Abstract
Grape byproducts are a rich source of phenolics having immense medicinal properties, but usually wasted from juice/wine processing industries. The present study investigates the phenolic antioxidants and the insulinotropic effect of extracts prepared from seed, skin and stems of two red wine grape cultivars: Pusa Navarang and Merlot. Pusa Navarang cultivar has shown high amounts of total phenolics (95.8 mg/ml), flavonoids (30.5 mg/ml) and flavan-3-ols (21.8 mg/ml) in seed extract and total anthocyanin (4.9 mg/ml) in its skin extract as compared to Merlot cultivar. As determined using HPLC, higher amounts of catechin hydrate (14909 mg/l) and epicatechin (9299 mg/l) were observed in its seed extract, while quercetin hydrate (5849 mg/l) was abundant in its skin extract. Similarly, ferric reducing antioxidant power (FRAP) and ABTS+. [2,2′-azinobis (3-ethylbenzothiazoline)-6-sulfonic acid] and DPPH. (1,1-diphenyl-2-picrylhy- drazyl) radicals scavenging, were higher in its seed extract, respectively being 134.8 mg/ml of Quercetin equivalent (QE), 18.7 mM of trolox equivalent (TE) and 33.5 mM of TE. Strong correlation was obtained between FRAP and total phenolics, flavonoids and flavan-3-ols contents with correlation coefficients (r2) of 0.915, 0.738 and 0.838 respectively. Interestingly, there was a 2–8 fold increase in insulin secretion by isolated mice pancreatic islets at 5.5 mM and 16.5 mM glucose concentration in presence of various extracts. Overall, the seed, skin and stem byproducts of both cultivars are rich sources of phenolics and antioxidants and represent a source of new insulin secretagogues.
Keywords: FRAP, Free radical scavenging, Total phenolics, Flavonoids, Flavan-3-ols, In vitro insulin secretion
Introduction
Liquid and solid wastes produced by the food processing industries are increasing now a days. Disposal of these waste materials containing biodegradable organic matter can create environmental problems. Efficient, inexpensive and environmentally rational utilization of agricultural byproducts is of undisputed importance for higher profitability and minimal environmental impact. One of the higher value options is the recovery of bioactive plant food constituents, which could be used in pharmaceutical, cosmetics and food industry (Makris et al. 2007). Grape (Vitis vinifera) is one of the world’s largest fruit crops with a global production of around 68 million tons in 2008 (OIV 2009). Wine as the main product of this crop reached about 26 million tons in 2008, generating large quantities of waste including grape skins and seeds (OIV 2009). These winery byproducts are rich in high-added-value compounds including phenolic acids, flavanols and anthocyanins (Amico et al. 2008; Bustamante et al. 2008; de Campos et al. 2008; Llobera and Canellas 2008), identified also in grapes and wine (Alonso et al. 2002; Goda et al. 2007; Kammerer et al. 2004; Makris et al. 2007; Spigno and De Faveri 2007; Shrikhande 2000). The grape byproducts either seeds or pomace, constitute a very cheap source for the extraction of antioxidants with potential health promoting and disease protective qualities (Zhang et al. 2007; Louli et al. 2004; Shi et al. 2003), which can be used as dietary supplements, or in the production of phytochemicals, thus providing an important economic advantage (Alonso et al. 2002; Negro et al. 2003). Phenolic compounds with their antioxidant capacity can preserve flavor and color, avoid vitamin destruction in foods and, more importantly, protect living systems from oxidative damage (Aliakbarian et al. 2009; Moure et al. 2001) and protection against cardiovascular diseases, anti-inflammatory activities and anticarcinogenic effects (Spigno and De Faveri 2007). In addition, antioxidants can be added to the food products containing oil and fat to increase the shelf life of the products (Aliakbarian et al. 2008). In particular, t-resveratrol (trans- 3, 5, 4′-trihydroxystilbene), one of the most important phenolic compounds present in grape, has shown anti-artherosclerosis, anticoronary diseases and anticancer properties, which make it particularly attractive for food and human health (Pascual-Marti et al. 2001).
Dietary antioxidants have been associated with the reduced risk of type II diabetes by inhibiting peroxidation chain reactions. Dietary supplements have been used extensively both as pharmacological supplements, food ingredients, in processed foods to aid weight control, and the regulation of glucose control for diabetic patients (Charles 2005). Flavonoids may also have antidiabetic activity (Vessal et al. 2003; Kao et al. 2000; Ong and Khoo 1996; Ahmad et al. 1989). Studies of the in vivo and in vitro effects of various flavonoids on glucose metabolism have shown opposite and often controversial results. This is probably because of the different structural characteristics of the molecules and the different experimental designs used (Harmon and Patel 2003; Kamei et al. 2003; Jarvill-Taylor et al. 2001). The treatment of diabetes mellitus has spent vast amounts of resources in all countries. However, very few studies have investigated the potential of grape pomace as an alternative bioresource for diabetes management (de Campos et al. 2008; Goda et al. 2007; Sehm et al. 2007; Thimothe et al. 2007; Bobek 1999).
Quantitative and qualitative distribution of polyphenols in grape pomace may show significant differences, depending on several factors, such as grape varieties, vinification conditions, the location of cultures and the winemaking procedures (Ruberto et al. 2007). Studies have been carried out in order to try to correlate the variety with the chemical composition of the grapes and wines obtained from them (Dopico-García et al. 2007; Tounsi et al. 2009). However, uses of grape pomace are limited but have been recycled as organic fertilizers, manure, and animal feed (Lafka et al. 2007). For that reason, treatment of winemaking wastes is a serious environmental problem and other uses than as fertilizers have to be found for these by-products. Additionally, little attention has been paid to grape stems, despite that these contain important amounts of polyphenols (Kallay and Kerenyi 1999; Alonso et al. 2002; Doshi et al. 2006; Llobera and Canellas 2007; Makris et al. 2007).
Thus, in the present study the antioxidant capacity and the insulinotropic activity of the phenolic extracts prepared from grape seed, skin and stem of two red wine grape cultivars namely Pusa Navarang and Merlot were determined. The various analyses involved total phenolics, flavonoids, anthocyanin and individual phenolic compounds in the extracts prepared from grape byproducts; seed, skin and stems followed by antioxidant capacity determination in terms of FRAP, ABTS and DPPH assay as well as in vitro insulin secretion by isolated mice pancreatic islets.
Materials and methods
Chemicals
The standard reference chemicals used; (+)-catechin hydrate, gallic acid, quercetin (−) epicatechin, epicatechin gallate, protocatechuic acid, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, rutin hydrate, quercetrin hydrate, trans-resveratrol were obtained from Sigma (St. Lous, MO, USA). Also, other chemicals like 2, 4, 6-tripyridyl-s-triazine (TPTZ), ABTS [2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammoinium salt], DPPH (2, 2-diphenyl-picrylhydrazyl) and Trolox were obtained from Sigma (St. Lous, MO, USA). p-dimethylaminocinnamaldehydde (analytical reagent grade) was obtained from the S.D Fine Chemicals, Ltd, India. All other solvents (HPLC grade) and chemicals used in this study were obtained from Merck, India Ltd.
Sample preparation
The studies were carried out on two colored grape cultivars Pusa Navrang and Merlot (Vitis vinifera L.), obtained from vineyard of National Research Centre for Grapes, Pune (M.S) India. The samples were separated into berries and stem (rachis, branches and pedicles) immediately after harvest on the same day. Further, berries were selected from bunches on the basis of their physico-chemical characteristics;., size (12–14 mm), pH (3.5–4.0), TSS (18–20 ° Brix) and titrable acidity (0.7 %–0.8 % tartaric acid equivalent). The skin and seeds were separated from berries pulp. The seeds were air dried and skin and stem samples were subsequently lyophilized using a Freeze Drier (Benchtop 4 K VIRTIS, NY, USA) at −78 °C, powdered and stored at −40 °C for few days till further analyses.
Sample extraction
5.0 g of the different lyophilized samples were extracted by overnight shaking on a mechanical shaker in the dark at RT. The solvent used was 80 % aqueous methanol, as reported to be a better solvent for polyphenol extraction (Bonilla et al. 2003). The grape skin samples were extracted with 0.01 % HCl in 80 % aqueous methanol. The extracts were centrifuged at 12,000 rpm for 15 min at 4 °C. The residues were re-extracted (2 times, 3 h each) in similar conditions. The filtrates were pooled and concentrated using a rotary evaporator till the methanol gets completely evaporated. The residue was dissolved in 5.0 ml distilled water, filtered through 0.45 μm filters and stored in different aliquots at −40 °C till further analysis.
Spectroscopic analysis of phenolics
Total phenolics, flavonoids, procyanidin monomers (flavan-3-ols) and total anthocyanins contents in the crude extracts were estimated following the previously reported methods (Doshi et al. 2006).
Specific phenolic compounds analysis using HPLC
Separation of individual phenolic components in various extracts was performed on a C-18 column 250 mm x 4.6 mm i.d., 5 μm particle size (Perkin Elmer) using a Dionex system equipped with autosampler (ASI-100), quaternary pump system (P 680) and PDA detector (UVD340U). The mobile phase for chromatographic separation consisted of solvent A: 2 % acetic acid in water v/v, Solvent B: acetonitrile and solvent C: 1 % acetic acid in water and acetonitrile (1:1). The gradients were 0.0–25 min, 88 % solvent A, 2 % solvent B and 10 % solvent C; 38 min, 57 % solvent A, 3 % solvent B and 40 % solvent C; 45–48 min 35 % solvent A, 10 % solvent B and 55 % solvent C; 52–60 min 88 % solvent A, 2 % solvent B and 10 % solvent C. The flow rate was 1.0 ml/min and injection volume was 10 μl of the diluted extracts as per requirement. The identification and quantification of individual phenolics was done by comparison with characteristic spectra, their retention times and calibration curves obtained for the respective external standards.
Antioxidant capacity evaluation
The antioxidant capacity was assessed using one method based on reduction of a ferric tripyridyl-s-triazine complex to its ferrous form, namely FRAP assay (ferric reducing antioxidant power) as described by Benzie and Strain (1996). And two other methods based on measurement of free radical scavenging using ABTS [2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammoinium salt] as reported by Re et al. (1999) and DPPH (2,2-diphenyl-picrylhydrazyl) as reported by Arnous et al. (2001) were also used.
FRAP assay
FRAP assay was carried out following the previously reported procedure (Doshi et al. 2006). In brief, a volume of 100 μl of the diluted samples was added to 1 ml FRAP solution, which was prepared by mixing 25 ml of 300 mM acetate buffer (pH 3.6), 2.5 ml of 2,4,6-tripyridyl-s-triazine (TPTZ) solution (10 mM TPTZ in 40 mM HCl) and 2.5 ml of 20 mM ferric chloride (FeCl3). Antioxidant capacity was calculated as the difference in the absorbance at 593 nm after 0 and 4 min (ΔA). The results were expressed as quercetin equivalents (QE) as determined from linear regression obtained by plotting (ΔA) of known quercetin standard solution against concentration (1–5 μg, r2 = 0.9982).
Free radical scavenging (antiradical activity)
In the first method, ABTS radical cations (ABTS.+) were generated by adding ABTS (7 mM) and potassium persulfate (2.45 mM) in distilled water and kept for 12–24 h in the dark. The ABTS solution was diluted with 50 % ethanol to an absorbance of 0.70 (± 0.05) at 734 nm. 1950 μl of this solution were added to 50 μl of each sample, mixed and absorbance at 734 nm was recorded exactly after 10 min. The suppression of absorbance of ABTS.+ Fig. radical cations by sample antioxidants was compared with that of Trolox. Results were expressed as mM Trolox equivalent (TE) as determined from linear regression obtained after plotting absorbance of known Trolox standard solutions against the concentration (100 to 500 μM, r2 = 0.9823).
In the second method, 50 μl aliquots of diluted samples were added to 950 μl of DPPH. solution (60 μM in methanol) and the absorbance was read at 515 nm after 30 minutes. The suppression of absorbance of DPPH. radical by sample antioxidants were compared with that of Trolox standard. Results was expressed as mM Trolox equivalent (TE) as determined from linear regression obtained after plotting absorbance of known Trolox standards solutions against the concentration (200 to 600 μM, r2 = 0.9862).
Insulin secretion in vitro
Pancreatic Islets isolation
The assay was carried out using mice pancreatic islet following the procedure reported by Shewade et al. (1999), at National Center for Cell Science (NCCS), Pune, India. In brief, Balb/C mice of both sexes (6 to 8 week old) were obtained from an inbred colony maintained at the animal house of NCCS. The islets were purified from a collagenase (Sigma, St Louis, MO, USA) digested pancreas on a Ficoll gradient and cultured in RPMI 1640 media (Gibco, Grand Island, NY, USA) supplemented with 10 % FCS (Trace Bioscience Pvt. Ltd., New South Wales, Australia) and antibiotics (Penicillin-200 U/ml and Streptomycin—0.2 mg/ml) in culture grade Nunclon flasks at 37 °C, 5 % CO2 for 48 h.
In vitro insulin secretion assay
The islets were washed twice with Krebs Ringer bicarbonate buffer (pH 7.4) supplemented with 0.5 mg/ml bovine serum albumin (Sigma, St Louis, MO, USA), 1 mM glucose and 10 mM HEPES. The islets were then transferred into 24 well plates (approximately 250 islets per well) and incubated at 37 °C with the same fresh modified Krebs buffer with 0.5 % bovine serum albumin, separately with basal 5.5 mM and 16.5 mM glucose for 1 h. The supernatant was removed and the islets were again incubated at 37 °C with addition of sample extracts at 5.5 mM and 16.5 mM glucose for 1 h. The supernatants collected after both the incubation periods i.e. with and without extracts addition were stored at −65 °C and assayed later by using insulin ELISA kit (Bioscience Europe S.A., Nivelles, Belgium).
Statistics
All experiments were repeated at least three times. The results were analyzed by student’s unpaired t test and a p value of ≤0.05 was taken to be significantly different.
Results and discussion
Phenolic compounds
The contents of total phenolics, flavonoids and flavan-3-ols (procyanidin monomers) in the extracts of skin, stem and seeds of Pusa Navarang and Merlot grape cultivars obtained were as given in Table 1. The total phenolic content determined in terms of gallic acid equivalents (GAE) was higher in seed extracts as compared to stem and skin extracts, being 65.8 mg/ml in Pusa Navarang cultivar and 41.7 mg/ml in Merlot cultivar. The flavonoids and flavan-3-ol contents as catechin equivalents (CE) were also higher in seed extracts followed by stems and skin of both the cultivars. Grape seeds make about 15 % of solid waste in wine industry and contain approximately (60–70 %) of total extractible grape phenolic compounds (Luque-Rodrıguez et al. 2005; Nawaz et al. 2006). These seeds are an excellent source of monomeric phenolic compounds such as catechin, epicatechin, epicatechin-gallate, dimeric, trimeric and tetrameric procyanidins as well as highly polymerized proanthocyanidins (condensed tannins), which act as anti-aging, anti-mutagenic, anti-proliferative, anti-atherogenic and antiviral agents (Lazzè et al. 2009; Jayaprakasha et al. 2003; Shi et al. 2003a, b). Grape seed extracts were recently reported to have hepatoprotective (Dogan and Celik 2011) and antiallergic properties (Bing-Hung Chen et al. 2012) also.
Table 1.
Total phenolics, flavonoids, flavan-3-ols and total anthocyanin contents in seed, skin and berry stem extracts of the two grape cultivars Pusa Navarang and Merlot
| Samples | Total phenolics (GAE) mg/ml | Flavonoids (CE) mg/ml | Flavan-3-ols (CE) mg/ml | Total anthocyanin (Cy-3-G) mg/ml |
|---|---|---|---|---|
| Pusa Navarang | ||||
| Seed | 65.8 ± 1.26a | 30.5 ± 0.70a | 21.8 ± 0.27a | NA |
| Skin | 12.5 ± 0.57b | 2.8 ± 0.11b | 0.92 ± 0.03b | 4.9 ± 0.11a |
| Berry stem | 11.6 ± 1.99c | 8.6 ± 0.28c | 3.7 ± 0.10c | NA |
| Merlot | ||||
| Seed | 41.7 ± 0.09d | 26.2 ± 0.70d | 15.5 ± 0.19d | NA |
| Skin | 6.1 ± 0.04e | 2.9 ± 0.04b | 0.83 ± 0.03e | 1.0 ± 0.03b |
| Berry stem | 20.5 ± 0.12f | 14.3 ± 0.31e | 6.8 ± 0.09f | NA |
The values are mean ± standard deviation of three replicates and those marked with different letters in same column are significantly different at p ≤ 0.05 NA Not applicable, GAE Gallic acid equivalent, CE Catechin equivalent, Cy-3-G Cyanidine-3-glucoside equivalent
The total anthocyanin content determined as cyanidine-3-glucoside equivalents (Cy-3-G) was relatively higher in skin of Pusa Navarang cultivar (4.9 mg/ml) as compared to the Merlot cultivar (1.0 mg/ml). This difference could be attributed to purple-red colored skin of Pusa Navarang cultivar and bronze colored skin of Merlot cultivar, which is in agreement with the reported literature that purple skinned grapes have higher anthocyanin content as compared to the bronze skinned grapes (Bonilla et al. 2003). It is also reported that red grape cultivars contain high levels of cyanidin and peonidin in skin tissues, whereas black cultivars contain high levels of delphinidin and malvidin (Shiraishi and Watanabe 1994). Anthocyanin pigments not only contributes towards sensory and organoleptic characteristics as well as antioxidant properties in red grapes and wines (Ghiselli et al. 1998; Pellegrini et al. 2000), but also play an important role in protection towards fungal and bacterial infections (Piermattei et al. 1999).
Specific phenolic compounds
Efforts were made to identify the individual phenolics in the extracts of seed, skin and stem of both grape cultivars using HPLC. Grape phenolic compounds can be divided into two groups: non-flavonoid (hydroxybenzoic and hydroxycinnamic acids, stilbenes) and flavonoid compounds (anthocyanins, flavan-3-ols and flavonols) (Gómez-Alonso et al. 2007). Fourteen phenolic compounds classified as hydroxybenzoic acids: gallic acid, vanillic acid, caffeic acid and syringic acid; hydroxycinnamic acids: protocatechuic acid, chlorogenic acid and p-coumaric acid; flavan-3-ols: catechin, epicatechin, epicatechin gallate; flavonols: quercetrin hydrate and rutin hydrate and stilbenes: trans-resveratrol and trans-piceatannol could be separated using optimized conditions as mentioned in the methodology section. The detection was performed at 280 nm for hydroxybenzoic acids and flavonols; at 320 nm for hydroxycinnamic acids and at 360 nm for flavan-3-ols. Varying levels of different classes of phenolic compounds could be detected from seed, skin and stem of different cultivars as shown in Table 2. Among the hydroxybenzoic acids, gallic acid were detected in high amount in seed extracts of Pusa Navarang cultivar followed by Merlot cultivar respectively being 1332 mg/l and 689 mg/l. Also, caffeic acid and syringic acid was detected higher in seeds as compared to skin and stem. Whereas, vanillic acid content was found to be more in stem extracts of both cultivars respectively being 76 mg/l and 66 mg/l. Among the hydroxycinnamic acids good amount of chlorogenic acid followed by p-coumaric acid could be detected in seeds as compared to skin and stem in both cultivars. The chlorogenic acid was found to be higher in seed extract of Pusa Navarang cultivar (1013 mg/l) as compared to the Merlot cultivar (258 mg/l). Whereas, protocatechuic acid was found to be high in stem extracts of both the cultivars being 946 mg/l in Pusa Navarang cultivar and 838 mg/l in Merlot cultivar. The flavan-3-ols compounds; catechin, epicatechin and epicatechin gallate which are well established for their high antioxidant capacity could be detected in high amounts in seeds followed by stems & least in skin of both the cultivars. The flavonol compounds; quercetrin hydrate having a role in protection & stabilization of color pigment (Chen and Hrazdina 1981) could be detected in higher amount in skin followed by stem and least in seeds, maximum being 5849 mg/l in skin of Pusa Navarang cultivar. Also, the compound rutin hydrate was higher in skin (468 mg/l) followed by stem (119 mg/l) of Merlot cultivar. Similarly, the stilbene compounds; trans-resveratrol & trans-piceatannol, which are phytoalexins (Jeandet et al. 1991; Hart 1981), could be detected in higher amounts in skin and stem extracts, whereas they could not be detected in seed extracts of both cultivars. The compounds catechin and epicatechin (flavan-3-ols), quercetin and its glycoside rutin (flavonols), and trans-Resveratrol (stilbene) have been proven to be potent antioxidants and to have important biological, pharmacological and medicinal properties (Maier et al. 2009; Villano et al. 2007; Auger et al. 2004; Kammerer et al. 2004; Rice-Evans et al. 1996).
Table 2.
Specific phenolics in the extracts of seed, skin and berry stems of the two grape cultivars Pusa Navarang and Merlot
| Grapes | Pusa Navarang | Merlot | ||||
|---|---|---|---|---|---|---|
| Specific phenolics (mg/l) | seed | skin | berry stem | seed | skin | berry stem |
| Hydroxybenzoic acids | ||||||
| Gallic acid | 1332.0 ± 90.2 a | 21.1 ± 2.1b | 24.3 ± 2.6b | 689.8 ± 22.0a | 15.0 ± 2.8b | 36.1 ± 1.2c |
| Vanillic acid | 3.4 ± 1.3a | 7.5 ± 0.62b | 76.8 ± 11.6c | 2.8 ± 0.27a | ND | 66.9 ± 2.5b |
| Caffeic acid | 243.7 ± 2.4a | 52.9 ± 3.9b | 56.1 ± 7.7b | 306.7 ± 2.0a | 48.7 ± 2.1b | 50.3 ± 0.58b |
| Syringic acid | 44.0 ± 2.0a | 4.9 ± 1.6b | 5.2 ± 0.18b | 52.6 ± 3.7a | 4.8 ± 0.08b | 8.9 ± 0.52c |
| Hydroxycinnamic acids | ||||||
| Protocatechuic Acid | 78.8 ± 1.2a | 17.0 ± 0.58b | 946.4 ± 67.8c | 87.4 ± 71.0a | 372.4 ± 45.6b | 838.5 ± 49.9c |
| Chlorogenic Acid | 1013.2 ± 91.6a | 38.5 ± 3.6b | 57.9 ± 0.82c | 258.8 ± 5.7a | 44.4 ± 2.5b | 27.1 ± 3.8c |
| p-Coumaric acid | 341.3 ± 4.6a | ND | 9.1 ± 2.8b | 212.2 ± 4.8a | 2.9 ± 0.53b | 11.7 ± 0.59c |
| Flavan-3-ols | ||||||
| catechin hydrate | 14909.2 ± 82.3a | 59.9 ± 0.83b | 392.5 ± 0.93c | 3633.9 ± 14.0a | 885.2 ± 15.5b | 2380.1 ± 14.7c |
| Epicatechin | 9299.4 ± 82.2a | 34.0 ± 9.5b | 59.5 ± 0.74c | 7980.5 ± 2.2a | 48.8 ± 2.7b | 85.5 ± 4.5c |
| Epicatechin gallate | 1975.1 ± 2.8a | 21.0 ± 1.3b | 39.4 ± 2.2c | 748.9 ± 9.4a | 26.2 ± 2.7b | 110.2 ± 1.2c |
| Flavonols | ||||||
| Quercetrin hydrate | 28.7 ± 1.2a | 5849.7 ± 89.9b | 56.9 ± 1.5c | 124.7 ± 1.9a | 94.9 ± 1.4b | 85.2 ± 1.7c |
| Rutin hydrate | ND | 232.3 ± 21.3a | ND | ND | 468.4 ± 21.5a | 119.5 ± 30.5b |
| Stilbenes | ||||||
| trans-resveratrol | ND | 34.5 ± 4.9a | 7.8 ± 9.4b | ND | 37.5 ± 3.6a | ND |
| trans-piceatannol | ND | 5.7 ± 5.8a | 1521.7 ± 51.6b | ND | 18.9 ± 2.4a | ND |
The values are mean ± standard deviation (SD) of three replicates and those marked with different letters in same column are significantly different at p ≤ 0.05. ND: not detected
Antioxidant capacity
The results obtained for antioxidant capacity in terms of free radical scavenging (antiradical activity) using ABTS.+ and DPPH. radicals and FRAP were as given in (Table 3). The maximum antiradical activity was detected in seed extract of Pusa Navarang cultivar being 18.7 and 33.5 mM of Trolox equivalents (TE) respectively for ABTS.+ and DPPH. radicals. Also, FRAP value was higher in its seed extract, being 134.8 of QE in mg/ml. This result was correlating well with the high content of procyanidin monomers (flavan-3-ols) in the seeds of these cultivars (Table 1). This is in agreement with the reported literature that the flavan-3-ols classified under flavonoid group exhibit substantial antioxidant activity (Cao et al. 1997). Since the majority of medicinal properties proven for the phenolic compounds are attributed to the flavan-3-ols and other polymeric procyanidins (Liu et al. 1998; Lotito et al. 2000), this observation is significant from nutraceutical and functional food point of view. So also, the polyphenolic compounds from grape seeds or the alcohol extracts have been reported to show a vitamin E sparing effect (Simonetti et al. 2002) as well as suppression of oxidative stress (Soo-Kyong et al. 2012).
Table 3.
Antiradical activity (AR) and ferric reducing antioxidant power (FRAP) in phenolics extracts of seed, skin and berry stems of grape cultivars Pusa Navarang and Merlot
| Samples | ARABTS (TE mM/ml) | ARDPPH (TE mM/ml) | FRAP (QE mM/ml) |
|---|---|---|---|
| Pusa Navarang | |||
| Seed | 18.7 ± 0.37a | 33.5 ± 1.49a | 134.8 ± 8.46a |
| Skin | 4.7 ± 0.01b | 4.2 ± 0.19b | 18.6 ± 0.09b |
| Berry stem | 4.0 ± 0.12c | 3.8 ± 0.22c | 20.6 ± 0.57c |
| Merlot | |||
| Seed | 8.0 ± 0.12d | 11.7 ± 0.11d | 47.1 ± 2.68d |
| Skin | 2.3 ± 0.09e | 1.8 ± 0.13e | 5.1 ± 0.48e |
| Berry stem | 6.1 ± 0.11f | 7.9 ± 0.09f | 29.5 ± 0.72f |
All the values are mean ± standard deviation of three replicates and those marked with different letters in same column are significantly different at p ≤ 0.05 AR ABTS Antiradical activity using ABTS+. [2,2′-azinobis (3-ethylbenzothiazoline)-6-sulfonic acid] radicals, AR DPPH Antiradical activity using DPPH. (1,1-diphenyl-2-picrylhy- drazyl) radicals, TE Trolox equivalent, QE Quercetin equivalent
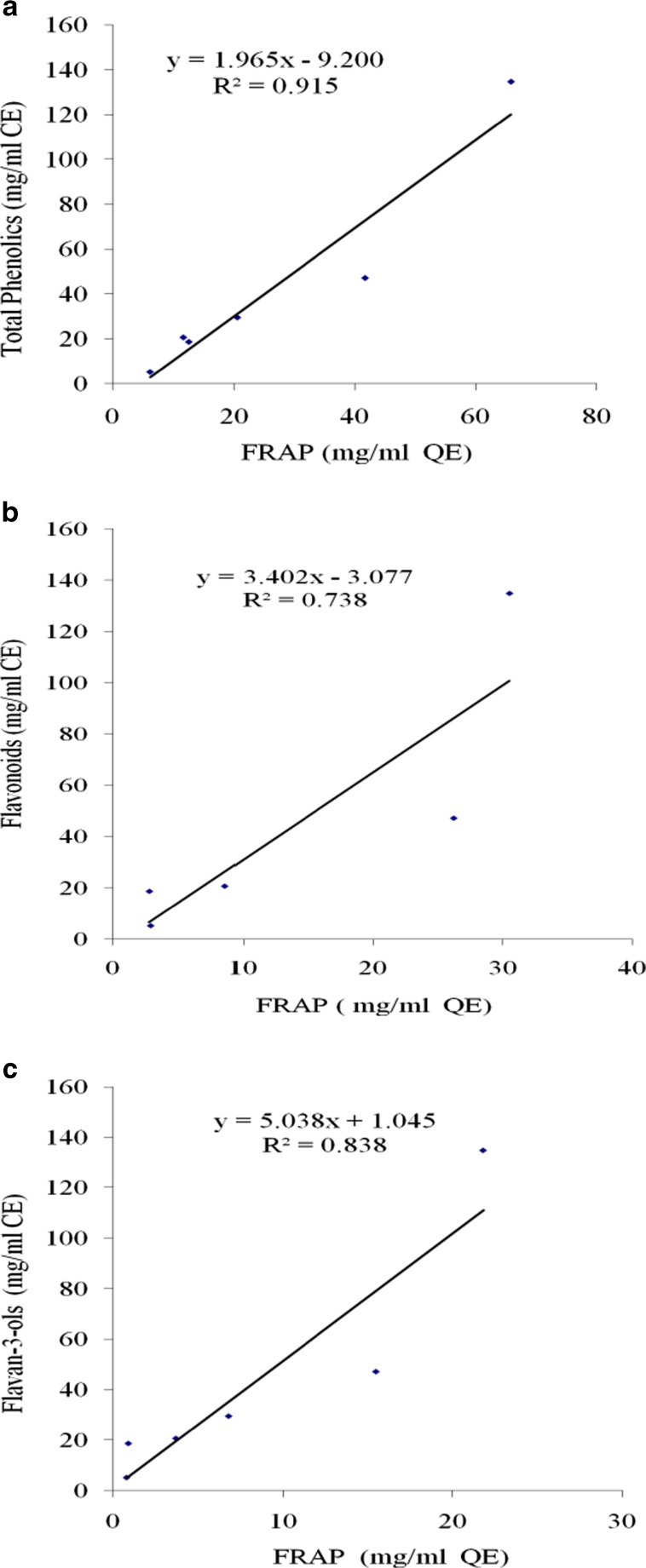
Plotting total phenolics, flavonoids and flavan-3-ols contents with (FRAP), the corresponding correlation coefficients (r2) obtained were 0.9157, 0.7386 and 0.8385 respectively, as shown (Fig. 1a,b and c). This shows the strong correlation of total phenolic compounds and good correlation of flavonoids as well flavan-3-ols to the antioxidant capacity. This is supported by the reported literature that the antioxidant activity has been highly correlating with the amount of total phenolics and could be attributed to the synergistic effect of overall phenolics composition (Yemis et al. 2008; Meyer et al. 1997). However, the reported literature also suggests that the grape seed extract, usually considered as an antioxidant nutritive supplement, may have prooxidant activity as well, depending on dose, duration of administration, and other dietary components, which was supported by an observation using in vitro primary leukocyte culture, that antioxidant activity of grape seed extract might be mediated by prooxidant quinones and oxidation products of the polyphenols from grape seeds (Chedea et al. 2010)
Fig. 1.
Correlation of (a) Total phenolics, (b) Flavonoids and (c) Flavan-3-ols with ferric reducing antioxidant power (FRAP) of seed, skin and berry stem of Pusa Navrang and Merlot cultivars. CE: catechin equivalent; QE: Quercetin equivalent
In vitro Insulin secretion
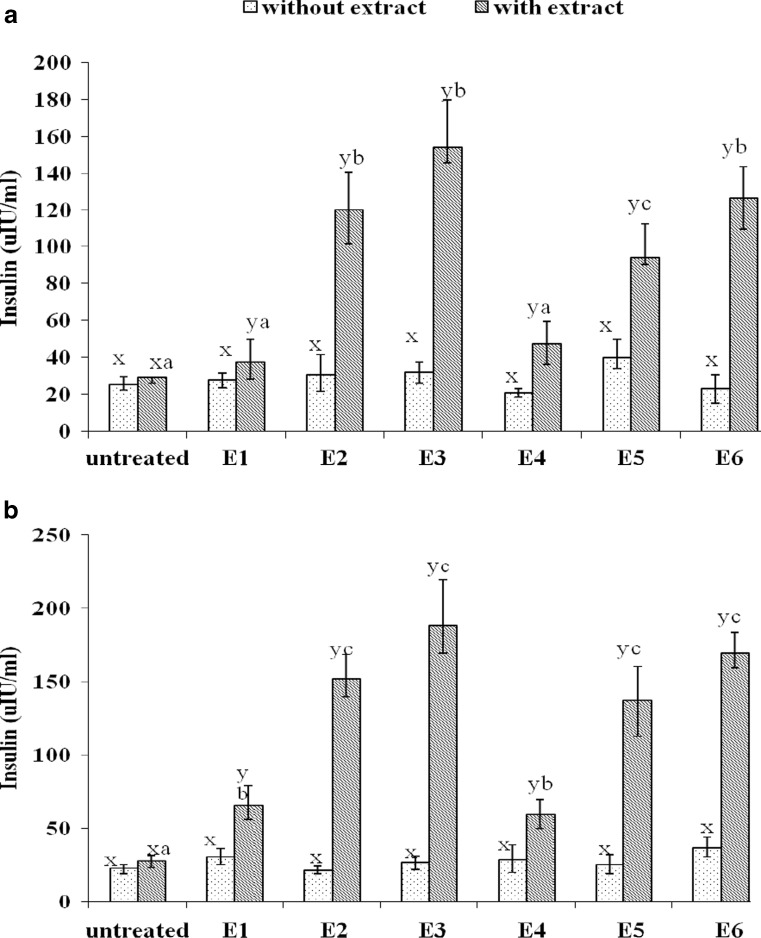
Interestingly, the extracts prepared from seed, skin and stems of both the grape cultivars have shown insulinotropic effects on isolated mice pancreatic islets. The amount of insulin secreted by islets at different glucose concentration basal (5.5 mM glucose) as well as enhanced glucose concentration (16.5 mM glucose) without extracts and with extracts was represented as (μIU/ml) as given in Fig. 2a and b. Tremendous stimulation in the insulin secretion by a set of pancreatic islets was observed with the presence of crude extracts of all the samples as compared to the insulin secreted by the same set of islets without the extracts at both glucose concentrations. The percentage increase in insulin secreted by mice pancreatic islets in presence of various extracts as compared to the insulin secretion by islets without extracts was in the range of 135 % to 543 % at 5.5 mM glucose concentration as given in Fig. 2a. Whereas, the insulinotropic effect of all the extracts were better evident at higher glucose concentration (16.5 mM) with increase in the range of 206 % to 807 % as compared to the islets without extracts as given in Fig. 2b.
Fig. 2.
Stimulation of mice pancreatic islets insulin secretion with extracts of grape seed, skin and berry stems at (a) basal glucose level (5.5 mM) and (b) enhanced glucose level (16.5 mM) as compared to without extracts.(n = 3). E1, E2 and E3 respectively represent extracts of seed, skin and berry stem of Pusa Navarang grape cultivar and E4, E5, E6 respectively represent extracts of seed, skin and berry stem of Merlot grape cultivar. Different letters (a, b, c) on top of bar (mean±SD) indicates significance difference (p ≤ 0.05), between different parts seed, skin and berry stem and letters x and y indicates significance difference between without extract and with extract respectively
According to the reported literature, the extracts of a traditional antidiabetic plant in India Asparagus adscendens (Shweta musali) have shown 19–248 % increase in insulin secretion by clonal pancreatic β cell line, BRIN-BD11 (Mathews et al. 2006). The another traditional antidiabetic plant Teucrium polium extracts have shown 135 % increase in insulin secretion by isolated rat islets at 16.0 mM glucose concentration (Razieh et al. 2005). Also, the well documented traditional antidiabetic plant Viscum album (mistletoe) has shown 1.1 to 12.2 fold stimulation in insulin secretion by clonal pancreatic β-cell at 16.7 mM glucose concentration (Gray and Flatt 1999). Thus, in the present study the 2 to 8 fold stimulation in insulin secretion by isolated pancreatic islets at 16.5 mM glucose concentration in presence of grape seed, skin and stems extracts is highly appreciating and represents a source of potential new oral hypoglycemic agent.
The grape seed extracts are well established as potent antioxidants and the recent literature has also reported that grape seed extracts have antihyperglycemic and insulinomimetic properties as the grape seed procyanidins mimic or influence insulin effects by directly acting on specific components of the insulin signaling transduction pathway (Meeprom et al. 2011; Pinent et al. 2004). The grape pomace extracts exerted a significant anti-postprandial hyperglycemic effect, suggesting that grape pomace could be a valuable food derived bioresource that is rich in antioxidants and anti-hyperglycemic compounds. These dual bioactive attributes derived from the grape pomace could play a complementary and alternative role in managing the poorly regulated blood glucose levels and oxidative stress associated with Type 2 diabetes (Pinent et al. 2004; Al-Awwadi et al. 2004). Also, grape skin extract has been recently reported to improve glycemia and inflammation (Shelly et al. 2011). Though the flavan-3-ol (procyanidin) contents were relatively less in grape skin and stem the higher insulin secreting activity could be attributed to the other specific phenolic components like flavonols and stilbenes present in abundant in these grape parts. Also, the higher insulinotropic effect of grape skin could also be attributed to anthocyanin pigments which are reported to have property of insulin secretion when exposed to pancretic β-cells (Jayaprakasam et al. 2005). However, to our knowledge this is the first report of an insulinotropic effect of grape stem extracts.
Conclusion
In conclusion, our results suggest that besides grape seeds the grape skin and stem extracts not only have the high antioxidant properties but also have the immense potential as insulin secretagogues and may be useful in the treatment of type II diabetes. However, in vivo studies and clinical evaluations of these compounds must be carried out to further validate our in vitro results. Also, exploration of these grape tissues awaits further research and purification of the active components from the crude extracts. Future work assessing its use as a dietary adjunct or as a source of active components may provide new opportunities for the treatment of diabetes.
Acknowledgments
We are grateful to Department of Biotechnology (DBT), Government of India for funding the project. We are also grateful to Dr Ramesh. R Bhonde, National Centre for Cell Science, Pune for helping us to provide facility for carrying out in vitro insulin secretion assay.
References
- Ahmad FKP, Khan MM, Rastogi AK, Kidwai JR. Insulin like activity in (−) epicatechin. Acta Diabetol Lat. 1989;26:291–300. doi: 10.1007/BF02624640. [DOI] [PubMed] [Google Scholar]
- Al-Awwadi NA, Poucheret P, Cassanas G, Krosniak M, Auger C, Gasc F, Rouanet JM, Cros G, Teissedre PL. Antidiabetic activity of red wine polyphenolic extract, ethanol, or both in streptozotocin-treated rats. J Agric Food Chem. 2004;52(4):1008–1016. doi: 10.1021/jf030417z. [DOI] [PubMed] [Google Scholar]
- Aliakbarian B, De Faveri D, Converti D, Perego P. Optimisation of olive oil extraction by means of enzyme processing aids using response surface methodology. Biochem Eng J. 2008;42(1):34–40. doi: 10.1016/j.bej.2008.05.006. [DOI] [Google Scholar]
- Aliakbarian B, Dehghani F, Perego P. The effect of citric acid on the phenolic contents of olive oil. Food Chem. 2009;116(3):617–623. doi: 10.1016/j.foodchem.2009.02.077. [DOI] [Google Scholar]
- Alonso AM, Guillen DA, Barroso CG, Puertas B, Garcıa A. Determination of antioxidant activity of wine by-products and it correlation with polyphenolic content. J Agri Food Chem. 2002;50(21):5832–5836. doi: 10.1021/jf025683b. [DOI] [PubMed] [Google Scholar]
- Amico V, Chillemi R, Mangiafico S, Spatafora C, Tringali C. Polyphenol- enriched fractions from Sicilian grape pomace: HPLC–DAD analysis and antioxidant activity. Bioresour Technol. 2008;99(13):5960–5966. doi: 10.1016/j.biortech.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Arnous A, Makris DP, Kefalas P. Effect of principle polyphenolic components in relation to antioxidant characteristics of aged red wines. J Agric Food Chem. 2001;49(12):5736–5742. doi: 10.1021/jf010827s. [DOI] [PubMed] [Google Scholar]
- Auger C, Al Awwadi N, Bornet A, Rouanet JM, Gasc F, Cros G, Teissedre PL (2004) Catechins and procyanidins in Mediterranean diets. Food Res Int 37:233–245
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bobek P. Dietary tomato and grape pomace in rats: Effect on lipids in serum and liver and on antioxidant status. Br J Biomed Sci. 1999;56(2):109–113. [PubMed] [Google Scholar]
- Bonilla EP, Akoh CC, Sellapan S, Krewer G. Phenolics content and antioxidant capacity of Muscadine grapes. J Agric Food Chem. 2003;51(18):5497–5503. doi: 10.1021/jf030113c. [DOI] [PubMed] [Google Scholar]
- Bustamante MA, Moral R, Paredes C, Perez-Espinosa A, Moreno-Caselles J, Perez-Murcia MD. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008;28:372–380. doi: 10.1016/j.wasman.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Cao G, Sofic E, Prior RL. Antioxidant capacity and prooxidant behavior of flavonoids: Structure activity relationships. Free Rad Biol Med. 1997;22(5):749–760. doi: 10.1016/S0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- Charles SB. Dietary fibre, glycaemic response and diabetes. Mol Nutr Food Res. 2005;49:560–570. doi: 10.1002/mnfr.200500025. [DOI] [PubMed] [Google Scholar]
- Chedea VS, Braicu C, Socaciu C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010;121(1):132–139. doi: 10.1016/j.foodchem.2009.12.020. [DOI] [Google Scholar]
- Chen L, Hrazdina G. Structural aspects of anthocyanin-flavonoid complex formation and its role in plant color. Phytochemistry. 1981;20:297–303. doi: 10.1016/0031-9422(81)85111-4. [DOI] [Google Scholar]
- Chen B-H, Hung M-H, Chen JY-F, Chang H-W, Meng-Lung Y, Wan L, Tsai FJ, Wang T-P, Tzu-Fun F, Chiu C-C. Anti- allergic activity of grape seed extract (GSE) on RBL-2H3 mast cells. Food Chem. 2012;132(2):968–974. doi: 10.1016/j.foodchem.2011.11.079. [DOI] [Google Scholar]
- de Campos LM, Leimann FV, Pedrosa RC, Ferreira SR. Free radical scavenging of grape pomace extracts from cabernet sauvingnon (Vitis vinifera) Bioresour Technol. 2008;99(17):8413–8420. doi: 10.1016/j.biortech.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Dopico-García MS, Valentao P, Jagodzinska A, Klepczynska J, Guerra L, Andrade PB, Seabra RM. Solid-phase extraction versus matrix solid-phase dispersion: Application to white grapes. Talanta. 2007;74(1):20–31. doi: 10.1016/j.talanta.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Doshi PJ, Adsule PG, Banerjee K. Phenolic composition and antioxidant activity in grape (Vitis venifera L) c v. Sharad seedless berries and different vine parts during maturation. Int J Food Sci Technol. 2006;41(S1):1–9. doi: 10.1111/j.1365-2621.2006.01214.x. [DOI] [Google Scholar]
- Ghiselli A, Nardini M, Baldi A, Scaccini C. Antioxidant activity of different phenolics fractions separated from an Italian red wine. J Agric Food Chem. 1998;46(2):361–367. doi: 10.1021/jf970486b. [DOI] [PubMed] [Google Scholar]
- Goda T, Suruga K, Komori A, Kuranuki S, Mochizuki K, Makita Y, Kumazawa T. Effects of miglitol, an alpha-glucosidase inhibitor, on glycaemic status and histopathological Changes in islets in non-obese, non-insulin dependent diabetic goto-kakizaki rats. Br J Nutr. 2007;98(4):702–710. doi: 10.1017/S0007114507742678. [DOI] [PubMed] [Google Scholar]
- Gómez-Alonso S, García-Romero E, Hermosín-Gutiérrez I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J Food Compos Anal. 2007;20:618–626. doi: 10.1016/j.jfca.2007.03.002. [DOI] [Google Scholar]
- Gray AM, Flatt PR. Insulin-secreting activity of the traditional antidiabetic plant Viscum album (mistletoe) J Endocrinol. 1999;160(3):409–414. doi: 10.1677/joe.0.1600409. [DOI] [PubMed] [Google Scholar]
- Harmon A, Patel YM. Naningerin inhibits phosphoinositide 3-kinase activity and glucose uptake in 3 T3–L1 adipocytes. Biochem Biophys Res Commun. 2003;305:229–234. doi: 10.1016/S0006-291X(03)00720-4. [DOI] [PubMed] [Google Scholar]
- Hart JH. Role of phytostilbenes in decay and disease resistance. Annual Rev Phytopathol. 1981;19:437–458. doi: 10.1146/annurev.py.19.090181.002253. [DOI] [Google Scholar]
- Abdulahad Dogan and Ismail Celik (2011) Hepatoprotective and antioxidant activities of grape seeds against ethanol-induced oxidative stress in rats. Bri J Nutr 1–7. doi: 10.1017/S0007114511002650 [DOI] [PubMed]
- Jarvill-Taylor JK, Anderson RA, Graves DJJ. A hydroxychalcone derived from Cinnamon functions as a mimetic for insulin in 3 T3–L1 adipocytes. J Am Coll Nutr. 2001;20(4):327–336. doi: 10.1080/07315724.2001.10719053. [DOI] [PubMed] [Google Scholar]
- Jayaprakasam B, Vareed SK, Olison KL, Nair GM. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem. 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Selvi T, Sakariah KK. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res Int. 2003;36(2):117–122. doi: 10.1016/S0963-9969(02)00116-3. [DOI] [Google Scholar]
- Jeandet P, Bessis R, Gautheron B. The production of resveratrol (3, 5, 4-trihydroxy- stilbene) by grape berries in different developmental stages. Am J Enol Vitic. 1991;42:41–46. [Google Scholar]
- Kallay M, Kerenyi Z. Occurrence and identification of procyanidins in hungarian grapes and wines. Hungarian Agric Res. 1999;4:17–20. [Google Scholar]
- Kamei RKM, Kitagawa Y, Hazeki O, Oikawa S. 2-Benzyloxychalcone derivatives stimulate glucose uptake in 3 T3–L1 adipocytes. Life Sci. 2003;73:2091–2099. doi: 10.1016/S0024-3205(03)00563-0. [DOI] [PubMed] [Google Scholar]
- Kammerer D, Claus A, Carle R, Schieber A. Polyphenol screening of pomace from Red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J Agric Food Chem. 2004;52:4360–4367. doi: 10.1021/jf049613b. [DOI] [PubMed] [Google Scholar]
- Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- Lafka TI, Sinanoglou V, Lazos ES. On the extraction and antioxidant activity of phenolic compounds from winery waste. Food Chem. 2007;104:1206–1214. doi: 10.1016/j.foodchem.2007.01.068. [DOI] [Google Scholar]
- Lazzè MC, Pizzala R, Pecharroman FJG, Garnica PG, Rodríguez JMA, Fabris N, Bianchi L. Grape waste extract obtained by supercritical fluid extraction contains bioactive antioxidant molecules and induces antiproliferative effects in human colon adenocarcinoma cells. J Med Food. 2009;12(3):561–568. doi: 10.1089/jmf.2008.0150. [DOI] [PubMed] [Google Scholar]
- Liu FJ, Zhang YX, Lau BH. Pycnogenol enhances immune and haemopoietic function in enescence-accelerated mice. Cell Mol Life Sci. 1998;54:1168–1172. doi: 10.1007/s000180050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobera A, Canellas J. Dietary fibre and antioxidant activity of Manto Negro red grape (Vitis vinifera): pomace and stem. Food Chem. 2007;101(2):659–666. doi: 10.1016/j.foodchem.2006.02.025. [DOI] [Google Scholar]
- Llobera A, Canellas J. Antioxidant activity and dietary fibre of Prensal Blanc white grape (Vitis vinifera) by-products. Int J Food Sci Technol. 2008;43(11):1953–1959. doi: 10.1111/j.1365-2621.2008.01798.x. [DOI] [Google Scholar]
- Lotito SB, Actis-Goretta RML, Caliguiri M, Rein D, Schmitz HH, Steinberg FM, Keen CL, Fraga CG. Influence of oligomeric chain length on the antioxidant activity of procyanidins. Biochem Biophysic Res Comm. 2000;276:945–951. doi: 10.1006/bbrc.2000.3571. [DOI] [PubMed] [Google Scholar]
- Louli V, Ragoussis N, Magoulas K. Recovery of phenolic antioxidants from wine industry byproducts. Bioresour Technol. 2004;92:201–208. doi: 10.1016/j.biortech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Luque-Rodrıguez JM, Luque de Castro MD, Perez-Juan P. Extraction of fatty acids from grape seed by superheated hexane. Talanta. 2005;68:126–130. doi: 10.1016/j.talanta.2005.04.054. [DOI] [PubMed] [Google Scholar]
- Maier T, Schieber A, Kammerer DR, Carle R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009;112(3):551–559. doi: 10.1016/j.foodchem.2008.06.005. [DOI] [Google Scholar]
- Makris DP, Boskou G, Andrikopoulos NK. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J Food Compos Anal. 2007;20:125–132. doi: 10.1016/j.jfca.2006.04.010. [DOI] [Google Scholar]
- Mathews JN, Flatt PR, Abdel-Wahab YH. Asparagus adscendens (Shweta Musali) insulin secretion, insulin action and inhibits starch digestion. Bri J Nutr. 2006;95(3):576–581. doi: 10.1079/BJN20051650. [DOI] [PubMed] [Google Scholar]
- Meeprom A, Sompong W, Suwannaphet W, Yibchok-anun S, Adisakwattana S. Grape seed extract supplementation prevents high-fructose diet-induced insulin resistance in rats by improving insulin and adiponectin signalling pathways. Bri J Nutr. 2011;106(8):1173–1181. doi: 10.1017/S0007114511001589. [DOI] [PubMed] [Google Scholar]
- Meyer AS, Yi OS, Pearson DA, Waterhouse AL, Frankel EN. Inhibition of human low-density lipoprotein oxidation in relation to composition of phenolic antioxidants in grapes (Vitis Vinifera) J Agric Food Chem. 1997;45:1638–1643. doi: 10.1021/jf960721a. [DOI] [Google Scholar]
- Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H. Review: Natural antioxidants from residual sources. Food Chem. 2001;72(2):145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Nawaz H, Shi J, Mittal GS, Kakuda Y. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Separation and Purification Technol. 2006;48(2):176–181. doi: 10.1016/j.seppur.2005.07.006. [DOI] [Google Scholar]
- Negro CL, Tommasi, Miceli A. Phenolic compounds and antioxidative activity from red grape marc extracts. Bioresour Technol. 2003;87:431–444. doi: 10.1016/S0960-8524(02)00202-X. [DOI] [PubMed] [Google Scholar]
- OIV (2009) Organisation internationale de la vigne et du vin. http://www.oiv.int (accessed December 26, 2010).
- Ong KC, Khoo H. Insulinomimetic effects of myricetin on lipogenesis and Glucose transport in rat adipocytes but not glucose transporter translocation. Biochem Pharmacol. 1996;51:423–429. doi: 10.1016/0006-2952(95)02195-7. [DOI] [PubMed] [Google Scholar]
- Pascual-Marti MC, Salvador A, Chafer A, Berna A. Supercritical fluid extraction of resveratrol from grape skin of Vitis vinifera and determination by HPLC. Talanta. 2001;54:735–74. doi: 10.1016/S0039-9140(01)00319-8. [DOI] [PubMed] [Google Scholar]
- Pellegrini N, Simonetti P, Gardana C, Brenna O, Brighenti F, Pietta P. Polyphenol content and total antioxidant activity of Vini Noveli (young red wines) J Agric Food Chem. 2000;48(3):732–735. doi: 10.1021/jf990251v. [DOI] [PubMed] [Google Scholar]
- Piermattei B, Piva A, Castellari M, Arfelli G, Amati A. The phenolics composition of red grapes and wines as infected by Oidium tuckeri development. Res Note Vitis. 1999;2:85–86. [Google Scholar]
- Pinent M, Blay M, Blade MC, Salvado MJ, Arola L, Ardevol A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology. 2004;145(11):4985–4990. doi: 10.1210/en.2004-0764. [DOI] [PubMed] [Google Scholar]
- Razieh Y, Esmaeili MA, Helen JA. Teucrium polium extract effects pancreatic function of streptozotocin diabetic Rats: A Histological Examination. Iranian Biomed J. 2005;9(2):81–85. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical Cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationship of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Ruberto G, Renda A, Daquino C, Amico V, Spatafora C, Tringali C, De Tommasi N. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007;100(1):203–210. doi: 10.1016/j.foodchem.2005.09.041. [DOI] [Google Scholar]
- Sehm J, Lindermayer H, Dummer C, Treutter D, Pfaffl MW. The influence of polyphenol rich apple pomace or red-wine pomace diet on the gut morphology in weaning piglets. J Anim Physiol Anim Nutr (Berl) 2007;91(7–8):289–296. doi: 10.1111/j.1439-0396.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- Shelly H, Cornea C, Shi S, Xiuxiu S, Hoda K, Kequan Z. Dietary supplementation of grape skin extract improves glycemia and inflammation in diet-induced obese mice fed a western high fat diet. J Agri Food Chem. 2011;7(59):3035–3041. doi: 10.1021/jf1042773. [DOI] [PubMed] [Google Scholar]
- Shewade Y, Umarani M, Bhonde RR. Large-scale isolation of islets by tissue culture of adult mouse pancreas. Transplant Proc. 1999;31(3):1721–1723. doi: 10.1016/S0041-1345(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Shi J, Yu J, Pohorly J, Young JC, Bryan M, Wu Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J Food Agri Envir. 2003;1(2):42–47. [Google Scholar]
- Shi J, Yu J, Pohorly JE, Yukio K. Polyphenolics in Grape seeds biochemistry and functionality. J Med Food. 2003;6(4):291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Watanabe Y. Anthocyanin composition in grape skin. Bulletin: Kyushu University, Farm. 1994;7:3–17. [Google Scholar]
- Shrikhande AJ. Wine by-products with health benefits. Food Res Int. 2000;33:469–474. doi: 10.1016/S0963-9969(00)00071-5. [DOI] [Google Scholar]
- Simonetti P, Ciappellano S, Gardana C, Bramati L, Pietta P. Procyanidinis from Vitis vinifera seeds: in vivo effects on oxidative stress. J Agric Food Chem. 2002;50:6217–6221. doi: 10.1021/jf011412+. [DOI] [PubMed] [Google Scholar]
- Soo-Kyong C, Xian-Hua Z, Jung-Sook S. Suppression of oxidative stress by grape seed supplementation in rats. Nutr Res Practice. 2012;6(1):3–8. doi: 10.4162/nrp.2012.6.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigno G, De Faveri DM. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J Food Eng. 2007;78(2):793–801. doi: 10.1016/j.jfoodeng.2005.11.020. [DOI] [Google Scholar]
- Thimothe J, Bonsi IA, Padilla-Zakour OI, Koo H. Chemical characterization of red wine grape (Vitis vinifera and Vitis interspecific hybrids) and pomace phenolic extracts and their biological activity against streptococcus mutans. J Agric Food Chem. 2007;55(25):10200–10207. doi: 10.1021/jf0722405. [DOI] [PubMed] [Google Scholar]
- Tounsi MS, Ouerghemmi I, Wannes WA, Ksouri R, Zemni H, Marzouk B, Kchouk ME. Valorization of three varieties of grape. Ind Crop Prod. 2009;30:292–296. doi: 10.1016/j.indcrop.2009.05.007. [DOI] [Google Scholar]
- Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozotocin induced diabetic rats. Comp Biochem Physiol Toxicol Pharmacol. 2003;135C(3):357–364. doi: 10.1016/S1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- Villano D, Fernandez-Pachon MS, Moya ML, Troncoso AM, Garcıa-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71(1):230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Yemis O, Bakkalbasi E, Artik N. Antioxidative activities of grape (Vitis vinifera) seed extracts obtained from different varieties grown in Turkey. Int J Food Sci Technol. 2008;43(1):154–159. doi: 10.1111/j.1365-2621.2006.01415.x. [DOI] [Google Scholar]
- Zhang FL, Gao HQ, Shen L. Inhibitory effect of grape on rage expression induced by advanced glycation end products in endothelial cells. J Cardiovasc Pharmacol. 2007;50(4):434–440. doi: 10.1097/FJC.0b013e3181342bfa. [DOI] [PubMed] [Google Scholar]