pathology. FTLD is largely seen as a grey matter disease, but FTLD-tau is known to result in inclusions in white matter [41,42]. Diffusion tensor imaging (DTI) using measures such as fractional anisotropy (FA) has shown significant reductions in white matter integrity in anterior cortical regions [43–46] generally, and anterior callosal regions specifically in FTD patients [43,47] and the FTLD group as a whole [48]. Anterior white matter changes do appear to occur more significantly in FTLD-tau than FTLD-TDP patients [48], and also appear to be present in presymptomatic patients who carry known mutations of the MAPT or GRN genes – particularly in the anterior half of the callosum – prior to the onset of grey matter changes [49]. When language variants are examined, anterior callosal differences have been reported in the anterior half of the callosum in PNFA, but only the most anterior region of the genu in SD [47], in a very similar pattern to our results. Very few of these studies examined white matter in concert with grey matter, so the spatiotemporal pattern of change across grey and white matter compartments remains unclear. White matter FA changes may precede grey matter atrophy across the FTLD spectrum [47], which further suggests that white matter imaging may be sensitive to early illness prior to grey matter atrophy. Altered patterns of network connectivity, encompassing grey and white matter, are apparent in FTLD subtypes in vivo [50]. In this context, our callosal morphometry methods may help to identify up- and downstream structures affected in FTLD, as we have proposed for neostriatal morphometry [15].
Neuroimaging measures such as callosal morphometry are one of a number of biomarkers that may be used to assist in cleaving dementia subtypes, which is important not just in understanding an individual patient’s prognosis but in choosing current and emerging treatments that may be tailored to different disease subtypes. As our knowledge of the causes of FTLD syndromes expands, we need to understand the relationship between clinical phenotype, neuroimaging characteristics, and the underpinning neurobiology of these groups. Subtypes based on neuronal protein inclusions seen in FTD – microtubule-associated protein tau (MAPT), tar-DNA binding protein 43 (TDP43) and fused-in-sarcoma (FUS) – may however only partially map to clinically and neuroradiologically defined syndromes [34]. Syndromes associated with MAPT inclusions show asymmetrical dorsolateral and orbitomedial frontal atrophy [51,52]; TDP43 with asymmetrical or symmetrical frontotemporal atrophy or anterior temporal atrophy [53,54]; and FUS with orbitofrontal, anterior cingulate, insula and caudate atrophy [55]. Similarly, mutations in the genes for MAPT, progranulin (GRN), the non-coding region of chromosome nine open reading frame 72 (C9ORF72), and the less common valosin-containing protein (VCP), charged multivesicular body protein 2B (CHMP2B), FUS and TDP43 show differing patterns of atrophy: MAPT mutations appear to be associated with symmetrical atrophy of anterior temporal regions, and GRN mutations with asymmetrical anterior temporal and inferior frontal atrophy [56,57]; whereas C9ORF72 hexanucleotide expansions are associated with symmetrical frontotemporal changes but also posterior cortical, cerebellar and thalamic volume reductions [58].
CONCLUSION
This analysis shows that different FTLD subtypes have unique callosal morphologic signatures that largely correspond with known regions of grey matter change. This suggests that neuroimaging analyses of FTLD patients need to separate disease subtypes when undertaking structural and functional neuroimaging analyses to minimise group heterogeneity, to ensure that generalizable differences are detected in between-group analysis. As in our work on subcortical structures, the shape profile of the corpus callosum may be a promising illness biomarker that helps to separate disease subtypes based on callosal morphometry. Extending this work to determine how different proteinopathic and genetic FTLD subtypes differ in callosal shape may allow for this MRI morphometric parameter to be used as an adjunct to diagnosis.
RESEARCH IN CONTEXT.
SYSTEMATIC REVIEW
We examined all available studies through a MedLine literature review supplemented by hand-searching of additional manuscripts using the terms “corpus callosum”, “MRI”, “dementia”, “Alzheimer’s disease” and “frontotemporal dementia” to extract all studies that examined the MRI-derived morphology of the corpus callosum in Alzheimer’s disease (AD) and frontotemporal lobar degeneration (FTLD) patients. The methodological approaches of the highlighted articles were reviewed.
INTERPRETATION
We found that no prior study had examined callosal morphology FTLD subgroups, in spite of clear differences in the pattern of cortical atrophy across these subgroups. Our study corroborated previous work that showed differences between the AD and FTLD populations as a whole, but also showed that the FTLD subtypes could be further differentiated on the basis of regional callosal measures. This also extends on our previous work showing that FTLD subtypes show different patterns of atrophy in the hippocampus and basal ganglia.
FUTURE DIRECTIONS
These findings re-inforce the need for future research to carefully consider diagnostic subtypes of dementing disorders, and their differential neuroimaging changes, when utilising neuroimaging methodology at a group and individual level. It also adds to our own body of work and that of the field, demonstrating that shape analysis can allow differentiation of dementia subtypes and can yield insights into the nature of cortical processes that occur in these disorders.
Acknowledgments
FUNDING SOURCES
This research has made use of the SMILE medical imaging laboratory at Karolinska University Hospital, Stockholm, Sweden. Dr Eva Örndahl, formerly of the Karolinska University Hospital, contributed to a previous poster version of this paper. A/Prof J.C.L. Looi self-funded travel costs to assist in coordination of this research in Australia, USA and Sweden as part of the Australian United States Scandinavian Imaging Exchange (AUSSIE). E.L., P.R., and P.T. are funded by grants from the U.S. National Institutes of Health, including a P41 resource grant from the NIBIB.
Footnotes
CONFLICTS
The authors report no conflicts of interest.
References
- 1.Lippa CF. Synaptophysin immunoreactivity in Pick’s disease: comparison with Alzheimer’s disease and dementia with Lewy bodies. Am J Alzheimers Dis Other Demen. 2004;19:341–344. doi: 10.1177/153331750401900606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teipel SJ, Bayer W, Alexander GE, Zebuhr Y, Teichberg D, et al. Progression of corpus callosum atrophy in Alzheimer disease. Arch Neurol. 2002;59:243–248. doi: 10.1001/archneur.59.2.243. [DOI] [PubMed] [Google Scholar]
- 3.Pogarell O, Teipel SJ, Juckel G, Gootjes L, Moller T, et al. EEG coherence reflects regional corpus callosum area in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76:109–111. doi: 10.1136/jnnp.2004.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teipel SJ, Hampel H, Pietrini P, Alexander GE, Horwitz B, et al. Region-specific corpus callosum atrophy correlates with the regional pattern of cortical glucose metabolism in Alzheimer disease. Arch Neurol. 1999;56:467–473. doi: 10.1001/archneur.56.4.467. [DOI] [PubMed] [Google Scholar]
- 5.Di Paola M, Spalletta G, Caltagirone C. In vivo structural neuroanatomy of corpus callosum in Alzheimer’s disease and mild cognitive impairment using different MRI techniques: a review. Journal of Alzheimer’s disease: JAD. 2010;20:67–95. doi: 10.3233/JAD-2010-1370. [DOI] [PubMed] [Google Scholar]
- 6.Varma AR, Adams W, Lloyd JJ, Carson KJ, Snowden JS, et al. Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer’s disease, frontotemporal dementia and vascular dementia. Acta Neurol Scand. 2002;105:261–269. doi: 10.1034/j.1600-0404.2002.1o148.x. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovici GD, Seeley WW, Kim EJ, Gorno-Tempini ML, Rascovsky K, et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen. 2007;22:474–488. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufer DI, Miller BL, Itti L, Fairbanks LA, Li J, et al. Midline cerebral morphometry distinguishes frontotemporal dementia and Alzheimer’s disease. Neurology. 1997;48:978–985. doi: 10.1212/wnl.48.4.978. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi H, Fukuyama H, Nagahama Y, Katsumi Y, Hayashi T, et al. Comparison of the pattern of atrophy of the corpus callosum in frontotemporal dementia, progressive supranuclear palsy, and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69:623–629. doi: 10.1136/jnnp.69.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hensel A, Ibach B, Muller U, Kruggel F, Kiefer M, et al. Does the pattern of atrophy of the Corpus callosum differ between patients with frontotemporal dementia and patients with Alzheimer’s disease? Dement Geriatr Cogn Disord. 2004;18:44–49. doi: 10.1159/000077734. [DOI] [PubMed] [Google Scholar]
- 11.Wiltshire K, Foster S, Kaye JA, Small BJ, Camicioli R. Corpus callosum in neurodegenerative diseases: findings in Parkinson’s disease. Dement Geriatr Cogn Disord. 2005;20:345–351. doi: 10.1159/000088526. [DOI] [PubMed] [Google Scholar]
- 12.Di Paola M, Luders E, Di Iulio F, Cherubini A, Passafiume D, et al. Callosal atrophy in mild cognitive impairment and Alzheimer’s disease: different effects in different stages. NeuroImage. 2010;49:141–149. doi: 10.1016/j.neuroimage.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindberg O, Walterfang M, Looi JC, Malykhin N, Ostberg P, et al. Hippocampal shape analysis in Alzheimer’s disease and frontotemporal lobar degeneration subtypes. Journal of Alzheimer’s disease: JAD. 2012;30:355–365. doi: 10.3233/JAD-2012-112210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Looi JC, Walterfang M, Styner M, Niethammer M, Svensson LA, et al. Shape analysis of the neostriatum in subtypes of frontotemporal lobar degeneration: neuroanatomically significant regional morphologic change. Psychiatry research. 2011;191:98–111. doi: 10.1016/j.pscychresns.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Looi JC, Walterfang M. Striatal morphology as a biomarker in neurodegenerative disease. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.54. [DOI] [PubMed] [Google Scholar]
- 16.Looi JC, Walterfang M, Velakoulis D, Macfarlane MD, Svensson LA, et al. Frontotemporal dementia as a frontostriatal disorder: neostriatal morphology as a biomarker and structural basis for an endophenotype. Aust N Z J Psychiatry. 2012;46:422–434. doi: 10.1177/0004867411432076. [DOI] [PubMed] [Google Scholar]
- 17.Lindberg O, Ostberg P, Zandbelt BB, Oberg J, Zhang Y, et al. Cortical morphometric subclassification of frontotemporal lobar degeneration. AJNR American journal of neuroradiology. 2009;30:1233–1239. doi: 10.3174/ajnr.A1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Looi JC, Lindberg O, Zandbelt BB, Ostberg P, Andersen C, et al. Caudate nucleus volumes in frontotemporal lobar degeneration: differential atrophy in subtypes. AJNR American journal of neuroradiology. 2008;29:1537–1543. doi: 10.3174/ajnr.A1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looi JC, Svensson L, Lindberg O, Zandbelt BB, Ostberg P, et al. Putaminal volume in frontotemporal lobar degeneration and Alzheimer disease: differential volumes in dementia subtypes and controls. AJNR American journal of neuroradiology. 2009;30:1552–1560. doi: 10.3174/ajnr.A1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Looi JC, Walterfang M, Styner M, Svensson L, Lindberg O, et al. Shape analysis of the neostriatum in frontotemporal lobar degeneration, Alzheimer’s disease, and controls. NeuroImage. 2010;51:970–986. doi: 10.1016/j.neuroimage.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 22.Association AP. Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 23.Organisation WH. International Classification of Diseases. Geneva: 2007. [Google Scholar]
- 24.Chan TF, Vese LA. Active contours without edges. IEEE Trans Image Process. 2001;10:266–277. doi: 10.1109/83.902291. [DOI] [PubMed] [Google Scholar]
- 25.Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, et al. Parasagittal asymmetries of the corpus callosum. Cerebral cortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- 26.Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, et al. Positive correlations between corpus callosum thickness and intelligence. NeuroImage. 2007;37:1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frederiksen KS, Garde E, Skimminge A, Ryberg C, Rostrup E, et al. Corpus callosum atrophy in patients with mild Alzheimer’s disease. Neurodegener Dis. 2011;8:476–482. doi: 10.1159/000327753. [DOI] [PubMed] [Google Scholar]
- 28.Hampel H, Teipel SJ, Alexander GE, Horwitz B, Teichberg D, et al. Corpus callosum atrophy is a possible indicator of region- and cell type-specific neuronal degeneration in Alzheimer disease: a magnetic resonance imaging analysis. Arch Neurol. 1998;55:193–198. doi: 10.1001/archneur.55.2.193. [DOI] [PubMed] [Google Scholar]
- 29.Kabay SC, Gulbandilar E, Ozden H, Ozbag D, Guven G, et al. Evaluation of the size and area of the corpus callosum with the Osiris method in Alzheimer’s disease. Neurodegener Dis. 2009;6:148–153. doi: 10.1159/000225375. [DOI] [PubMed] [Google Scholar]
- 30.Pandya D, Seltzer B. The topography of commissural fibres. In: Lepore F, Pitto M, Jasper H, Liss A, editors. Two Hemispheres - One Brain Functions of the Corpus Callosum. New York: Alan Liss; 1986. [Google Scholar]
- 31.Bermudez P, Zatorre RJ. Sexual dimorphism in the corpus callosum: methodological considerations in MRI morphometry. NeuroImage. 2001;13:1121–1130. doi: 10.1006/nimg.2001.0772. [DOI] [PubMed] [Google Scholar]
- 32.Walterfang M, Malhi GS, Wood AG, Reutens DC, Chen J, et al. Corpus callosum size and shape in established bipolar affective disorder. Aust N Z J Psychiatry. 2009;43:838–845. doi: 10.1080/00048670903107534. [DOI] [PubMed] [Google Scholar]
- 33.Pereira JM, Williams GB, Acosta-Cabronero J, Pengas G, Spillantini MG, et al. Atrophy patterns in histologic vs clinical groupings of frontotemporal lobar degeneration. Neurology. 2009;72:1653–1660. doi: 10.1212/WNL.0b013e3181a55fa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohrer JD, Rosen HJ. Neuroimaging in frontotemporal dementia. Int Rev Psychiatry. 2013;25:221–229. doi: 10.3109/09540261.2013.778822. [DOI] [PubMed] [Google Scholar]
- 35.Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, et al. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 36.Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 37.Di Virgilio G, Clarke S, Pizzolato G, Schaffner T. Cortical regions contributing to the anterior commissure in man. Exp Brain Res. 1999;124:1–7. doi: 10.1007/s002210050593. [DOI] [PubMed] [Google Scholar]
- 38.Peuskens D, van Loon J, Van Calenbergh F, van den Bergh R, Goffin J, et al. Anatomy of the anterior temporal lobe and the frontotemporal region demonstrated by fiber dissection. Neurosurgery. 2004;55:1174–1184. doi: 10.1227/01.neu.0000140843.62311.24. [DOI] [PubMed] [Google Scholar]
- 39.Jones M, Neary D, Embleton K, Snowden J, Herholz K. White matter connectivity in semantic dementia. J Neurol Neurosurg Psychiatry. 2010;81:e45. [Google Scholar]
- 40.Moon WJ, Kim HJ, Roh HG, Han SH. Atrophy measurement of the anterior commissure and substantia innominata with 3T high-resolution MR imaging: does the measurement differ for patients with frontotemporal lobar degeneration and Alzheimer disease and for healthy subjects? AJNR American journal of neuroradiology. 2008;29:1308–1313. doi: 10.3174/ajnr.A1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forman MS, Zhukareva V, Bergeron C, Chin SS, Grossman M, et al. Signature tau neuropathology in gray and white matter of corticobasal degeneration. Am J Pathol. 2002;160:2045–2053. doi: 10.1016/S0002-9440(10)61154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66:180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, et al. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One. 2012;7:e43993. doi: 10.1371/journal.pone.0043993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, et al. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain. 2009;132:2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuo K, Mizuno T, Yamada K, Akazawa K, Kasai T, et al. Cerebral white matter damage in frontotemporal dementia assessed by diffusion tensor tractography. Neuroradiology. 2008;50:605–611. doi: 10.1007/s00234-008-0379-5. [DOI] [PubMed] [Google Scholar]
- 46.Borroni B, Brambati SM, Agosti C, Gipponi S, Bellelli G, et al. Evidence of white matter changes on diffusion tensor imaging in frontotemporal dementia. Arch Neurol. 2007;64:246–251. doi: 10.1001/archneur.64.2.246. [DOI] [PubMed] [Google Scholar]
- 47.Agosta F, Scola E, Canu E, Marcone A, Magnani G, et al. White matter damage in frontotemporal lobar degeneration spectrum. Cerebral cortex. 2012;22:2705–2714. doi: 10.1093/cercor/bhr288. [DOI] [PubMed] [Google Scholar]
- 48.McMillan CT, Brun C, Siddiqui S, Churgin M, Libon D, et al. White matter imaging contributes to the multimodal diagnosis of frontotemporal lobar degeneration. Neurology. 2012;78:1761–1768. doi: 10.1212/WNL.0b013e31825830bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dopper EG, Rombouts SA, Jiskoot LC, Heijer T, de Graaf JR, et al. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology. 2013;80:814–823. doi: 10.1212/WNL.0b013e31828407bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohrer JD, Geser F, Zhou J, Gennatas ED, Sidhu M, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75:2204–2211. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitwell JL, Jack CR, Jr, Parisi JE, Senjem ML, Knopman DS, et al. Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology. 2010;75:2212–2220. doi: 10.1212/WNL.0b013e31820203c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitwell JL, Jack CR, Jr, Boeve BF, Parisi JE, Ahlskog JE, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology. 2010;75:1879–1887. doi: 10.1212/WNL.0b013e3181feb2e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain. 2011;134:2565–2581. doi: 10.1093/brain/awr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rohrer JD, Lashley T, Holton J, Revesz T, Urwin H, et al. The clinical and neuroanatomical phenotype of FUS associated frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2011;82:1405–1407. doi: 10.1136/jnnp.2010.214437. [DOI] [PubMed] [Google Scholar]
- 56.Whitwell JL, Jack CR, Jr, Boeve BF, Senjem ML, Baker M, et al. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology. 2009;72:813–820. doi: 10.1212/01.wnl.0000343851.46573.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohrer JD, Ridgway GR, Modat M, Ourselin S, Mead S, et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. NeuroImage. 2010;53:1070–1076. doi: 10.1016/j.neuroimage.2009.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012;135:794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]