Abstract
In the present study, resveratrol content and antioxidant properties of underutilized fruits such as Jamun (Syzygium cumini L.), Jackfruit (Artocarpus heterophyllus) and Mulberry (Morus rubra) were investigated keeping Grape (Vitis vinifera) as a reference. Ethanol/water (80:20 v/v) extracts of different parts of fruit samples including skin, pulp and seeds were analyzed by HPLC and MS for the quantification of resveratrol. Total polyphenols, flavonoids, DPPH scavenging activity and total antioxidant capacity were also investigated. Among the samples analyzed, mulberry fruit (whole) showed highest resveratrol content (50.61 μg g−1 dry weight) followed by jamun seed (34.87 μg g−1 dry weight), jamun pulp (13.70 μg g−1 dry weight) and skin of jamun (11.19 μg g−1 dry weight). Jamun seed extract exhibited the highest polyphenol content (55.54 mg gallic acid equivalent g−1 dry weight) and highest antioxidant property (IC50 value-0.40 mg ml−1). The results suggest that underutilized fruits high in resveratrol and other polyphenols can be used as functional beverages.
Keywords: Resveratrol, Polyphenols, Antioxidant, Mulberry, Jamun, Jackfruit
Introduction
Oxygen centered free radicals and reactive oxygen species (ROS) are highly toxic resulting in cell death and tissue damage leading to diseases such as atherosclerosis, diabetes, cancer, cirrhosis and neurodegenerative disorders (Lobo et al. 2010). Antioxidants are compounds which can inhibit or delay the oxidative damage preventing many diseases (Heim et al. 2002). Synthetic antioxidants like butylated hydroxyl anisole (BHA) and butylated hydroxyl toluene (BHT) are widely used but they have been restricted recently because of serious concerns about their side effects (Botterweck et al. 2000) Today there is interest in finding natural antioxidants without undesirable side effects and they should aid the endogenous protective system. The value of antioxidants in food has long been recognized for their health benefits. It is believed that higher intake of antioxidant rich fruits are associated with decreased risk of degenerative diseases particularly coronary heart disease (CHD) and cancer (Ruxton et al. 2006). The beneficial effect of fruits is mainly associated with the radical scavenging activity of polyphenols, which are largely present in fresh fruits and their processed products.
Stilbenes are one of important class of polyphenols synthesised by a wide range of plant species including Dipterocarpaceae, Cyperaceae, Gnetaceae, Pinaceae, Leguminosae, Myrtaceae, Moraceae, Fagaceae, Liliaceae and Vitaceae and are commonly found in the roots, barks, rhizomes and leaves. Resveratrol (3, 5, 4′-trihydroxystilbene) is one of the major stilbene phytoalexin produced by a wide variety of plants in response to stress, injury, ultraviolet (UV) irradiation, and fungal (e.g., Botrytis cinerea) infection (Burns et al. 2002)
There are numerous reports on resveratrol content of grapes (Vitis vinifera) (Pezzuto 2008). peanuts (Arachis hypogaea) (Tokusoglu et al. 2005), berries (Rimando et al. 2004) and legumes (Duenas et al. 2006). While these plants have been reported as the primary dietary sources of resveratrol, several other sources have also been identified, including Itadori tea (from the root of Polygonum cuspidatum) (Vastano et al. 2000). Resveratrol occurs in both cis and trans isomeric forms. Cis-resveratrol has not been reported in V. vinifera however, it has been shown to be present in wines (Vitrac et al. 2005). All of the research investigations focused only upon the trans-isomer, due to the unstability of cis-isomer and of the fact that it is not available commercially. The major studies on biological activities of resveratrol have considered only trans- resveratrol which is one of the important compounds involved in the cardioprotective effects of red wine. Biological benefits of resveratrol against cancer, cardiovascular disease, inflammation and ischaemic injuries, as well as enhancement of stress resistance and life span extension of various organisms from yeast to vertebrates has been reviewed (Baur and Sinclair 2006). It has been found that resveratrol modulates vascular cell function, inhibits LDL oxidation, suppresses platelet aggregation and reduces myocardial damage during ischemia-reperfusion (Bradamante et al. 2004). Physiological activity of cis-resveratrol was shown to inhibit kinase activity, a factor related to cancer (Jayatilake et al. 1993).
Since a number of taxonomically unrelated plant families have been reported to produce marked levels of resveratrol including grapes, peanuts, berries, pine trees, a detailed study on the screening of polyphenols and resveratrol in underutilized as well as poorly reported fruits of India would be of great interest. Bael (Aegle marmelos), Custard Apple (Annona squamosa), Indian gooseberry (Emblica officinalis), Sea buckthorn (Hippophae rhamnoided), Tamarind (Tamarindus indiaca), Mulberry (Morus rubra), Indian blackberry (Syzygium cumini L.), Jackfruit (Artocarpus heterophyllus), Wild pomegranate (Punica granatum), are traditional Indian fruits rich in polyphenols, closely linked to the cultural heritage of India which are being underutilized (Ravindran et al. 2007). This work would result in the development of fruit functional beverages as India is the second largest country producing 45.203 million tonnes of fruits in the world. In the present study, Mulberry, Indian blackberry and Jackfruit of Indian origin were explored for their resveratrol content and antioxidant properties.
Materials and methods
Samples and reagents
Grapes- blue Concord grapes (Vitis labrusca), Jamun (Syzygium cumini L.) and Jackfruit (Arterocarpus heterophyllus) were purchased from local markets. Mulberry (M. rubra) fruits were provided by Central Sericulture Research & Training Institute (CSRTI), Central Silk Board, Mysore. Samples were stored at −20 °C and protected from light until use.
BHT (butylated hydroxyl toluene), 2, 2-diphenyl-1-picrylhydrazyl (DPPH), L-ascorbic acid, α-tocopherol, gallic acid, p-coumaric acid, caffeic acid, (+)-catechin, (−)-epicatechin, and trans-resveratrol were purchased from Sigma-Aldrich. All the solvents used were from Sisco Research Laboratory, Mumbai, India.
Extraction of fruits for antioxidant assays
The experimental procedure was performed as described with slight modifications (Soong and Barlow 2006). Frozen fruit samples were thawed and different parts of fruits- skin, pulp and seeds were separated and lyophilized. Mulberry was used as a whole as it was tough to separate the parts. 1–2 g of lyohilized fruit samples were homogenized in mortar and pestle with 25 ml of ethanol/water (80:20 v/v) and kept in shaking water bath at 60 °C for 30 min. The samples were filtered through Whatman No.1 filter paper by applying vacuum. The crude extracts were then concentrated by flash evaporation to 10 ml and used for antioxidant assays. All the assays were carried out in triplicates.
DPPH radical scavenging activity
A different concentration of fruit extracts (0.2–1.0 mg) in methanol (300 μl) were treated with 2.7 ml of methanolic solution containing DPPH radicals (6 × 10−5 mol/l). The mixture was vortexed and incubated in dark for 60 min. The reduction of the DPPH radical was determined by reading the absorbance at 517 nm. The radical-scavenging activity (RSA) was calculated as a percentage of DPPH discoloration, using the equation: 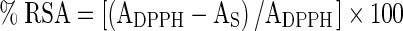 , where AS is the absorbance of the solution when the sample extract is added at a particular level and ADPPH is the absorbance of the DPPH solution. The extract concentration providing 50 % of radical-scavenging activity (IC50) was calculated from the graph of RSA percentage against extract concentration (Barros et al. 2007). BHT was used as standard.
, where AS is the absorbance of the solution when the sample extract is added at a particular level and ADPPH is the absorbance of the DPPH solution. The extract concentration providing 50 % of radical-scavenging activity (IC50) was calculated from the graph of RSA percentage against extract concentration (Barros et al. 2007). BHT was used as standard.
Estimation of total polyphenols
Total phenolic content of each crude extract was estimated by Folin-Ciocalteau method (Singleton and Rossi 1965). To 6.0 ml triple distilled water 0.1 ml sample and 0.5 ml Folin-Ciocalteau reagent was mixed followed by the addition of 1.5 ml Na2CO3 (20 g/100 ml water) and the volume was made up to 10.0 ml with distilled water. The reaction mixture was kept in dark for 30 min at 25 °C, the absorbance was measured at 760 nm and the phenolic contents were expressed as gallic acid equivalents.
Determination of flavonoids
Flavonoid contents in the crude extracts were determined by a colorimetric method (Zhishen et al. 1999). 50 μl of each extract were taken and made up to 5 ml and 0.3 ml of 5 % NaNO2 solution was added. After 5 min of incubation 0.5 ml 10 % AlCl3.H2O solution was added. After 6 min, 2 ml 1 M NaOH was added and the total volume was made up to 10 ml with distilled water. The solution was mixed well and the absorbance was measured against a blank at 510 nm. (+)-Catechin was used to prepare the standard curve (20–200 μg) and the results were expressed as mg of (+)-catechin equivalents (CEs) g−1 of extract.
Determination of total antioxidant capacity
The assay is based on the reduction of Mo (VI)-Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at acidic pH (Prieto et al. 1999). Reagent solution was prepared which constituted of 0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate.10 μl extract was taken in test tubes and 3 ml reagent solution was added. The tubes were capped and incubated in boiling water bath at 95 °C for 90 min. After the mixture had cooled to room temperature, the absorbance of the solution was measured at 695 nm against a blank. A typical blank solution contained 3 ml of reagent solution and the appropriate volume of the same solvent used for the sample. This was incubated under similar conditions as the rest of the samples. The antioxidant activity was expressed as the number of equivalents of gallic acid.
Extraction of resveratrol and other polyphenols
Solid Phase Extraction of fruit samples was carried out as described by Rodriguez-Saona and Wrolstad (2001). Lyophilized fruit samples (100 mg) were homogenized with 10 ml acidified methanol [0.01 % (v/v) HCl in methanol]. The slurry was transferred to 100 ml stoppered flasks and incubated in shaker at 200 rpm for 3 h at room temperature. The extract was filtered through Whatman No.1 filter paper. The filtrate was transferred to tared round bottom flask and methanol was evaporated under vaccum. The dried extract was dissolved in distilled water and 1 ml extract was subjected to solid phase extraction.
C18 cartridge (Waters Inc, USA) was conditioned by passing two column volumes of methanol through the sorbent bed. Further three column volumes of acidified water was passed through the column and the sample was loaded into the cartridge. Then cartridge was washed with two volumes of acidified water to remove sugars and acids. Residual water was removed by passing a stream of nitrogen gas through the cartridge for 2 to 3 min. Polyphenolic compounds such as phenolic acids, flavonols and resveratrol were eluted with ethyl acetate followed by the removal of anthocyanin pigments using acidified methanol. Ethyl acetate fraction was concentrated in a rotary evaporator under vacuum to the dryness, redissolved in methanol and filtered through 0.45 μm PTFE filter (Whatman) prior to HPLC injection.
HPLC analysis
Separation and quantification of resveratrol and other polyphenols was performed on a reversed-phased HPLC system (Shimadzu model LC10A) with a photodiode array UV-visible detector (280 nm). A Discovery C18 column (25 cm × 4.6 mm; 5 μm) from Supelco was used as the stationary phase with a flow of 0.8 ml/min. The gradient elution conditions are as follows: 0 min, 20 % A, 80 % B; 4 min, 30 % A, 70 % B; 8 min, 40 % A, 60 % B; 12 min, 65 % A, 35 % B; 16 min, 80 % A, 20 % B; 20 min, 95 % A, 5 % B; 21.8 min, 97 % A, 3 % B; 24 min, 100 % A, 0 % B, where solvent A was water/acetonitrile/acetic acid (67:32:1 v/v/v) and solvent B was water/acetic acid (99:1 v/v) (Quiros et al. 2007). Chromatographic peaks were identified by comparing their retention times and spectra data with those of the pure standards.
MS analysis of resveratrol
The presence of resveratrol was also confirmed by positive ion atmospheric pressure chemical ionization (APCI) mass spectrometry. The APCI mass spectrometry was performed using a Q-Tof Ultima Instrument from Waters Corporation (Micromass, Manchester, UK). Tof penning pressure (mbar) of 4.34e-7 was used. The source temperature was 120 °C. The corona voltage was 10 V and the cone voltage was of 150 V. Nitrogen was used as desolving and nebulizing gas with flow rates of about 300 L/h and 50 L/h, respectively. APCI mass spectra were recorded over the range m/z 140–600 in a scan time of 1 s (Wang et al. 2002). For system control and data processing, Mass Lynx software (Micromass) was used.
Statistical analysis
The experimental measurements were carried out in triplicate and results were presented as average of three analyses ± standard deviation. Statistical analysis of the data was done using the SPSS statistical package (7.5.1 version, SPSS Inc., 1996). One way ANOVA was used for testing statistical significance between different extracts, and individual pair difference was tested by means of Tukey’s multiple range tests. All data are presented as mean ± S.D. A P value of < 0.05 was taken as statistically significant.
Results and discussion
Fruits, vegetables, cereals and pulses are good sources of polyphenols and dietary fibers. Fruits are rich in bioactive phenolic compounds such as flavonoids, phenolic acids, stilbenes, coumarins and tannins. Polyphenols play a significant role as dietary antioxidant compounds. Phenolics exert antioxidant properties through various mechanisms of actions including the scavenging of free radicals and inhibition of the generation of reactive species during the course of normal cell metabolism, thereby preventing damage to lipids, proteins, and nucleic acids and eventually preventing cell damage (Gutteridge and Halliwell 2010)
Major research work has been done on antioxidant properties of fruits such as mango, citrus fruits, pomegranate and berries like blueberry, raspberry and cranberry (Kaur and Kapoor 2001). The present work describes the antioxidant properties and polyphenol content along with the resveratrol content of different underutilized fruits.
Total polyphenols
The total polyphenol content of ethanol extracts of underutilized fruits is presented in Table 1. The total polyphenols varied significantly (P < 0.05) among the different fruit extracts ranging between 1.00 and 55.54 mg gallic acid equivalents (GAE) g−1 dry weight. Jamun seed was found to have significantly higher polyphenols (55.54 mg GAE g−1) compared to other fruit extracts. However it has been detected 108.7 mg GAE g−1 of total polyphenols in jamun seed extracts (Bajpai et al. 2005). On the other hand, jackfruit seed (1.00 mg GAE g−1), jackfruit pulp (1.27 mg GAE g−1) and grape pulp (1.04 mg GAE g−1) extracts contain less amount of polyphenols when compared to others. Similar to jamun, seed extract of grapes (26.88 mg GAE g−1) was having significantly higher polyphenols followed by skin (10.76 mg GAE g−1) and pulp (1.04 mg GAE g−1) extracts. It was reported that seed extracts of Cabernet Sauvignon and Baco22A varieties of grapes showed total phenolic content of 43.69 and 37.47 mg g−1 respectively suggesting the variety of fruit may influence the phenolic content (Sung and Lee 2010). In contrast to jamun and grapes, skin extracts of jackfruit was found to have significantly more polyphenols (13.38 mg GAE g−1) compared to pulp (1.27 mg GAE g−1) and seed (1.00 mg GAE g−1) extracts. But this does not corroborate with the findings of Soong and Barlow (2004) who reported that seeds of jackfruit showed higher amounts of total phenolics content (27.7 mg GAE g−1). It is reported that ethanol extract of jackfruit pulp resulted in total phenolics content of 0.46 mg GAE g−1 (Jagtap et al. 2010). These differences in total phenolics of same fruits may be due to the complexity of these compounds and the methods of extraction and analysis (Kalt et al. 2001). Moreover, phenolics contents of plant foods depend on a number of intrinsic and extrinsic factors (Thomas-Barberan and Espin 2001). Mulberry extract was reported to contain 14.35 mg GAE g−1 which was significantly lower than jamun skin, jamun seed and grape seed extracts. However Bae and Suh (2007) have reported about 2570.4 μg GAE g−1 in mulberry extracts which is approximately six times lower than reported in the present study.
Table 1.
Total polyphenols, total flavonoids and antioxidant capacity of underutilized fruits
| Extract | Total polyphenolsA (mg GAEg−1 dry weight) | Total flavonoidsB (mg CEg−1 dry weight) | DPPH IC50 value (mg ml−1)C | Total antioxidant capacityD mM GAE g−1 of extract |
|---|---|---|---|---|
| Mulberry | 14.35 ± 0.41 a | 4.01 ± 0.13 a | 0.60 ± 0.01a | 3.10 ± 0.07 a |
| Jamun skin | 29.22 ± 1.95 b | 7.26 ± 0.68 b | 0.41 ± 0.00 a | 4.21 ± 0.12 d |
| Jamun pulp | 4.84 ± 0.50 c | 0.63 ± 0.03 c | 1.06 ± 0.07 a | 3.23 ± 0.31 a,b |
| Jamun seed | 55.54 ± 2.06 d | 5.09 ± 0.28 a | 0.40 ± 0.00 a | 3.33 ± 0.10 a,b |
| Jackfruit skin | 13.38 ± 0.29 a,e | 7.50 ± 0.18 b | 0.43 ± 0.00 a | 1.32 ± 0.11 c |
| Jackfruit pulp | 1.27 ± 0.33 f | 0.11 ± 0.03 c | 11.45 ± 0.46 b | 3.25 ± 0.25 a,b |
| Jackfruit seed | 1.00 ± 0.09 f | 0.61 ± 0.04 c | 6.07 ± 0.605 c | 0.19 ± 0.00 e |
| Grape skin | 10.76 ± 0.64 e | 2.63 ± 0.12 e | 0.70 ± 0.00 a | 3.21 ± 0.07 a |
| Grape pulp | 1.04 ± 0.08 f | 0.23 ± 0.02 c | 15.50 ± 1.4327 d | 3.66 ± 0.10 b |
| Grape seed | 26.88 ± 1.52 b | 16.15 ± 0.70 f | 0.41 ± 0.00 a | 1.53 ± 0.03 c |
Values are Mean ± SD of three independent analyses (n = 3)
a,b,c,d,e,f Values followed by different superscripts within each column are statisticaly different (p < 0.05)
ATotal phenolic contents expressed as gallic acid equivalents; BTotal flavonoids contents are expressed as (+)-catechin equivalents; CConcentration required to inhibit 50 % of DPPH radicals; DTotal antioxidant capacity expressed as gallic acid equivalents
Total flavonoids
Flavonoids are widely distributed group of polyphenolic compounds naturally present in most edible fruit and vegetable plants. They constitute most of the yellow, red and blue colors in the fruits. Table 1 shows the flavonoid contents of different extracts of underutilized fruits. Grape seed extracts showed significantly higher flavonoids (16.15 mg catechin equivalents (CE) g−1) compared to other fruit extracts. Sung and Lee (2010) reported that grape seed extracts of Cabernet Sauvignon and Baco22A varieties showed flavonoid content of 30.73 and 20.33 mg CE g−1 respectively. No significant difference was observed in flavonoid content of jamun skin (7.26 mg CE g−1) and jackfruit skin (7.50 mg CE g−1) extracts which showed relatively similar flavonoid content next to grape seed extracts. Jackfruit pulp and grape pulp extracts were found to have significantly lower flavonoids (0.11 and 0.23 mg CE g−1 respectively) as compared to the others. Jagtap et al. (2010) reported that the water extract of jackfruit pulp showed more flavonoid content (1.20 mg of rutin equivalents g−1). The flavonoids content of whole mulberry fruit extract was 4.01 mg CE g−1 which was significantly higher than jamun pulp, jackfruit pulp, jackfruit seed, grape skin and grape pulp extracts. In contrast, Bae and Suh (2007) found that the ethanol extract of mulberry fruit resulted in only 65.4 μg CE g−1.
DPPH scavenging activity
Antioxidants on interaction with DPPH, a stable free radical, either transfer electrons or hydrogen atoms of DPPH, thus neutralizing free radical ability (Naik et al. 2003). During reaction, the colour of the mixture changes from purple to yellow and its absorbance at wavelength 517 nm decreases. All the fruits analyzed were able to scavenge DPPH radicals at a dose dependent manner. Mulberry fruit extracts quenched 20.08 % DPPH free radicals at 0.2 mg ml−1 concentration and scavenging activity increased to 77.75 % with 1.0 mg ml−1 concentration. Among all extracts, jamun seed extract showed highest scavenging activity (74.27 %) with the lowest concentration (0.2 mg ml−1) and the activity increased to 93.9 at 1.0 mg ml−1. When considering jamun fruit extracts, skin and seed extracts exhibited 94.55 % and 93.90 % activity followed by pulp extracts (44.48 %). Skin extracts of jackfruit showed more DPPH quenching activity of 94.59 % at 1.0 mg ml−1 concentration followed by seed and pulp extracts. Seed extracts of grapes expressed more scavenging activity (92.78) followed by skin and pulp extracts and pulp extract is the least in quenching DPPH radicals (3.65 %) when compared to the others.
IC50 value
IC50 values (concentration of sample required to scavenge 50 % of free radicals) were calculated from the regression equations prepared from the concentrations of the extracts and percentage inhibition of free radical formation in DPPH assay. A lower IC50 value indicates greater antioxidant activity. All the extracts analyzed except grape pulp, jack fruit pulp and jack fruit seed showed no significance in effective concentration for DPPH scavenging activity. The effective concentration required to quench at least 50 % radicals was found to be 0.40 mg ml−1 with jamun seed, which was the lowest IC50 value but no significant difference was observed with extracts of jamun skin, jamun pulp, mulberry, jackfruit skin, grape skin and grape seed (Table 1). The IC50 value for mulberry extract was 0.60 mg ml−1. The IC50 value obtained with grape pulp extract was 15.50 mg ml−1 which was 39 times greater than that of jamun seed extracts. Among jamun fruit extracts, seed extract showed IC50 value of 0.40 mg ml−1 followed by skin and pulp extracts (0.41 and 1.06 mg ml−1 respectively). Banerjee et al. (2005) found that skin extract of jamun fruit showed IC50 value of 168 μg ml−1. Skin extracts of jackfruit were found to be effective and showed IC50 value of 0.43 mg ml−1 followed by seed and pulp extracts. Similar to jamun fruit, seed extracts of grapes were found to be effective and showed IC50 of 0.41 mg ml−1 followed by skin and pulp extracts (0.70 and 15.50 mg ml−1 respectively).
Total antioxidant capacity
Total antioxidant capacity of fruit extracts were expressed as gallic acid equivalents (Table 1). Among all extracts, jamun skin extract had significantly more total antioxidant capacity of 4.21 GAE mM g−1 when compared to the others. Jackfruit seed extract was found to have significantly lesser antioxidant capacity (0.19 GAE mM g−1) than the others. Interestingly, pulp extracts of jackfruit and grapes were found to contain more total antioxidant capacity of 3.25 and 3.66 GAE mM g−1 respectively followed by skin and seed extracts. Seed extracts of Cabernet Sauvignon varieties of grapes showed total antioxidant capacity of 121.2 mg Trolox equivalent (TEAC) g−1 (Sung and Lee 2010).
Resveratrol and phenolics
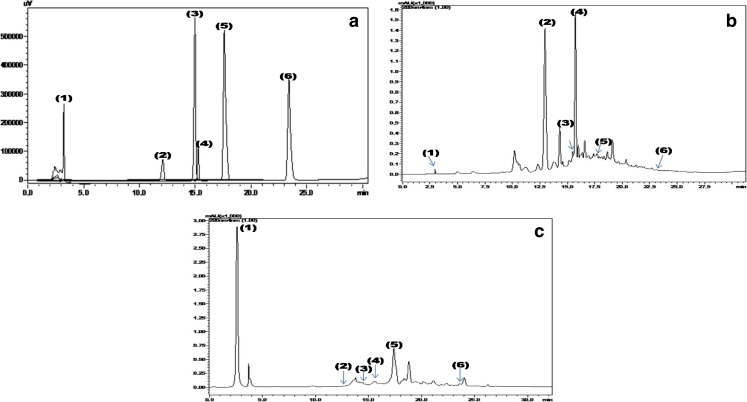
Resveratrol and other phenolics present in underutilized fruits were quantified by HPLC and the results presented in Table 2 and Fig. 1. Mulberry whole fruit extracts showed highest concentration of resveratrol (50.61 μg g−1) followed by Jamun seed extracts and their pulp and skin extracts. The resveratrol content of jackfruit skin was 3.56 μg g−1, which was comparatively similar to that of skin extracts of grapes. Jackfruit pulp and seed extracts showed very least amount of resveratrol content whereas grape seed extract showed 5.89 μg g−1 dry weight.
Table 2.
Phenolic compounds in underutilized fruits
| Sample | Phenolics and resveratrol content (μg g−1 dry weight) | |||||
|---|---|---|---|---|---|---|
| Gallic acid | Caffeic acid | (+)-Catechin | (−)-Epicatechin | p-Coumaric acid | Resveratrol | |
| Mulberry | 1996.55 | nd | nd | 99.69 | 34.37 | 50.61 |
| Jamun skin | 1398.07 | 33.97 | nd | 381.54 | 35.43 | 11.19 |
| Jamun pulp | 3471.64 | 57.84 | 14.78 | 342.46 | 216.3 | 13.70 |
| Jamun seed | 6243.36 | nd | nd | 129.20 | 14.06 | 34.87 |
| Jackfruit skin | NA | NA | NA | NA | NA | 3.56 |
| Jackfruit pulp | NA | NA | NA | NA | NA | 0.07 |
| Jackfruit seed | NA | NA | NA | NA | NA | 0.87 |
| Grape skin | 4.259 | 129.9 | 30.13 | 201.09 | 16.05 | 3.54 |
| Grape pulp | 4.95 | 6.05 | 1.08 | 20.63 | 2.90 | 1.44 |
| Grape seed | 38.79 | 223.47 | 6348.8 | 4577.8 | 123.30 | 5.89 |
nd not detected, NA not analyzed
Fig. 1.
HPLC chromatrogram of a Standard polyphenols, b Grape seed extract and c Jamun pulp extract at 280 nm. (1) Gallic acid, (2) (+)-Catechin, (3) Caffeic acid, (4) (−)-Epicatechin, (5) p-Coumaric acid and (6) trans – Resveratrol
Grape seed extracts were found to have more phenolics content when compared to other extracts analyzed. (+)-Catechin and (−)-epicatechin content of grape seed extracts were 6348.8 and 4577.8 μg g−1 respectively. Jamun seed extract exhibited more gallic acid content of 6243.36 μg g−1 among the extracts analyzed. In contrast, Bajpai et al. (2005) have detected very less amount (646 μg g−1) of gallic acid in jamun seed extracts.
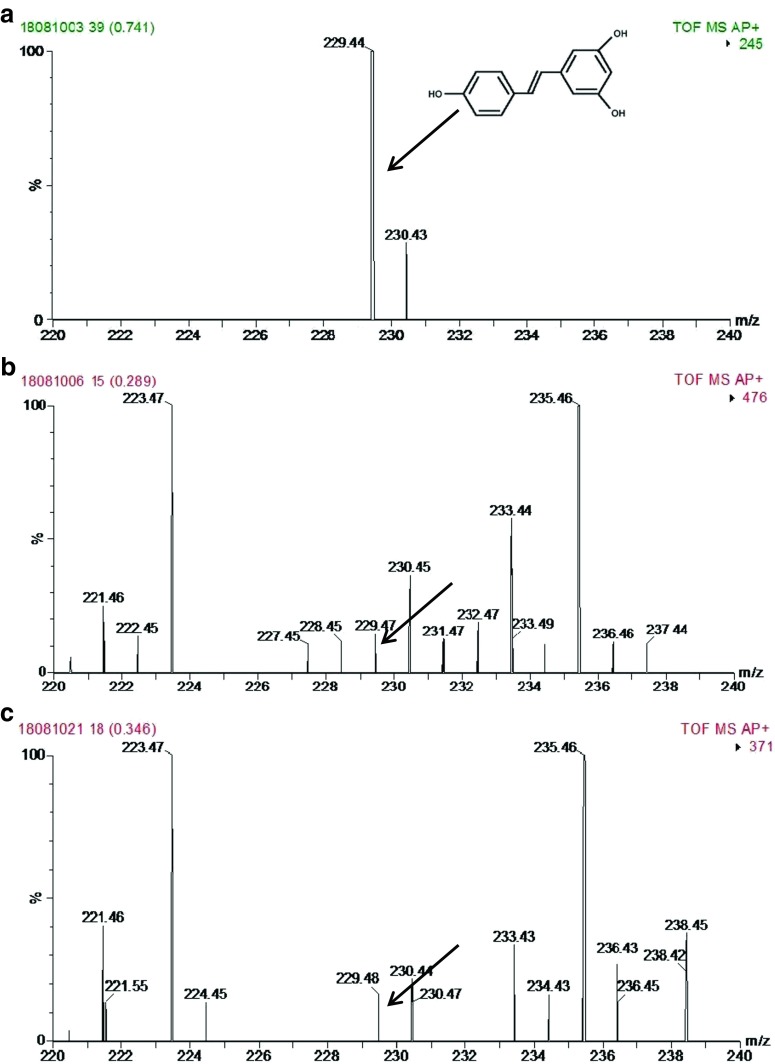
In mulberry, caffeic acid and (+)-catechin were not detected but (−)-epicatechin and p-coumaric acid were present in considerable amounts (99.69 and 34.37 μg g−1 respectively). In a HPLC-DAD analysis caffeoyl quinic acid, kaempferol-3-rutinoside, quercetin and rutin were detected in mulberry fruit extracts (Isabelle et al. 2008). The presence of resveratrol was also confirmed by positive ion atmospheric pressure chemical ionization (APCI) mass spectrometry (Fig. 2).
Fig. 2.
MS spectra of a Standard resveratrol, b Mulberry extract and c Jamun seed extract at positive APCI mode
The presence of cis-resveratrol, trans-resveratrol, trans-resveratrol glucoside and other related stilbenes were reported earlier in grapes (Langcake and Pryce 1976), red wines (Siemann and Creasy 1992), peanuts (Sanders et al. 2000), berries (Rimando et al. 2004), and other plants. However, presence of resveratrol is not reported in underutilized fruits of Indian origin such as jackfruit, jamun fruit and mulberry so far.
Conclusion
In conclusion, underutilized fruits rich in phytochemicals including phenolic compounds exhibit antioxidant activity thereby offer opportunities for developing value-added products, such as functional drinks to enhance health benefits. The present study clearly suggested that Mulberry (Morus rubra), Indian blackberry (Syzygium cuminii) and Jackfruit (Artocarpus heterophyllus) may be considered as sources of natural antioxidants and consumption of these fruits may supply substantial antioxidants which may provide health promoting and disease preventing effects. The present study is the first in the series to establish the quantitative detection of resveratrol in underutilized fruits of Indian origin. Detailed characterization of such phytoalexins and their bioactive properties are in progress. This study suggests that underutilized fruits rich in resveratrol and other antioxidants would help fight against heart related diseases and cancer and hence has tremendous scope for development of functional beverages.
Acknowledgments
The authors Akshatha H.S and Anbarasu K thank Indian Council of Medical Research (ICMR) and Council of Scientific and Industrial Research-Central Food Technological Research Institute (CSIR-CFTRI), Mysore-570020, INDIA for funding the fellowships.
References
- Bae SH, Suh HJ. Antioxidant activities of five different mulberry cultivars in Korea. Lebensm Wiss Technol. 2007;40:955–962. doi: 10.1016/j.lwt.2006.06.007. [DOI] [Google Scholar]
- Bajpai M, Pande A, Tewari SK, Prakash D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr. 2005;56:287–291. doi: 10.1080/09637480500146606. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Dasgupta N, De B. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem. 2005;90:727–733. doi: 10.1016/j.foodchem.2004.04.033. [DOI] [Google Scholar]
- Barros L, Baptista P, Ferreira ICFR. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem Toxicol. 2007;45:1731–1737. doi: 10.1016/j.fct.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Botterweck AAM, Verhagen H, Goldbohm RA, Kleinjans J, Van Den Brandt PA. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: results from analyses in the Netherlands Cohort Study. Food Chem Toxicol. 2000;38(7):599–605. doi: 10.1016/S0278-6915(00)00042-9. [DOI] [PubMed] [Google Scholar]
- Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22:169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Burns J, Yokota T, Ashihara H. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- Duenas M, Hernandez T, Estrella I. Assessment of in vitro antioxidant capacity of the seed coat and the cotyledon of legumes in relation to their phenolic contents. Food Chem. 2006;98:95–103. doi: 10.1016/j.foodchem.2005.05.052. [DOI] [Google Scholar]
- Gutteridge J, Halliwell B. Antioxidants: molecules, medicines and myths. Biochem Biophys Res Commun. 2010;393(4):561–564. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilye DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Isabelle M, Lee BL, Ong CN. Peroxyl radical scavenging capacity, polyphenolics, and lipophilic antioxidant profiles of mulberry fruits cultivated in southern china. Food Chem. 2008;56:9410–9416. doi: 10.1021/jf801527a. [DOI] [PubMed] [Google Scholar]
- Jagtap UB, Panaskar SN, Bapat VA. Evaluation of antioxidant capacity and phenol content in Jackfruit (Artocarpus heterophyllus Lam.) fruit pulp. Plant Foods Hum Nutr. 2010;65:99–104. doi: 10.1007/s11130-010-0155-7. [DOI] [PubMed] [Google Scholar]
- Jayatilake GS, Jayasuriya H, Lee ES. Kinase inhibitors from Polygonum cuspidatum. J Nat Prod. 1993;56:1805–1810. doi: 10.1021/np50100a021. [DOI] [PubMed] [Google Scholar]
- Kalt W, Ryan DAJ, Duy JC. Interspecific variation in anthocyanins, phenolics and antioxidant capacity among genotypes of high bush and low bush blueberries (Vaccinium section Cyanococcus spp.) J Agric Food Chem. 2001;49:4761–4767. doi: 10.1021/jf010653e. [DOI] [PubMed] [Google Scholar]
- Kaur C, Kapoor HC. Antioxidants in fruits and vegetables- the millennium’s health. Int J Food Sci Technol. 2001;36:703–725. doi: 10.1046/j.1365-2621.2001.00513.x. [DOI] [Google Scholar]
- Langcake P, Pryce RJ. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Plant Pathol. 1976;9:77–86. doi: 10.1016/0048-4059(76)90077-1. [DOI] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik GH, Priyadarsini KI, Satav JG. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry. 2003;63:97–104. doi: 10.1016/S0031-9422(02)00754-9. [DOI] [PubMed] [Google Scholar]
- Pezzuto JM. Grapes and human health: a perspective. J Agric Food Chem. 2008;56:6777–6784. doi: 10.1021/jf800898p. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Quiros AR, Lopez-Hernandez J, Ferraces-Casais P. Analysis of non-anthocyanin phenolic compounds in wine samples using high performance liquid chromatography with ultraviolet and fluorescence detection. J Sep Sci. 2007;30:1262–1266. doi: 10.1002/jssc.200600489. [DOI] [PubMed] [Google Scholar]
- Ravindran C, Kohli A, Murthy BNS. Fruit production in India. Chronica Horticuturae. 2007;47:21–26. [Google Scholar]
- Rimando AM, Kalt W, Magee JB. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem. 2004;52:4713–4719. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Saona LE, Wrolstad RE (2001) Unit F2.1, Anthocyanins. Extraction, isolation and purification of anthocyanins. In: King S, Gates M, Scalettar L (eds) Current protocols in food analytical chemistry. John Wiley & Sons, Inc, New York, p 1–11
- Ruxton CHS, Gardner JE, Walker D. Can pure fruit and vegetable juice protect against cancer and cardiovascular disease too? A review of the evidence. Int J Food Sci Nutr. 2006;57:249–272. doi: 10.1080/09637480600858134. [DOI] [PubMed] [Google Scholar]
- Sanders TH, McMichael RW, Hendrix KW. Occurrence of resveratrol in edible peanuts. J Agric Food Chem. 2000;48:1243–1246. doi: 10.1021/jf990737b. [DOI] [PubMed] [Google Scholar]
- Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Enol Viticult. 1992;43:49–52. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Soong YY, Barlow PJ. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88:411–417. doi: 10.1016/j.foodchem.2004.02.003. [DOI] [Google Scholar]
- Soong YY, Barlow PJ. Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chem. 2006;97:524–530. doi: 10.1016/j.foodchem.2005.05.033. [DOI] [Google Scholar]
- Sung J, Lee J. Antioxidant and antiproliferative activities of grape seeds from different cultivars. Food Sci Biotechnol. 2010;19:321–326. doi: 10.1007/s10068-010-0046-6. [DOI] [Google Scholar]
- Thomas-Barberan FA, Espin JC. Phenolic compounds and related enzymes as determinants of quality of fruits and vegetables. J Sci Food Agric. 2001;81:853–876. doi: 10.1002/jsfa.885. [DOI] [Google Scholar]
- Tokusoglu O, Una MK, Yemis F. Determination of the phytoalexin resveratrol (3, 5, 4′ trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatography–mass spectrometry (GC-MS) J Agric Food Chem. 2005;53:5003–5009. doi: 10.1021/jf050496+. [DOI] [PubMed] [Google Scholar]
- Vastano BC, Chen Y, Zhu N. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 2000;48:253–256. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- Vitrac X, Bornet AL, Vanderlinde R (2005) Determination of Stilbenes (α-viniferin, trans-astringin, trans-piceid, cis- and trans-resveratrol, €-viniferin) in Brazilian Wines. J Agric Food Chem 53:5664–5669 [DOI] [PubMed]
- Wang Y, Catana F, Yang Y. An LC-MS method for analysing total resveratrol in grape juice, cranberry juice, and in wine. J Agric Food Chem. 2002;50:431–435. doi: 10.1021/jf010812u. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]