Abstract
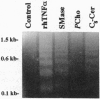
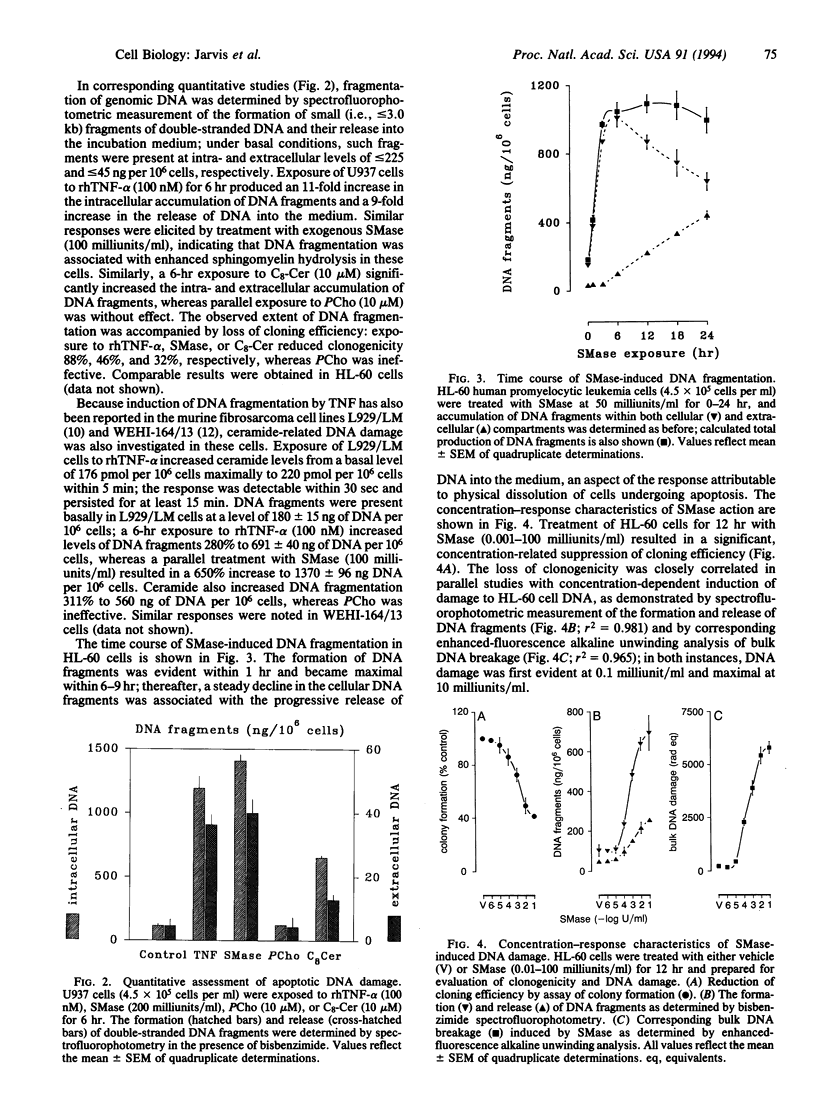
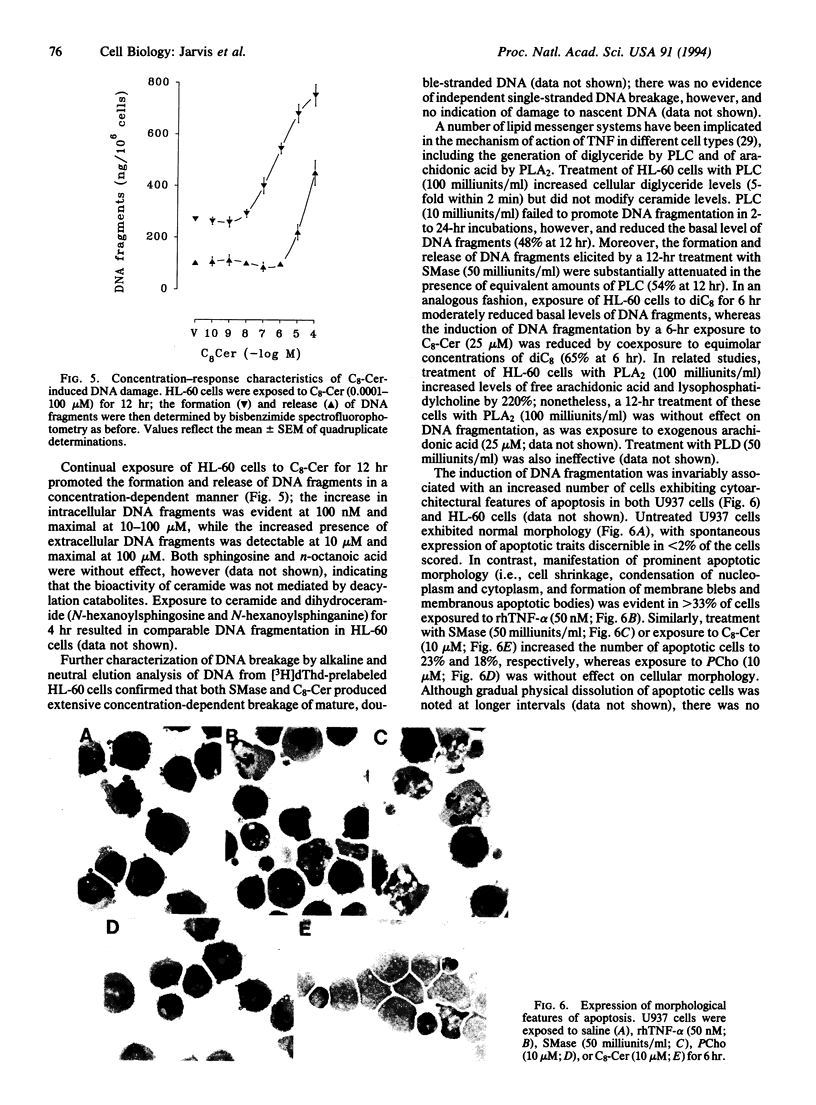
The potential involvement of ceramide-related signaling processes in the induction of apoptosis by tumor necrosis factor alpha was assessed by multiple biochemical strategies in the human leukemic cell lines HL-60 and U937 and the murine fibrosarcoma cell lines L929/LM and WEHI 164/13. Exposure of these cells to tumor necrosis factor alpha resulted in internucleosomal cleavage of genomic DNA, yielding laddered patterns of oligonucleosomal fragments characteristic of apoptosis when resolved by agarose gel electrophoresis; similar responses were observed after exposure to exogenous sphingomyelinase or synthetic ceramides. Quantitative spectrofluorophotometry demonstrated that these treatments promoted time- and concentration-dependent degradation of DNA, resulting in the formation of and eventual release of small DNA fragments (< or = 3.0 kb). Corresponding damage to bulk DNA was demonstrated by enhanced-fluorescence alkaline unwinding analysis. DNA fragmentation was not induced by phospholipase C or synthetic diglyceride; in fact, the effects of sphingomyelinase and ceramide were substantially reduced by coexposure to these agents, suggesting opposing roles for diglyceride- and ceramide-mediated signals in the regulation of apoptosis. Phospholipase A2 and arachidonic acid failed to promote DNA fragmentation, as did phospholipase D. Characterization of DNA strand breaks by alkaline and neutral elution analyses confirmed that ceramide action was restricted to breakage of mature, double-stranded DNA but not of nascent DNA. The induction of DNA damage was associated with appearance of apoptotic morphology and decreased clonogenicity. These results demonstrate that the ceramide-dependent signaling system selectively induces apoptosis and raise the possibility that ceramide-activated enzymes represent important components in a signaling cascade involved in the regulation of programmed cell death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Dobrowsky R. T., Hannun Y. A. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992 Mar 15;267(8):5048–5051. [PubMed] [Google Scholar]
- Dressler K. A., Mathias S., Kolesnick R. N. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992 Mar 27;255(5052):1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- Elias L., Berry C. O. Induction of differentiation by tumour necrosis factor in HL-60 cells is associated with the formation of large DNA fragments. Leukemia. 1991 Oct;5(10):879–885. [PubMed] [Google Scholar]
- Elias L., Moore P. B., Rose S. M. Tumor necrosis factor induced DNA fragmentation of HL-60 cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):963–969. doi: 10.1016/s0006-291x(88)80968-9. [DOI] [PubMed] [Google Scholar]
- Ellis A. L., Randolph J. K., Conway B. R., Gewirtz D. A. Biochemical lesions in DNA associated with the antiproliferative effects of mitoxantrone in the hepatoma cell. Biochem Pharmacol. 1990 May 15;39(10):1549–1556. doi: 10.1016/0006-2952(90)90520-u. [DOI] [PubMed] [Google Scholar]
- Fehsel K., Kolb-Bachofen V., Kolb H. Analysis of TNF alpha-induced DNA strand breaks at the single cell level. Am J Pathol. 1991 Aug;139(2):251–254. [PMC free article] [PubMed] [Google Scholar]
- Fulton A. M., Chong Y. C. The role of macrophage-derived TNFa in the induction of sublethal tumor cell DNA damage. Carcinogenesis. 1992 Jan;13(1):77–81. doi: 10.1093/carcin/13.1.77. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Rotello R. J. Apoptosis: a different type of cell death. FASEB J. 1992 Apr;6(7):2450–2455. doi: 10.1096/fasebj.6.7.1563596. [DOI] [PubMed] [Google Scholar]
- Grant S., Jarvis W. D., Swerdlow P. S., Turner A. J., Traylor R. S., Wallace H. J., Lin P. S., Pettit G. R., Gewirtz D. A. Potentiation of the activity of 1-beta-D-arabinofuranosylcytosine by the protein kinase C activator bryostatin 1 in HL-60 cells: association with enhanced fragmentation of mature DNA. Cancer Res. 1992 Nov 15;52(22):6270–6278. [PubMed] [Google Scholar]
- Greenblatt M. S., Elias L. The type B receptor for tumor necrosis factor-alpha mediates DNA fragmentation in HL-60 and U937 cells and differentiation in HL-60 cells. Blood. 1992 Sep 1;80(5):1339–1346. [PubMed] [Google Scholar]
- Gromkowski S. H., Mama K., Yagi J., Sen R., Rath S. Double-stranded RNA and bacterial lipopolysaccharide enhance sensitivity to TNF-alpha-mediated cell death. Int Immunol. 1990;2(9):903–908. doi: 10.1093/intimm/2.9.903. [DOI] [PubMed] [Google Scholar]
- Kanter P. M., Schwartz H. S. A fluorescence enhancement assay for cellular DNA damage. Mol Pharmacol. 1982 Jul;22(1):145–151. [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Kyprianou N., Alexander R. B., Isaacs J. T. Activation of programmed cell death by recombinant human tumor necrosis factor plus topoisomerase II-targeted drugs in L929 tumor cells. J Natl Cancer Inst. 1991 Mar 6;83(5):346–350. doi: 10.1093/jnci/83.5.346. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Mathias S., Dressler K. A., Kolesnick R. N. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger C., Ellis A., Woods K., Randolph J., Yanovich S., Gewirtz D. Evidence for inhibition of growth related to compromised DNA synthesis in the interaction of daunorubicin with H-35 rat hepatoma. Cancer Res. 1988 May 1;48(9):2404–2411. [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Schwartzman R. A., Cidlowski J. A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993 Apr;14(2):133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Weber R. F., Figari I. S., Reynolds C., Palladino M. A., Jr, Goeddel D. V. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Lee T. H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991 Apr 25;266(12):7313–7316. [PubMed] [Google Scholar]
- Wright S. C., Kumar P., Tam A. W., Shen N., Varma M., Larrick J. W. Apoptosis and DNA fragmentation precede TNF-induced cytolysis in U937 cells. J Cell Biochem. 1992 Apr;48(4):344–355. doi: 10.1002/jcb.240480403. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yanaga F., Watson S. P. Tumor necrosis factor alpha stimulates sphingomyelinase through the 55 kDa receptor in HL-60 cells. FEBS Lett. 1992 Dec 21;314(3):297–300. doi: 10.1016/0014-5793(92)81493-6. [DOI] [PubMed] [Google Scholar]
- Yang Z., Costanzo M., Golde D. W., Kolesnick R. N. Tumor necrosis factor activation of the sphingomyelin pathway signals nuclear factor kappa B translocation in intact HL-60 cells. J Biol Chem. 1993 Sep 25;268(27):20520–20523. [PubMed] [Google Scholar]