Abstract
This study measured the time courses of concentration changes following administration of the catalytic antioxidants Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP) and Mn (III) 3-methoxy N, N' bis (salicyclidene) ethylenediamine chloride (EUK-134) in blood and cerebrospinal fluid (CSF) of rats with a spinal cord injury (SCI) and sham controls. Parallel measurements were made for methylprednisolone, the only drug presently used clinically for treating SCI. The time courses kinetically characterized the agents in their stability, disposition, and ability to penetrate the blood–spinal cord barrier (BSB). In both the SCI and control groups, MnTBAP was stable in CSF and in blood across the collection periods (10 h and 24 h, respectively) following administration. In the blood, [EUK-134] and [methylprednisolone] rapidly declined to near basal concentrations at 4 h and 2 h, respectively, post-administration. Therefore the order of stability in CSF and blood was MnTBAP >> EUK-134 > methylprednisolone. The maximum CSF/blood concentration ratios for EUK-134, methylprednisolone and MnTBAP post-administration were: 32 ± 3.1%, 19.2 ± 6.4%, and 4.42 ± 0.73% in the injured rats, and 22 ± 6.5%, 17.8 ± 2.9%, and 1.0 ± 0.5% in the sham control animals. This suggests an order of BSB penetration of EUK-134 > methylprednisolone >> MnTBAP. Despite much lower penetration by MnTBAP compared with EUK-134 and methylprednisolone, a lower dose of MnTBAP because of its stability provided a higher concentration in CSF than did the other agents given at higher doses. This finding supports further exploration of MnTBAP as a potential treatment for SCI.
Keywords: MnTBAP, EUK-134, Methylprednisolone, Blood–spinal cord barrier, Spinal cord injury, Stability and disposition, Antioxidant therapy, Mn-containing catalytic antioxidants
INTRODUCTION
Traumatic spinal cord injury (SCI) is worsened by secondary damage processes caused by the overproduction of endogenous deleterious substances [1, 2]. Reactive species (RS), including free radicals and non-radical oxidants, are believed to contribute to secondary destruction after central nervous system (CNS) injury by oxidatively damaging the major cellular components proteins, DNA, and phospholipids [3–5]. Substantial experimental evidence supports RS as important mediators of secondary destruction after SCI through induction of oxidative damage [6–16] and other mechanisms [17]. Therefore, antioxidant therapy with a broad-spectrum RS scavenger may significantly reduce the secondary damage of SCI.
Three structural classes of Mn-containing catalytic antioxidants have been synthesized as selective catalytic antioxidants - the macrocyclics, and non-selective catalytic antioxidants - the salens and porphyrins [18–20]. One of the non-selective catalytic Mn-porphyrins, Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), scavenges a wide range of RS [21, 22] and inhibits membrane lipid peroxidation [23]. Intracerebroventricular injection of MnTBAP inhibited kainate-induced mitochondrial superoxide production, DNA oxidation, and neuronal loss in the rat hippocampus [24]. We demonstrated that intrathecal administration of MnTBAP to rats significantly reduced oxidative damage to proteins, lipids and cell death after SCI [25]. Mn-salens are another class of non-selective antioxidants. They scavenge superoxide, hydrogen peroxide [26, 27], and peroxynitrite [28]. The salen Mn (III) 3-methoxy N, N' bis (salicyclidene) ethylenediamine chloride (EUK-134) has been tested in animal models of neurological degenerative diseases, and in a cerebral ischemia model. It has proven protective in spongiform encephalopathy [29], amyotrophic lateral sclerosis [30], and stroke [31] in rodent models. Like MnTBAP, it reduces the oxidation of lipids and proteins [30]. These results indicate that the antioxidants MnTBAP and EUK-134 may be beneficial for SCI and warrant closer examination.
To evaluate the therapeutic potential of MnTBAP and EUK-134 for treating CNS injuries and disorders, it is critical to know their stability, disposition, and ability to penetrate the blood–brain barrier (BBB) after administration. The protective effect of EUK-134 in neurological disorders suggests that EUK-134 penetrates the BBB into the CNS. It has been suggested that MnTBAP poorly penetrates the BBB, thereby making it less effective than EUK-134 in treating neuronal disorders [32]. However, one of the earliest events following SCI is the disruption of the blood–spinal cord barrier (BSB). A number of studies demonstrated that BSB disruption lasts up to 28 days following the initial injury and spreads along the length of the cord [33, 34]. Using dynamic contrast-enhanced magnetic resonance imaging, Cohen et al., found that the BSB remains compromised even at 56 days post-SCI [35]. This pathological event may provide an opportunity for MnTBAP to cross the disrupted BSB into the CNS. This is reinforced by our finding that MnTBAP is neuron protective even when administered intraperitoneally (ip) following SCI [36]. Thus MnTBAP may also have potential for treating SCI. Unfortunately, sound in vivo measurement to address these important issues in treating CNS injuries and neurological disorders is still lacking.
The objective of this study was to kinetically characterize in vivo the stability, disposition and BSB penetrating ability of the two catalytic antioxidants MnTBAP and EUK-134 by measuring their concentrations over time in the cerebrospinal fluid (CSF) and blood of injured and sham control animals following administration. Parallel measurements of methylprednisolone (MP), the only drug used clinically to treat SCI, were performed to provide reference information. Our results provide information for designing routes and frequencies of the administration of MnTBAP and EUK-134 for further exploration of their potential for the treatment of SCI.
MATERIALS AND METHODS
Animal Preparation and Spinal Cord Injury
Male Sprague-Dawley rats (300–400 g) were used in all animal experiments. The procedures on the rats were approved by the University of Texas Medical Branch Animal Care and Use Committee and were conducted in accord with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All efforts were made to minimize animal suffering and the number of animals used.
The rats were anesthetized (ip) with sodium pentobarbital (35 mg/kg body weight) followed by urethane (500 mg/kg) for maintenance. When a rat was fully anesthetized, a laminectomy was performed on vertebra T13. A polyethylene (PE)-10 tube filled with heparin solution (Heparin Sodium Injection 1,000 USP units/ml) was inserted into the tail artery for blood collection. Another PE-10 tube was inserted into the femoral vein to deliver the treatment agents. Then, the rat was clamped with an ear bar adaptor and positioned in a frame by attachment to its dorsal vertebral processes (David Kopf Instruments, Tujunga, CA, USA). Body temperature was maintained at 37–38°C throughout the experiment with a heating blanket regulated by a rectal probe (Harvard homeothermic blanket control unit; Les Ulis, France). The skin covering the occipital bone and the cervical dorsum was incised, exposing the occipital bone and the upper cervical vertebra. The atlanto-occipital membrane was identified and carefully freed from other tissue. A PE-10 tube was affixed to the membrane with Super Glue hardened prior to perforation of the membrane. A specially prepared needle with a stopper at an appropriate position was inserted through the tube to perforate the membrane without injuring the cisterna magna, as reported [37]. CSF flowed through the hole in the atlanto-occipital membrane into the tube.
Impact injury was performed by dropping a 10 g weight 2.5 cm through a guide tube onto the exposed cord (25 g·cm) at vertebra T13. Then, the incision was surgically repaired. The procedures for anesthesia, surgery, and impact injury are described in detail in our previous publications [6–10, 25, 36]. The only difference in animal preparation between the sham-operated controls and the injured group was that no impact injury was performed on the sham control rats.
Administration of Treatment Agents
Six groups of rats were used (n = 4 or 5 for each group): three injured groups treated with MnTBAP (Calbiochem, San Diego, CA, USA), EUK-134 (Cayman Chemical, Ann Arbor, Michigan, USA), or methylprednisolone sodium succinate (MPSS, Upjohn, Bridgewater, NJ, USA) and three sham control groups treated with MnTBAP, EUK-134, or MPSS.
MPSS, a water soluble derivative of MP, is the only drug used clinically for treating SCI. MPSS quickly breaks down to MP, the effective component of MPSS, following its administration. The standard regimen of MPSS for clinical treatment after acute SCI includes a bolus dose of 30 mg/kg followed by a maintenance dose of 5.4 mg/kg/h [38] given intravenously (iv). This regimen has also been used in experimental SCI in rats [39–41]. In the present study, therefore, a bolus dose of 30 mg/kg MPSS was administered iv for a 10-min period immediately following injury through the PE-10 tube implanted into the femoral vein.
MnTBAP was dissolved in 0.1 M NaOH, which was diluted with 0.9 % saline, and the pH was adjusted to 7.1–7.3, as reported [32]. We previously showed that MnTBAP at 10 mg/kg (ip) significantly increased the number of surviving neurons and significantly reduced the number of apoptotic neurons after SCI in rats [36]. In the present study, MnTBAP at 6.4 mg/kg was administered iv immediately following injury by the same route used for MPSS.
Daily treatment with EUK compounds (including EUK-134) at 30 mg/kg (ip) extended the lifespans of superoxide dismutase 2 nullizygous mice beyond those of untreated mice and mice treated with MnTBAP at 5 mg/kg [29]. In an amyotrophic lateral sclerosis mouse model, 33 mg/kg (ip) of EUK-134 given three times a week beginning at 60 days of age reduced the oxidation of cell membrane lipids and proteins and increased the survival time of the animals by 68% [30]. EUK-134 at 2.5 mg/kg given iv reduced the infarct volume by 90% compared with vehicle treatment in a rat cerebral ischemia model [31]. One the basis of the reported doses, in the present study, EUK-134 at 15 mg/kg in saline was administered to determine its stability and disposition. EUK-134 at 15 mg/kg was not detectable in the CSF and blood of either injured or control rats a few minutes after iv administration; therefore, it was given by ip injection immediately following SCI.
Blood and CSF Sampling
Blood samples were collected through the PE-10 tube implanted into the tail artery at 0.5, 1, 2, 4, 6, 8, 10, and 24 h post-administration, and CSF samples were collected through the PE-10 tube affixed to the atlanto-occipital membrane at 0.5, 1, 2, 4, 6, 8, and 10 h post-administration. Saline (1 ml) was injected (ip) every 2 h during the first 10 h to supplement the loss of liquid due to blood and CSF withdrawal. The animals were kept anesthetized during the surgery and sampling by injections of urethane.
Sample Preparation and Analysis by High Performance Liquid Chromatography (HPLC)
MnTBAP, EUK-134, and MP collected from blood and CSF were analyzed according to published methods [42–45] with some modifications. Blood samples were placed in 0.6 ml tubes and left at room temperature to clot for 30 min. Serum was removed after spinning at 1,000 g for 10 min for antioxidants or 5,000 g for 5 min for MPSS and MP. Serum and CSF samples were stored at −80°C until analysis. For analysis of the antioxidants, 400 µl or 200 µl of methanol was added to 20 µl of serum or CSF, respectively. For analysis of MPSS and MP, 90 µl of methanol was added to 30 µl of serum or CSF. The extracts were vortexed for 10 min and centrifuged at 7,000 g for 5 min. The supernatant was pipetted into a centrifugal filtration tube (NANOSEP®, molecular weight cutoff 10 kD, Pall Corporation, Ann Arbor, MI, USA) and spun at 10,000 g for 20 min. Fifty microliters of CSF or 20 µl of serum filtrates containing the MnTBAP or EUK-134 or 30 µl of one or the other containing MPSS and MP was injected into a HPLC column for analysis.
All samples were analyzed on a Shimadzu (Shimadzu Scientific Instruments Inc., Columbia, MD, USA) SCL-6A HPLC with a UV-SPD detector (SPD-6A) and a Spherisorb ODS2 column (4.6 × 150 mm, 3 µm particles, Waters, Milford, MA, USA). The mobile phase for analyzing MnTBAP was 60% solution A (0.1% trifluoroacetic acid in water) and 40% solution B (0.1% trifluoroacetic acid in acetonitrile/water, 90/10 v/v). EUK-134 was analyzed isocratically using a mobile phase composed of 15% acetonitrile and 85% phosphate buffer (pH 6.05, 25 mM). The detector wavelength for MnTBAP or EUK-134 was set to 468 nm. The flow rate was 1.0 ml/min for analysis of both agents. The retention time of MnTBAP was 10.5 min. The detection limit of MnTBAP was 0.1 ng.
The concentrations of both MP and MPSS were measured in the blood and CSF samples. However, only the concentrations of MP - the effective component of MPSS - are presented in the results. Gradient elution was used to separate MP, MPSS, and other metabolites. The mobile phase was a mixture of acetonitrile and a 0.067 M KH2PO4 buffer, pH 4.5. At t = 0, the mobile phase was 25% acetonitrile and 75% buffer (v/v). During the first 15 min the mobile phase changed to 35% acetonitrile and 65% buffer (v/v) over a linear gradient. The composition then changed linearly over 3 min to 50% acetonitrile/50% buffer and remained there for 5 min. The mobile phase was then returned to its initial composition over 3 min and held there for 7 min before the next run was started. The flow rate was 1.5 ml/min and the detection wavelength was 250 nm. The retention times for MP and MPSS were 8.2 min and 11.4 min, respectively.
The recoveries of our analytic methods were determined by adding the standard agents into blood samples and then analyzing their concentrations by the procedures described. The recovery was calculated and presented as a percentage of added agents by comparing the amount measured from blood extracts to the original added amount. MnTBAP 2.0, 10.0, or 20.0 mg/mL was added to blood samples; recoveries were 93.0 ± 1.7%, 97.9 ± 4.9%, and 98.3 ± 2.0% (n = 3, mean ± SD). Average recovery of 2.0 mg/L MP or MPSS added to blood samples was 106.7 ± 11.5% or 98.91 ± 8.06% (n=3, mean ± SD). These values demonstrate excellent recovery and the reproducibility of our analysis methods.
Statistical Analyses
Two-way repeated measures analysis of variance (RMANOVA) was used to compare the time courses of concentration changes between blood and CSF and between injured and sham groups, and the time courses of the CSF/blood concentration ratios between injured and sham groups for MnTBAP and MP. One-way analysis of variance (ANOVA) followed by the Tukey test was used to compare results among different times post-administration for each time course measured. A paired t-test was performed to compare the differences in the CSF/blood concentration ratios of EUK-134 between injured and sham control groups at each time point. P < 0.05 was considered a statistically significant difference.
RESULTS
The time courses of concentration changes of the catalytic antioxidants MnTBAP and EUK-134 were measured in CSF and blood of injured and sham control rats following agent administration. Results were compared with those for MP to provide reference information. The concentrations of the three agents in the injured animals are given in Table 1 (mg/L, mean ± SEM). The time dependencies of concentration changes of these three agents following their administration reveal their stability and disposition in CSF and blood, and provide information regarding their ability to penetrate the BSB from the blood to the CSF. The time courses of the CSF/blood concentration ratios of these agents are also shown in Table 1. The scales of the concentration ratios differ greatly for different agents, so the data are presented as percentage of agent concentration in CSF to its concentration in blood at each time point for each animal. Using the percentage of CSF/blood concentration ratios facilitates comparison and discussion the ability to penetrate the BSB between agents.
Table 1.
Time Courses of MnTBAP, EUK-134 and MP Concentrations in CSF and Blood and their CSF/Blood Concentration Ratios in Injured Rats Following Administration
| Times (h) |
M nTBAP (6.4 mg/kg) | EUK-134 (15 mg/kg) | MP (MPSS 30 mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CSF | Blood | CSF/Blood (%) |
CSF | Blood | CSF/Blood (%) |
CSF | Blood | CSF/Blood (%) |
|
| (mg/L, mean±SEM) | (mg/L, mean±SEM) | (mg/L, mean±SEM) | |||||||
| 0.5 | 0.42 ± 0.2 | 35.6 ± 2.2 | 1.3 ± 0.8 | 0.11 ± 0.01 | 0.33 ± 0.02 | 32 ± 1.4 | 0.60 ± 0.2 | 3.5 ± 0.7 | 19.2 ± 6.4 |
| 1.0 | 0.81 ± 0.3 | 25.9 ± 1.0 | 3.2 ± 1.2 | - | - | - | 0.45 ± 0.1 | 1.5 ± 0.2 | 28.7 ± 9.1 |
| 2.0 | 0.81 ± 0.2 | 20.3 ± 1.1 | 4.0 ± 1.0 | 0.09 ± 0.01 | 0.20 ± 0.01 | 43 ± 2.8 | 0.24 ± 0.1 | 0.64 ± 0.1 | 35.6 ± 2.0 |
| 4.0 | 0.78 ± 0.1 | 18.8 ± 0.9 | 4.2 ± 0.8 | N/D | 0.11 ± 0.01 | - | 0.12 ± 0.04 | 0.22 ± 0.05 | 48.7 ± 8.8 |
| 6.0 | 0.91 ± 0.1 | 20.5 ± 1.1 | 4.4 ± 0.4 | N/D | 0.07 ± 0.01 | - | 0.11 ± 0.04 | 0.25 ± 0.2 | 44.1 ± 18.0 |
| 8.0 | 0.80 ± 0.1 | 23.6 ± 1.6 | 3.5 ± 0.7 | N/D | N/D | - | 0.08 ± 0.07 | 0.07 ± 0.04 | 55.5 ± 30.2 |
| 10.0 | 0.74 ± 0.3 | 26.8 ± 1.8 | 2.9 ± 1.2 | N/D | N/D | - | N/D | N/D | - |
| 24.0 | - | 27.5 ± 0.9 | - | N/D | N/D | - | N/D | N/D | - |
N/D: not detectable.
Time courses of MnTBAP Concentration Changes in CSF and in Blood Following its Administration
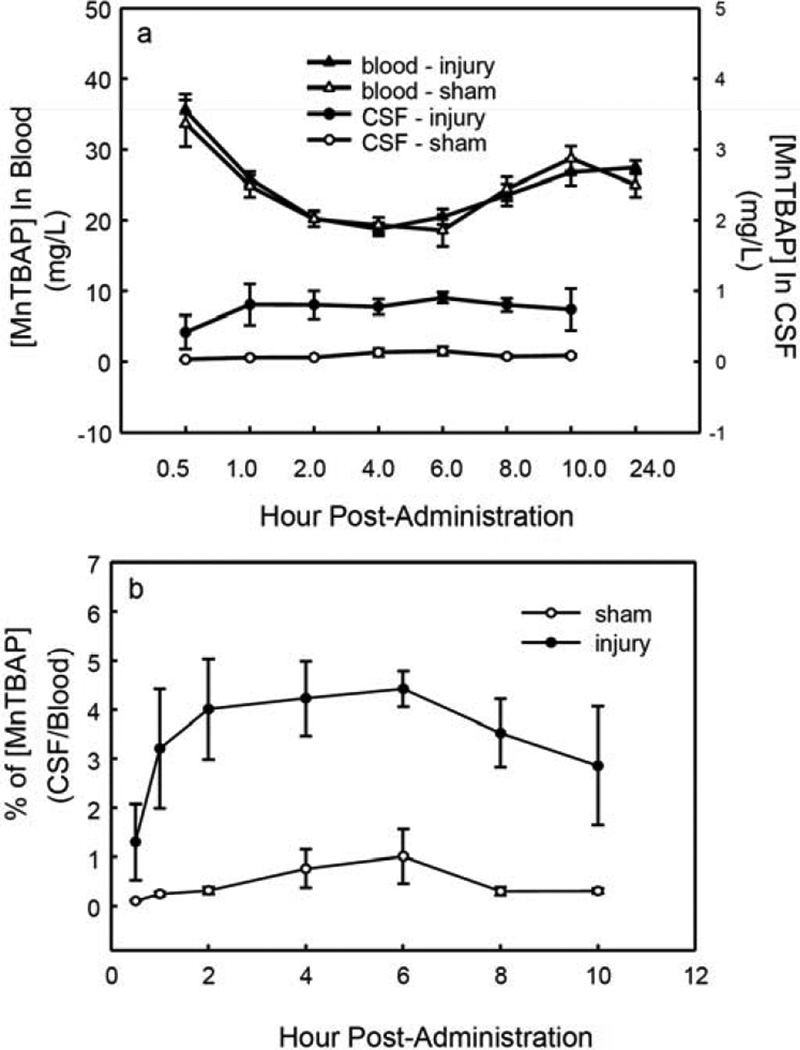
The time courses of [MnTBAP] in blood and in CSF from SCI and sham control animals following iv injection are shown in Fig. (1a). In the injured group, MnTBAP at 6.4 mg/kg injected into blood produced the maximum concentration—35.6 ± 2.2 mg/L—in the first sample, collected at 0.5 h post-administration. The concentration in blood slowly declined thereafter but remained near the maximum throughout the time course. At 10 h it was 26.8 ± 1.8 mg/L (75% of the maximum concentration), and at the 24-h final measurement it was 27.5 ± 0.9 mg/L (77% of the maximum concentration). Although [MnTBAP] in CSF was much lower than in blood, it remained almost unchanged (~ 0.8 mg/L) from 1 h post-injection to the final measurement at 10 h. This demonstrated that MnTBAP is quite stable in blood for 24 h and in CSF for 10 h post-administration. This feature is valuable in a therapeutic agent for CNS injury.
Fig. (1).
The time courses of [MnTBAP] changes following administration. The animal experiment, agent administration, sample collection, and HPLC analysis are described in Materials and Methods. MnTBAP (6.4 mg/kg) was administered (iv) immediately after SCI (25 g․cm). Blood samples were drawn through a PE-10 tube from the tail artery and CSF was sampled through another PE-10 tube mounted on the atlanto-occipital membrane. MnTBAP in collected blood or in CSF was analyzed by HPLC with UV detection. a. The time courses of [MnTBAP] changes in blood and CSF samples from sham control and SCI animals, which show stable MnTBAP concentrations in CSF and blood post-administration. b. The CSF/blood ratios of [MnTBAP] over time in injured and sham control animals. The time courses of the ratios show stability and low penetration of BSB in both injured and sham control rats.
There were no significant differences in the time courses of [MnTBAP] in blood between SCI and sham control animals (p = 0.7, two-way RMANOVA), i.e., there were no effects of injury on blood concentrations of i.v-administered MnTBAP. However, the concentrations of MnTBAP in the CSF of injured animals were significantly higher than those in the CSF of sham control animals (p = 0.004, two-way RMANOVA). [MnTBAP] was significantly higher in blood than in CSF for the duration of the measurements in both the SCI and control animals (p < 0.001 for both, two-way RMANOVA). The maximum [MnTBAP] in CSF in the sham control group was very low (0.15 ± 0.06 mg/L at 6 h post-injection) compared with that in injured rats (0.91 ± 0.1 mg/L). These results suggest that a BSB exists for MnTBAP in both injured and control animals, and indicate that little MnTBAP crosses the normal BSB. Disruption of the BSB by injury apparently increases the permeability of the BSB to MnTBAP in response to SCI.
The CSF/blood concentration ratios for MnTBAP over time in MnTBAP-treated injured and sham control animals are shown in Fig. (1b). The concentration ratio for MnTBAP in injured animals was 1.3 ± 0.8% at 0.5 h post-injection. The ratio increased about 3-fold in injured animals during the first 2 h after administration. It was significantly higher at all time points after 1 h compared with 0.5 h post-injection (p = 0.02 to < 0.001, one-way ANOVA followed by the Tukey test) and remained there for the remaining 9 h of measurements, further demonstrating the stability of MnTBAP. Stability was also observed in sham control animals. The concentration ratios for MnTBAP was significantly higher in injured animals than in sham control animals (P = 0.007, two-way RMANOVA), again suggesting that injury increased the penetration of the BSB. However, the maximum CSF/blood concentration ratio for MnTBAP at 6 h post-SCI was not very high even in injured animals (4.4 ± 0.4%, mean ± SEM), and it was much lower in sham control animals at the same time point (1.0 ± 0.5%, mean ± SEM).
Time Courses of EUK-134 Concentration Changes in CSF and in Blood Following its Administration
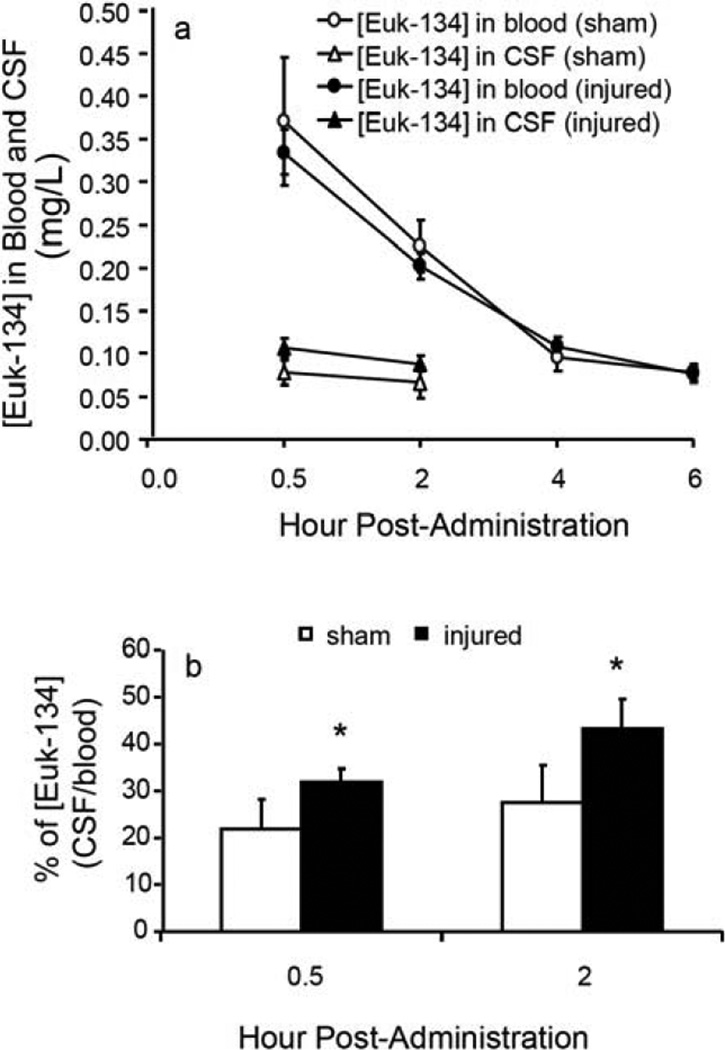
The time courses of [EUK-134] in CSF and blood from SCI and sham control animals following ip injection are given in Fig. (2a). The dose of 15 mg/kg EUK-134 produced the maximum concentration in the first blood sample, collected at 0.5 h post-injection at 0.33 ± 0.02 in injured rats and 0.37 ± 0.07 mg/L in sham control animals. The [EUK-134] in blood declined rapidly and throughout the entire time period assessed. At 4 h post-administration, they were 0.11 ± 0.01 mg/L for injured and 0.09 ± 0.02 mg/L for sham control animals. In CSF, the EUK-134 concentrations at 0.5 h post-administration were 0.11 ± 0.01 mg/L in the injured and 0.08 ± 0.01 mg/L in the sham control groups, and they declined gradually as shown in Fig. (2a). Owing to its low concentrations and rapid decline, EUK-134 in CSF was detectable for only the first 2 h after administration. [EUK-134] was significantly higher in blood samples than in CSF samples, indicating that a BSB exists. [EUK-134] was not significantly different between injured and sham control samples either in blood or CSF. The CSF/blood concentration ratios for EUK-134 were 32 ± 1.4% at 0.5 h and 43 ± 2.8 % at 2 h in injured rats and 22 ± 2.9% at 0.5 h and 28 ± 3.4% at 2 h in sham control rats after its administration, suggesting a great penetrating ability Fig. (2b). The differences in the concentration ratios for EUK-134 between the injured and sham control groups were slight but significant (p = 0.04 at both 0.5 and 2 h post-administration, paired t-test), suggesting that injury increased the penetration of the BSB by EUK-134.
Fig. (2).
The time courses of [EUK-134] changes following administration. The animal experimental procedures were the same as in Fig. 1, and the HPLC analysis is described in Materials and Methods. EUK-134 (15 mg/kg) was administered (ip) immediately after SCI (25 g․cm). a. The time courses of [EUK-134] changes in blood and CSF samples from injured and sham control animals. A sharp decline in [EUK-134] is observed in blood. b. The CSF/blood ratios of [EUK-134] in injured and sham control rats, showing greater penetration of the BSB compared with MnTBAP in Fig. 1b in both injured and sham control animals.
Time Courses of MP Concentration Changes in CSF and in Blood Following Administration of MPSS
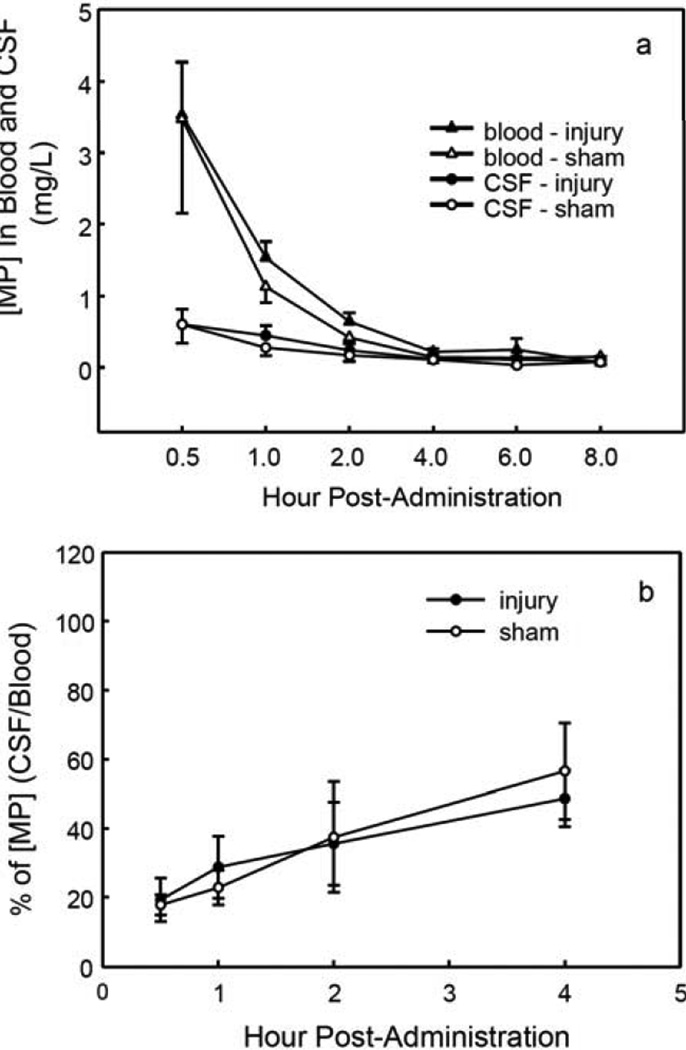
The time courses of [MP] changes in CSF and in blood in injured and control rats following iv administration of MPSS are shown in Fig. (3a). In blood, MPSS quickly decomposes to MP, the active form of MPSS. The dose of 30 mg/kg MPSS (iv) yielded a maximum blood [MP] of 3.5 ± 0.7 mg/L at 0.5 h post-injection in injured animals, as shown in Table 1. The blood [MP] decreased sharply to 0.6 ± 0.1 mg/L at 2 h and 0.07 ± 0.04 mg/L at 8 h post-injection. The same pattern was found in sham control animals. The maximum [MP] in CSF samples from injured animals was 0.6 ± 0.2 mg/L at 0.5 h post-injection. The CSF [MP] decreased slowly, to 0.2 ± 0.1 mg/L at 2 h and 0.08 ± 0.07 mg/L at 8 h post-administration. There were no significant differences in the time courses of [MP] changes between injured and sham control animals in blood or CSF (p = 0.9 and 1.0, respectively; two-way RMANOVA), suggesting that MP penetrates the BSB even when the BSB is not disrupted by SCI. This feature is valuable in a therapeutic agent for CNS injury. There were significant differences in the time courses of [MP] changes between CSF and blood for both injured and control animals (p = 0.008 and 0.02, respectively; two-way RMANOVA), suggesting that a BSB for MP exists in both injured and control animals.
Fig. (3).
The time courses of [MP] changes following administration. The animal experimental procedures were the same as in Fig. 1, but MPSS (30 mg/kg) was administered (iv). The HPLC analysis method is described in Materials and Methods. a. The time courses of [MP] changes in blood and CSF samples from SCI and sham control rats. A dramatic decline of [MP] in blood samples is shown with a comparatively slower reduction of [MP] in CSF. b. The CSF/blood ratios of [MP] over time in injured and sham control animals, showing greater penetration of the BSB by MP in both injured and sham control animals compared with MnTBAP in Fig. 1b.
The CSF/blood concentration ratios for MP over time in MPSS-treated injured and sham control rats are presented in Fig. (3b). The ratio of [MP] gradually increased, from 19.2 ± 6.4% at 0.5 h to 48.7 ± 8.8% at 4 h in injured rats and from 17.8 ± 2.9% at 0.5 h to 56.6 ± 13.9% at 4 h in control rats; there was no significant difference between the groups (p = 0.45, two-way RMANOVA). The [MP] ratio at 0.5 h post-administration of MPSS was nearly 20% for both injured (19.2%) and sham control (17.8%) rats. The MP concentration ratio gradually increased in both the SCI and sham control groups without the development of a significant difference between them. The increase in the MP concentration ratio may not indicate accumulation of MP, but may instead reflect that the decline in its concentration was faster in blood than in CSF.
DISCUSSION
We measured the time courses of changes in [MnTBAP], [EUK-134] and [MP] across 10 h in CSF and across 24 h in blood following their administration. We found the Mn-porphyrin MnTBAP to be quite stable in both CSF and blood after administration and throughout the periods assessed. Although a U-shaped time course of [MnTBAP] in blood was observed, it may not indicate the instability of this agent. It might be attributed to the following events: 1) The iv administration maximized [MnTBAP] at the first time point. 2) Uptake of MnTBAP by tissues, including those of the CNS, led to a gradual slow reduction of [MnTBAP] in blood. 3) The slight, albeit statistically significant, increases in the [MnTBAP] in blood after 6 h post-administration might represent stabilization due to the distribution of the agent to various tissues in conjunction with a reduction in its distribution to CNS upon the gradual recovery of the BSB. The fact that [MnTBAP] in blood maximized (35.6 ± 2.2 mg/L) in the first sample collected at 0.5 h post-administration and remained near the maximum throughout the time course with a 27.5 ± 0.9 mg/L (77% of the maximum concentration) at the 24-h post-administration supports the above explanation. It appears from our data that [MnTBAP] would have remained stable for days in both blood and CSF had the experiments lasted longer. Blood concentrations of Mn-selen EUK-134 and the steroid MP dramatically declined to near the basal values by 6 h and 4 h, respectively, following administration Figs. (2a and 3a). Consonant with EUK-134’s low concentration and its rapid decline in blood, it was detectable in the CSF for only the first 2 h after its ip administration. The [MP] in CSF was also lower than those of [MnTBAP], but the stability was greater than in blood samples. Thus, as reported above, the order of stability in both CSF and blood was MnTBAP >> EUK-134 > MP.
The maximum CSF/blood concentration ratio for MnTBAP was 4.4% for injured animals and 1.0% for sham controls. As indicated in the Results, the increases in the CSF/blood concentration ratios for MP and EUK-134 over time may be due to the faster decline in their concentration in blood than in CSF; therefore, the first sample, collected at 0.5 h post-administration, may more accurately represent their ability to penetrate the BSB. At 0.5 h post-administration, the CSF/blood concentration ratio was 19% for MP and 32% for EUK-134 in the injured rats, and 18% for MP and 22% for EUK-134 in the sham control rats. In the injured rats, the maximum concentration ratio for MnTBAP (4.4%) was much lower than those for EUK-134 and MP (approximately 30% and 20%), suggesting that the order of penetration of the BSB by the three agents was EUK-134 > MP >> MnTBAP. Thus, direct injection into the intrathecal space may be an effective route for administering MnTBAP to treat CNS injuries and disorders.
The concentrations of MnTBAP, EUK-134, and MP in blood were all significantly higher than their concentrations in CSF in both the injured and in sham control animals. These findings indicate that a BSB exists in both groups of animals for all three compounds. The finding that [MnTBAP] in the CSF was significantly higher in injured than in sham control animals suggests that the injury disrupted the BSB and thereby increased its penetrability, allowing more MnTBAP to enter the CSF. In comparison, only a small amount of MnTBAP crossed the normal BSB. The CSF/blood concentration ratios for MnTBAP were significantly higher in injured than in control rats, which further supports this presumption. In contrast, there were no significant differences in the CSF concentrations of EUK-134 and MP between injured and control rats. Consistent with the concentration results, the CSF/blood concentration ratios were slightly different for EUK-134 and not different for MP between injured and sham control animals. Because of a good penetrative ability, EUK-134 and MP could easily cross the BSB even without SCI; slight differences between the injured and control groups in the CSF concentrations of the two agents could be obscured by the high influx through the BSB, particularly at very low concentrations of the two agents. Additionally, considering the uptake of these agents by the CNS tissue from CSF after passing through the BSB, the actual influx of MP and EUK-134 from blood into the CSF should be much higher than their concentrations measured in the CSF. This great influx of the two agents might be sufficiently high to cloak a slighter influx stemming from disruption of the BSB by injury.
Although our data suggest that EUK-134 and MP penetrated the BSB better than did MnTBAP, this beneficial feature would in part be offset by their instability. Owing to their instability in blood, MPSS and EUK-134 at doses higher than the dose of MnTBAP (30 and 15 mg/kg versus 6.4 mg/kg) generated a maximum concentrations in CSF (0.6 and 0.1 mg/L) lower than that of MnTBAP provided in CSF: ~ 0.8 mg/L at all times 1 h post-administration in the injured rats. To overcome the instability, bolus and maintenance doses of MPSS and EUK-134 have to be used to yield effective concentrations in blood. These findings support the standard clinical regimen of continuously administering MPSS for treating SCI and multiple administrations of EUK-134 for treating neurological disorders. We previously demonstrated that MnTBAP at 10 mg/kg is neuron protective even when administered by only one ip injection following SCI in rats [36]. This finding suggests that enough MnTBAP entered the blood and then crossed the BSB following SCI, contributing to its cytoprotective properties. Since MnTBAP can permeate cell membranes, this protection may arise from its actions in CSF and inside blood vessels. In the present study, we found that MnTBAP concentrations remained near the maximum value in the CSF of injured rats for at least 10 h after its administration, a duration substantially exceeding those of EUK-134 and MP. This too may contribute to the effectiveness of MnTBAP. MnTBAP concentrations remained near the maximum concentration in the blood of both injured and control animals for at least 24 h, providing a reservoir of MnTBAP to maintain its nearly maximum concentrations in the CSF. This further demonstrates the persistence of MnTBAP, so it should be effective without follow-up infusion.
The CSF concentration of an agent measured at a certain time point represents its current influx from the blood to the CSF plus the amount accumulated from the preceding time-span, minus the amount taken up by neural tissues from the CSF and the amount metabolized over that time. Therefore, an agent’s concentration in CSF is not an exact indicator of its penetration of the BSB at each time point. It provides information of an agent regarding its penetration, accumulation (according to stability), breakdown (metabolic clearance), and uptake by nerve tissue. Uptake and breakdown may have less influence on the CSF concentration of MnTBAP compared with EUK-134 and MP because of its greater stability and hydrophilicity. Therefore, penetration and accumulation may be the major contributors for the [MnTBAP] measured in CSF at each time point following administration. Because of the greater instability and lipophilicity of EUK and MP, fast uptake and breakdown may contribute to the rapid decline in their blood concentrations, which may in part account for their very low concentrations in CSF, even with their greater penetrating ability compared with MnTBAP. [MP] and [EUK-134] in CSF measured at a given time point are each a net reflection of their penetration of the BSB minus uptake and breakdown at that time. The maximum concentrations of MP and EUK-134 were seen at the first sampling time point of 30 min in both the blood and CSF of the SCI and control groups, suggesting their rapid penetration of the BSB after administration. The maximum concentrations in blood declined about twofold between 30 min and 1 h after administration of MPSS, and between 2 and 4 h after EUK-134 administration. It was reported from an animal study that [MP] in the injured spinal cord tissue peaked at 30 min to 1 h after a single 30-mg/kg iv injection of MPSS and then rapidly declined [46]. The [MP] in CSF in our study and that reported for spinal cord tissue suggests that MP rapidly penetrates the BSB into both CSF and central nervous tissue to reach a maximum concentration at 30 min, then declined nearly equally approaching basal values at 8 h. Thus, over time MP appears to equilibrate between the spinal cord tissue and the CSF while it is removed from both. The half-time for clearance of MP from humans is 3.5 ± 2.2 h [47]. The fairly rapid rate of MP clearance supports the validity of administering a bolus followed by ongoing infusion of MP. Direct in vivo measurements of uptake and breakdown are not available for MnTBAP and EUK-134 in experimental animals, particularly in CNS. EUK-134’s apparently penetration of the BSB compared with MnTBAP makes it a promising agent for treating SCI. However, it also declines fairly rapidly in the CSF; thus, its effectiveness may be limited by its short duration of presence. Combined administration of MnTBAP and EUK-134 might therefore compensate for the possible short-comings of each agent.
CONCLUSIONS
By measuring the time courses of [MnTBAP], [EUK-134], and [MP] in CSF and blood following agent administration to SCI and sham control rats, we demonstrated that the order of stability of these agents was MnTBAP >> EUK-134 > MP, and that their order of penetration of the BSB was EUK-134 > MP>>MnTBAP. To our knowledge, this study presents the first in vivo measurements of the stability, disposition, and BSBpenetrating ability of the catalytic antioxidants MnTBAP and EUK-134 in CSF and blood, providing critical information for designing further animal experiments to test the two agents for treating SCI. Despite the much lower penetration of the BSB by MnTBAP compared with EUK-134 and MP, because of its higher stability a lower dose of MnTBAP produces a higher concentration in the CSF than attained by EUK-134 and MP given at higher doses. This supports further exploration of MnTBAP as a potential agent for treatment of SCI. The time courses of [MP] changes in CSF and blood following administration are new and supplementary to reports of the uptake and clearance of MP in spinal cord tissue following SCI. The present study demonstrates that it is advantageous to measure the post-administration time courses of concentration changes of an agent by continuously sampling CSF and blood in one group of animals. This provides information regarding the agent’s stability, accumulation, disposition, and penetration after administration, and obviates the need to sacrifice separate groups of animals at every post-administration time point to obtain spinal cord tissue.
ACKNOWLEDGEMENTS
The authors thank the National Institutes of Health (National Institute of Neurological Disorders and Stroke, RO1 NS 44324) for financial support, D. J. McAdoo for his helpful advice and S. L. Simpson for her editorial assistance in the preparation of the manuscript.
ABBREVIATIONS
- ANOVA
Analysis of variance
- BBB
Blood–brain barrier
- BSB
blood–spinal cord barrier
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- EUK-134
Mn (III) 3-methoxy N, N' bis (salicyclidene) ethylenediamine chloride
- HPLC
High-performance liquid chromatograph
- ip
Intraperitoneal
- iv
Intravenous
- MnTBAP
Mn (III) tetrakis (4-benzoic acid) porphyrin
- MP
Methylprednisolone
- MPSS
Methylprednisolone sodium succinate
- PE
Polyethylene
- RMANOVA
Repeated measures of analysis of variance
- RS
Reactive species
- SCI
Spinal cord injury
Footnotes
CONFLICT OF INTEREST
No competing financial interests exist.
REFERENCES
- 1.Balentine JD. Hypotheses in spinal cord trauma research. In: Becker DP, Povlishock JT, editors. Central Nervous System Trauma Status Report. Bethesda, Maryland: NIH; 1985. pp. 455–461. [Google Scholar]
- 2.Young W. Secondary injury mechanisms in acute spinal cord injury. J. Emerg. Med. 1993;11:13–22. [PubMed] [Google Scholar]
- 3.Genovese T, Cuzzocrea S. Role of free radicals and poly(ADP-ribose) polymerase-1 in the development of spinal cord injury: new potential therapeutic targets. Curr. Med. Chem. 2008;15:477–487. doi: 10.2174/092986708783503177. [DOI] [PubMed] [Google Scholar]
- 4.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J. Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 5.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cerebr. Blood F. Met. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Sybert TE, Qian H, Liu J. Superoxide production after spinal injury detected by microperfusion of cytochrome c. Free Radic. Biol. Med. 1998;25:298–304. doi: 10.1016/s0891-5849(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Liu J, Wen J. Elevation of hydrogen peroxide after spinal cord injury detected by using the Fenton reaction. Free Radic. Biol. Med. 1999;27:478–482. doi: 10.1016/s0891-5849(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 8.Liu D, Ling X, Wen J, Liu J. The role of reactive nitrogen species in secondary spinal cord injury: formation of nitric oxide, peroxynitrite, and nitrated protein. J. Neurochem. 2000;75:2144–2154. doi: 10.1046/j.1471-4159.2000.0752144.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Liu J, Sun D, Wen J. The time course of hydroxyl radical formation following spinal cord injury: the possible role of the iron-catalyzed Haber-Weiss reaction. J. Neurotrauma. 2004;21:805–816. doi: 10.1089/0897715041269650. [DOI] [PubMed] [Google Scholar]
- 10.Leski ML, Bao F, Wu L, Qian H, Sun D, Liu D. Protein and DNA oxidation in spinal injury: neurofilaments--an oxidation target. Free Radic. Biol. Med. 2001;30:613–624. doi: 10.1016/s0891-5849(00)00500-1. [DOI] [PubMed] [Google Scholar]
- 11.Springer JE, Azbill RD, Mark RJ, Begley JG, Waeg G, Matson MP. 4-hydroxynonenal, a lipid peroxidation product, rapidly accumulates following traumatic spinal cord injury and inhibits glutamate uptake. J. Neurochem. 1997;68:2469–2476. doi: 10.1046/j.1471-4159.1997.68062469.x. [DOI] [PubMed] [Google Scholar]
- 12.Scott GS, Jakeman LB, Stokes BT, Szabo C. Peroxynitrite production and activation of poly (adenosine diphosphate-ribose) synthetase in spinal cord injury. Ann. Neurol. 1999;45:120–124. [PubMed] [Google Scholar]
- 13.Carrico KM, Vaishnav R, Hall ED. Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. J. Neurotrauma. 2009;26:1369–1378. doi: 10.1089/neu.2008-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aksenova M, Butterfield D, Zhang S, Underwood M, Geddes J. Increased protein oxidation and decreased creatine kinase BB expression and activity after spinal cord contusion injury. J. Neurotrauma. 2002;19:491–502. doi: 10.1089/08977150252932433. [DOI] [PubMed] [Google Scholar]
- 15.Genovese T, Mazzon E, Esposito E, Muià C, Rosanna Di Paola, Bramanti P, Cuzzocrea S. Beneficial effects of FeTSPP, a peroxynitrite decomposition catalyst, in a mouse model of spinal cord injury. Free Radic Biol. Med. 2007;43:763–780. doi: 10.1016/j.freeradbiomed.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Genovese T, Mazzon E, Esposito E, Muià C, Rosanna Di Paola, Murthy K, Neville L, Bramanti P, Cuzzocrea S. Effects of a metalloporphyrinic peroxynitrite decomposition catalyst, ww-85, in a mouse model of spinal cord injury. Free Rad. Biol. Med. 2009;43:631–645. doi: 10.1080/10715760902954126. [DOI] [PubMed] [Google Scholar]
- 17.Titsworth WL, Liu NK, Xu XM. Role of secretory phospholipase A2 in CNS inflammation: Implications in traumatic spinal cord injury. CNS Neurol. Disord. Drug Targets. 2008;7:254–269. doi: 10.2174/187152708784936671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day BJ. Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov. Today. 2004;9:557–566. doi: 10.1016/S1359-6446(04)03139-3. [DOI] [PubMed] [Google Scholar]
- 19.Day BJ. Catalase and glutathione peroxidase mimics. Biochem. Pharmacol. 2009;77:285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batinić-Haberle I, Rebouças JS, Spasojević I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 22.Zingarelli B, Day B, Crapo JD, Salzman AL, Szabo C. The potential role of peroxynitrite in the vascular contractile and cellular energetic failure in endotoxic shock. Br. J. Pharmacol. 1997;120:259–267. doi: 10.1038/sj.bjp.0700872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day BJ, Batinic-Haberle I, Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic. Biol. Med. 1999;26:730–736. doi: 10.1016/s0891-5849(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 24.Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate induced hippocampal damage. Neuroscience. 2000;101:563–570. doi: 10.1016/s0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- 25.Hachmeister JE, Valluru L, Bao F, Liu D. Mn (III) tetrakis (4-benzoic acid) porphyrin administered into the intrathecal space reduces oxidative damage and neuron death after spinal cord injury: a comparison with methylprednisolone. J. Neurotrauma. 2006;23:1766–1778. doi: 10.1089/neu.2006.23.1766. [DOI] [PubMed] [Google Scholar]
- 26.Doctrow SR, Huffman K, Marcus CB, Musleh W, Bruce A, Baudry M, Malfroy B. Salen-manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv. Pharmacol. 1997;38:247–269. doi: 10.1016/s1054-3589(08)60987-4. [DOI] [PubMed] [Google Scholar]
- 27.Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Mascarenhas J, Malfroy B. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J. Med. Chem. 2002;45:4549–4558. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 28.Sharpe MA, Ollosson R, Stewart VC, Clark JB. Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem. J. 2002;366:97–107. doi: 10.1042/BJ20020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci. Lett. 2001;304:157–160. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- 31.Baker K, Marcus C, Huffman K, Kru H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- 32.Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat. Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- 33.Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp. Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- 34.Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J. Neurosci. Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu S-J, Narayana PA. Blood-spinal cord barrier permeability in experimental spinal cord injury: Dynamic contrast-enhanced magnetic resonance imaging NMR. Biomed. 2009;22:332–341. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling X, Liu D. Temporal and spatial profiles of cell loss after spinal cord injury: Reduction by a metalloporphyrin. J. Neurosci. Res. 2007;85:2175–2185. doi: 10.1002/jnr.21362. [DOI] [PubMed] [Google Scholar]
- 37.Huang YL, Saljo A, Suneson A, Hansson HA. A new approach for multiple sampling of cisternal cerebrospinal fluid in rodents with minimal trauma and inflammation. J. Neurosci. Methods. 1995;63:13–22. doi: 10.1016/0165-0270(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 38.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 39.De Ley G, Leybaert L. Effect of flunarizine and methylprednisolone on functional recovery after experimental spinal injury. J. Neurotrauma. 1993;10:25–35. doi: 10.1089/neu.1993.10.25. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Espejo MA, Haghighi SS, Adelstein EH, Madsen R. The effects of taxol, methylprednisolone, and 4-aminopyridine in compressive spinal cord injury: A qualitative experimental study. Surg. Neurol. 1996;46:350–357. doi: 10.1016/s0090-3019(96)00200-5. [DOI] [PubMed] [Google Scholar]
- 41.Koc RK, Akdemir H, Karakucuk EI, Oktem IS, Menku A. Effect of methylprednisolone, tirilazad mesylate and vitamin E on lipid peroxidation after experimental spinal cord injury. Spinal Cord. 1999;1:29–32. doi: 10.1038/sj.sc.3100732. [DOI] [PubMed] [Google Scholar]
- 42.Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of Bleomycin-Induced Pulmonary Fibrosis by a Catalytic Antioxidant Metalloporphyrin. Am. J. Respir. Cell Mol. Biol. 2001;25:164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 43.Kachadourian R, Menzleev R, Agha B, Bocckino SB, Day BJ. High-performance liquid chromatography with spectrophotometric and electrochemical detection of a series of manganese (I I I) cationic porphyrins. J. Chromatogr. 2002;767:61–67. doi: 10.1016/s0378-4347(01)00531-x. [DOI] [PubMed] [Google Scholar]
- 44.Smith MD. High-performance liquid chromatographic determination of hydrocortisone and methylprednisolone and their hemisuccinate esters in human serum. J. Chromatogr. 1979;164:129–137. doi: 10.1016/s0378-4347(00)81182-2. [DOI] [PubMed] [Google Scholar]
- 45.Vree TB, Lagerwerf AJ, Verwey-van Wissen CP, Jongen PJ. High-performance liquid chromatography analysis, preliminary pharmacokinetics, metabolism and renal excretion of methylprednisolone with its C6 and C20 hydroxy metabolites in multiple sclerosis patients receiving high-dose pulse therapy. J. Chromatogr. 1999;732:337–348. doi: 10.1016/s0378-4347(99)00292-3. [DOI] [PubMed] [Google Scholar]
- 46.Braughler JM, Hall ED. Uptake and elimination of methylprednisolone from contused cat spinal cord following intravenous injection of the sodium succinate ester. J. Neurosurgery. 1983;58:538–542. doi: 10.3171/jns.1983.58.4.0538. [DOI] [PubMed] [Google Scholar]
- 47.Segal JL, Maltby BF, Langdorf MI, Jacobson R, Brunnemann SR, Jusko WJ. Methylprednisolone disposition kinetics in patients with acute spinal cord injury. Pharmacotherapy. 1998;18:16–22. [PubMed] [Google Scholar]