Introduction
There is considerable evidence that stress worsens allergies1-4, asthma5, 6, as well as skin diseases7. Prenatal stress has been associated with increased cord blood imunnoglobulin (IgE), and this correlation was stronger between mothers with a history of atopy and offspring sensitive to dust mites8. Acute stress is also implicated in cardiovascular pathology1, especially in eliciting myocardial ischemia (MI) in patients with coronary artery disease (CAD)9. MI occurring without angina on presentation appears to make up a sizable portion of the MI population10-12. Recent papers have confirmed that psychological and social stressors contribute to CAD13. However, the mechanism of this effect is not well understood.
In a prospective cohort study (Whitehall II) of 7,268 subjects, the perception that stress worsens health was significantly associated with increased CAD risk14. Results from the same study indicated that job insecurity was associated with higher incidence of CAD-associated events15. Moreover, mental-stress-induced ischemia was more common than exercise-induced ischemia in patients with clinically stable CAD16. A cohort study of 4,204 patients with acute MI showed that perceived stress was associated with adverse one year health outcomes17. An independent Meta analysis of 6 studies with 118,696 total subjects reported a significant association between high perceived stress and increased risk of CAD18.
A Meta analysis of 13 European studies (1985-2003) concluded that job strain increased the risk for CAD19. Another prospective study of 8,838 healthy participants, reported that “burnout” was as independent risk factor for future CAD over a 3.4 year period20. A ten year prospective Women's Health study reported a significant correlation between high strain jobs, but not job insecurity, and CAD21. Job strain was also associated with high blood C-reactive protein (CRP)22. CAD-related events were higher in US firefighters during strenuous duties and more so in those subjects with underlying CAD resulting in CAD being the leading cause of death (45%)23.
Here we review the relevant literature and propose that activation of cardiac mast cells (MC) by stress plays a key role in stress-induced CAD, especially since beta-blockers do not prevent the effect of stress. Moreover, MC have also been implicated in obesity 24 and obesity-related asthma25, which are known risk factors for CAD26. ACS clinically manifest themselves as unstable angina or acute MI and are most commonly caused by the rupture of atherosclerotic plaques. However, a key component of CAD is local inflammation 27, 28 not only of the intima, but also of the arterial adventitia that may be more important than simple cholesterol accumulation because the inflammatory plaque is more likely to break off and cause MI29.
Regulation of the stress response
CRH activates the hypothalamic-pituitary-adrenal (HPA) axis typically leading to anti-inflammatory actions through release of adrenal steroids. The effect of CRH is mediated through two main types of G protein-coupled receptors, CRHR-1 and CRHR-2. The CNS and the anterior pituitary express primarily CRHR-1, activation of which leads to release of adrenocorticotropic hormone. In addition to CRH, these receptors are activated by urotensin, sauvagine and the urocortins (Ucn), Ucn-II and Ucn-III, which are stronger agonists for CRHR-230. CRHR-2 has three different spliced forms (α, β and γ) of which CRHR-2α is found mainly in the CNS and on MC31, while CRHR-2β, along with Ucn mRNA, is predominantly expressed in the heart and on cardiomyocytes, with CRHR-2β being predominantly expressed in the left ventricle32.
CRH can also be released outside the central nervous system (CNS) where it has pro-inflammatory actions33. Human skin expresses CRH and CRHR-1 that may act as a “peripheral HPA axis” outside the brain30. In addition to the hypothalamus, CRH is synthesized by skin cells immune cells, and MC34. CRH secreted from MC can decrease the ability of T-regulatory (Treg) cells to produce the immunosuppressant interleukin-10 (IL-10), thus further increasing inflammation35. This has led to the proposal that CRH may be involved in the pathophysiology of skin and other inflammatory diseases36, 37, especially when worsened by stress, through MC involvement33.
CRH is often released together with another brain peptide, neurotensin (NT), which is vasoactive and has also been implicated in inflammation38. NT is increased in the skin following acute stress, stimulates skin MC and increases vascular permeability in rodents, an effect synergistic with CRH39. NT stimulates rodent MC to secrete histamine and elevates histamine plasma levels through activation of NT receptors (NTR) 40.
Acute stress leads to increased skin vascular permeability, mimicked by intradermal injection of CRH, effects absent in MC deficient mice41. CRH also increases microvasculature permeability of human skin in an MC-dependent manner42. CRHR-1 gene is expressed on human cultured MC, activation of which induces production of vascular endothelial growth factor (VEGF)31 . We recently reported that serum CRH was increased in psoriasis (Ps) and atopic dermatitis (AD)43 patients, and so was NT in Ps 43 and AD patients44.
CRH and Ucn secreted under acute stress, have been implicated in the pathophysiology of neuroinflammatory disorders45 and MI9, 46. Ucn mediates stress-induced IL-6 release in vivo, and administration of Ucn causes elevation of plasma IL-6 in rats. Ucn also stimulates IL-6 secretion from human peripheral mononuclear cells in vitro, as well as increase IL-6 mRNA levels through CRHR-2 activation in rat aortic smooth muscle cells47. Moreover, Ucn can stimulate IL-6 release from neonatal cardiomyocytes48. CRHR-2 could have pro-inflammatory actions 45 through a mechanism that involves MC 33. On the other hand, Ucn has been generally considered to be cardioprotective, especially in ischemia-reperfusion (IR) injury49, through upregulation of the p42/p44 MAPK pathway50. Ucn-II and Ucn-III are also cardioprotective against IR injury51, 52. Stimulation of CRHR-2β by Ucn-II and III reduced infarct size51. However, the effects of CRH and related peptides may not always be the same and may depend on the stage of maturation of the target cells and/or activation of specific CRHR isoforms, documented in keratinocytes and MC31. For instance, a soluble CRHR-2α isoform was shown to neutralize the effect of CRH agonists53. Moreover, in macrophages, CRHR-1 and CRHR-2 agonists have an early stimulating effect, but a later inhibitory effect, on TNF-α release54.
Cardiovascular mast cells and CAD
MC are well-known for their role in the pathogenesis of allergic reactions55, but MC are now considered important in innate 56 and acquired immunity57, antigen presentation58, and inflammation59. MC originate from haemopoietic stem cells that differentiate in tissues under the influence of various tissue microenvironmental conditions, including nerve growth factor (NGF) and mainly stem cell factor (SCF)60 . MC are also present in the heart61, and cardiac MC were shown to differ from other connective tissue MC in that they were not stimulated by morphine62. MC are present especially in coronary arteries during spasm, they accumulate in the rupture prone shoulder region of the coronary atheromas63 (Fig. 1), and are associated specifically with plaque erosion and rupture64. Degranulated MC were also identified in the adventitia of vulnerable and ruptured lesions in patients with MI64. MC can be triggered by many molecules relevant to CAD such as oxidized low density lipoprotein (LDL)65, and complement fragment 5a (C5a), which is implicated in ruptured coronary plaques in MI66. Adventitial MC are localized close to nerve endings in atherosclerotic coronary arteries and correlated with the number of nerve fibers67. Nerve fibers immunoreactive for NT are also present in the heart, and NT can trigger coronary vasoconstriction68. Stress-induced cardiac MC degranulation was blocked by preatreatment with a NT-receptor antagonist69. Reactive oxygen species (ROS) can also activate MC70 and release substance P (SP) from sensory nerves. The mitochondrial Uncoupling Protein 2 (UCP2), known to regulate ROS production, was reported to inhibit mast cell activation71. SP treatment significantly enhanced the number and extent of degranulation of adventitial MC compared to controls, and promoted intraplaque hemorrhage; this was prevented by the neurokinin-1 receptor antagonist Spantide I and was absent in MC deficient ApoE−/− mice67, which develop hyperlipidemia and spontaneous atherosclerosis. In addition to stimulating the secretion of histamine and other inflammatory mediators from human MC, SP also induced release of VEGF, an action augmented by IL-3372. MC activation by SP or NT also results in mitochondrial translocation to the cell surface73, and extracellular release of mitochondrial, but not genomic, DNA, that acts as an “innate pathogen” inducing potent autocrine and paracrine inflammatory effects74 .
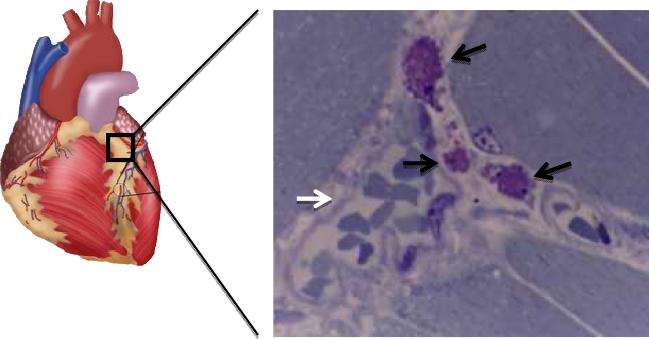
Figure 1.
MC (black arrows) stained with toluidine blue close to a coronary blood vessel (white arrow) containing many erythrocytes from a mouse exposed to restraint stress for 30 min. Magnification= x 400
Mast cell deficient LDLr−/− mice had decreased atheroma size, lipid deposition, as well as T-cell and macrophage numbers as compared to atherosclerosis-prone LDLr−/− mice75. Adoptive transfer of bone marrrow-derived MC precursors from normal wild type mice to LDLr−/− kitw-sh/w-sh mice restored atherogenesis; however when IL-6 and interferon-γ (IFN-γ) deficient MC were reconstituted, the atherogenesis failed to occur75. MC-deficient kitw-sh/w-sh mice had significantly lower serum cholesterol and triglyceride levels with a concomitant decrease in atherogenic apoB-containing particles76.
Cardiac MC could, therefore, participate in the development of atherosclerosis, coronary inflammation and cardiac ischemia (Table 1), in addition to their activation with stress.
Table 1.
Mast cell mediators and their pro-ACS effects
| Mediator | Cardiovascular effect |
|---|---|
| Chymase | Generates angiotensin II, vasoconstriction, MMP-1 activation, endothelial cell apoptosis, formation of foam cells |
| CRH | Autocrine MC and immune cell stimulation |
| Histamine | Coronary artery constriction, stimulation of endothelial cell IL-6 and IL-8 release, P-selectin upregulation, potentiation of the effect of PAF, induction of microvascular permeability and deposition of LDL in the intima |
| IL-6 | Pro-inflammatory, CRP induction, Th17 maturation, leukocyte recruitment |
| IL-8 | Immune cell chemoattraction |
| Leukotrienes | Coronary vasoconstriction |
| MMP-9 | Matrix and vascular integrity degradation |
| PAF | Platelet activation and aggregation, peo-inflammatory |
| Neurotensin | Pro-inflammatory, vasoconstriction |
| Thromboxanes | Platelet aggregation, vasoconstriction |
| Renin | Angiotensin I synthesis, vasoconstriction |
| TNF | Pro-inflammatory, IL-6 upregulation, MMP activation, endothelial cell apoptosis |
| Tryptase | Pro-inflammatory, PAR-2 activation, HDL degradation, endothelial apoptosis, induction of microvascular permeability and deposition of LDL in the intima |
| Ucn | Cardiomyocyte IL-6 release |
Mast cell mediators and CAD
Many MC-derived mediators have profounf effects on the cardiovascular system (Table 1). The pro-inflammatory cytokine IL-6 is thought to contribute to the development of CAD, ACS77 and MI78. Increased serum levels of CRP79 and IL-679, especially intracardiac IL-6 77 are considered independent risk factors for CAD. High plaque levels of CRP and IL-6 were significantly correlated to increased risk of CAD80. The Health ABC study showed that plasma IL-6 levels had a stronger association with CRP than CAD, while the PRIME study showed that only IL-6 remained significantly associated with MI. The incidence of future acute coronary events and mortality of patients with stable CAD or healed MI was also strongly correlated with serum IL-6 levels over a 6 year observation period81. Acute restraint stress increased plasma levels of IL-6 uniquely in a mast cell-dependent manner82. Serum IL-6 was also increased in I/R in mice and the levels correlated with the extent of cardiac tissue necrosis, but were again absent in mast cell deficient mice83. Cardiomyocytes released IL-6 in response to hypoxic stress and to cytokines. Moreover, IL-1 expressed from the secondary inflammatory plaque could stimulate MC to release IL-6 84 selectively without degranulation85. MC-derived IL-1 was shown to drive skin inflammation86; this IL-1 also induced vascular leakage and recruited neutrophils in histamine-dependent urticaria87.
Human coronary artery specimens contain MC that also store and release TNF 88. MC can secrete preformed TNF, while they also release newly synthesized TNF in response to LPS73. In fact, MC are the only immune cells that store preformed TNF in their secretory granules and can secrete it rapidly89. Obviously, endothelial cells and other immune cells participate. MC-derived TNF contributes to the upregulation of IL-6 in infiltrating leukocytes and initiates the cytokine cascade responsible for myocyte intercellular adhesion molecule-1 (ICAM-1) induction and subsequent neutrophil-induced injury. The fact that TNF is degraded quickly supports the importance of the local TNF secretion. Cardiac MC also secrete renin during IR, thus initiating local angiotensin formation90. Moreover, chymase is the main cardiac source of converting enzyme generating angiotensin II, which has potent vasoconstrictor and pro-arrhythmogenic actions90. MC chymase also activates pro-matrix metaloproteinase-1 (MMP-1), and human MC also secrete MMP-9 and can enhance T cell activation91 on contact with activated T-cells and through TNF92. Chymase, tryptase, and cathepsin G can degrade vascular endothelial cadherin, a molecule involved in the survival signaling of endothelial cells93. Even though one study reported that there was no correlation between serum chymase level and CAD94, it is local release of chymase and other mediators that would be important. Tryptase further leads to inflammation through protease activated receptors (PARs), that are also present on MC and can be stimulated by thrombin.95. Persistent serum tryptase elevations were detected in patients with both acute ACS and stable CAD96. Serum tryptase and chymase were higher in nonallergic patients with acute MI and unstable angina than in patients without substantial CAD97. Elevated tryptase was also noted in coronary syndrome and hypersensitivity reactions97.
Histamine levels were increased in the great cardiac vein in patients suffering from attacks of variant angina unrelated to an allergic event 98. Histamine is a coronary vasoconstrictor and blood concentrations were more than twice that of age- and sex-matched controls in patients with ACS in the absence of any allergies99. Histamine blood levels were also significantly higher in patients with unstable angina and acute MI when compared with control normal subjects100. Histamine induces endothelial cell release of IL-6 and IL-8, production of which is enhanced by LPS and TNF-α, which can also contribute to endothelial apoptosis101.
MC-derived leukotrienes exhibit strong pro-inflammatory activities in cardiovascular tissues. Leukotrienes are also powerful vasoconstrictors and their biosynthesis is enhanced in the acute phase of unstable angina102. Expression of the 5-lipoxygenase (5-LO) pathway is increased in arterial walls of patients with various stages of atherosclerosis, and MC in atherosclerotic plaques express 5-LO103. Deficiency of one 5-LO allele potent protection against atherosclerosis development of LDLr −/− mice, and leukotriene B4 receptor antagonism was also protective in several atherosclerosis susceptible mouse strains103.
Platelet Activating Factor (PAF) is another molecule generated from arachidonic acid, much like the leukotrienes, but from the conversion of ether-linked phospholipids104. PAF has been implicated in allergic inflammation, especially asthma105, 106 and anaphylaxis107. PAF can be released from mast cells108 and also stimulates mast cells109. PAF has been implicated in the pathogenesis of CAD110-112 In particular, elevated PAF acetylhydrolase levels have been reported in ACS113-118. Mast cell activation syndrome (MCAS), which presents with signs and symptoms of mastocytosis without elevated serum or urine markers119, has been associated with cerebral vasospasm- a Kounis- like syndrome120.
Coronary hypersensitivity syndromes
There is evidence pointing to a possible association between allergy and the cardiovascular system121, 122, as well as between asthma and CAD123. Moreover, air pollution was found to be associated with increased incidence of deaths from CAD124. Patients with elevated serum tryptase are diagnosed with mastocytosis, a rare disease characterized by high number of hyperresponsive MC and cardiovascular problems119, 125, 126.
ACS, coronary spasm, acute MI and stent thrombosis in the setting of allergic or anaphylactic reactions has been termed Kounis syndrome 127-129. This syndrome is increasingly recognized in different clinical settings and has been associated with gelofusin130, Latex exposure131 ceftriaxone132 eosinophilic periarteritis 133 and coronary stents132, 134-136. Whether MC are activated upong contact with metal or drug-coated stents remaines to be investigated.
Cardiovascular symptoms are also present in many patients with ME/CFS137, 138, characterized by debilitating fatigue for over 3 months, as well as neurohormonal and sleep disturbances. Such patients show high heart rate and peripheral resistance on 20 degree “tilt-table” test as compared to controls139. ME/CFS symptoms worsen with stress and may be associated with brain MC activation140.
Given the above, it is apparent that inhibiting MC activation would be beneficial in coronary hypersensitivity syndromes, but also in CAD even though coronary MC may be one of the many cell types involved. The ability of MC to secrete a number of mediators selectively85, allows MC to participate in different types of reactions59, as well as serve as immunomodulatory cells141-144. Clearly, such actions need not be addressed in the acute setting
Clinical Implications
Treatment of the allergic event with intravenous hydrocortisone and histamine-receptor-1,2 antagonists usually also reduces cardiovascular symptoms. Subcutaneous allergen-specific immunotherapy used for treatment of IgE-mediated allergic diseases was associated with lower risk of acute MI and autoimmune disease145. Endothelin-1 (ET-1) is increased in patients with atherosclerosis and coronary endothelial dysfunction. Administration of ET-1 to blood-perfused, isolated rat hearts resulted in extensive MC degranulation and increased MMP-2 activity146. Long term administration of ET-1 receptor antagonists improves coronary endothelial function in patients with early atherosclerosis147.
For those patients with documented CAD, statins have been helpful in reducing atherosclerosis148. Statins have also been shown to have anti-inflammatory effects27, 109, 148, 149. Niacin reduces total cholesterol and LDL, while increasing HDL150, and also prevents release of inflammatory mediators from adipocytes150. However, compliance with niacin is severely limited by “flush”, characterized by erythema, itching and a sense of warmth and discomorft, that occurs even in slow or extended release forms151. Nevertheless, use of statins and niacin to address underlying atherosclerosis is likely to also reduce the risk of coronary hypersensitivity, especially due to stress.
Unfortunately, there is no effective human MC inhibitor clinically available. Disodium cromoglycate (cromolyn) inhibits histamine secretion from rat peritoneal152, but not intestinal 153, 154 MC. Cromolyn was reported to improve only gastrointestinal symptoms in patients with mastocytosis155, even though it could not inhibit human gastrointestinal or lung mucosal MC156. More recently, cromolyn was reported to not even inhibit mouse MC157, 158. Cromolyn is a weak inhibitor of contact dermatitis and phtosensitivity in humans157, 158. In fact, it was recently shown that a cromolyn cream was able to reduce itching in patients with mastocytosis, but apparently through an action on sensory nerve endings, rather than on skin MC159.
Some H1-receptor antagonists have MC blocking actions and could be used prophylactically. Rupatadine is a histamine-1 (H1) -receptor antagonist, which also inhibits the actions of PAF160,161 and is particularly useful in allergic rhinitis and urticaria161, 162. Rupatadine can inhibit mediator release from human MC163 and can also block the ability of PAF to stimulate human MC through an action unrelated to its H1-receptor blocking properties164.
IL-10 is produced mostly by Th2 cells, macrophages and CD8+ cell clones. It can inhibit the synthesis and release of several pro-inflammatory cytokines in antigen or mitogen-activated rodent MC165. IL -10 also inhibits IL-6 166 and TNF167, but not preformed mediator release from rat peritoneal MC166. Moreover, IL-10 gene transfer apparently protects against acute myocarditis in rats168, and downregulates the expression of the IgE receptor in mouse MC169. However, the effect of IL-10 on human MC mast cells is not clear because IL-10 does not inhibit tryptase and IL-6 from human leukemic mast cells170.
The naturally occurring flavonoids, luteolin and quercetin, have potent anti-oxidant and anti-inflammatory actions 171-173, and are generally considered safe174, 175. Flavonols have been proposed as possible therapeutic agents for CAD 176-178. Meta analysis of epidemiological studies shows an inverse relationship between flavonol/flavone intake and CAD179. A review of twenty publications from twelve prospective cohorts in European and US populations reported that consumption of flavonoids and flavones were most strongly associated with lower CAD mortality180. A double-blind, placebo-controlled, randomized clinical study using the polyhenolic compound Pycnogenol showed improved endothelial function in patients with CAD109. A pilot study of 2 week consumption of a polyphenolic drink lowered urinary biomarkers of CAD181.
The flavonol quercetin was shown to inhibit rat mucosal mast cells, when quercetin was ieffective156, 182. Quercetin also inhibits human mast cell release of pro-inflammatory cytokines176, including IL-684. The flavone luteolin also inhibits human MC183, suppresses adipocyte activation of macrophages, inhibits inflammation 184, 185, increases insulin sensitivity of the endothelium184, and inhibits MC-dependent T cell stimulation91. Moreover, luteolin prevented niacin-induced flush 186, 187.
Stress reduction through transcedental medication in a randomized control trial significantly reduced risk of mortality MI and stroke in patients with CAD188. The Responses of Mental Stress-Induced Myocardial Ischemia to Escitalopram Ttreatment (REMIT) trial concluded that administration of escitalopram (5 mg/day titrated up to 20 mg/day) for 6 weeks resulted in lower rate of mental stress-induced, but not exercise-induced, MI compared to controls189.
Concluding remarks
Increasing evidence indicates that stress worsens or precipitates CAD through stimulation of coronary MC leading to local inflammation. This effect may be more pronounced in patients with atherosclerosis or during acute MC activation by allergic or non-allergic triggers. Combining anti-inflammatory and MC inhibitory agents, along with reduction of atheroscleorsis and stress may be novel treatment approaches. Certain natural flavonoids may be particularly useful in this respect and should be tested in appropriate clinical trials.
Figure 2.
Diagrammatic representation of the possible triggers of cardiovascular MC and their key mediators with CAD-relevant actions and major pathological sequellae.
Acknowledgements
Aspects of our work discussed here were supported in part by the US National Institutes of Health (NIH) grants to TCT: AR47652; NS66205; NS71361 and NS071361.
Abbreviations
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- CPR
C-reactive protein
- CRH
corticotropin-releasing hormone
- ET-1
endothelin-1
- HPA
hypothalamic-pituitary adrenal
- IgE
immunoglobulin E
- IL-6
interleukin-6
- IR
ischemia-reperfusion
- MC
mast cells
- ME/CFS
myalgic encephalopathy/chronic fatigue syndrome
- MI
myocardial ischemia
- NGF
nerve growth factor
- NT
neurotensin
- NF-κB
nuclear factor-kappa B
- PAF
platelet activating factor
- ROS
reactive oxygen species
- SP
substance P
- TNF
tumor necrosis factor
- UCP-2
uncoupling protein-2
- Ucn
urocortin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146(1-2):1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5(1):23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Slattery MJ. Psychiatric comorbidity associated with atopic disorders in children and adolescents. Immunol Allergy Clin North Am. 2005;25(2):407–20. viii. doi: 10.1016/j.iac.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. 2008;70(1):102–116. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- 5.Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21(8):993–999. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theoharides TC, Enakuua S, Sismanopoulos N, Papadimas E, Angelidou A, Alysandratos K. Stress contributes to asthma worsening through mast cell activation. Annals of Allergy, Asthma and Immunology. 2012;109(1):14–19. doi: 10.1016/j.anai.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Seiffert K, Hilbert E, Schaechinger H, Zouboulis CC, Deter HC. Psychophysiological reactivity under mental stress in atopic dermatitis. Dermatology. 2005;210(4):286–293. doi: 10.1159/000084752. [DOI] [PubMed] [Google Scholar]
- 8.Peters JL, Cohen S, Staudenmayer J, Hosen J, Platts-Mills TA, Wright RJ. Prenatal negative life events increases cord blood IgE: interactions with dust mite allergen and maternal atopy. Allergy. 2012;67(4):545–551. doi: 10.1111/j.1398-9995.2012.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang W, Babyak M, Krantz DS, et al. Mental stress-induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 10.Deanfield JE, Shea M, Kensett M, et al. Silent myocardial ischaemia due to mental stress. Lancet. 1984;2:1001–1005. doi: 10.1016/s0140-6736(84)91106-1. [DOI] [PubMed] [Google Scholar]
- 11.Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 12.Deedwania PC. Mental stress, pain perception and risk of silent ischemia. J Am Coll Cardiol. 1995;25:1504–1506. doi: 10.1016/0735-1097(95)00190-f. [DOI] [PubMed] [Google Scholar]
- 13.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9(6):360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 14.Nabi H, Kivimaki M, Batty GD, et al. Increased risk of coronary heart disease among individuals reporting adverse impact of stress on their health: the Whitehall II prospective cohort study. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrie JE, Kivimaki M, Shipley MJ, Davey SG, Virtanen M. Job insecurity and incident coronary heart disease: the Whitehall II prospective cohort study. Atherosclerosis. 2013;227(1):178–181. doi: 10.1016/j.atherosclerosis.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W, Samad Z, Boyle S, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Coll Cardiol. 2013;61(7):714–722. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: long-term mortality and health status outcomes. J Am Coll Cardiol. 2012;60(18):1756–1763. doi: 10.1016/j.jacc.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, Edmondson D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol. 2012;110(12):1711–1716. doi: 10.1016/j.amjcard.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kivimaki M, Nyberg ST, Batty GD, et al. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380(9852):1491–1497. doi: 10.1016/S0140-6736(12)60994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toker S, Melamed S, Berliner S, Zeltser D, Shapira I. Burnout and risk of coronary heart disease: a prospective study of 8838 employees. Psychosom Med. 2012;74(8):840–847. doi: 10.1097/PSY.0b013e31826c3174. [DOI] [PubMed] [Google Scholar]
- 21.Slopen N, Glynn RJ, Buring JE, Lewis TT, Williams DR, Albert MA. Job strain, job insecurity, and incident cardiovascular disease in the Women's Health Study: results from a 10-year prospective study. PloS One. 2012;7(7):e40512. doi: 10.1371/journal.pone.0040512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emeny R, Lacruz ME, Baumert J, et al. Job strain associated CRP is mediated by leisure time physical activity: results from the MONICA/KORA study. Brain Behav Immun. 2012;26(7):1077–1084. doi: 10.1016/j.bbi.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Soteriades ES, Smith DL, Tsismenakis AJ, Baur DM, Kales SN. Cardiovascular disease in US firefighters: a systematic review. Cardiol Rev. 2011;19(4):202–215. doi: 10.1097/CRD.0b013e318215c105. [DOI] [PubMed] [Google Scholar]
- 24.Theoharides TC, Sismanopoulos N, Delivanis DA, Zhang B, Hatziagelaki EE, Kalogeromitros D. Mast cells squeeze the heart and stretch the gird: Their role in atherosclerosis and obesity. Trends Pharmacol Sci. 2011;32(9):534–542. doi: 10.1016/j.tips.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Sismanopoulos N, Delivanis DA, Mavrommati D, Hatziagelaki E, Conti P, Theoharides TC. Do mast cells link obesity and asthma? Allergy. 2013;68(1):8–15. doi: 10.1111/all.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chrostowska M, Szyndler A, Hoffmann M, Narkiewicz K. Impact of obesity on cardiovascular health. Best Pract Res Clin Endocrinol Metab. 2013;27(2):147–156. doi: 10.1016/j.beem.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Matusik P, Guzik B, Weber C, Guzik TJ. Do we know enough about the immune pathogenesis of acute coronary syndromes to improve clinical practice? Thromb Haemost. 2012;108(3):443–456. doi: 10.1160/TH12-05-0341. [DOI] [PubMed] [Google Scholar]
- 28.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 29.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 30.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013 doi: 10.1210/er.2012-1092. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174(12):7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 32.Markel H. “Who's on first?” - medical discoveries and scientific priority. N Engl J Med. 2004;351:2792–2794. doi: 10.1056/NEJMp048166. [DOI] [PubMed] [Google Scholar]
- 33.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25(11):563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Kempuraj D, Papadopoulou NG, Lytinas M, et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 35.Oh SH, Park CO, Wu WH, et al. Corticotropin-releasing hormone downregulates IL-10 production by adaptive forkhead box protein 3-negative regulatory T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129(1):151–159. doi: 10.1016/j.jaci.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 36.O'Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp Dermatol. 2006;15(3):143–153. doi: 10.1111/j.1600-0625.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 37.Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol. 2009;160(2):229–232. doi: 10.1111/j.1365-2133.2008.08958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mustain WC, Rychahou PG, Evers BM. The role of neurotensin in physiologic and pathologic processes. Curr Opin Endocrinol Diabetes Obes. 2011;18(1):75–82. doi: 10.1097/MED.0b013e3283419052. [DOI] [PubMed] [Google Scholar]
- 39.Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Theoharides TC. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA. 2006;103:7759–7764. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldberg RS, Cochrane DE, Carraway RE, et al. Evidence for a neurotensin receptor in rat serosal mast cells. Inflamm Res. 1998;47:245–250. doi: 10.1007/s000110050325. [DOI] [PubMed] [Google Scholar]
- 41.Theoharides TC, Singh LK, Boucher W, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its pro-inflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 42.Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J Clin Endocrinol Metab. 2003;88:5427–5432. doi: 10.1210/jc.2003-030377. [DOI] [PubMed] [Google Scholar]
- 43.Vasiadi M, Therianou A, Alysandratos KD, et al. Serum neurotensin (NT) is increased in psoriasis and NT induces VEGF release from human mast cells. Br J Dermatol. 2012;166(6):1349–1352. doi: 10.1111/j.1365-2133.2012.10843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasiadi M, Mondolfi A, Alysandratos K-D, et al. Neurotensin serum levels and skin gene expression are increased in atopic dermatitis. Br J Dermatol. 2013;166(6):1349–1352. doi: 10.1111/bjd.12413. [DOI] [PubMed] [Google Scholar]
- 45.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 46.Krantz DS, Sheps DS, Carney RM, Natelson BH. Effects of mental stress in patients with coronary artery disease: evidence and clinical implications. JAMA. 2000;283:1800–1802. doi: 10.1001/jama.283.14.1800. [DOI] [PubMed] [Google Scholar]
- 47.Kageyama K, Suda T. Urocortin-related peptides increase interleukin-6 output via cyclic adenosine 5′-monophosphate-dependent pathways in A7r5 aortic smooth muscle cells. Endocrinology. 2003;144:2234–2241. doi: 10.1210/en.2002-0023. [DOI] [PubMed] [Google Scholar]
- 48.Huang M, Kempuraj D, Papadopoulou N, et al. Urocortin induces interleukin-6 release from rat cardiomyocytes through p38 MAP kinase, ERK and NF-kappaB activation. J Mol Endocrinol. 2009;42(5):397–405. doi: 10.1677/JME-08-0120. [DOI] [PubMed] [Google Scholar]
- 49.Latchman DS. Urocortin protects against ischemic injury via a MAPK-dependent pathway. Trends Cardiovasc Med. 2003;11(167):169. doi: 10.1016/s1050-1738(01)00093-7. [DOI] [PubMed] [Google Scholar]
- 50.Schulman D, Latchman DS, Yellon DM. Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am J Physiol Heart Circ Physiol. 2003;283:H1481–H1488. doi: 10.1152/ajpheart.01089.2001. [DOI] [PubMed] [Google Scholar]
- 51.Brar BK, Jonassen AK, Egorina EM, et al. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145:24–35. doi: 10.1210/en.2003-0689. [DOI] [PubMed] [Google Scholar]
- 52.Woodcock EA. Ucn-II and Ucn-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for CRFR2 in the murine heart. Endocrinology. 2004;145:21–23. doi: 10.1210/en.2003-0689. [DOI] [PubMed] [Google Scholar]
- 53.Chen AM, Perrin MH, Digruccio MR, et al. A soluble mouse brain splice variant of type 2alpha corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci U S A. 2005;102(7):2620–2625. doi: 10.1073/pnas.0409583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsatsanis C, Androulidaki A, Dermitzaki E, Gravanis A, Margioris AN. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-alpha release from macrophages via induction of COX-2 and PGE2. J Cell Physiol. 2007;210(3):774–783. doi: 10.1002/jcp.20900. [DOI] [PubMed] [Google Scholar]
- 55.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39(1):11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 57.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong J, Yang NS, Croft M, et al. The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcepsilonRI and MHC II. BMC Immunol. 2010;11:34. doi: 10.1186/1471-2172-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochim Biophys Acta. 2010;1822(1):21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gurish MF, Boyce JA. Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell. J Allergy Clin Immunol. 2006;117(6):1285–1291. doi: 10.1016/j.jaci.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Dvorak AM. Mast-cell degranulation in human hearts. N Engl J Med. 1986;315(15):969–970. doi: 10.1056/nejm198610093151515. [DOI] [PubMed] [Google Scholar]
- 62.Patella V, de Crescenzo G, Ciccarelli A, Marino I, Adt M, Marone G. Human heart mast cells: a definitive case of mast cell heterogeneity. Int Arch Allergy Immunol. 1995;106:386–393. doi: 10.1159/000236871. [DOI] [PubMed] [Google Scholar]
- 63.Constantinides P. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92:1083–1088. doi: 10.1161/01.cir.92.5.1083. [DOI] [PubMed] [Google Scholar]
- 64.Laine P, Kaartinen M, Penttilä A, Panula P, Paavonen T, Kovanen PT. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation. 1999;99:361–369. doi: 10.1161/01.cir.99.3.361. [DOI] [PubMed] [Google Scholar]
- 65.Kelley J, Hemontolor G, Younis W, Li C, Krishnaswamy G, Chi DS. Mast cell activation by lipoproteins. Methods Mol Biol. 2006;315:341–348. doi: 10.1385/1-59259-967-2:341. [DOI] [PubMed] [Google Scholar]
- 66.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 67.Bot I, de Jager SC, Bot M, et al. The neuropeptide substance P mediates adventitial mast cell activation and induces intraplaque hemorrhage in advanced atherosclerosis. Circ Res. 2010;106(1):89–92. doi: 10.1161/CIRCRESAHA.109.204875. [DOI] [PubMed] [Google Scholar]
- 68.Quirion R, Rioux F, Regoli D, St.-Pierre S. Selective blockade of neurotensin-induced coronary vessel constriction in perfused rat hearts by a neurotensin analogue. Eur J Pharmacol. 1980;61:309–312. doi: 10.1016/0014-2999(80)90133-8. [DOI] [PubMed] [Google Scholar]
- 69.Pang X, Alexacos N, Letourneau R, et al. A neurotensin receptor antagonist inhibits acute immobilization stress-induced cardiac mast cell degranulation, a corticotropin-releasing hormone-dependent process. J Pharm & Exp Therap. 1998;287:307–314. [PubMed] [Google Scholar]
- 70.Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev. 2007;217:186–205. doi: 10.1111/j.1600-065X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 71.Tagen M, Elorza A, Boucher W, Kepley CL, Shirihai O, Theoharides TC. Mitochondrial uncoupling protein 2 (UCP2) inhibits mast cell activation and reduces histamine content. J Immunol. 2009;183(19):6313–6319. doi: 10.4049/jimmunol.0803422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theoharides TC, Zhang B, Kempuraj D, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107(9):4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang B, Alysandratos KD, Angelidou A, et al. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: Relevance to atopic dermatitis. J Allergy Clin Immunol. 2011;127(6):1522–1531. doi: 10.1016/j.jaci.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang B, Asadi S, Weng Z, Sismanopoulos N, Theoharides TC. Stimulated human mast cells secrete mitochondrial components that have autocrine and paracrine inflammatory actions. PloS One. 2012;7(12):e49767. doi: 10.1371/journal.pone.0049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13(6):719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 76.Heikkila HM, Trosien J, Metso J, et al. Mast cells promote atherosclerosis by inducing both an atherogenic lipid profile and vascular inflammation. J Cell Biochem. 2010;109(3):615–623. doi: 10.1002/jcb.22443. [DOI] [PubMed] [Google Scholar]
- 77.Deliargyris EN, Raymond RJ, Theoharides TC, Boucher WS, Tate DA, Dehmer GJ. Sites of interleukin-6 release in patients with acute coronary syndromes and in patients with congestive heart failure. Am J Cardiol. 2000;86:913–918. doi: 10.1016/s0002-9149(00)01121-8. [DOI] [PubMed] [Google Scholar]
- 78.Miyao Y, Yasue H, Ogawa H, et al. Elevated plasma interleukin-6 levels in patients with acute myocardial infarction. Am Heart J. 1993;126(6):1299–1304. doi: 10.1016/0002-8703(93)90526-f. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki M, Inaba S, Nagai T, Tatsuno H, Kazatani Y. Relation of C-reactive protein and interleukin-6 to culprit coronary artery plaque size in patients with acute myocardial infarction. Am J Cardiol. 2003;91(3):331–333. doi: 10.1016/s0002-9149(02)03162-4. [DOI] [PubMed] [Google Scholar]
- 80.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 81.Fisman EZ, Benderly M, Esper RJ, et al. Interleukin-6 and the risk of future cardiovascular events in patients with angina pectoris and/or healed myocardial infarction. Am J Cardiol. 2006;98(1):14–18. doi: 10.1016/j.amjcard.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 82.Huang M, Pang X, Karalis K, Theoharides TC. Stress-induced interleukin-6 release in mice is mast cell-dependent and more pronounced in Apolipoprotein E knockout mice. Cardiovasc Res. 2003;59(1):241–249. doi: 10.1016/s0008-6363(03)00340-7. [DOI] [PubMed] [Google Scholar]
- 83.Bhattacharya K, Farwell K, Huang M, et al. Mast cell deficient W/Wv mice have lower serum IL-6 and less cardiac tissue necrosis than their normal littermates following myocardial ischemia-reperfusion. Int J Immunopath Pharmacol. 2007;20(1):69–74. doi: 10.1177/039463200702000108. [DOI] [PubMed] [Google Scholar]
- 84.Kandere-Grzybowska K, Kempuraj D, Cao J, Cetrulo CL, Theoharides TC. Regulation of IL-1-induced selective IL-6 release from human mast cells and inhibition by quercetin. Br J Pharmacol. 2006;148:208–215. doi: 10.1038/sj.bjp.0706695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 86.Nakamura Y, Franchi L, Kambe N, Meng G, Strober W, Nunez G. Critical role for mast cells in interleukin-1beta-driven skin inflammation associated with an activating mutation in the nlrp3 protein. Immunity. 2012;37(1):85–95. doi: 10.1016/j.immuni.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamura Y, Kambe N, Saito M, et al. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J Exp Med. 2009;206(5):1037–1046. doi: 10.1084/jem.20082179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaartinen M, Penttilä A, Kovanen PT. Mast cells in rupture-prone areas of human coronary atheromas produce and store TNF-α. Circulation. 1996;94:2787–2792. doi: 10.1161/01.cir.94.11.2787. [DOI] [PubMed] [Google Scholar]
- 89.Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991;174(1):103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mackins CJ, Kano S, Seyedi N, et al. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia-reperfusion. J Clin Invest. 2006;116(4):1063–1070. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakae S, Suto H, Iikura M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176(4):2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 92.Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA. Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNF-alpha. J Immunol. 2001;167(7):4008–4016. doi: 10.4049/jimmunol.167.7.4008. [DOI] [PubMed] [Google Scholar]
- 93.Mayranpaa MI, Heikkila HM, Lindstedt KA, Walls AF, Kovanen PT. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coron Artery Dis. 2006;17(7):611–621. doi: 10.1097/01.mca.0000224420.67304.4d. [DOI] [PubMed] [Google Scholar]
- 94.Jin CN, Ma H, Lin Y, Wang JA, Xiang MX. Association between SNP rs1800875, serum chymase and immunoglobulin E levels in patients with coronary heart disease. J Zhejiang Univ Sci B. 2011;12(8):660–667. doi: 10.1631/jzus.B1101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Molino M, Barnathan ES, Numerof R, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 96.Deliargyris EN, Upadhya B, Sane DC, et al. Mast cell tryptase: a new biomarker in patients with stable coronary artery disease. Atherosclerosis. 2005;178:381–386. doi: 10.1016/j.atherosclerosis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Kounis NG, Tsigkas G, Almpanis G, Kounis GN, Mazarakis A, Hahalis G. Tryptase levels in coronary syndromes and in hypersensitivity episodes: a common pathway towards Kounis syndrome. Atherosclerosis. 2011;219(1):28–29. doi: 10.1016/j.atherosclerosis.2011.07.101. [DOI] [PubMed] [Google Scholar]
- 98.Sakata Y, Komamura K, Hirayama A, et al. Elevation of the plasma histamine concentration in the coronary circulation in patients with variant angina. Am J Cardiol. 1996;77(12):1121–1126. doi: 10.1016/s0002-9149(96)00147-6. [DOI] [PubMed] [Google Scholar]
- 99.Clejan S, Japa S, Clemetson C, Hasabnis SS, David O, Talano JV. Blood histamine is associated with coronary artery disease, cardiac events and severity of inflammation and atherosclerosis. J Cell Mol Med. 2002;6:583–592. doi: 10.1111/j.1582-4934.2002.tb00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zdravkovic V, Pantovic S, Rosic G, et al. Histamine blood concentration in ischemic heart disease patients. J Biomed Biotechnol. 2011;2011:315709. doi: 10.1155/2011/315709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y, Chi L, Stechschulte DJ, Dileepan KN. Histamine-induced production of interleukin-6 and interleukin-8 by human coronary artery endothelial cells is enhanced by endotoxin and tumor necrosis factor-alpha. Microvas Res. 2001;61:253–262. doi: 10.1006/mvre.2001.2304. [DOI] [PubMed] [Google Scholar]
- 102.Riccioni G, Zanasi A, Vitulano N, Mancini B, D'Orazio N. Leukotrienes in atherosclerosis: new target insights and future therapy perspectives. Mediators Inflamm. 2009;2009:737282. doi: 10.1155/2009/737282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spanbroek R, Grabner R, Lotzer K, et al. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci U S A. 2003;100(3):1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Demopoulos CA, Pinckard RN, Hanahan DJ. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979;254(19):9355–9358. [PubMed] [Google Scholar]
- 105.Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet-activating factor (PAF): a review of its role in asthma and clinical efficacy of PAF antagonists in the disease therapy. Recent Pat Inflamm Allergy Drug Discov. 2008;2(1):72–76. doi: 10.2174/187221308783399306. [DOI] [PubMed] [Google Scholar]
- 106.Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet activating factor as a mediator and therapeutic approach in bronchial asthma. Inflammation. 2008;31(2):112–120. doi: 10.1007/s10753-007-9056-9. [DOI] [PubMed] [Google Scholar]
- 107.Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358(1):28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 108.Rumore MM, Kim KS. Potential role of salicylates in type 2 diabetes. Ann Pharmacother. 2010;44(7-8):1207–1221. doi: 10.1345/aph.1M483. [DOI] [PubMed] [Google Scholar]
- 109.Enseleit F, Sudano I, Periat D, et al. Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: a double-blind, randomized, placebo-controlled, cross-over study. Eur Heart J. 2012;33(13):1589–1597. doi: 10.1093/eurheartj/ehr482. [DOI] [PubMed] [Google Scholar]
- 110.Caslake MJ, Packard CJ. Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr Opin Lipidol. 2003;14(4):347–352. doi: 10.1097/00041433-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 111.Sutton BS, Crosslin DR, Shah SH, et al. Comprehensive genetic analysis of the platelet activating factor acetylhydrolase (PLA2G7) gene and cardiovascular disease in case-control and family datasets. Hum Mol Genet. 2008;17(9):1318–1328. doi: 10.1093/hmg/ddn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xue M, Chen KJ, Yin HJ. Relationship between platelet activation related factors and polymorphism of related genes in patients with coronary heart disease of blood-stasis syndrome. Chin J Integr Med. 2008;14(4):267–273. doi: 10.1007/s11655-008-0267-1. [DOI] [PubMed] [Google Scholar]
- 113.Blankenberg S, Stengel D, Rupprecht HJ, et al. Plasma PAF-acetylhydrolase in patients with coronary artery disease: results of a cross-sectional analysis. J Lipid Res. 2003;44(7):1381–1386. doi: 10.1194/jlr.M300086-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Winkler K, Winkelmann BR, Scharnagl H, et al. Platelet-activating factor acetylhydrolase activity indicates angiographic coronary artery disease independently of systemic inflammation and other risk factors: the Ludwigshafen Risk and Cardiovascular Health Study. Circulation. 2005;111(8):980–987. doi: 10.1161/01.CIR.0000156457.35971.C8. [DOI] [PubMed] [Google Scholar]
- 115.Ninio E, Letter regarding article by Winkler et al. Platelet-activating factor acetylhydrolase activity indicates angiographic coronary artery disease independently of systemic inflammation and other risk factors: the Ludwigshafen Risk and Cardiovascular Health Study. Circulation. 2005;112(8):e108–e109. doi: 10.1161/CIRCULATIONAHA.105.547257. [DOI] [PubMed] [Google Scholar]
- 116.Samsamshariat S, Basati G, Movahedian A, Pourfarzam M, Sarrafzadegan N. Elevated plasma platelet-activating factor acetylhydrolase activity and its relationship to the presence of coronary artery disease. J Res Med Sci. 2011;16(5):674–679. [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng GH, Xiong SQ, Mei LJ, Chen HY, Wang T, Chu JF. Elevated plasma platelet activating factor, platelet activating factor acetylhydrolase levels and risk of coronary heart disease or blood stasis syndrome of coronary heart disease in Chinese: a case control study: a case-control study. Inflammation. 2012;35(4):1419–1428. doi: 10.1007/s10753-012-9455-4. [DOI] [PubMed] [Google Scholar]
- 118.Mazereeuw G, Herrmann N, Bennett SA, et al. Platelet activating factors in depression and coronary artery disease: A potential biomarker related to inflammatory mechanisms and neurodegeneration. Neurosci Biobehav Rev. 2013;37(8):1611–1621. doi: 10.1016/j.neubiorev.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 119.Picard M, Giavina-Bianchi P, Mezzano V, Castells M. Expanding spectrum of mast cell activation disorders: monoclonal and idiopathic mast cell activation syndromes. Clin Ther. 2013;35(5):548–562. doi: 10.1016/j.clinthera.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 120.Gonzalez-de-Olano D, varez-Twose I, Matito A, Sanchez-Munoz L, Kounis NG, Escribano L. Mast cell activation disorders presenting with cerebral vasospasm-related symptoms: a “Kounis-like” syndrome? Int J Cardiol. 2011;150(2):210–211. doi: 10.1016/j.ijcard.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 121.Ozben B, Erdogan O. The role of inflammation and allergy in acute coronary syndromes. Inflamm Allergy Drug Targets. 2008;7(3):136–144. doi: 10.2174/187152808785748128. [DOI] [PubMed] [Google Scholar]
- 122.Triggiani M, Patella V, Staiano RI, Granata F, Marone G. Allergy and the cardiovascular system. Clin Exp Immunol. 2008;153(Suppl 1):7–11. doi: 10.1111/j.1365-2249.2008.03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iribarren C, Tolstykh IV, Miller MK, Sobel E, Eisner MD. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol. 2012;176(11):1014–1024. doi: 10.1093/aje/kws181. [DOI] [PubMed] [Google Scholar]
- 124.Brunekreef B, Hoek G, Fischer P, Spieksma FT. Relation between airborne pollen concentrations and daily cardiovascular and respiratory-disease mortality. Lancet. 2000;355(9214):1517–1518. doi: 10.1016/S0140-6736(00)02168-1. [DOI] [PubMed] [Google Scholar]
- 125.Bridgman DE, Clarke R, Sadleir PH, Stedmon JJ, Platt P. Systemic mastocytosis presenting as intraoperative anaphylaxis with atypical features: a report of two cases. Anaesth Intensive Care. 2013;41(1):116–121. doi: 10.1177/0310057X1304100120. [DOI] [PubMed] [Google Scholar]
- 126.Mueller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7(4):337–341. doi: 10.1097/ACI.0b013e328259c328. [DOI] [PubMed] [Google Scholar]
- 127.Biteker M. Current understanding of Kounis syndrome. Expert Rev Clin Immunol. 2010;6(5):777–788. doi: 10.1586/eci.10.47. [DOI] [PubMed] [Google Scholar]
- 128.Kounis NG, Mazarakis A, Tsigkas G, Giannopoulos S, Goudevenos J. Kounis syndrome: a new twist on an old disease. Future Cardiol. 2011;7(6):805–824. doi: 10.2217/fca.11.63. [DOI] [PubMed] [Google Scholar]
- 129.Kounis NG. Coronary hypersensitivity disorder: the kounis syndrome. Clin Ther. 2013;35(5):563–571. doi: 10.1016/j.clinthera.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 130.Shah G, Scadding G, Nguyen-Lu N, et al. Peri-operative cardiac arrest with ST elevation secondary to gelofusin anaphylaxis - Kounis syndrome in the anaesthetic room. Int J Cardiol. 2013;164(3):e22–e26. doi: 10.1016/j.ijcard.2012.09.166. [DOI] [PubMed] [Google Scholar]
- 131.Marcoux V, Nosib S, Bi H, Brownbridge B. Intraoperative myocardial infarction: Kounis syndrome provoked by latex allergy. BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-007581. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yurtdas M, Aydin MK. A case of coronary spasm with resultant acute myocardial infarction: likely the result of an allergic reaction. Intern Med. 2012;51(16):2161–2164. doi: 10.2169/internalmedicine.51.7852. [DOI] [PubMed] [Google Scholar]
- 133.Kounis NG, Mazarakis A, Tsigkas G. Eosinophilic coronary periarteritis presenting with vasospastic angina and sudden death: a new cause and manifestation of Kounis syndrome? Virchows Arch. 2013;462(6):687–688. doi: 10.1007/s00428-013-1406-4. [DOI] [PubMed] [Google Scholar]
- 134.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathways to induce proinflammatory Th17 cells. Nature. 2007;448:484–488. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bohm I. Kounis syndrome in a patient with allergy to iodinated contrast media. Int J Cardiol. 2011;151(1):102–103. doi: 10.1016/j.ijcard.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 136.Kocabay G, Karabay CY, Kounis NG. Myocardial infarction secondary to contrast agent. Contrast effect or type II Kounis syndrome? Am J Emerg Med. 2012;30(1):255–2. doi: 10.1016/j.ajem.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 137.Miwa K, Fujita M. Cardiovascular dysfunction with low cardiac output due to a small heart in patients with chronic fatigue syndrome. Intern Med. 2009;48(21):1849–1854. doi: 10.2169/internalmedicine.48.2347. [DOI] [PubMed] [Google Scholar]
- 138.Azevedo A, Bettencourt P, Pimenta J, et al. Clinical syndrome suggestive of heart failure is frequently attributable to non-cardiac disorders--population-based study. Eur J Heart Fail. 2007;9(4):391–396. doi: 10.1016/j.ejheart.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 139.Hollingsworth KG, Jones DE, Taylor R, Blamire AM, Newton JL. Impaired cardiovascular response to standing in chronic fatigue syndrome. Eur J Clin Invest. 2010;40(7):608–615. doi: 10.1111/j.1365-2362.2010.02310.x. [DOI] [PubMed] [Google Scholar]
- 140.Theoharides TC. Atopic conditions in search of pathogenesis and therapy. Clin Ther. 2013;35(5):544–547. doi: 10.1016/j.clinthera.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 141.Theoharides TC, Conti P. Mast cells: the JEKYLL and HYDE of tumor growth. Trends Immunol. 2004;25:235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 142.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8(6):478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ozdemir O. Mast cells and the tumor-associated neoangiogenesis. Med Sci Monit. 2006;12(6):LE9–11. [PubMed] [Google Scholar]
- 144.Ozdemir O. Immunosurveillance function of human mast cell? World J Gastroenterol. 2005;11(44):7054–7056. doi: 10.3748/wjg.v11.i44.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Linneberg A, Jacobsen RK, Jespersen L, Abildstrom SZ. Association of subcutaneous allergen-specific immunotherapy with incidence of autoimmune disease, ischemic heart disease, and mortality. J Allergy Clin Immunol. 2012;129(2):413–419. doi: 10.1016/j.jaci.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 146.Murray DB, Gardner JD, Brower GL, Janicki JS. Endothelin-1 mediates cardiac mast cell degranulation, matrix metalloproteinase activation, and myocardial remodeling in rats. Am J Physiol Heart Circ Physiol. 2004;287(5):H2295–H2299. doi: 10.1152/ajpheart.00048.2004. [DOI] [PubMed] [Google Scholar]
- 147.Reriani M, Raichlin E, Prasad A, et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 2010;122(10):958–966. doi: 10.1161/CIRCULATIONAHA.110.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koch CG. Statin therapy. Curr Pharm Des. 2012;18(38):6284–6290. doi: 10.2174/138161212803832335. [DOI] [PubMed] [Google Scholar]
- 149.Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18(11):1519–1530. doi: 10.2174/138161212799504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Montecucco F, Quercioli A, Dallegri F, Viviani GL, Mach F. New evidence for nicotinic acid treatment to reduce atherosclerosis. Expert Rev Cardiovasc Ther. 2010;8(10):1457–1467. doi: 10.1586/erc.10.116. [DOI] [PubMed] [Google Scholar]
- 151.Jacobson TA. A “hot” topic in dyslipidemia management--“how to beat a flush”: optimizing niacin tolerability to promote long-term treatment adherence and coronary disease prevention. Mayo Clin Proc. 2010;85(4):365–379. doi: 10.4065/mcp.2009.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Theoharides TC, Sieghart W, Greengard P, Douglas WW. Antiallergic drug cromolyn may inhibit histamine secretion by regulating phosphorylation of a mast cell protein. Science. 1980;207(4426):80–82. doi: 10.1126/science.6153130. [DOI] [PubMed] [Google Scholar]
- 153.Barrett KE, Metcalfe DD. The histologic and functional characterization of enzymatically dispersed intestinal mast cells of nonhuman primates: effects of secretagogues and anti-allergic drugs on histamine secretion. J Immunol. 1985;135:2020–2026. [PubMed] [Google Scholar]
- 154.Pearce FL, Befus AD, Gauldie J, Bienenstock J. Mucosal mast cells. II: Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982;128:2481–2486. [PubMed] [Google Scholar]
- 155.Horan RF, Sheffer AL, Austen KF. Cromolyn sodium in the management of systemic mastocytosis. J Allergy Clin Immunol. 1990;85(5):852–855. doi: 10.1016/0091-6749(90)90067-e. [DOI] [PubMed] [Google Scholar]
- 156.Fox CC, Wolf EJ, Kagey-Sobotka A, Lichtenstein LM. Comparison of human lung and intestinal mast cells. J Allergy Clin Immunol. 1988;81:89–94. doi: 10.1016/0091-6749(88)90225-4. [DOI] [PubMed] [Google Scholar]
- 157.Oka T, Kalesnikoff J, Starkl P, Tsai M, Galli SJ. Evidence questioning cromolyn's effectiveness and selectivity as a ‘mast cell stabilizer’ in mice. Lab Invest. E. 2012;92(10):1472–1482. doi: 10.1038/labinvest.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Weng Z, Zhang B, Asadi S, et al. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PloS One. 2012;7(3) doi: 10.1371/journal.pone.0033805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vieira Dos SR, Magerl M, Martus P, et al. Topical sodium cromoglicate relieves allergen and histamine-induced dermal pruritus. Br J Dermatol. 2010;162(3):674–676. doi: 10.1111/j.1365-2133.2009.09516.x. [DOI] [PubMed] [Google Scholar]
- 160.Merlos M, Giral M, Balsa D, et al. Rupatadine, a new potent, orally active dual antagonist of histamine and platelet-activating factor (PAF). J Pharmacol Exp Ther. 1997;280(1):114–121. [PubMed] [Google Scholar]
- 161.Metz M, Maurer M. Rupatadine for the treatment of allergic rhinitis and urticaria. Expert Rev Clin Immunol. 2011;7(1):15–20. doi: 10.1586/eci.10.85. [DOI] [PubMed] [Google Scholar]
- 162.Picado C. Rupatadine: pharmacological profile and its use in the treatment of allergic disorders. Expert Opin Pharmacother. 2006;7(14):1989–2001. doi: 10.1517/14656566.7.14.1989. [DOI] [PubMed] [Google Scholar]
- 163.Vasiadi M, Kalogeromitros K, Kempuraj D, et al. Rupatadine inhibits pro-inflammatory mediator secretion from human mast cells triggered by different stimuli. Int Arch Allergy Immunol. 2010;151(1):38–45. doi: 10.1159/000232569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alevizos M, Karagkouni A, Vasiadi M, et al. Rupatadine inhibits inflammatory mediator release from human LAD2 cultured mast cells stimulated by PAF. Annals of Allergy, Asthma and Immunology. 2013 doi: 10.1016/j.anai.2013.08.025. in press. [DOI] [PubMed] [Google Scholar]
- 165.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32(1):23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marshall JS, Leal-Berumen I, Nielsen L, Glibetic M, Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. Clin Invest. 1996;97:1122–1128. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin TJ, Befus AD. Differential regulation of mast cell function by IL-10 and stem cell factor. J Immunol. 1997;159:4015–4023. [PubMed] [Google Scholar]
- 168.Palaniyandi SS, Watanabe K, Ma M, Tachikawa H, Kodama M, Aizawa Y. Inhibition of mast cells by interleukin-10 gene transfer contributes to protection against acute myocarditis in rats. Eur J Immunol. 2004;34(12):3508–3515. doi: 10.1002/eji.200425147. [DOI] [PubMed] [Google Scholar]
- 169.Gillespie SR, DeMartino RR, Zhu J, et al. IL-10 inhibits FcepsilonRI expression in mouse mast cells. J Immunol. 2004;172:3181–3188. doi: 10.4049/jimmunol.172.5.3181. [DOI] [PubMed] [Google Scholar]
- 170.Conti P, Kempuraj D, Kandere K, et al. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86:123–129. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 171.Guo W, Kong E, Meydani M. Dietary polyphenols, inflammation, and cancer. Nutr Cancer. 2009;61(6):807–810. doi: 10.1080/01635580903285098. [DOI] [PubMed] [Google Scholar]
- 172.Middleton EJ, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52(4):673–751. [PubMed] [Google Scholar]
- 173.Xiao ZP, Peng ZY, Peng MJ, Yan WB, Ouyang YZ, Zhu HL. Flavonoids health benefits and their molecular mechanism. Mini Rev Med Chem. 2011;11(2):169–177. doi: 10.2174/138955711794519546. [DOI] [PubMed] [Google Scholar]
- 174.Harwood M, nielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45(11):2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 175.Kawanishi S, Oikawa S, Murata M. Evaluation for safety of antioxidant chemopreventive agents. Antioxid Redox Signal. 2005;7(11-12):1728–1739. doi: 10.1089/ars.2005.7.1728. [DOI] [PubMed] [Google Scholar]
- 176.Kempuraj D, Madhappan B, Christodoulou S, et al. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yap S, Qin C, Woodman OL. Effects of resveratrol and flavonols on cardiovascular function: Physiological mechanisms. Biofactors. 2010;36(5):350–359. doi: 10.1002/biof.111. [DOI] [PubMed] [Google Scholar]
- 178.Perez-Vizcaino F, Duarte J. Flavonols and cardiovascular disease. Mol Aspects Med. 2010;31(6):478–494. doi: 10.1016/j.mam.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 179.Perez-Vizcaino F, Duarte J. Flavonols and cardiovascular disease. Mol Aspects Med. 2010;31(6):478–494. doi: 10.1016/j.mam.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 180.Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev. 2012;70(9):491–508. doi: 10.1111/j.1753-4887.2012.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mullen W, Gonzalez J, Siwy J, et al. A pilot study on the effect of short-term consumption of a polyphenol rich drink on biomarkers of coronary artery disease defined by urinary proteomics. J Agric Food Chem. 2011;59(24):12850–12857. doi: 10.1021/jf203369r. [DOI] [PubMed] [Google Scholar]
- 182.Pearce FL, Befus AD, Bienenstock J. Effect of quercetin and other flavonoids on antigen-induced histamine secretion from rat intestinal mast cells. J Allergy Clin Immunol. 1984;73:819–823. doi: 10.1016/0091-6749(84)90453-6. [DOI] [PubMed] [Google Scholar]
- 183.Asadi S, Zhang B, Weng Z, et al. Luteolin and thiosalicylate inhibit HgCl(2) and thimerosal-induced VEGF release from human mast cells. Int J Immunopathol Pharmacol. 2010;23(4):1015–1020. doi: 10.1177/039463201002300406. [DOI] [PubMed] [Google Scholar]
- 184.Deqiu Z, Kang L, Jiali Y, Baolin L, Gaolin L. Luteolin inhibits inflammatory response and improves insulin sensitivity in the endothelium. Biochimie. 2011;93(3):506–512. doi: 10.1016/j.biochi.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 185.Ando C, Takahashi N, Hirai S, et al. Luteolin, a food-derived flavonoid, suppresses adipocyte-dependent activation of macrophages by inhibiting JNK activation. FEBS Lett. 2009;583(22):3649–3654. doi: 10.1016/j.febslet.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 186.Papaliodis D, Boucher W, Kempuraj D, Theroharides TC. The flavonoid luteolin inhibits niacin-induced flush. Brit J Pharmacol. 2008;153:1382–1387. doi: 10.1038/sj.bjp.0707668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kalogeromitros D, Makris M, Chliva C, Aggelides X, Kempuraj D, Theoharides TC. A quercetin containing supplement reduces niacin-induced flush in humans. Int J Immunopathol Pharmacol. 2008;21(3):509–514. doi: 10.1177/039463200802100304. [DOI] [PubMed] [Google Scholar]
- 188.Schneider RH, Grim CE, Rainforth MV, et al. Stress reduction in the secondary prevention of cardiovascular disease: randomized, controlled trial of transcendental meditation and health education in Blacks. Circ Cardiovasc Qual Outcomes. 2012;5(6):750–758. doi: 10.1161/CIRCOUTCOMES.112.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139–2149. doi: 10.1001/jama.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]