Abstract
Psychological insulin resistance (PIR) affects patients’ self-care behaviors and quality of life due to the delay of insulin treatment for optimal glycemic control. Although effective patient-provider communication and relationships have been shown to improve patients’ overall treatment adherence and attitude toward treatment, little is known about the potential mechanisms by which effective patient-provider communication and relationships decrease PIR and whether these relationships are mediated by diabetes self-efficacy. The purpose of this study is to examine whether diabetes self-efficacy among patients with type 2 diabetes (T2D) mediates the relationships between PIR and perceived patient-provider relationships. A total of 178 patients with T2D participated in a cross-sectional study. Data were obtained by patient interview using validated measures of diabetes attitude, diabetes knowledge, self-efficacy, and patient-provider communication. PIR was measured by using a validated measure, Barriers to Insulin Treatment. A structural equation model was developed to estimate direct and indirect effects of patient-provider relationship on PIR when self-efficacy was controlled as a mediator. Diabetes knowledge and attitude were not significantly associated with PIR. Better patient-provider relationship was directly associated with lower PIR (β = −.40, p = 0.008). When diabetes self-efficacy was included as a mediator, the direct effect between patient-provider relationship and PIR changed (β = −.27, p = 0.034), indicating that better patient-provider relationship that reduces PIR is due to greater diabetes self-efficacy. The findings suggest that development of intervention programs aimed at improving diabetes self-efficacy—which may be positively correlated with better patient-provider relationship—is needed to reduce PIR.
Keywords: psychological insulin resistance, self-efficacy, patient-provider relationship, mediator
1. Introduction
Type 2 diabetes (T2D) is a chronic illness that requires continual health care and active patient self-management to prevent long-term complications and acute complications. Although substantial research has shown that diabetes treatment through oral agents and lifestyle modification improves glycemic control and prevents diabetes complications, a large percentage of T2D patients will eventually require exogenous insulin therapy to achieve and maintain recommended targets for glycemic control given the progressive nature of this disease (U.K. prospective diabetes study 16. overview of 6 years' therapy of type II diabetes: A progressive disease. U.K. prospective diabetes study group.1995; Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group.1998). Unfortunately, many T2D patients are reluctant to start insulin treatments—often termed “psychological insulin resistance” (PIR)(Polonsky, Fisher, Guzman, Villa-Caballero, & Edelman, 2005). Prevalence of PIR has been reported as a range from 30% to 50%, across various settings (e.g., community and hospitals) and in studies including observational studies and randomized controlled trials (United kingdom prospective diabetes study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years.1995; Peyrot et al., 2005).
The delay of insulin initiation for significant periods of time in patients with T2D not meeting glycemic target goals resulting from PIR prolongs poor glycemic control, may increase diabetes complications and impair quality of life. Factors affecting PIR include patients’ demographics: Women and ethnic minorities tend to have more PIR compared to their counterparts, and patient beliefs, attitudes, and knowledge about diabetes and its treatment (Machinani, Bazargan-Hejazi, & Hsia, 2013; Nam, Chesla, Stotts, Kroon, & Janson, 2010; Polonsky et al., 2005). There are also misconceptions about insulin treatment. Many patients perceive insulin treatment as a personal failure of their diabetes self-management and that insulin treatment may cause serious negative consequences such as blindness or amputation (Polonsky et al., 2005). Not only patients’ diabetes knowledge and attitude toward diabetes but also health care providers’ attitude toward diabetes and their communication style influence patients’ self-management adherence (Egede & Michel, 2002; Puder & Keller, 2003). Some providers reported that they doubted the efficacy of diabetes treatment and a lack of confidence in insulin treatment and treatment for other diabetes related complications (Brown et al., 2002).
Studies show that patients receive a message from providers, implying that insulin would be the last resort in diabetes treatment (Meece, 2006). Accepting insulin treatment seems to be challenging for patients who have worked hard for their diabetes care for many years. A good patient-provider relationship predicts better diabetes self-management and outcomes (Heisler, Bouknight, Hayward, Smith, & Kerr, 2002; Schillinger et al., 2003). Similarly, ineffective provider communication is associated with more barriers to patients’ diabetes self-management and poorer treatment adherence (Ciechanowski, Katon, Russo, & Walker, 2001).
Patient’s diabetes self-efficacy is shown to be a predictor of medication adherence and associated with other psychosocial variables such as attitudes, perceived relationship with health care providers, perceived social support, and quality of life (Celano, Beale, Moore, Wexler, & Huffman, 2013; Sarkar, Fisher, & Schillinger, 2006). Studies have also found that self-efficacy is a potential mediator in the relationship among treatment adherences and various psychosocial variables such as social support and depression (Kadden & Litt, 2011; Sacco et al., 2007; Tovar, Rayens, Gokun, & Clark, 2013); however, the mechanism in PIR and its relationship with other modifiable factors— self-efficacy and patient-provider relationship remains unknown. Understanding such factors associated with PIR may improve patients’ ability to agree to the therapy and to participate in self-management of T2D using the prescribed therapy.
Current approaches to address PIR are largely educational, using classes and workshops to change attitudes and behavior; therefore, it is important to understand potential mechanisms by which effective patient-provider communication and relationships decrease PIR and whether the relationships are mediated by diabetes self-efficacy.
Based on the literature reviewed, we have the following hypotheses: (1) Each of diabetes attitude, diabetes knowledge, diabetes self-efficacy and patient-provider relationship is associated with PIR. (2) The relationship between patient-provider relationship and PIR is mediated by diabetes self-efficacy, where a better patient-provider relationship is associated with greater diabetes self-efficacy. (3) The mediating effect of diabetes self-efficacy would differ across gender and racial groups.
2. Methods
2.1 Sample and Setting
A descriptive correlational cross-sectional survey was conducted of 82 men and 96 women recruited from urban residential areas of the San Francisco Bay Area. The participants were recruited through flyers posted at the two adult general internal medicine clinics and at the Diabetes Teaching Center at the University of California, San Francisco, two local community clinics and three churches. Ethical approval for the study was obtained from University Institutional Review Board, and all participants provided written informed consent. Inclusion criteria were as follows: 18 years or older, diagnosed with T2D, being treated with diabetic oral agents, and able to speak English. Patients with the following conditions were excluded: type 1 diabetes, severe psychiatric disease, such as active schizophrenia and drug dependency, dementia or on current insulin treatment were excluded. Data were collected by face-to-face interview in doctors’ offices or phone interview and medical records were reviewed for clinical data related to diabetes.
2.2 Variables and Measures
Demographic and biomedical data
Demographic data were obtained by patient self-report. HbA1c level (the most recent value within the last 4 months), chronic disease co-morbidities (e.g. congestive heart failure, chronic obstructive pulmonary disease, or arthritis) and diabetes-related complications (nephropathy, retinopathy, neuropathy) were obtained from the patient’s self-report and medical records.
Diabetes attitude
Attitude toward diabetes was measured with the Diabetes Attitude Scale (DAS-3)(Anderson, Fitzgerald, Funnell, & Gruppen, 1998). The DAS-3 is a self-reported measure whose items are scored on a five-point Likert scale that ranges from “strongly disagree” (scored 1) to “strongly agree” (scored 5). A high score reflects positive attitudes toward diabetes. The possible score of each subscale ranges from 1 to 5; it is calculated by summing the score and dividing by the total number of items in that subscale. The five subscales are as follows: Need for special training to provide diabetes care (5 items), Seriousness of type 2 diabetes (7 items), Values of tight control (7 items), Psychosocial impact of diabetes (6 items), and Patient autonomy (8 items). The internal consistency reliability for this study as measured by Cronbach’s alpha coefficient was 0.78.
Diabetes knowledge
Knowledge was measured with the validated Diabetes Knowledge Test (DKT)(Fitzgerald et al., 1998). The DKT has two components: a 14-item general test and a 9-item insulin use subscale. Only the 14-item general test was administered to these participants. The test is scored as a percentage of questions answered correctly.
Diabetes self-efficacy
Self-efficacy was measured with the Diabetes Self-Efficacy Scale
(DSES)(Rapley, Passmore, & Phillips, 2003). The response options are rated on a Likert scale ranging from 1 (strongly agree) to 6 (strongly disagree). The 13 positively worded items are reverse-scored, so higher scores indicate a higher level of self-efficacy. The range of possible scores is 1 to 6. Either the total scale score or the following five subscales scores can be used: Diabetic routine (4 items), Self-treatment (5 items), Certainty about self-care (4 items), Diet (3 items), and Exercise (2 items). Cronbach’s alpha internal consistency reliability coefficient for this study was 0.84.
Provider-patient relationship
Provider-patient relationship was measured with the Interpersonal Processes of Care Survey-18 (IPC-18)(Stewart, Napoles-Springer, & Perez-Stable, 1999). Respondents reported on the care they had received from their providers over the past 12 months. The measurement included three broad domains: communication, decision making, and interpersonal style; each has several subdomains. Communication has three subscales (lack of clarity, elicited concerns, and explained results). Decision making has one subscale (worked together) and interpersonal style has three subscales (provider’s compassionate and respectful interpersonal style, discrimination due to race/ethnicity, and disrespectful office staff). For each item, respondents are asked how often that type of care had been provided using a five-point scale (1, never; 2, rarely; 3, sometimes; 4, usually; 5, always). Higher scores indicate “better” processes (e.g., decided together). The items that are worded negatively (e.g., lack of clarity) were reversed prior to summary scoring. Internal consistency reliability for this study was 0.88.
Psychological insulin resistance (PIR)
PIR was measured with the Barriers to Insulin Treatment (BIT)(Petrak et al., 2007). The BIT scale measures various aspects of psychological barriers to insulin treatment and attitude toward insulin treatment in T2D patients. The BIT questionnaire includes 14 items. Each item is measured with a 10-point numerical rating scale with the following five subscales: Fear of injection and self-testing, Expectations regarding positive insulin-related outcomes, Expected hardship from insulin treatment, Stigmatization by insulin injection, and Fear of hypoglycemia. The numerical values for a set of items in a particular subscale are added and the total is divided by the number of items in the subscale. The resulting value is the score for that subscale. An overall score for the BIT can be calculated by adding all of the item scores and dividing by 14. The coefficient alpha values for this study was 0.88.
2.3 Data analysis
Descriptive statistics, univariate and bivariate analyses, and multiple linear regression were performed using Statistical Package for the Social Science (SPSS). All hypothesis testing was 2-sided; type I error was controlled at the 0.05 level. To analyze the mediating effect of diabetes self-efficacy, descriptive statistics, Pearson’s correlation, and path diagram were carried out using SPSS and Analysis of Moment Structure (AMOS) software.
Mediation occurs when X (referring to the exogenous causal influence) is significantly related to M (referring to mediator or endogenous causal influence), M is significantly related to Y (referring to the dependent variable), and the relationship of X to Y diminishes when M is in the model (Baron & Kenny, 1986). Correlation structure among the three variables using Pearson’s correlation r was calculated to check if the first prerequisite for mediation effect was established. If this correlational structure is observed, a mediation analysis will provide support for mediation. Then, a structure equation model (SEM) with a bootstrapping approach was used to test whether diabetes self-efficacy mediated the relationship between IPC and BIT. A mediating effect was found when the association between the variables in the SEM became reduced or disappeared as the mediating variables are introduced into the equation. If the effect of X on Y was no longer significant after controlling for M, complete mediation was inferred. If the direct effect of the mediator construct, M, accounted for a significant amount of variance in Y, but XY path remained significant, a partial mediated relationship was indicated.
The model fit was performed using the AMOS program to confirm the models. Chi-square goodness- of- fit (χ2/df), normed fit index (NFI), root mean square error of approximation (RMSEA), standardized root mean square residual (SRMR), and comparative fit index (CFI) were applied to estimate the model fit. The chi-square goodness- of- fit can test the difference between the observed covariance matrix and the one predicted by the specified model; however this index is sensitive to sample size; therefore, the relative goodness- of- fit indices such as NFI, CFI, and SRMR are strongly recommended (Bentler, 1990). The low value of chi-square with non-significance indicates that the hypothesized model is a good fit (Schaufeli, Salanova, González-romá, & Bakker, 2002). An acceptable fit of other indices were the RMSEA ≤0.10, NFI ≥0.80, CFI ≥0.80, and SRMR ≤0.08 (Hu & Bentler, 1999).
3. Results
3.1 Demographics of participants
The characteristics of participants (n=178) are presented in Table 1. Mean age of participants was 64.3 years and gender 54% females. The ethnically diverse sample included 32.6% Asians, 31.5% Whites, 25.3% African Americans and 10.6% others. The mean HbA1c level was 6.98% (SD = 0.99; range = 5.2 to 11.0). There were no significant differences in HbA1c level by different racial groups. Mean duration of diabetes diagnosis was 7.03 (± 4.07) years. All participants were taking diabetes oral medication.
Table 1.
Demographics of participants (n=178)
| Characteristics | Mean ±SD | N (%) | |
|---|---|---|---|
| Age | Range: | 64.3 ± 13.54 | |
| Gender | Female | 96(53.9) | |
| Race | Asians | 58(32.6) | |
| Blacks | 45(25.3) | ||
| Whites | 56(31.5) | ||
| Others (American Indians or Alaska Natives, Native Hawaiian or Pacific Islander) | 19(10.6) | ||
| Education | Less than high school | 14 (7.9) | |
| High school graduate | 36(20.2) | ||
| Some college 1–3years | 65(36.5) | ||
| Bachelor’s degree | 36(20.2) | ||
| Graduate degree | 27(15.2) | ||
| HbA1C* | Range: 5.2–11.0 | 6.98 ± 0.99 | |
| Number of comorbidities | None | 118(66.3) | |
| 1 | 42 (23.6) | ||
| 2 | 7(3.9) | ||
| 3 | 1(0.6) | ||
| Number of microvascular diabetic complications‡ | None | 146(82.0) | |
| 1 | 16(9.0) | ||
| 2 | 5(2.8) | ||
| 3 | 1(0.6) |
HbA1C: glycosylated hemoglobin, *n=158
n=168
Comorbidities: congestive heart failure, chronic obstructive pulmonary disease, and arthritis
3.2 Correlations among variables
Test the correlation matrix among variables is described in Table 2. BIT was negatively correlated with DSES (r=−.294, p<0.001) and IPC (r=−.409, p<0.001). DSES was positively correlated with IPC (r=.292, p<0.001); however, no significant correlations were found in our study between BIT and HbA1c (r=.025, p=.755), between BIT and DAS (r=−.032, p=.673), or between BIT and DKT (r=.035, p=.642). Therefore, the first prerequisite for mediation analysis was met among three variables such as DSES, IPC and BIT but not among DAS and DKT.
Table 2.
Correlation matrix among variables
| r-value | BIT | DSES | IPC | DAS | DKT |
|---|---|---|---|---|---|
| BIT | - | ||||
| DSES | −.294** | - | |||
| IPC | −.409** | .292** | - | ||
| DAS | −.032 | .057 | −.047 | - | |
| DKT | .035 | .117 | −.022 | .445** | - |
p<.001
BIT: Barriers to Insulin Treatment
DSES: Diabetes Self-Efficacy Scale
IPC: Interpersonal Process of Care
DAS: Diabetes Attitude Scales
DKT: Diabetes Knowledge
3.3 Mediational Analysis
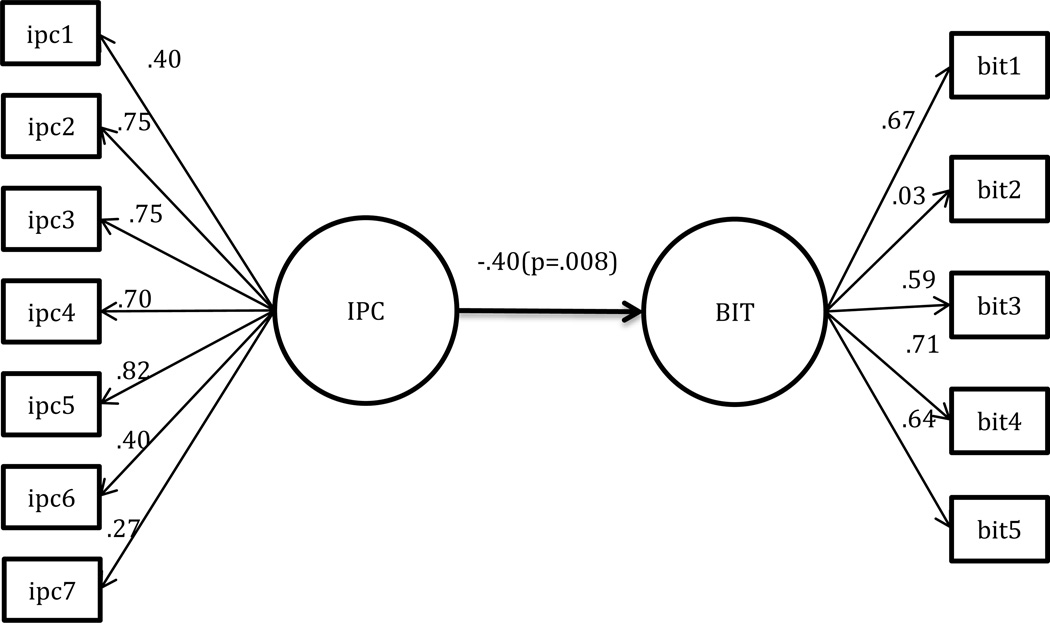
The path model with direct standardized coefficients is shown in Figure 1. The direct standardized effect of IPC on BIT was −.40 (p = 0.008), indicating that participants with better patient-provider relationship are directly associated with fewer barriers to insulin treatment. The direct effect model fit indices included chi-square goodness- of- fit (χ2/df) (1.929), RMR (1.304), NFI (0.834), RMSEA (0.072), CFI (0.873), and SRMR (0.078) (Table 3).
Figure 1.
Direct Path Model predicting Barriers to Insulin Treatment (BIT)
Interpersonal Processes of Care Survey (IPC)
ipc 1: Communication, Lack of clarity
ipc 2: Communication, Elicited concern
ipc 3: Communication, Explained results
ipc 4: Decision making, Worked together
ipc 5: Interpersonal style, Compassionate, respectful
ipc 6: Interpersonal style, Discriminated due to race/ethnicity
ipc 7: Interpersonal style, disrespectful office staff
Barriers to Insulin Treatment (BIT)
bit1: Fear of injection
bit 2: Expectations regarding positive outcome
bit 3: Expected hardship
bit 4: Stigmatization
bit 5: Fear of hypoglycemia
Table 3.
Goodness of Fit Statistics for the Model 1 and Model 2
| Model | χ2 | df | p | χ2/df | NFI | RMSEA | SRMR | CFI |
|---|---|---|---|---|---|---|---|---|
| Direct Effect Model | 102.244 | 53 | <.01 | 1.929 | 0.834 | 0.072 | 0.078 | 0.873 |
| Partial Mediation Model | 195.908 | 116 | <.01 | 1.689 | 0.784 | 0.062 | 0.073 | 0.896 |
RMR: Root Mean square Residual
NFI: Normed Fit Index
RMSEA: Root Mean Square Error of Approximation
SRMR: Standardized RMR
CFI: Comparative Fit Index
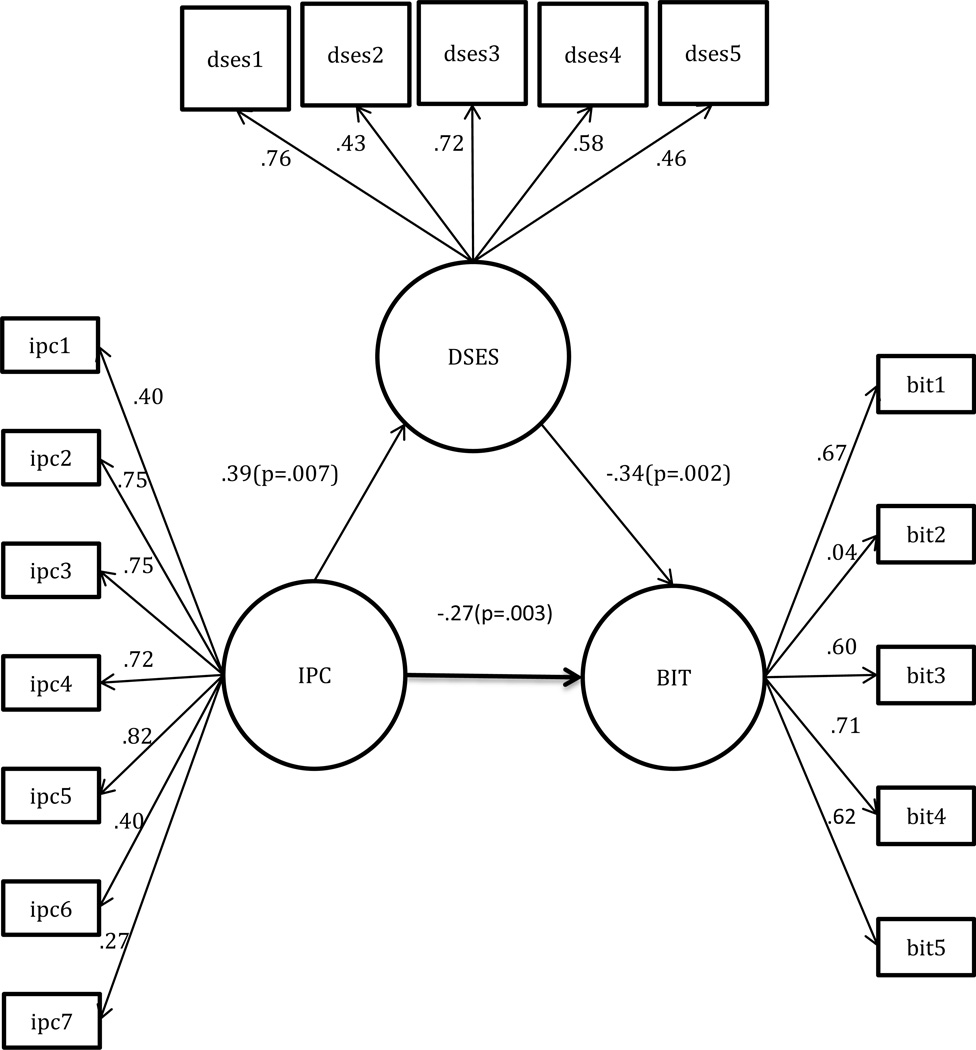
An indirect effect between IPC and BIT was identified in the path diagram. As shown in Figure 2, the standardized indirect effect of IPC on BIT mediated by self-efficacy was −.27 (p =.003), indicating that better patient-provider relationship that reduces PIR is in part due to greater diabetes self-efficacy. Better perceived patient-provider relationship was associated with greater diabetes self-efficacy (β = 0.39, p = .007). Participants who had greater self-efficacy perceived fewer barriers to insulin treatment (β = −0.34, p = .002). That is, a negative relationship between BIT and IPC remained significant after adjusting for diabetes self-efficacy and there was a significant reduction in magnitude (β reduction in magnitude from −0.40 to −0.27) predicting the negative relationship between BIT and IPC (β = −0.27, p = .003); therefore, diabetes self-efficacy partially mediated the prediction of barriers to insulin treatment by patient-provider relationship. Overall, the mediation model showed better fit compared to the direct model as with chi-square goodness of fit (χ2/df) (1.689), NFI (0.784), RMSEA (0.062), CFI (0.896), and SRMR (0.073) (Table 3).
Figure 2.
Standardized Path Estimates for the Mediating Impact of Diabetes Self-Efficacy (DSES)
Interpersonal Processes of Care Survey (IPC)
ipc 1: Communication, Lack of clarity
ipc 2: Communication, Elicited concern
ipc 3: Communication, Explained results
ipc 4: Decision making, Worked together
ipc 5: Interpersonal style, Compassionate, respectful
ipc 6: Interpersonal style, Discriminated due to race/ethnicity
ipc 7: Interpersonal style, disrespectful office staff
Barriers to Insulin Treatment (BIT)
bit1: Fear of injection
bit 2: Expectations regarding positive outcome
bit 3: Expected hardship
bit 4: Stigmatization
bit 5: Fear of hypoglycemia
Diabetes Self-Efficacy Scale (DSES)
dses 1: Diabetes routine
dses 2: Self-treat
dses 3: Certainty
dses 4: Diet
dses 5: Exercise
Our findings did not support the hypothesis that the mediating effect of diabetes self-efficacy on PIR would differ across gender and racial groups. That is, there was no significant moderating effect of gender and race groups.
4. Discussion
The findings from the our study show that better patient-provider relationship is associated with lower PIR and that patients’ diabetes self-efficacy partially mediates the relationship between patient-provider relationship and PIR. Studies have shown that effective patient-provider relationship and communication affect patient’s psychosocial and biomedical outcomes (Ciechanowski et al., 2001; Heisler et al., 2002; Schillinger et al., 2003). Yet, the mechanism in the patient-provider relationship and PIR has been unknown. There may be several pathways to explain our findings in the role of diabetes self-efficacy between patient-provider relationship and PIR. With the findings from the our study, we argue that some healthcare providers may convey more effective message to patients about how to practice diabetes self-care, or provide better emotional and informational support to patients than other providers and in turn increase patient’s diabetes self-efficacy. Patients with high self-efficacy will be more persistent in the face of obstacles (Bandura, 1977); that is, patients with strong self-efficacy in their own ability to manage diabetes may feel they can also handle insulin treatment and have low insulin-related distress through diabetes self-efficacy.
A low level of self-efficacy is shown to be associated with either overprotection or lack of support (Schokker et al., 2011). For example, in a study of the role of overprotection by the partner in coping with diabetes, the relationship between overprotection and diabetes self-efficacy was stronger for patients with worse glycemic control, implying that in patients with poor glycemic control, overprotection by a partner is more strongly associated with self-efficacy than in patients with good glycemic control (Schokker et al., 2011). HbA1c in most of our study sample, however, showed that their diabetes was under good control with their oral medication and was not associated with the BIT score; therefore, we did not see additional moderating effect of glucose control. Future study including more patients with poorly controlled diabetes may be helpful to understand whether the relationship between patient-provider relationship and PIR would apply more strongly for patients with worse glycemic control by interacting with the patients’ self-efficacy.
Diabetes knowledge was not associated with either diabetes self-efficacy, patient-provider relationship, or patients’ PIR. Studies that examined the relationship between diabetes knowledge and adherence to self-care behaviors have shown mixed findings (Holmstrom & Rosenqvist, 2005; Murata et al., 2003; Pace, Ochoa-Vigo, Caliri, & Fernandes, 2006), indicating that having better diabetes knowledge does not always lead to better patient diabetes self-care behaviors.
Studies have shown that patients who have positive attitudes toward managing their diabetes are more likely to change their behavior in order to control their blood glucose levels than those with negative attitudes (Farmer, Kinmonth, & Sutton, 2006; Nam, Chesla, Stotts, Kroon, & Janson, 2011). In our study, the diabetes attitude was not associated with PIR. Our findings may stem from the instrument that measured diabetes attitude that included the following aspects: value of tight diabetes control, seriousness of diabetes, or psychosocial impact of diabetes. For example, participants who value the importance of diabetes control and take diabetes as a serious illness may think that insulin treatment is necessary. At the same time, the participants may also anticipate more psychosocial impact from using insulin. Therefore, these conflicting attitudes may contribute to the strength of association between diabetes attitude and PIR toward the null.
Women have been found to report more diabetes-related distress than men (Fisher et al., 2008; Helgeson & Novak, 2007). Studies have shown that women tend to be affected or rely on interpersonal relationship-oriented aspects such as support from others and interpersonal relationships have a stronger impact on illness perception among women compared to men(Kawachi & Berkman, 2001). In our previous study, women also had more fear of insulin injection than men (Nam et al., 2010); therefore, we hypothesized that the association between patient-provider relationship and PIR is stronger for women. The findings concluded by the moderating analysis, however, contradicted our hypothesis of the group difference by gender and race.
There are several limitations in the current study. Although the study sample includes various ethnic/racial groups, these data from a small convenience sample, whose diabetes is relatively under control, may not accurately represent individuals with T2D and are not of sufficient sample size to test multiple hypotheses. Patients with high HbA1c may have lower PIR than those who with better diabetes control by seeking more effective diabetes treatment after experiencing the effect of oral agents. Therefore our study may not generalize to patients with severe diabetes or diabetes complications. Another limitation is that the cross-sectional study design cannot establish a causal relationship between patient-provider relationship and self-efficacy. For example, whether ineffective patient-provider relationship contributes to poor diabetes self-efficacy or whether patients with poorer diabetes self-efficacy may perceive their relationship with healthcare providers as less effective and may feel that they receive insufficient support from healthcare providers which they needed, including the skills and knowledge that insulin treatment requires. Despite the limitations, the findings from this study provide an important insight on which factor is needed to target to reduce PIR by examining a mediator in the relationship between patient-provider relationships and PIR, and can guide future intervention development for this population. Our study findings suggest that training and support for diabetes self-efficacy should be incorporated into diabetes self-management education. Directions for future research include developing interventions to improve diabetes self-efficacy based on various health behavior and chronic illness models. It is equally important to develop interventions that can provide healthcare providers with education and system support to better serve patients with complicated chronic illnesses such as T2D.
5. Conclusion
This study demonstrates that self-efficacy is a mediator of the relationship between patient-provider relationships and PIR. This finding has important clinical implications as interventions are designed to improve self-efficacy among individuals with T2D for optimal glucose control by introducing insulin in a timely manner. It also suggests the need for more attention on the role of healthcare providers, specifically the importance of their communication style, and support in bolstering patients’ self-efficacy and decreasing barriers to insulin treatment.
Acknowledgments
Funding
Soohyun Nam was supported by a National Institutes of Health (NIH), National Institute of Nursing Research (1F31NR010450) and the Sigma Theta Tau International Honor Society of Nursing.
Footnotes
Conflicts of Interest
The authors declare that they have no potential conflicts of interest relevant to this article.
References
- Anderson RM, Fitzgerald JT, Funnell MM, Gruppen LD. The third version of the diabetes attitude scale. Diabetes Care. 1998;21(9):1403–1407. doi: 10.2337/diacare.21.9.1403. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Brown JB, Harris SB, Webster-Bogaert S, Wetmore S, Faulds C, Stewart M. The role of patient, physician and systemic factors in the management of type 2 diabetes mellitus. Family Practice. 2002;19(4):344–349. doi: 10.1093/fampra/19.4.344. [DOI] [PubMed] [Google Scholar]
- Celano CM, Beale EE, Moore SV, Wexler DJ, Huffman JC. Positive psychological characteristics in diabetes: A review. Current Diabetes Reports. 2013;13(6):917–929. doi: 10.1007/s11892-013-0430-8. 10.1007/s11892-013-0430-8. [DOI] [PubMed] [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE, Walker EA. The patient-provider relationship: Attachment theory and adherence to treatment in diabetes. The American Journal of Psychiatry. 2001;158(1):29–35. doi: 10.1176/appi.ajp.158.1.29. [DOI] [PubMed] [Google Scholar]
- Egede LE, Michel Y. Attitudes of internal medicine physicians toward type 2 diabetes. Southern Medical Journal. 2002;95(1):88–91. [PubMed] [Google Scholar]
- Farmer A, Kinmonth AL, Sutton S. Measuring beliefs about taking hypoglycaemic medication among people with type 2 diabetes. Diabetic Medicine : A Journal of the British Diabetic Association. 2006;23(3):265–270. doi: 10.1111/j.1464-5491.2005.01778.x. [DOI] [PubMed] [Google Scholar]
- Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabetic Medicine : A Journal of the British Diabetic Association. 2008;25(9):1096–1101. doi: 10.1111/j.1464-5491.2008.02533.x. 10.1111/j.1464-5491.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JT, Funnell MM, Hess GE, Barr PA, Anderson RM, Hiss RG, Davis WK. The reliability and validity of a brief diabetes knowledge test. Diabetes Care. 1998;21(5):706–710. doi: 10.2337/diacare.21.5.706. [DOI] [PubMed] [Google Scholar]
- Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. Journal of General Internal Medicine. 2002;17(4):243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson VS, Novak SA. Illness centrality and well-being among male and female early adolescents with diabetes. Journal of Pediatric Psychology. 2007;32(3):260–272. doi: 10.1093/jpepsy/jsl018. [DOI] [PubMed] [Google Scholar]
- Holmstrom IM, Rosenqvist U. Misunderstandings about illness and treatment among patients with type 2 diabetes. Journal of Advanced Nursing. 2005;49(2):146–154. doi: 10.1111/j.1365-2648.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. [Google Scholar]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- Kadden RM, Litt MD. The role of self-efficacy in the treatment of substance use disorders. Addictive Behaviors. 2011;36(12):1120–1126. doi: 10.1016/j.addbeh.2011.07.032. 10.1016/j.addbeh.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Berkman LF. Social ties and mental health. Journal of Urban Health : Bulletin of the New York Academy of Medicine. 2001;78(3):458–467. doi: 10.1093/jurban/78.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machinani S, Bazargan-Hejazi S, Hsia SH. Psychological insulin resistance among low-income, U.S. racial minority patients with type 2 diabetes. Primary Care Diabetes. 2013;7(1):51–55. doi: 10.1016/j.pcd.2012.11.003. 10.1016/j.pcd.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meece J. Dispelling myths and removing barriers about insulin in type 2 diabetes. The Diabetes Educator. 2006;32(1 Suppl):9S–18S. doi: 10.1177/0145721705285638. [DOI] [PubMed] [Google Scholar]
- Murata GH, Shah JH, Adam KD, Wendel CS, Bokhari SU, Solvas PA, Duckworth WC. Factors affecting diabetes knowledge in type 2 diabetic veterans. Diabetologia. 2003;46(8):1170–1178. doi: 10.1007/s00125-003-1161-1. [DOI] [PubMed] [Google Scholar]
- Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care. 2010;33(8):1747–1749. doi: 10.2337/dc10-0099. 10.2337/dc10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: Patient and provider factors. Diabetes Research and Clinical Practice. 2011;93(1):1–9. doi: 10.1016/j.diabres.2011.02.002. 10.1016/j.diabres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Pace AE, Ochoa-Vigo K, Caliri MH, Fernandes AP. Knowledge on diabetes mellitus in the self care process. Revista Latino-Americana De Enfermagem. 2006;14(5):728–734. doi: 10.1590/s0104-11692006000500014. [DOI] [PubMed] [Google Scholar]
- Petrak F, Stridde E, Leverkus F, Crispin AA, Forst T, Pfützner A. Development and validation of a new measure to evaluate psychological resistance to insulin treatment. Diabetes Care. 2007;30(9):2199–2204. doi: 10.2337/dc06-2042. [DOI] [PubMed] [Google Scholar]
- Peyrot M, Rubin RR, Lauritzen T, Skovlund SE, Snoek FJ, Matthews DR International DAWN Advisory Panel. Resistance to insulin therapy among patients and providers: Results of the cross-national diabetes attitudes, wishes, and needs (DAWN) study. Diabetes Care. 2005;28(11):2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: The scope of the problem. Diabetes Care. 2005;28(10):2543–2545. doi: 10.2337/diacare.28.10.2543. [DOI] [PubMed] [Google Scholar]
- Puder JJ, Keller U. Quality of diabetes care: Problem of patient or doctor adherence? Swiss Medical Weekly. 2003;133(39–40):530–534. doi: 10.4414/smw.2003.10290. [DOI] [PubMed] [Google Scholar]
- Rapley P, Passmore A, Phillips M. Review of the psychometric properties of the diabetes self-efficacy scale: Australian longitudinal study. Nursing & Health Sciences. 2003;5(4):289–297. doi: 10.1046/j.1442-2018.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- Sacco WP, Wells KJ, Friedman A, Matthew R, Perez S, Vaughan CA. Adherence, body mass index, and depression in adults with type 2 diabetes: The mediational role of diabetes symptoms and self-efficacy. Health Psychology : Official Journal of the Division of Health Psychology, American Psychological Association. 2007;26(6):693–700. doi: 10.1037/0278-6133.26.6.693. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Fisher L, Schillinger D. Is self-efficacy associated with diabetes self-management across race/ethnicity and health literacy? Diabetes Care. 2006;29(4):823–829. doi: 10.2337/diacare.29.04.06.dc05-1615. [DOI] [PubMed] [Google Scholar]
- Schaufeli W, Salanova M, González-romá V, Bakker A. The measurement of engagement and burnout: A two sample confirmatory factor analytic approach. Journal of Happiness Studies. 2002;3(1):71–92. [Google Scholar]
- Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, Bindman AB. Closing the loop: Physician communication with diabetic patients who have low health literacy. Archives of Internal Medicine. 2003;163(1):83–90. doi: 10.1001/archinte.163.1.83. [DOI] [PubMed] [Google Scholar]
- Schokker MC, Links TP, Bouma J, Keers JC, Sanderman R, Wolffenbuttel BH, Hagedoorn M. The role of overprotection by the partner in coping with diabetes: A moderated mediation model. Psychology & Health. 2011;26(1):95–111. doi: 10.1080/08870440903342325. 10.1080/08870440903342325. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Napoles-Springer A, Perez-Stable EJ. Interpersonal processes of care in diverse populations. The Milbank Quarterly. 1999;77(3):305–339. 274. doi: 10.1111/1468-0009.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar E, Rayens MK, Gokun Y, Clark M. Mediators of adherence among adults with comorbid diabetes and depression: The role of self-efficacy and social support. Journal of Health Psychology. 2013 doi: 10.1177/1359105313512514. [DOI] [PubMed] [Google Scholar]
- U.K. prospective diabetes study 16. overview of 6 years' therapy of type II diabetes: A progressive disease. U.K. prospective diabetes study group. Diabetes. 1995;44(11):1249–1258. [PubMed] [Google Scholar]
- United kingdom prospective diabetes study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ (Clinical Research Ed.) 1995;310(6972):83–88. [PMC free article] [PubMed] [Google Scholar]