Abstract
Background
Cerebral vasospasm is an independent predictor of poor outcome after subarachnoid hemorrhage (SAH). The nitric oxide-cyclic GMP (NO-cGMP) vasodilatory pathway is strongly implicated in its pathophysiology. Preliminary studies suggest that phosphodiesterase 5 (PDE5) – an enzyme that degrades cGMP – may play a role, as the PDE5 inhibitor sildenafil was found to reduce vasospasm after SAH. However, several questions that are critical when considering translational studies remain unanswered.
Objective
To elucidate the mechanism of action of sildenafil against vasospasm, and to assess whether sildenafil attenuates SAH-induced neuronal cell death, improves functional outcome after SAH, or causes significant physiological side effects when administered at therapeutically relevant doses.
Methods
SAH was induced via endovascular perforation in male C57BL6 mice. Beginning two hours later, mice received sildenafil citrate (0.7, 2 or 5mg/kg P.O. BID) or vehicle. Neurological outcome was assessed daily. Vasospasm was determined on post-SAH Day 3. Brain PDE5 expression and activity, cGMP content, neuronal cell death, arterial blood pressure (BP), and intracranial pressure (ICP) were examined.
Results
We found that PDE5 activity (but not expression) is increased after SAH, leading to decreased cGMP levels. Sildenafil attenuates this increase in PDE5 activity and restores cGMP levels after SAH. Post-SAH initiation of sildenafil was found to reduce vasospasm, decrease neuronal cell death, and markedly improve neurological outcome, without causing significant physiological side effects.
Conclusion
Sildenafil–an FDA-approved drug with a proven track record of safety in humans –is a promising new therapy for vasospasm and neurological deficits following SAH.
INTRODUCTION
Vasospasm-induced delayed cerebral ischemia (DCI) is a major risk factor for poor outcome after SAH, and is the most common cause of secondary neurological injury in this population.1 Numerous studies suggest that a key contributing factor to vasospasm pathophysiology is downregulation of the NO-cGMP vasodilatory pathway. Evidence for this include reduced NO bioavailability, decreased endothelial nitric oxide synthase, and reduced cGMP (the downstream effector of NO) after SAH.2 The latter appears to occur (in part) from SAH-induced upregulation of PDE5 – an enzyme that selectively hydrolyzes cGMP to GMP.
Sildenafil citrate (Viagra™) is one of a number of highly selective PDE5 inhibitors that attenuate PDE5-mediated hydrolysis of cGMP leading to increased intracellular cGMP levels, smooth muscle relaxation, and arterial vasodilation.3 Several recent studies suggest sildenafil and other PDE5 inhibitors likely reduce vasospasm following SAH.4–8 Though these results are promising, additional investigation is required before considering translational studies in humans. For example, the following have yet to be examined: 1) whether sildenafil reduces neuronal cell death following SAH; 2) whether sildenafil improves neurological outcome following SAH; and 3) whether sildenafil causes systemic hypotension or exacerbates SAH-induced intracranial hypertension when administered at doses that attenuate vasospasm. The molecular pathway by which sildenafil exerts its anti-vasospasm effect has also not been elucidated. We sought to answer these critical questions utilizing an endovascular perforation mouse model of SAH.
MATERIALS AND METHODS
Experimental SAH
All experimental protocols were approved by the Animal Studies Committee at Washington University in St Louis. Three to four month old male C57BL/6J mice (Jackson Laboratories; Bar Harbor, Maine) were used. Endovascular perforation SAH and sham surgery were performed as described.9 Briefly, mice were anesthetized with Isoflurane (4% induction, 1.5% maintenance). A blunted 5-0 monofilament nylon suture was introduced into the ECA, and advanced through the internal carotid artery (ICA) until the anterior cerebral artery (ACA)–middle cerebral artery (MCA) bifurcation was encountered. For SAH surgery, the suture was advanced 3 mm further to perforate the ACA leading to SAH. For sham surgery, the suture was not advanced. The suture was then removed, the ECA ligated, and the skin incision closed. After recovery, mice were returned to their cages and allowed free access to water and rodent chow. To prevent dehydration post-operatively, saline containing 10% dextrose (0.5 ml) was intraperitoneally administered twice a day for 3 days.
Drug Administration
Sildenafil citrate (Viagra™, Pfizer Inc., New York, NY) was powdered and suspended in saline. Vehicle (saline) or sildenafil (0.7, 2 or 5 mg/kg) was administered by oral gavage twice daily starting 2h after surgery. Low dose was based on the effective dose for erectile dysfunction in humans: 0.7 mg/kg; higher doses were chosen because the elimination half-life in mice (1.3 h) is less than humans (3.7 h).10 The time point for initiating sildenafil was based on cerebral ischemia studies.11
Physiological Data
To examine BP, naive mice were anesthetized with isoflurane and a polyethylene catheter (PE10, Clay Adams) was inserted into the common carotid artery. Thirty minutes later, BP in awake mice was recorded before and after sildenafil (2 mg/kg) or vehicle administration. To examine ICP, a Mikro-tip pressure catheter (AD Instruments) was placed through a 1-mm right parietal burr hole; ICP was monitored for 10 minutes beginning 3 hours after SAH.
Behavioral Tests
Neurobehavioral outcome was examined daily using Neuroscore and Rotarod tests as described.9 Briefly, neurological function was graded based on a motor score (0–12) that evaluated spontaneous activity, symmetry of limb movements, climbing, balance and coordination; and a sensory score (4–12) that evaluated body proprioception and vibrissae, visual, and tactile responses. Balance and coordination were assessed by performance on Rotarod (Rotamex-5, Columbus Instruments, Columbus, OH).9 The latency on three trials of 180 seconds each was averaged daily on Days 0–3. Mice were pre-trained on the Rotarod one day prior to surgery. All assessments were performed by investigators who were blinded to the surgical procedure and treatment group. Functional outcome was assessed in the same groups of mice that were used for vasospasm assessment.
Vasospasm
MCA vasospasm was assessed by cerebrovascular casting method as described with modification.9, 12 Briefly, under isoflurane anesthesia, mice were transcardially perfused at a pressure of 75±5 mmHg with heparinized phosphate-buffered saline (PBS), followed by 10% formalin, and then casting medium containing 3% gelatin and 25% India ink. Mice were refrigerated overnight to allow gelatin solidification. Brains were then removed and blood vessels imaged using a CCD camera (CoolSNAP ES, Thotometrics, Tucson, AZ). Spasm of the ipsilateral proximal MCA was assessed by recording the narrowest diameter in the first 1000 µm of the MCA. To assess the time course of vasospasm in our model, mice were initially sacrificed on Days 2–4 after SAH. Since Day 3 was found to be the period of maximum vasospasm (see below), mice were sacrificed on Day 3 for subsequent efficacy studies with sildenafil. Vasospasm assessment was performed by investigators who were blinded to the treatment group.
PDE5 Activity
PDE5 activity was determined by measuring cGMP-hydrolyzing activity as described.7 Ten microliters of brain lysates were added to 90 µL of reaction buffer (20 mM HEPES, pH 7.4, 5 mM MgCl2, 1 mM DTT, 42 mM KCl, 10% sucrose and protease inhibitor cocktail) containing 1 µM cGMP with or without the PDE5 inhibitor, zaprinast (10µM).13 After incubating at 37°C for 30 min, the reaction was stopped by heating at 95°C for 5 min. The remaining cGMP content was determined by cGMP ELISA assay. Total PDE and PDE5 activities were calculated as pmol/min/mg protein. Investigators were blinded to the surgical procedure (Sham or SAH) and treatment group (Vehicle or Sildenafil).
Western Blot
Western blots were performed on cerebral cortices ipsilateral to endovascular perforation as described.9, 14 The following primary antibodies were used: rabbit polyclonal anti-PDE5 1:500 (Santa Cruz Biotechnology Inc) and mouse monoclonal anti alpha-Tubulin 1:20000 (Sigma). Blots were subsequently incubated with anti-rabbit or anti-mouse horseradish peroxidase-conjugated IgG (Biorad, Hercules, CA) and visualized using an enhanced chemiluminescence kit (Biorad, Hercules, CA). Alpha-tubulin was used as the internal control. Investigators were blinded to the surgical procedure (Sham or SAH) and treatment group (Vehicle or Sildenafil).
cGMP ELISA
Ipsilateral brain tissue was lysed in a buffer containing 10 mM HEPES, pH, 7.4, 1% Triton X-100, 5 mM MgCl2, 1 mM DTT, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO).14 Tissue lysates were centrifuged at 10,000 × g at 4°C for 10 min and the supernatants (10 µl) subjected to cGMP ELISA assay (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s protocol. Investigators were blinded to the surgical procedure (Sham or SAH) and treatment group (Vehicle or Sildenafil).
Immunohistochemistry
Free floating coronal brain sections (40 µm) were subjected to immunostaining with anti-cGMP antibody (1:500; Chemicon, Millipore, Billerica, MA)using a Vectastain Elite ABC-peroxidase kit (Vector Laboratories) and Tyramide signal amplification kit (Invitrogen), as described.14 Smooth muscle cells were labelled with Phalloidin-Alexa 568 (Invitrogen).
Terminal deoxynucleotidyl transferase-dUTP nick end labeling (TUNEL)
Equally-spaced brain sections (4 sections per mouse) were used. DNA fragmentation was detected using a TUNEL apoptosis detection kit (Invitrogen) as described.15 TUNEL-positive cells in the ipsilateral parietal cortex were quantified (4 fields per section). Investigators were blinded to the treatment group (Vehicle or Sildenafil).
Statistical analysis
Data are presented as the mean ± SEM. All data except functional outcome were analyzed by ANOVA followed by Newman-Keuls multiple comparison method. For functional outcome, Neuroscore (after rank transformation) and Rotarod data were analyzed using Repeated Measures ANOVA and post-hoc Fisher’s Least Significant Difference method;A p<0.05 was considered as statistically significant for all analyses.
RESULTS
SAH causes vasospasm
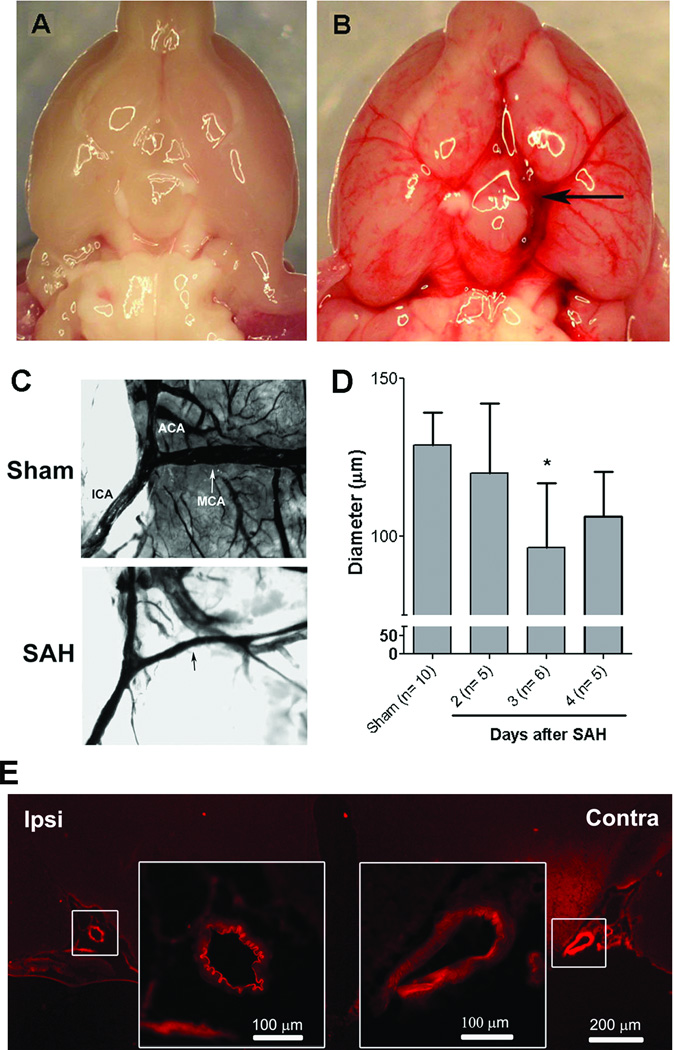
Endovascular suture-perforation consistently produced SAH around the left ICA, MCA, and ACA and the extended subarachnoid space with minimal post-operative mortality (<10%) (Fig. 1A,B). Vasospasm of the MCA was consistently noted, with peak vasospasm occurring on post-SAH Day 3 (n=10, 5, 6 and 5 for Sham, SAH:Day 2, SAH:Day 3 and SAH:Day 4 groups respectively) (Fig. 1C,D). Histology confirmed marked narrowing of the arterial lumen as well as intimal corrugation of the ipsilateral cerebral arteries (Fig. 1E).
Figure 1. Cerebral vasospasm following experimental SAH in mice.
Mice underwent sham operation or SAH by endovascular perforation of the left ACA. At 30 min post-surgery, ventral brain surfaces from mice subjected to sham (A) and SAH (B) were photographed. Extensive SAH centered at the site of arterial perforation was noted. On Days 2–4 post-surgery, brains from mice subjected to sham and SAH were cast with gelatin/india ink and imaged (C). Quantitation of MCA diameter demonstrated SAH-induced vasospasm peaked on Day 3 (D). Histological assessment on Day 3 also showed marked luminal narrowing and intimal corrugation in cross-sections of cerebral arteries ipsi- but not contra-lateral to endovascular perforation (E).
Sildenafil inhibits SAH-induced increase in PDE5 activity
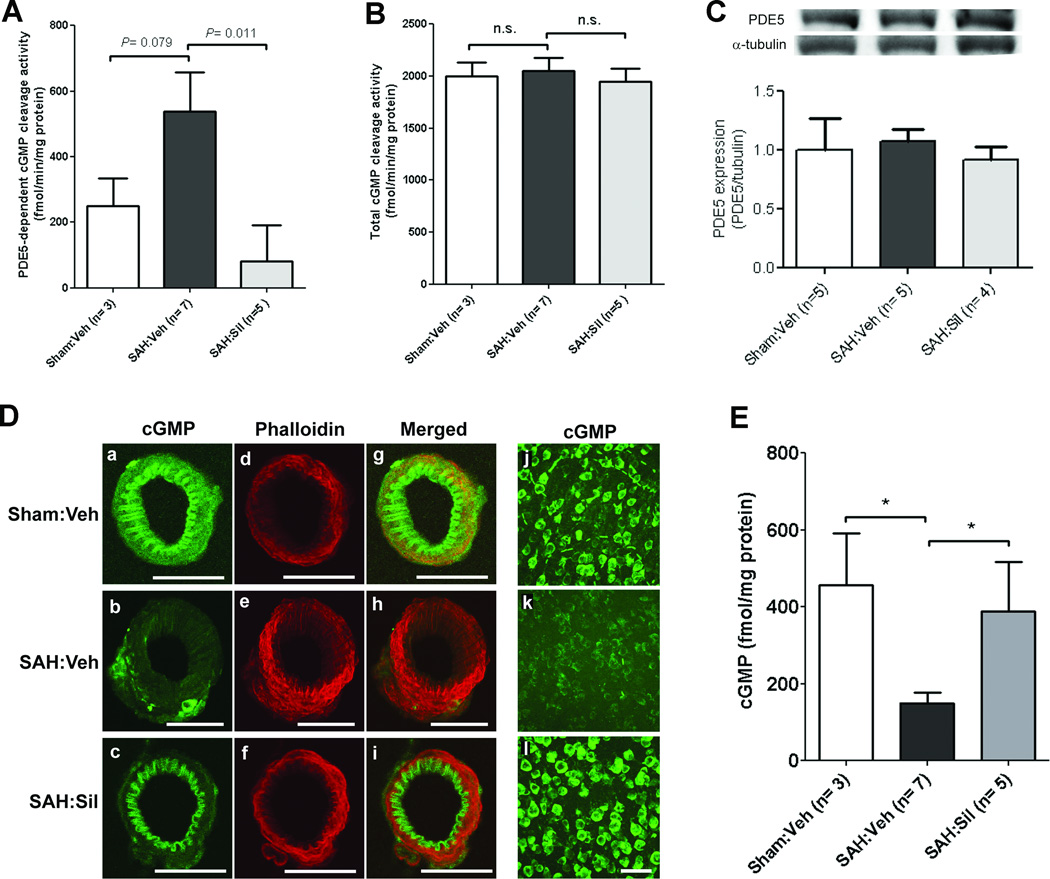
An increase in zaprinast-sensitive PDE5 activity was noted in the ipsilateral hemisphere 3 d post-SAH as compared with sham-operated mice [SAH:vehicle (n=7): 538.4±118.5 vs. Sham:vehicle (n=3): 250.0±84.6 fmol/min/mg protein, P=0.07] (Fig. 2A). Sildenafil strongly attenuates this increase in PDE5 activity [SAH:sildenafil (n=5): 81.5±110.5 vs. SAH:vehicle (n=7): 538.4±118.5 fmol/min/mg protein, P<0.05]. Total cGMP-hydrolyzing activity (by all PDEs) was unaltered in SAH mice ± sildenafil as compared with sham-operated mice (P>0.05) (Fig 2B). These data indicate that PDE5 is the predominant PDE isoform that is activated following SAH, and that sildenafil attenuates this activation. Western blot analysis showed no significant difference between groups in PDE5 expression (Fig 2C), suggesting that SAH-induced PDE5 upregulation is likely due to post-translational modifications.
Figure 2. Sildenafil attenuates SAH-induced alterations in PDE5 activity and cGMP.
Mice underwent SAH or sham operation. SAH mice were treated with vehicle or sildenafil (2 mg/kg P.O. BID) for 3d and brain tissues were prepared 3d post-SAH. Cyclic GMP-hydrolyzing activities were assayed with tissue lysates in the presence (A) or absence (B) of the PDE5 inhibitor zaprinast to determine the enzymatic activities of PDE5 (A) and total PDEs (B). Data indicate the mean±S.E.M. C. Representative western blot images and quantification data of PDE5 expression in the ipsilateral hemisphere. Expression levels of PDE5 were normalized to alpha-tubulin. Data indicate the mean±S.E.M. D. Representative images of MCA sections ipsilateral to SAH immunostained with cGMP (a–c) and co-labeled with the smooth muscle marker phalloidin conjugated with Alexa Fluor-568 (d–f) demonstrating that sildenafil attenuates SAH-induced drop in vascular cGMP. Superimposed images are also shown (g–i). Similar changes were seen in neuronal cGMP (j–l). Scale bar: 50 µm. E. Levels of cGMP in whole brains were determined by ELISA. Data represent the mean±S.E.M. Statistics were analyzed by ANOVA followed by Newman-Keuls multiple comparison test.
Sildenafil inhibits SAH-induced cGMP breakdown
Immunohistochemical labeling with anti-cGMP antibody and confocal microscopy revealed a marked decrease in cGMP immunoreactivity in cerebral arteries and neurons following SAH (n=6) vs. sham-operated mice (n=4) (Fig 2D); sildenafil (2mg/kg) (n=6) restores cGMP levels after SAH (Fig. 2D). These findings were confirmed by cGMP ELISA assay (Fig. 2E). cGMP content in the ipsilateral cortex was decreased 3 d post-SAH vs. sham-operated mice [SAH:vehicle (n=7): 150±27 vs. Sham:vehicle (n=3): 456±134 fmol/mg protein, P <0.05); sildenafil attenuates this SAH-induced drop in cGMP [SAH:sildenafil (n=5): 389±128 vs. SAH:vehicle (n=7): 150±27 fmol/mg protein, P<0.05).
Sildenafil attenuates vasospasm after SAH
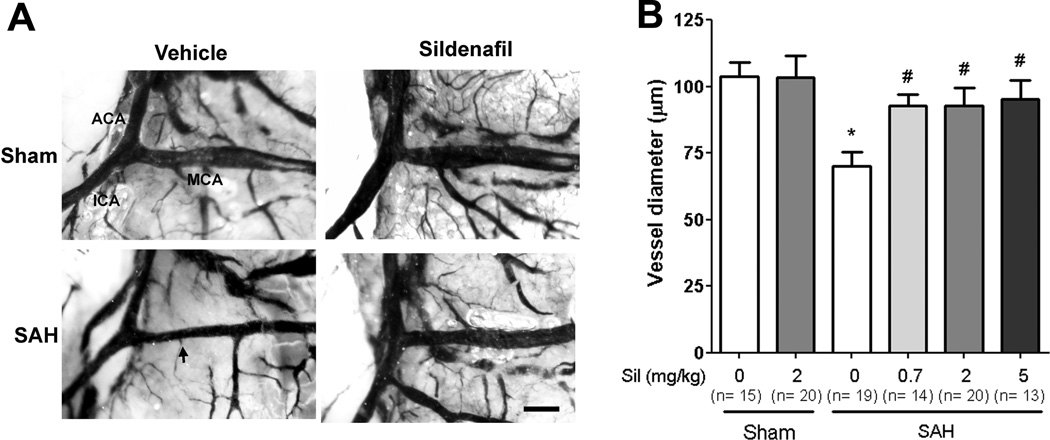
Sildenafil (2 mg/kg) did not alter MCA caliber in sham-operated mice [Sham:sildenafil (2mg/kg) (n=20): 103.4±8.1 vs. Sham:vehicle (n=15): 103.9±5.1, p>0.05). Importantly, all three doses of sildenafil reduced MCA vasospasm in SAH-operated mice [SAH:vehicle (n=19): 70.1±5.3 vs. SAH:sildenafil (0.7mg/kg) (n=14): 92.8±4.1, SAH:sildenafil (2mg/kg) (n=20): 92.9±6.6 µm, and SAH:sildenafil (5mg/kg) (n=13): 95.3±6.9; P<0.05 for all three groups vs. SAH:vehicle] (Fig. 3 A,B). No significant differences in mortality were noted between groups (data not shown).
Figure 3. Sildenafil attenuates vasospasm following SAH.
Mice underwent SAH or sham operation. Treatment with sildenafil (Sil) (0.7, 2 and 5 mg/kg P.O. BID) or vehicle (Veh) was initiated 2 hours later. On post-surgery day 3, mice were perfused with gelatin/india ink. A. Representative images of gelatin/India ink-casted cerebral vessels. Vasospasm of the MCA was evident in SAH mice compared with sham-operated mice; sildenafil attenuates this SAH-induced vasospasm. Scale bar: 200 µm. Abbreviations: ACA=anterior cerebral artery; ICA=internal carotid artery. B. Vessel caliber in the proximal MCA ipsilateral to suture perforation was determined. Data represent the mean±S.E.M. *P<0.05 vs. Sham:vehicle, #p<0.05 vs. SAH:vehicle. Data were analyzed by ANOVA followed by Newman-Keuls multiple comparison test.
Sildenafil improves functional outcome after SAH
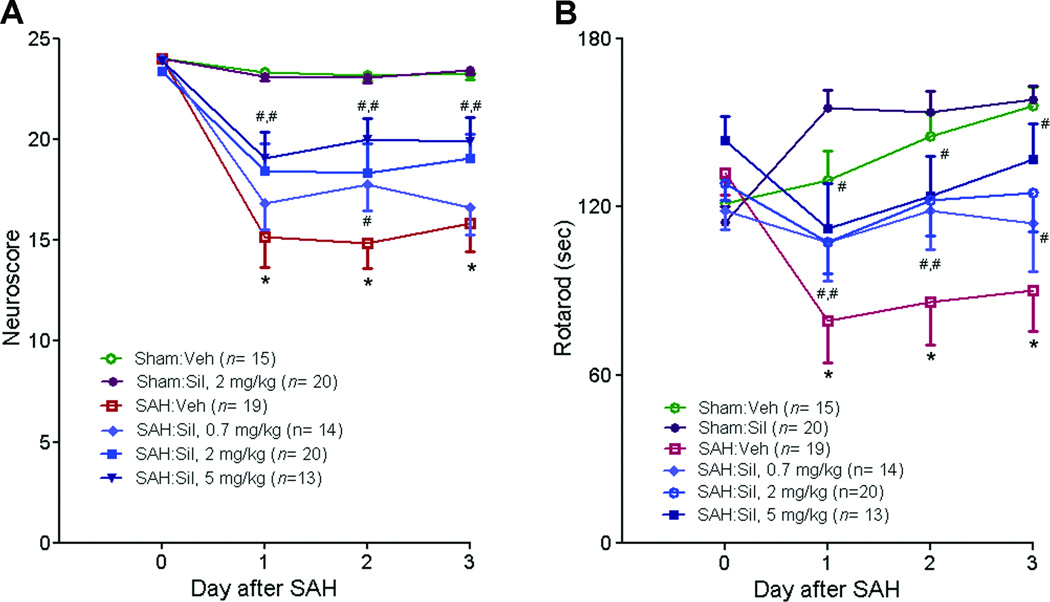
Sildenafil reduces SAH-induced neurological deficits in a dose dependent manner (P<0.05) as assessed by Neuroscore (Fig. 4A) and Rotarod test (Fig. 4B). Sildenafil (2mg/kg) has no effect on the performance of sham-operated mice.
Figure 4. Sildenafil improves neurological outcome following SAH.
Mice underwent SAH or sham operation. Treatment with sildenafil (Sil) (2 mg/kg P.O. BID) or vehicle (Veh) was initiated 2 hours later. Behavioral performance was assessed daily via sensorimotor neurological score (A) and rotarod latency test (B). Data represent the mean±S.E.M. Statistical analysis was performed by Repeated Measures ANOVA followed by a post-hoc test.
Sildenafil attenuates neuronal cell death after SAH
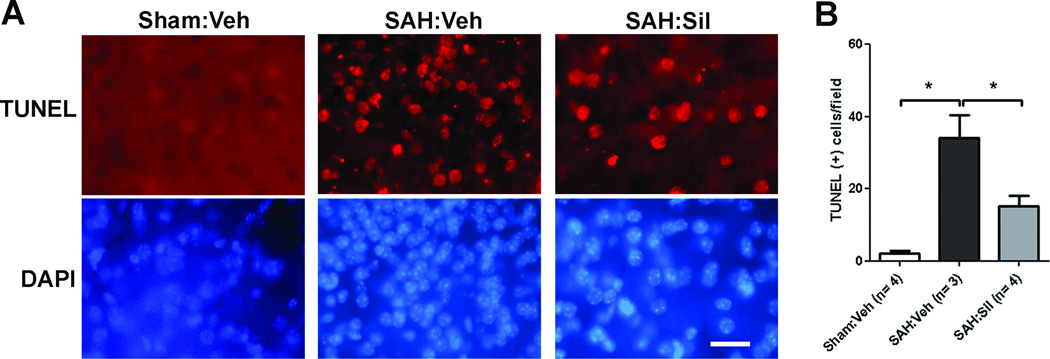
There was a significant increase in the number of TUNEL positive neurons in the ipsilateral parietal cortex after SAH [Sham:vehicle (n=4): 2.0±0.9 vs. SAH:vehicle (n=3): 35±6.3 cells/field (field = 83,083µm2), p<0.05). Sildenafil (2mg/kg) results in a 55% reduction in TUNEL positive neurons following SAH (SAH:vehicle (n=3): 35±6.3 cells/field (field = 83,083µm2) vs. SAH:sildenafil (2mg/kg) (n=4): 15.1±2.8 cells/field, p<0.05) (Fig 5).
Figure 5. Sildenafil reduces neuronal cell death after SAH.
Mice underwent SAH or sham operation. SAH mice were treated with sildenafil (2mg/kg P.O. BID) or vehicle (Veh); brain tissues were prepared 3d post-SAH. A. Brain sections were labeled by TUNEL (upper panels) and stained with DAPI (lower panels). Scale bar: 25µm. B. Quantitative analysis of TUNEL positive cells in the ipsilateral parietal cortex per field (83,083 µm2). Data represent the mean ± S.E.M. *P<0.05 vs. Sham:vehicle, # p<0.05 vs. SAH:vehicle.
Sildenafil has no effect on BP and ICP
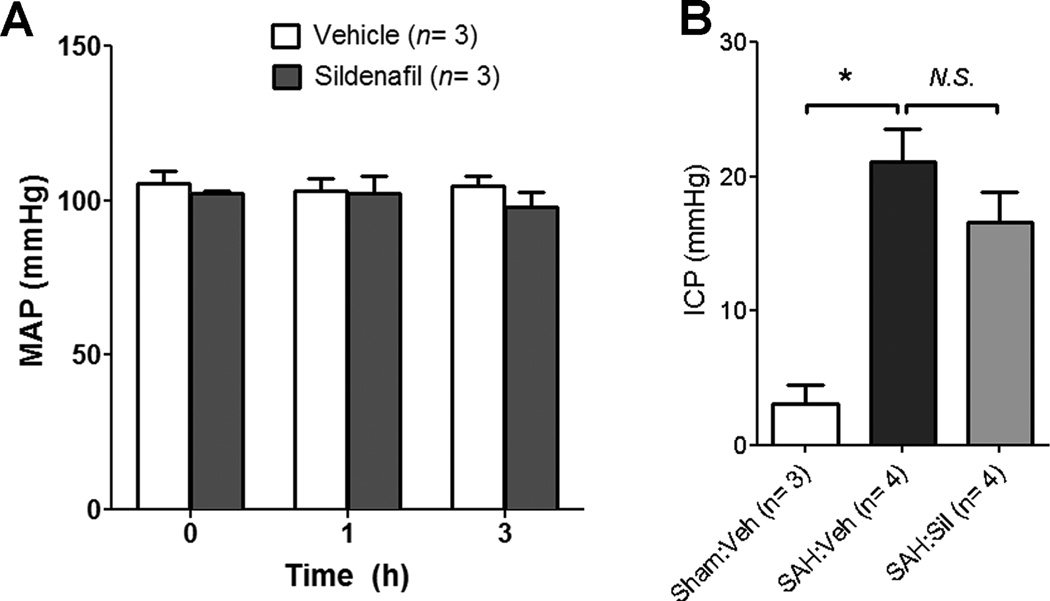
In normal conscious mice (n=3/group), sildenafil (2 mg/kg) does not affect mean arterial pressure for up to 3 hours post-injection (Fig 6A). In mice subjected to SAH, sildenafil (2 mg/kg) does not exacerbate SAH-induced intracranial hypertension [SAH:vehicle (n=4): 21.1±2.3 vs. SAH:sildenafil (n=4): 16.6±2.3 mmHg, P>0.05) (Fig 6B).
Figure 6. Sildenafil does not affect systemic blood pressure or intracranial pressure.
A. Mean arterial blood pressure (MAP) was monitored via catheterization of the common carotid artery before and after treatment with sildenafil (Sil) (2 mg/kg P.O.) or vehicle (Veh) in normal mice. B. In a separate experiment, mice were treated with sildenafil (2 mg/kg P.O.) or vehicle 2 hours after SAH surgery. One hour later, ICP was monitored using a Mikro-tip pressure catheter. Data represent the mean±S.E.M and were analyzed by ANOVA followed by Newman-Keuls multiple comparison test. *P<0.05 vs. Sham:vehicle, #p<0.05 vs. SAH:vehicle.
DISCUSSION
Following SAH, several molecular events occur that alter the balance of the NO-cGMP vasodilatory pathway such that it has become a prime target for anti-vasospasm therapy. First, SAH causes decreased NO bioavailability via a multitude of pathways.2 Based on this finding, many therapeutic strategies for increasing NO availability have been pursued; however, mixed results have thus far been encountered.16 This is likely due to the short half life17 and unpredictable distribution of NO,2 downstream alteration in PDE5 activity that could impede NO-induced vasodilation,7,18 and side effects of NO-directed therapy including systemic hypotension,19 vascular steal,20 and increased ICP.19
Second, SAH causes impaired vascular responsiveness to NO by decreasing cGMP availability. This has been attributed to increased PDE5 expression7 and/or activity,7, 18 which has made PDE5 inhibition a novel and attractive anti-vasospasm strategy. Studies to date show the following: 1) acute administration of sildenafil partially relaxes basilar artery vasospasm in dogs7; 2) pre-SAH initiation of sildenafil partially inhibits vasospasm in rabbits4; and 3) post-SAH initiation of sildenafil and other PDE5 inhibitors attenuates vasospasm in rat cisterna magna models of SAH.5,6,8
In the present study, we sought to not only confirm the anti-vasospasm effect of sildenafil in an endovascular perforation model of SAH, but also examine (for the first time) its mechanistic and functional consequences in the setting of SAH. First, we found that SAH increases PDE5 activity without altering PDE5 expression, and that sildenafil attenuates this SAH-induced augmentation of PDE5 activity. Two past studies have examined the effect of SAH on PDE5, with one showing increased PDE5 expression7 and both showing increased PDE5 activity.7,18 Our data corroborates that PDE5 activity is enhanced following SAH, but suggests post-translational modifications (rather than enhanced enzyme expression) is primarily responsible. Next, we found that cGMP content in brain and cerebral vessels is reduced following SAH, and that sildenafil markedly but incompletely prevents this SAH-induced drop in cGMP (the incomplete restoration of cGMP might indicate that other cGMP-hydrolyzing PDE’s are also contributing). Taken together, these data strongly suggest that enhanced PDE5 activity and subsequent augmentation of cGMP hydrolysis is responsible, at least in part, for SAH-induced downregulation of the NO-cGMP pathway. Moreover, we are the first to demonstrate that sildenafil attenuates SAH-induced downregulation of the NO-cGMP pathway by reducing PDE5 activity and restoring cGMP levels. A schematic illustration of these changes is shown in Figure 7.
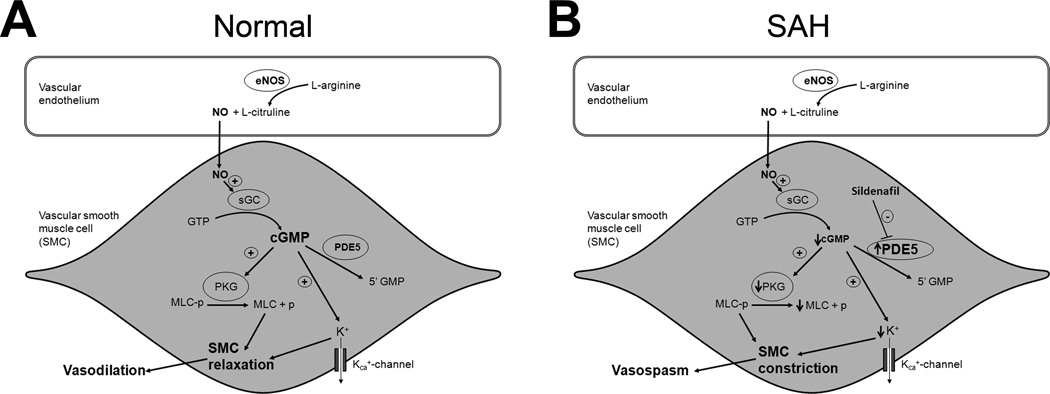
Figure 7. Schematic representation of NO-cGMP pathway.
A. Under normal physiological conditions nitric oxide (NO) produced in the vascular endothelium diffuses into the vascular smooth muscle cells (SMC) and activates soluble guanylate cyclase (sGC). sGC enhances the conversion of guanosine-5'-triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). cGMP activates a variety of downstream molecules including protein kinase G (PKG) and calcium dependent potassium channels (KCa+). Activation of PKG leads to dephosphorylation of myosin light chain (MLC), and activation of KCa+ leads to SMC hyperpolarization via potassium (K+) efflux. Both these changes lead to SMC relaxation and subsequent vasodilation. B. Increase in phosphodiesterase-5 (PDE5) activity after SAH reduces cGMP levels. Decreased cGMP levels result in maintenance of phosphorylated MLC (via decreased PKG activity) and SMC depolarization (via decreased K+ efflux) leading to SMC constriction and vasospasm. Inhibition of PDE5 with Sildenafil attenuates these pathophysiological changes and reduces vasospasm.
Next, we examined several key questions regarding the therapeutic potential of PDE5 inhibition for SAH. First, we found that post-SAH initiation of sildenafil attenuates vasospasm in an endovascular perforation SAH model, which confirms previous cisterna magna injection SAH studies.5–6, 8 Second, we document that sildenafil improves neurological outcome after SAH – a critical finding given that numerous studies have shown a dissociation between the vascular and functional benefits of certain SAH therapeutics.21–22 Third, we found that sildenafil reduces SAH-induced neuronal cell death, which may reflect a reduction in vasospasm-induced ischemia or a direct neuroprotective effect (or both). Finally, we found that a therapeutically relevant dose of sildenafil does not cause systemic hypotension, does not affect cerebral vessel caliber in sham-operated mice, and does not exacerbate intracranial hypertension in SAH-operated mice.
PDE5 inhibitors have been approved by the Food and Drug Administration for the treatment of erectile dysfunction since 1998.23 Over 100 manufacturer-sponsored trials of PDE5 inhibitors for the treatment of this condition have been completed, and an excellent safety profile has been consistently demonstrated.24 Consistent with our expectations, sildenafil (2mg/kg) had no effect on BP or ICP in our experiments. In humans, PDE5 inhibitors have been associated with a slight reduction in BP25; however, this is rarely clinically relevant except in patients who are concurrently taking nitrates. Other reported side effects are either benign in nature or decidedly rare.26
Our study has several limitations. First, the endovascular perforation SAH model has been criticized for its high mortality and hemorrhage variability; however, it has been applauded for its acute pathophysiological changes that closely mimic aneurysmal rupture.27–28 In our experience, mortality has been acceptably low (<10%). Also, we controlled for hemorrhage variability by distributing mice equally into different groups based on the severity of initial injury, before administration of vehicle or sildenafil. Second, rodent models of SAH do not completely recapitulate human SAH. Therefore, our findings regarding neuronal cell death, functional outcome, and side effects should be validated in other SAH models. Third, the improved neurological outcome noted in our study may reflect an activity of sildenafil other than its influence on vasospasm. For example, sildenafil inhibits platelet aggregation,29–30 and could thus reduce formation of microthrombi, which have also been implicated in the pathophysiology of DCI after SAH.31 Finally, the manner in which sildenafil reduces SAH-induced neuronal cell death was not addressed in our study.
Based on the findings that sildenafil prevents or reverses vasospasm following SAH in an endovascular perforation model in mice (this study) and a cisterna magna injection model in rats,5 rabbits,4 and dogs7; reduces neuronal cell death and improves neurological outcome after SAH in mice (present study); and causes no significant physiological side effects in mice subjected to SAH (present study); we conclude that sildenafil holds great promise as a strategy to prevent vasospasm and improve neurological outcome in patients with SAH. Though additional preclinical testing would be beneficial (e.g. to examine the effect of sildenafil on cerebral blood flow, neuronal cell death, and neurological outcome in larger animal models), we believe that the aforementioned excellent safety profile for sildenafil in humans indicates that a Phase I safety and dose-finding clinical trial examining sildenafil as a prophylactic strategy in patients with aneurysmal SAH is justified. Organization of such a trial is therefore currently underway.
Acknowledgements
This work was supported by grants from the McDonnell Center for Cellular and Molecular Neurobiology (B.H.H.), American Heart Association (G.J.Z.), Brain Aneurysm Foundation (G.J.Z. and B.H.H.), Neurosurgery Research and Education Foundation (G.J.Z.), McDonnell Center for Higher Brain Function (G.J.Z.), and NIH Neuroscience Blueprint Center Core Grant P30 NS057105 to Washington University (Alafi Neuroimaging Lab – Hope Center for Neurological Disorders). The authors thank Ernesto Gonzales for performing subarachnoid hemorrhage surgery.
REFERENCES
- 1.Bederson JB, Connolly ES, Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009 Mar;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 2.Pluta RM. Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacology & therapeutics. 2005 Jan;105(1):23–56. doi: 10.1016/j.pharmthera.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Medina P, Segarra G, Vila JM, Domenech C, Martinez-Leon JB, Lluch S. Effects of sildenafil on human penile blood vessels. Urology. 2000 Sep 1;56(3):539–543. doi: 10.1016/s0090-4295(00)00622-1. [DOI] [PubMed] [Google Scholar]
- 4.Atalay B, Caner H, Cekinmez M, Ozen O, Celasun B, Altinors N. Systemic administration of phosphodiesterase V inhibitor, sildenafil citrate, for attenuation of cerebral vasospasm after experimental subarachnoid hemorrhage. Neurosurgery. 2006 Nov;59(5):1102–1107. doi: 10.1227/01.NEU.0000245605.22817.44. discussion 1107–1108. [DOI] [PubMed] [Google Scholar]
- 5.Gokce C, Gulsen S, Yilmaz C, Guven G, Caner H, Altinors N. The effect of the sildenafil citrate on cerebral vasospasm and apoptosis following experimental subarachnoid hemorrhage in rats. J Neurosurg Sci. 2010 Mar;54(1):29–37. [PubMed] [Google Scholar]
- 6.Gul S, Bahadir B, Hanci V, et al. Effect of vardenafil on cerebral vasospasm following experimental subarachnoid hemorrhage in rats. J Clin Neurosci. 2010 Aug;17(8):1038–1041. doi: 10.1016/j.jocn.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Inoha S, Inamura T, Ikezaki K, Nakamizo A, Amano T, Fukui M. Type V phosphodiesterase expression in cerebral arteries with vasospasm after subarachnoid hemorrhage in a canine model. Neurol Res. 2002 Sep;24(6):607–612. doi: 10.1179/016164102101200447. [DOI] [PubMed] [Google Scholar]
- 8.Koktekir E, Erdem Y, Akif Bayar M, Gokcek C, Karatay M, Kilic C. A new approach to the treatment of cerebral vasospasm: the angiographic effects of tadalafil on experimental vasospasm. Acta Neurochir (Wien) 2010 Mar;152(3):463–469. doi: 10.1007/s00701-009-0540-x. [DOI] [PubMed] [Google Scholar]
- 9.Vellimana AK, Milner E, Azad TD, et al. Endothelial nitric oxide synthase mediates endogenous protection against subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2011 Mar;42(3):776–782. doi: 10.1161/STROKEAHA.110.607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker DK, Ackland MJ, James GC, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica: the fate of foreign compounds in biological systems. 1999 Mar;29(3):297–310. doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Wang Y, Zhang L, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002 Nov;33(11):2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 12.Parra A, McGirt MJ, Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Mouse model of subarachnoid hemorrhage associated cerebral vasospasm: methodological analysis. Neurol Res. 2002 Jul;24(5):510–516. doi: 10.1179/016164102101200276. [DOI] [PubMed] [Google Scholar]
- 13.Pauvert O, Lugnier C, Keravis T, Marthan R, Rousseau E, Savineau JP. Effect of sildenafil on cyclic nucleotide phosphodiesterase activity, vascular tone and calcium signaling in rat pulmonary artery. Br J Pharmacol. 2003 Jun;139(3):513–522. doi: 10.1038/sj.bjp.0705277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000 Aug 1;20(15):5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin DH, Bae YC, Kim-Han JS, et al. Polyphenol amentoflavone affords neuroprotection against neonatal hypoxic-ischemic brain damage via multiple mechanisms. J Neurochem. 2006 Jan;96(2):561–572. doi: 10.1111/j.1471-4159.2005.03582.x. [DOI] [PubMed] [Google Scholar]
- 16.Aihara Y, Jahromi BS, Yassari R, Sayama T, Macdonald RL. Effects of a nitric oxide donor on and correlation of changes in cyclic nucleotide levels with experimental vasospasm. Neurosurgery. 2003 Mar;52(3):661–667. doi: 10.1227/01.neu.0000048188.88980.86. discussion 666–667. [DOI] [PubMed] [Google Scholar]
- 17.Gabikian P, Clatterbuck RE, Eberhart CG, Tyler BM, Tierney TS, Tamargo RJ. Prevention of experimental cerebral vasospasm by intracranial delivery of a nitric oxide donor from a controlled-release polymer: toxicity and efficacy studies in rabbits and rats. Stroke. 2002 Nov;33(11):2681–2686. doi: 10.1161/01.str.0000033931.62992.b1. [DOI] [PubMed] [Google Scholar]
- 18.Sobey CG, Quan L. Impaired cerebral vasodilator responses to NO and PDE V inhibition after subarachnoid hemorrhage. The American journal of physiology. 1999 Nov;277(5 Pt 2):H1718–H1724. doi: 10.1152/ajpheart.1999.277.5.H1718. [DOI] [PubMed] [Google Scholar]
- 19.Egemen N, Turker RK, Sanlidilek U, et al. The effect of intrathecal sodium nitroprusside on severe chronic vasospasm. Neurol Res. 1993 Oct;15(5):310–315. doi: 10.1080/01616412.1993.11740153. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Isotani E, Mizuno Y, Azuma H, Hirakawa K. Effective improvement of the cerebral vasospasm after subarachnoid hemorrhage with low-dose nitroglycerin. J Cardiovasc Pharmacol. 2000 Jan;35(1):45–50. doi: 10.1097/00005344-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008 Nov;39(11):3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 22.Haley EC, Jr, Kassell NF, Torner JC. A randomized trial of nicardipine in subarachnoid hemorrhage: angiographic and transcranial Doppler ultrasound results. A report of the Cooperative Aneurysm Study. J Neurosurg. 1993 Apr;78(4):548–553. doi: 10.3171/jns.1993.78.4.0548. [DOI] [PubMed] [Google Scholar]
- 23.Uthayathas S, Karuppagounder SS, Thrash BM, Parameshwaran K, Suppiramaniam V, Dhanasekaran M. Versatile effects of sildenafil: recent pharmacological applications. Pharmacol Rep. 2007 Mar-Apr;59(2):150–163. [PubMed] [Google Scholar]
- 24.Gorkin L, Hvidsten K, Sobel RE, Siegel R. Sildenafil citrate use and the incidence of nonarteritic anterior ischemic optic neuropathy. Int J Clin Pract. 2006 Apr;60(4):500–503. doi: 10.1111/j.1368-5031.2006.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prisant LM. Phosphodiesterase-5 inhibitors and their hemodynamic effects. Curr Hypertens Rep. 2006 Aug;8(4):345–351. doi: 10.1007/s11906-006-0075-y. [DOI] [PubMed] [Google Scholar]
- 26.Cheitlin MD, Hutter AM, Jr, Brindis RG, et al. Use of sildenafil (Viagra) in patients with cardiovascular disease. Technology and Practice Executive Committee. Circulation. 1999 Jan 5–12;99(1):168–177. doi: 10.1161/01.cir.99.1.168. [DOI] [PubMed] [Google Scholar]
- 27.Titova E, Ostrowski RP, Zhang JH, Tang J. Experimental models of subarachnoid hemorrhage for studies of cerebral vasospasm. Neurol Res. 2009 Jul;31(6):568–581. doi: 10.1179/174313209X382412. [DOI] [PubMed] [Google Scholar]
- 28.Sozen T, Tsuchiyama R, Hasegawa Y, et al. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009 Jul;40(7):2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis GD, Witzke C, Colon-Hernandez P, Guerrero JL, Bloch KD, Semigran MJ. Sildenafil improves coronary artery patency in a canine model of platelet-mediated cyclic coronary occlusion after thrombolysis. J Am Coll Cardiol. 2006 Apr 4;47(7):1471–1477. doi: 10.1016/j.jacc.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 30.Berkels R, Klotz T, Sticht G, Englemann U, Klaus W. Modulation of human platelet aggregation by the phosphodiesterase type 5 inhibitor sildenafil. J Cardiovasc Pharmacol. 2001 Apr;37(4):413–421. doi: 10.1097/00005344-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008 Nov;28(11):1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]