Abstract
Interleukin-10 (IL-10), an anti-inflammatory cytokine, may have therapeutic potential in the fetal inflammatory response syndrome and its sequelae such as bronchopulmonary dysplasia (BPD). Our aim was to compare the effects of IL-10 versus dexamethasone (DEX) on important PMN functions of the newborn. PMNs were isolated into culture medium from cord blood after elective cesarean section deliveries. IL-10 and DEX were compared on an equimolar basis corresponding to previously measured plasma levels of DEX from infants treated for BPD. The endotoxin (LPS)-stimulated release of the pro-inflammatory cytokines, tumor necrosis factor (TNFα) and IL-1β, were markedly inhibited equally by IL-10 and DEX; the anti-inflammatory cytokine IL-4 was not released and IL-1 receptor antagonist (IL-1ra) was released less with DEX compared to IL-10. PMNs exposed to LPS, N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP), or S. aureus did not show a significant difference between control, DEX and IL-10 for apoptosis, respiratory burst, phagocytosis or killing respectively. Chemotaxis to fMLP or IL-8 was unaffected by DEX or IL-10. The principal effects of both IL-10 and DEX, on the PMN functions studied, are related to the control of pro- and anti-inflammatory cytokine release.
Polymorphonuclear leukocytes (PMNs) are the principal pro-inflammatory effector cells that contribute to the acute innate immune responses of the fetus and newborn (1). However, an excessive inflammatory response, mediated by PMNs may lead to collateral healthy tissue injury as exemplified by serious adverse sequelae of the fetal inflammatory response syndrome (2). Accordingly, there is increasing interest in developing safe and effective anti-inflammatory therapy other than glucocorticoids (3). During early development of bronchopulmonary dysplasia (BPD), airway fluid contains predominantly PMNs and proinflammatory cytokines; however, interleukin-10 (IL-10), an anti-inflammatory cytokine, is either undetectable or detectable at very low levels (4–9). In previous work, we have shown that the PMN of the newborn does not release IL-10 at detectable levels (10). In addition, we have shown that exogenous IL-10 is equipotent to dexamethasone (DEX) with regard to the inhibition of the pro-inflammatory cytokine, interleukin-8 (IL-8) from stimulated PMNs of the newborn (10). Therefore, IL-10 may have anti-inflammatory therapeutic potential for the fetus and newborn (10, 11). IL-10 has been used in chronic inflammatory disorders of adults (12).
The newborn is predisposed to a variety of inflammatory disorders in which the innate host defense is critical for survival. Before further studies are undertaken with regard to the potential therapeutic use of IL-10, it was considered important to elucidate the effect of IL-10 versus DEX on important PMN functions in the newborn. This study avoided preterm subjects because of the confounding variables of antenatal infection, antenatal steroids, and a variety of maternal conditions and medications associated with indicated premature delivery. While the fetal and neonatal inflammatory response syndrome and the role of the PMN is important for both term and preterm infants (1, 2), there are some known differences in neutrophil functions between these two stages of development (1, 13). Developmental differences regarding the effects of IL-10 versus DEX on PMN functions could exist. No previous studies have compared the equimolar effects of IL-10 and DEX on multiple PMN functions. The aims of this study were to determine the effect of IL-10 versus DEX on the release of pro-inflammatory and anti-inflammatory cytokines from PMNs of the newborn, as well as chemotaxis, phagocytosis, respiratory burst, bacterial killing and apoptosis.
METHODS
Subjects
Cord blood (approximately 30 ml) was obtained from placentas after elective, term, caesarean section deliveries. The deliveries were attended by one of the investigators. Deliveries were not associated with labor, rupture of membranes, meconium-stained fluid, or clinical evidence of chorioamnionitis. Blood was collected in heparinized preservative-free tubes for transport to the laboratory, followed by immediate PMN isolation. A total of 28 cord blood samples were used to study the six different PMN functions. This study was approved by the Institutional Review Board of the North Shore-Long Island Jewish Health System.
PMN isolation
PMNs were isolated under endotoxin-free conditions using Ficoll-Paque PLUS (GE Healthcare Bio-Sciences Corp, Piscataway, NJ, USA) centrifugation. The PMNs were subsequently purified by dextran (Sigma-Aldrich, St. Louis, MO) sedimentation and hypotonic lysis of residual red blood cells as previously described (10). The cells were 95–98% pure PMNs and the viability using trypan-blue exclusion was above 98%. At 18 hrs of culture, PMN viability was >94% for all experimental conditions.
Pro-inflammatory and anti-inflammatory cytokine measurements
In previous work, we demonstrated that LPS at a concentration of 10 ng/ml was a submaximal stimulus for IL-8 release (10). This physiologic and clinically relevant stimulus was used to stimulate PMNs, 5×106 PMNs/ml resuspended in RPMI-1640 supplemented with 10% fetal bovine serum (Gibco-Invitrogen, Grand Island, NY) for 18 hrs at 37°C, at which time aliquots of culture media were taken for ELISA. Prior to LPS stimulation, PMNs were pre-incubated with PBS (Phosphate Buffered Saline, Gibco-Invitrogen) (vehicle), IL-10 (R&D Systems, Minneapolis, MN) or DEX (Abraxis Pharmaceutical Products, Shaumburg, IL), both at 10−8 M, for 1 hr at 37°C. Under the same experimental conditions, we previously found that this level of IL-10 or DEX produced a marked inhibition of LPS-induced IL-8 release from PMNs of the newborn (10, 14). Furthermore, DEX at 10−8 M is in the therapeutic range of DEX levels measured in plasma from infants treated for BPD (15, 16). In the present study, we measured the pro-inflammatory mediators TNFα and IL-1β, as well as the anti-inflammatory mediators IL-4 and IL-1 receptor antagonist (IL-1ra), using commercially available ELISA kits (R&D Systems).
Chemotaxis
Detailed methods for PMN chemotaxis have been previously described by our laboratory (17). Chemotaxis was measured in a 3-tiered, 48-well chemotaxis chamber (Neuro Probe, Gaithersberg, MD) with a 5 µm polycarbonate filter between the upper and lower chambers, and a 2 µm filter at the base of the lower chamber and above the lowest tier. Prior to assembling the chamber, PMNs (3×106 PMNs/ml resuspended in serum-free RPMI-1640) were pre-incubated with serum-free RPMI-1640 (vehicle) or 10−10,10−9,10−8, or 10−7 M IL-10 or DEX at 37°C in pyrogen-free test tubes for 1 hr. The lower chemoattractant chamber was prepared with 50 µl of serum-free RPMI-1640 (control vehicle), IL-8 (R&D Systems) at 10−8 M or N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP, Sigma-Aldrich) at 10−7 M. The upper chamber contained 50 µl of pre-incubated cell suspension with and without IL-10 or DEX, and with and without IL-8 or fMLP. The assembled 48-well chamber was placed in an incubator at 37°C for 1 hr. The chamber was then disassembled. PMN drop-off from the underside of the 5 µm filter was assessed by cytocentrifuging the lower 2 tiers of the chamber at 500 × g for 10 min to collect cells for counting on the 2 µm filter. Filters were stained using a Hema 3 stain set (Fisher Scientific, Kalamazoo, MI). Cells were counted using a light microscope and averaging counts from 5 high-powered fields (hpf, 10 × 40). Total migration was derived from movement of cells from the upper to lower wells when no chemoattractant was placed in the upper chamber. Chemokinesis (random migration) was derived from the movement of cells from the upper to lower chamber when chemoattractant was placed in the upper and lower wells. Chemotaxis (directed movement) was derived by subtracting values of chemokinesis from the respective values of total migration. We corrected the values using migration without chemoattractant in the lower chamber.
Respiratory burst
PMN superoxide anion generation was measured by the superoxide dismutase (SOD) inhibitable reduction of ferricytochrome C at 550 nm (18, 19). Isolated PMNs were resuspended in Hank’s balanced salt solution containing Ca++ and Mg++ (HBSS+, Gibco-Invitrogen) at a concentration of 1×106 PMNs/ml in pyrogen-free test tubes. PMNs were pre-incubated with PBS (vehicle), IL-10 or DEX at 10−8 M concentration for 1 hr at 37°C. Immediately after pre-incubation, cytochrome c (from bovine heart, Sigma-Aldrich, 1 mg/ml) was added to all test samples. Reference samples included SOD (from bovine erythrocytes, Sigma-Aldrich, 50 U/ml) in addition to cytochrome c. Then both test and reference samples were stimulated with PBS (vehicle) or fMLP (10−7 M) for 0, 10, and 20 minutes at 37°C. Incubation was stopped by placing tubes on ice. Cells were then pelleted and the absorbance of the supernatant was measured at 550 nm by a spectrophotometer. Respiratory burst was determined from the difference in absorbance between the test samples and the corresponding reference samples. Results were expressed as nanomoles of O2− produced per 106 cells with an extinction coefficient of 2.1 × 104 M−1 cm−1.
Phagocytosis and killing
The rate of phagocytosis and killing was measured by a two-step assay as previously described in detail (20). Briefly, isolated PMNs were resuspended in HBSS+ at 1×107 PMNs/mL and pre-incubated with PBS (vehicle), IL-10 or DEX, both at 10−8 M, for 1 hr at 37°C. In the meantime, an overnight culture of bacteria (Staphylococcus aureus subsp. aureus ATCC® 27217™) was washed twice with PBS and resuspended in HBSS+. Turbidity was measured at 550 nm and the bacterial concentration was estimated by using a standard curve (turbidity at 550 nm versus the number of colony forming units (CFU) grown on agar). For opsonization, the bacteria were resuspended at 1×108 CFU/mL in HBSS+ containing 10% autologous serum in a glass tube, rotated for 20 min at 37°C, and used immediately. For the two-step assay, PMNs and bacteria were mixed at a 1:10 ratio in a glass tube (experimental tubes). A control tube was included where the PMNs were replaced with HBSS+. The tubes were rotated at 37°C, and 50 µL of sample was taken from the experimental tubes at 5, 10 and 20 min, and 0 and 20 min from the control tube. The 50 µL samples were added to 950 µL of ice-cold PBS, and centrifuged at low speed to pellet the PMNs only. Supernatant (containing extracellular bacteria) was collected without disturbing the PMN pellet (containing intracellular bacteria), and the pellet was washed twice with PBS, pooling the supernatants from the three washes. To release the intracellular bacteria, PMNs were lysed by incubating in 2.5 mL sterile H20, pH 11, for 10 min at room temperature, and then vortexing thoroughly. Samples were further diluted in H20, pH 11, and 20 µL was spread on half a nutrient agar plate (4 half plates/sample). Plates were incubated overnight at 37°C and the colonies formed were counted the next day. The half-lives of phagocytosis and killing were calculated by an Excel data analysis file provided by Green et al (20).
Apoptosis
Caspase-3 activity was used as a screening tool for evolving apoptosis as previously described (21). PMNs (5×106 PMNs/ml) were resuspended in RPMI-1640 supplemented with 10% fetal bovine serum and PMNs were pre-incubated with PBS, IL-10 or DEX (10−8 M) for 1 hr at 37°C. PMNs were then stimulated with LPS (10 ng/ml) for 0, 3, 6, or 18 hrs. Caspase-3 activity was measured by a commercially available colorimetric assay kit (R&D Systems). At the stopping time points PMNs were lysed by the lysing buffer provided by the kit, and the cell lysates stored at −80°C until measurements were made.
RESULTS
Cytokine release
The effect of equimolar levels of IL-10 and DEX (10−8 M) on pro-inflammatory and anti-inflammatory cytokine release from PMNs of the newborn, over 18 hours, is shown in Figure 1. LPS stimulation resulted in an increase in TNFα release that was markedly inhibited by IL-10 (77% reduction) or DEX (55% reduction), with no difference between IL-10 and DEX. LPS stimulation also resulted in an increase in IL-1β release that was markedly inhibited by IL-10 (90% reduction) and DEX (70% reduction), with no difference between IL-10 and DEX. Furthermore, LPS stimulation resulted in an increase in release of the anti-inflammatory mediator, IL-1ra, shown in panel C. In contrast to the pro-inflammatory mediators, IL-10 and DEX did not significantly affect IL-1ra release compared to LPS alone. We could not detect IL-4 release and therefore the data is not shown.
Figure 1.
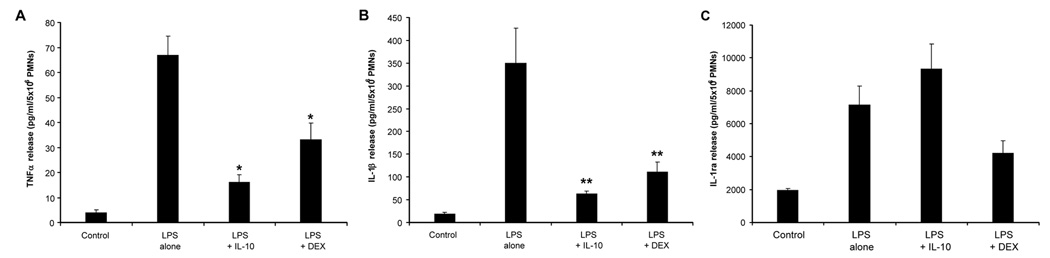
Pro-inflammatory and anti-inflammatory cytokine release from endotoxin (LPS)-stimulated polymorphonuclear leukocytes of the newborn over 18 hours (n=7, mean±SE): effects of pretreatment with equimolar (10−8 M) doses of interleukin-10 and dexamethasone. Panel A shows tumor necrosis factor release, * different from LPS alone, p<0.001. Panel B shows interleukin-1β release, ** different from LPS alone, p<0.01. Panel C shows interleukin-1 receptor antagonist release.
Chemotaxis
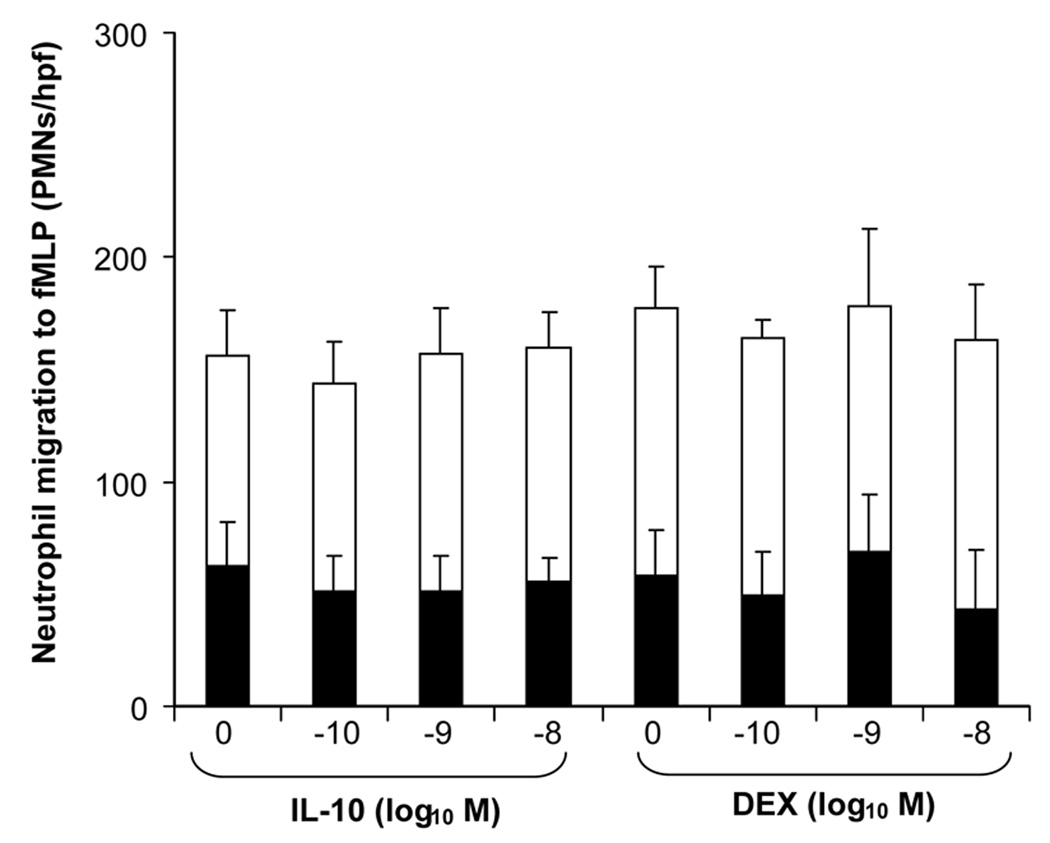
Figure 2 demonstrates the effect of different molar levels of IL-10 and DEX on neutrophil migration to the bacterial peptide chemoattractant fMLP (10−7 M). There was no effect on either total migration (total height of bar), chemokinesis (shaded lower area of bar), or chemotaxis (open, upper area of bar). The levels of DEX and IL-10 used in these experiments were designed to include 10−8 M, which markedly inhibited pro-inflammatory cytokine release as shown in Figure 1. We repeated the same chemotaxis study using IL-8 (10−8 M) instead of fMLP as a chemoattractant, and also found that IL-10 and DEX did not inhibit chemotaxis (data not shown).
Figure 2.
Total migration in vitro, divided into the components of chemotaxis ( ) and chemokinesis (
) and chemokinesis ( ), for polymorphonuclear leukocytes of the newborn using fMLP as the chemoattractant (n=5, mean±SE): effects of different equimolar doses of interleukin-10 and dexamethasone.
), for polymorphonuclear leukocytes of the newborn using fMLP as the chemoattractant (n=5, mean±SE): effects of different equimolar doses of interleukin-10 and dexamethasone.
Respiratory burst
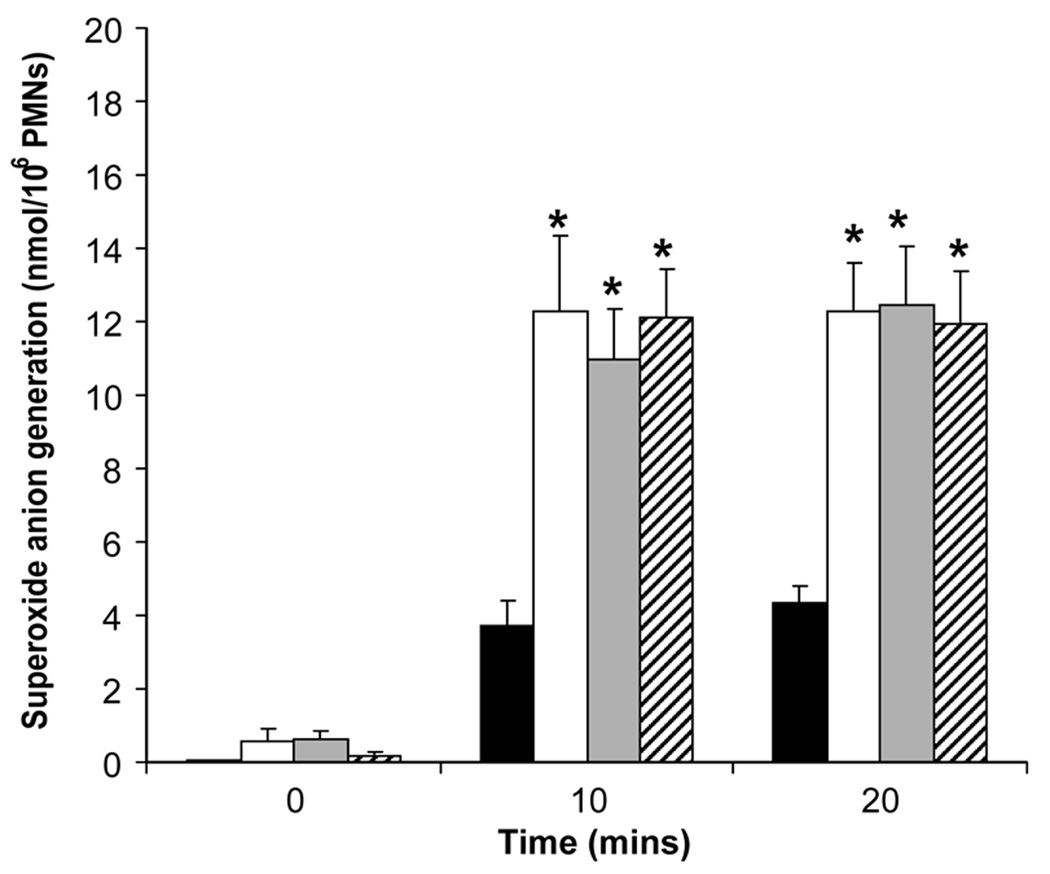
The effect of equimolar levels of IL-10 and DEX (10−8 M) on superoxide anion generation from fMLP-stimulated PMNs over 20 minutes is shown in Figure 3. A significant increase in superoxide anion generation (nmol/106 PMNs) was observed above baseline with stimulation by fMLP. IL-10 and DEX at 10−8 M had no effect on superoxide production at 10 or 20 minutes of fMLP stimulation.
Figure 3.
Superoxide anion generation from fMLP-stimulated polymorphonuclear leukocytes of the newborn over time (n=5, mean±SE): effects of equimolar (10−8 M) doses of interleukin-10 and dexamethasone. Control,
Control, fMLP alone,
fMLP alone, fMLP+IL-10,
fMLP+IL-10, fMLP+DEX; * different from control, p<0.05.
fMLP+DEX; * different from control, p<0.05.
Phagocytosis and killing
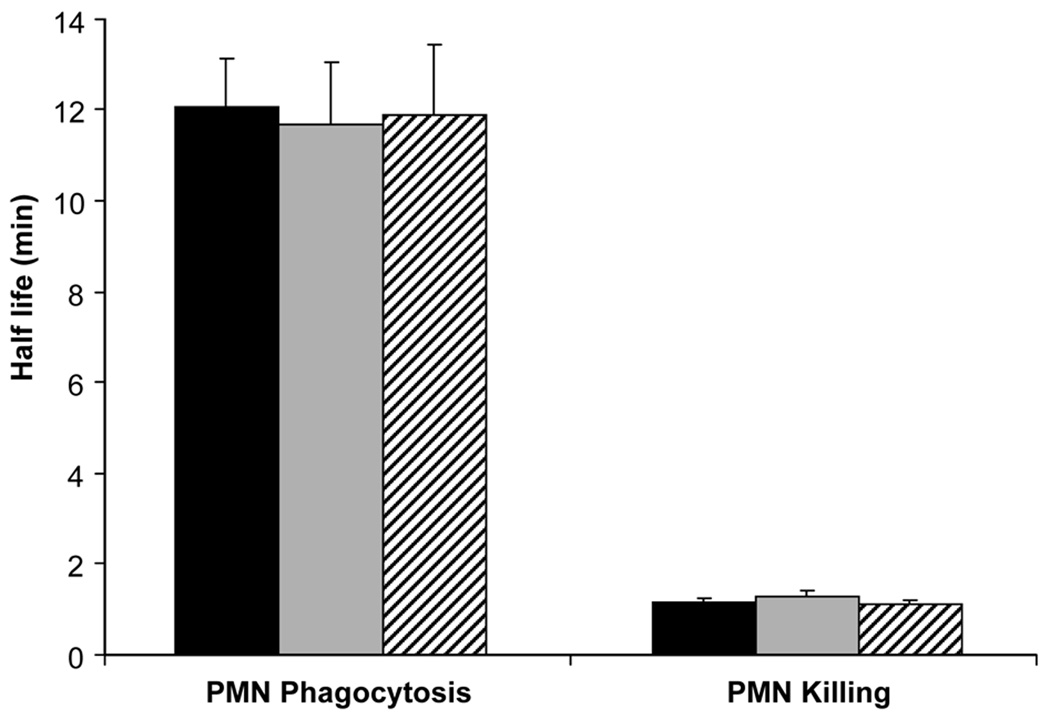
The effect of equimolar levels of IL-10 and DEX (10−8 M) on phagocytosis and killing of S. aureus by PMNs is shown in Figure 4. IL-10 and DEX at 10−8 M had no significant effect on the half-lives of phagocytosis and killing in comparison to untreated PMNs.
Figure 4.
Half-lives of phagocytosis and killing of Staphylococcus aureus by polymorphonuclear leukocytes of the newborn (n=5, mean±SE): effects of pretreatment with equimolar (10−8 M) doses of interleukin-10 ( ) and dexamethasone (
) and dexamethasone ( ) compared to control (
) compared to control ( ).
).
Caspase-3 activity
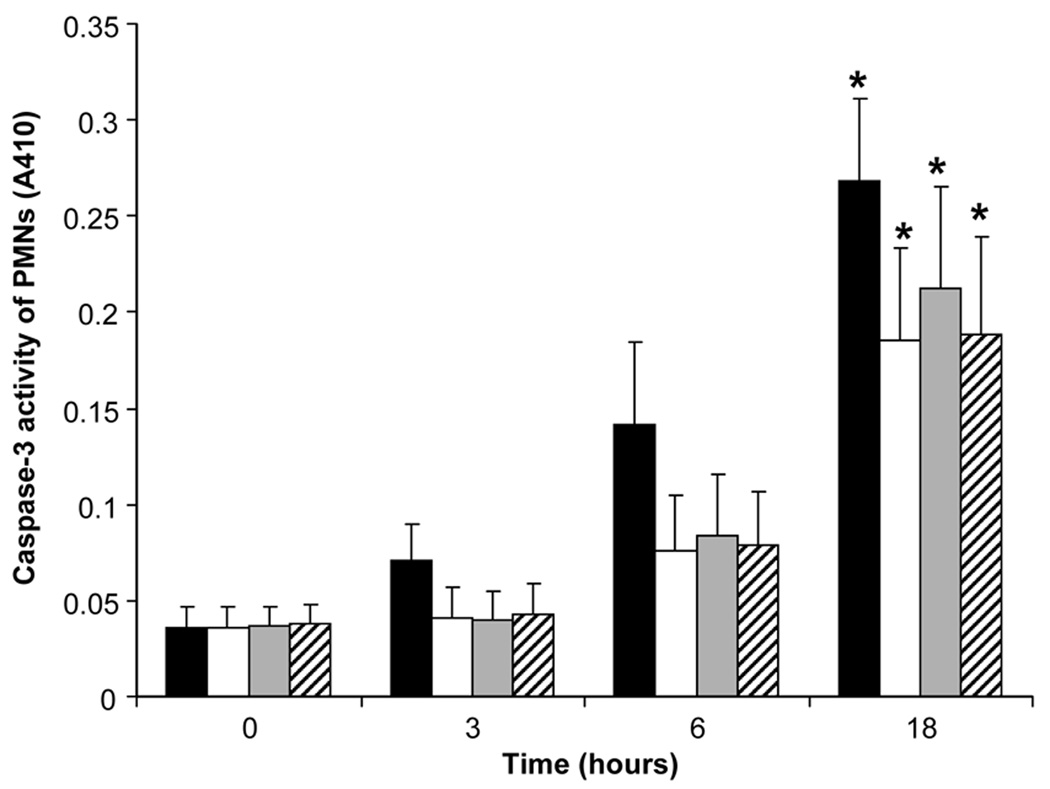
The effect of equimolar doses of IL-10 and DEX (10−8 M) on caspase-3 activity from PMNs alone, PMNs exposed to LPS, and PMNs exposed to LPS with either IL-10 or DEX (10−8 M) is shown in Figure 5. For PMNs without exposure to LPS, caspase-3 activity increased over time with statistical significance reached at 18 hours. LPS tended to reduce caspase-3 activity at each time point but the difference was not statistically significant compared to PMNs that were not exposed to LPS. IL-10 and DEX did not have any effect on caspase-3 activity.
Figure 5.
Caspase-3 activity from unstimulated ( ) and endotoxin (
) and endotoxin ( )-stimulated polymorphonuclear leukocytes of the newborn over 18 hours (n=6, mean±SE): effects of pretreatment with equimolar (10−8 M) of interleukin-10 (
)-stimulated polymorphonuclear leukocytes of the newborn over 18 hours (n=6, mean±SE): effects of pretreatment with equimolar (10−8 M) of interleukin-10 ( ) and dexamethasone (
) and dexamethasone ( ). * Results were different from baseline at 0 hr, p<0.05.
). * Results were different from baseline at 0 hr, p<0.05.
DISCUSSION
The PMN plays an important role in the fetal and neonatal inflammatory response syndrome and potential multi-organ injury such as bronchopulmonary dysplasia and brain injury (2). The aim of the present study was to compare the effect of IL-10 versus DEX on important PMN innate immune functions of the newborn. The plasma levels of DEX known to be associated with a reduction in BPD have been previously described and are in the range of 10−8 M (15, 16). In previous work, we demonstrated that endotoxin-stimulated release of IL-8 from PMNs of the newborn was markedly inhibited by equimolar levels (10−8 M) of DEX or IL-10 (10). Therefore, the present study focused on IL-10 and DEX, principally at 10−8 M level, based on these clinical and laboratory observations. Our intention was to minimize confounding effects such as chorioamnionitis, antenatal steroids and maternal medications for indicated deliveries. Chronic, clinically silent chorioamnionitis is still possible for term infants delivered by elective caesarean section (22). The results of this study may not extrapolate to some PMN functions of the premature infant, which differ from term infants (1, 13). While the separate effects of DEX and IL-10 on PMN functions of the adult have been described, comparative studies on a variety of PMN pro-inflammatory functions of the newborn have not been previously described (23, 24).
Pro-inflammatory and anti-inflammatory cytokine measurements have been made in the bloodstream and airway fluid of newborns (25–27), but the cell source of these mediators is incompletely understood and may involve different molecular mechanisms leading to their release or the inhibition of their release (21). In amniotic and airway fluid of premature infants at risk for BPD, there have been absolute elevations of pro-inflammatory cytokines such as IL-1β and TNFα; there have been elevated ratios of these cytokines to their inhibitors described as well (25–27). In the present study, we demonstrated that, like in previous work with IL-8 (10), IL-10 and DEX at 10−8 M markedly inhibited IL-1β and TNFα release from LPS-stimulated PMNs of the newborn. With regard to anti-inflammatory cytokines, we found no detectable levels of IL-4 upon LPS stimulation of PMNs of the newborn. In addition, previous studies have shown that PMNs from newborns and adults do not release IL-10 (10, 28). On the other hand, we found that the LPS-stimulated PMN of the newborn is capable of producing IL-1ra and interestingly, there was a different effect of IL-10 versus DEX on IL-1ra release. We found that IL-10 did not affect IL-1ra release but DEX did reduce IL-1ra release compared to IL-10.
PMN chemotaxis is reduced in the newborn compared to the adult (1). IL-10 has been shown to mildly reduce chemotaxis using PMNs from adults and the under-agarose method (29). In the present study, we used two well-studied PMN chemoattractants, IL-8 and fMLP, with a 48-well chemotaxis chamber. Doses of IL-10 and DEX below and up to 10−8 M had no direct effect on total migration, chemokinesis, or chemotaxis upon exposure to either IL-8 or fMLP.
Phagocytosis, bacterial killing, and respiratory burst are similar for the newborn compared to adult under healthy conditions, yet phagocytosis may be depressed in a variety of serious systemic disorders in the adult and newborn (1). In the present study, we found no effect of IL-10 or DEX on phagocytosis, bacterial killing or respiratory burst. Conflicting results, perhaps due to study conditions, have been reported with regard to IL-10 and inhibition of respiratory burst in PMNs from adults (30, 31). In preterm infants with evolving BPD, DEX reduced respiratory burst (32). The present study only compared IL-10 and DEX at an equimolar level, which is known to have marked inhibitory effects on PMN release of pro-inflammatory cytokines and which can be related to therapeutic levels of DEX used in the treatment of BPD.
PMNs leaving the circulation usually live no longer than 24 hours in the tissues. In the airspace, apoptotic PMNs are rapidly ingested by alveolar macrophages. Conditions that modulate apoptosis could have an important effect on the persistence or termination of inflammation in disorders such as BPD (33, 34). Previous work using PMNs from adults have demonstrated that endotoxin reduces apoptosis; in addition DEX promotes survival of LPS-stimulated PMN survival (35) while IL-10 inhibits LPS-induced survival of LPS-stimulated PMNs from adults (36). In the present study, we saw a trend towards the reduction in caspase-3 activity by endotoxin, indicating a reduction in the rate of apoptosis, but the change in activity did not reach statistical significance. In contrast to the studies using PMNs from adults, we found no appreciable effect of IL-10 or DEX at 10−8 M on caspase-3 activity.
The cell-specific effects of IL-10 versus DEX, at therapeutic levels, on PMN functions of the newborn have not been studied previously outside of reports from our laboratory (10, 14). Further work is needed in this area for a number of reasons. First, the in vitro conditions used in the present study may not reflect the complex in vivo environment seen in well or sick, term or preterm newborns. Secondly, it is known that as an inflammatory circulating cell moves from the circulation into the tissues, its functions may change (37). However, in the context of the fetal inflammatory response syndrome and its sequelae, our study on circulating PMNs has clinical relevance (2). There are also other PMN functions for example, adhesion, which should be studied; DEX is associated with decreased expression of adhesion molecules on PMNs and monocytes of the newborn (38), but the effect of IL-10 is unknown.
We conclude that the principal anti-inflammatory effects of IL-10 and DEX on the functions of PMNs of the newborn that were measured in this study occur via pro-inflammatory cytokine inhibition. Secondary indirect effects of IL-10 and DEX that are anti-inflammatory may then occur, such as decreased IL-8-induced PMN recruitment into a site of inflammation, without a direct effect on chemotaxis, phagocytosis, respiratory burst and apoptosis. IL-10 may have an anti-inflammatory advantage over DEX, owing to the inhibition of IL-1ra by DEX compared to IL-10. A limitation of this study may be the applicability of our results to neutrophil functions of the extremely preterm versus term human newborn. For example, the preterm infant under certain conditions has decreased neutrophil phagocytosis compared to the term infant (13). Further studies should be undertaken to understand the comprehensive developmental and physiologic effects of IL-10 versus DEX, as well as differences in their molecular mechanisms of action. Novel, safe and effective anti-inflammatory therapy for the fetus and newborn is urgently needed (3).
Acknowledgments
Financial Support: National Institute of Child Health and Human Development (NICHD), NIH Grant Number 5R03HD048508-02
ABBREVIATIONS
- BPD
bronchopulmonary dysplasia
- DEX
dexamethasone
- fMLP
N-formyl-L-methionyl-L-leucyl-L-phenylalanine
- IL-1ra
interleukin-1 receptor antagonist
- LPS
lipopolysaccharide
- PMN
polymorphonuclear leukocytes
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lewis DB, Wilson CB. Developmental immunology and role of host defenses in fetal and neonatal susceptibility to infection. In: Remington J, Klein J, Baker C, Wilson C, editors. Infectious Diseases of the Fetus and Newborn Infant. 6th ed. Philadelphia: Elsevier-Saunders; 2006. pp. 149–159. [Google Scholar]
- 2.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 3.Watterberg K. Anti-inflammatory therapy in the neonatal intensive care unit: present and future. Semin Fetal Neonatal Med. 2006;11:378–384. doi: 10.1016/j.siny.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Jones CA, Cayabyab RG, Kwong KY, Stotts C, Wong B, Hamdan H, Minoo P, deLemos RA. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr Res. 1996;39:966–975. doi: 10.1203/00006450-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McColm JR, Stenson BJ, Biermasz N, McIntosh N. Measurement of interleukin-10 in bronchoalveolar lavage from preterm ventilated infants. Arch Dis Child Fetal Neonatal Ed. 2000;82:F156–F159. doi: 10.1136/fn.82.2.F156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beresford MW, Shaw NJ. Detectable IL-8 and IL-10 in bronchoalveolar lavage fluid from preterm infants ventilated for respiratory distress syndrome. Pediatr Res. 2002;52:973–978. doi: 10.1203/00006450-200212000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Garingo A, Tesoriero L, Cayabyab R, Durand M, Blahnik M, Sardesai S, Ramanathan R, Jones C, Kwong K, Li C, Minoo P. Constitutive IL-10 expression by lung inflammatory cells and risk for bronchopulmonary dysplasia. Pediatr Res. 2007;61:197–202. doi: 10.1203/pdr.0b013e31802d8a1c. [DOI] [PubMed] [Google Scholar]
- 8.Merritt TA, Stuard ID, Puccia J, Wood B, Edwards DK, Finkelstein J, Shapiro DL. Newborn tracheal aspirate cytology: classification during respiratory distress syndrome and bronchopulmonary dysplasia. J Pediatr. 1981;98:949–956. doi: 10.1016/s0022-3476(81)80603-8. [DOI] [PubMed] [Google Scholar]
- 9.Huang HC, Tai FY, Wang FS, Liu CA, Hsu TY, Ou CY, Yang KD. Correlation of augmented IL-8 production to premature chronic lung disease: implication of posttranscriptional regulation. Pediatr Res. 2005;58:216–221. doi: 10.1203/01.PDR.0000175886.46201.D7. [DOI] [PubMed] [Google Scholar]
- 10.Davidson D, Miskolci V, Clark DC, Dolmaian G, Vancurova I. Interleukin-10 production after pro-inflammatory stimulation of neutrophils and monocytic cells of the newborn. Comparison to exogenous interleukin-10 and dexamethasone levels needed to inhibit chemokine release. Neonatology. 2007;92:127–133. doi: 10.1159/000101432. [DOI] [PubMed] [Google Scholar]
- 11.Kwong KY, Jones CA, Cayabyab R, Lecart C, Khuu N, Rhandhawa I, Hanley JM, Ramanathan R, deLemos RA, Minoo P. The effects of IL-10 on proinflammatory cytokine expression (IL-1beta and IL-8) in hyaline membrane disease (HMD) Clin Immunol Immunopathol. 1998;88:105–113. doi: 10.1006/clin.1997.4510. [DOI] [PubMed] [Google Scholar]
- 12.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy – review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 13.Urlichs F, Speer CP. Neutrophil function in preterm and term infants. NeoReviews. 2004;5:e417–e429. [Google Scholar]
- 14.Tryzmel J, Miskolci V, Castro-Alcaraz S, Vancurova I, Davidson D. Interleukin-10 inhibits pro-inflammatory chemokine release by neutrophils of the newborn without suppression of nuclear factor-kappa B. Pediatr Res. 2003;54:382–386. doi: 10.1203/01.PDR.0000077471.36217.6E. [DOI] [PubMed] [Google Scholar]
- 15.Schild PN, Charles BG. Determination of dexamethasone in plasma of premature neonates using high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1994;658:189–192. doi: 10.1016/0378-4347(94)00192-8. [DOI] [PubMed] [Google Scholar]
- 16.Lugo RA, Nahata MC, Menke JA, McClead RE., Jr Pharmacokinetics of dexamethasone in premature neonates. Eur J Clin Pharmacol. 1996;49:477–483. doi: 10.1007/BF00195934. [DOI] [PubMed] [Google Scholar]
- 17.Zentay Z, Sharaf M, Qadir M, Drafta D, Davidson D. Mechanism for dexamethasone inhibition of neutrophil migration upon exposure to lipopolysaccharide in vitro: role of neutrophil interleukin-8 release. Pediatr Res. 1999;46:406–410. doi: 10.1203/00006450-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 19.Ward RA, McLeish KR. Polymorphonuclear leukocyte oxidative burst is enhanced in patients with chronic renal insufficiency. J Am Soc Nephrol. 1995;5:1697–1702. doi: 10.1681/ASN.V591697. [DOI] [PubMed] [Google Scholar]
- 20.Green JN, Winterbourn CC, Hampton MB . Neutrophil Methods and Protocols. Analysis of neutrophil bactericidal activity. In: Quinn MT, DeLeo FR, Bokoch GM, editors. Methods in Molecular Biology. Vol 138. Totowa: Humana Press Inc; 2007. pp. 319–332. [Google Scholar]
- 21.Castro-Alcaraz S, Miskolci V, Kalasapudi B, Davidson D, Vancurova I. NF-kappa B regulation in human neutrophils by nuclear I kappa B alpha: correlation to apoptosis. J Immunol. 2002;169:3947–3953. doi: 10.4049/jimmunol.169.7.3947. [DOI] [PubMed] [Google Scholar]
- 22.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, Reynolds PR, Feldman RG, Sullivan MH. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57:404–411. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 23.Barnes PJ. Corticosteroids: The drugs to beat. Eur J Pharmacol. 2006;533:2–14. doi: 10.1016/j.ejphar.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 24.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 25.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, Campbell AB, Wilson PD, Hester L, Hasday JD. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 26.Viscardi RM, Hasday JD, Gumpper KF, Taciak V, Campbell AB, Palmer TW. Cromolyn sodium prophylaxis inhibits pulmonary proinflammatory cytokines in infants at high risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1997;156:1523–1529. doi: 10.1164/ajrccm.156.5.9611088. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson B, Li YH, Noack G, Brauner A, Tullus K. Downregulatory cytokines in tracheobronchial aspirate fluid from infants with chronic lung disease of prematurity. Acta Paediatr. 2000;89:1375–1380. doi: 10.1080/080352500300002606. [DOI] [PubMed] [Google Scholar]
- 28.Reglier H, Arce-Vicioso M, Fay M, Gougerot-Pocidalo MA, Chollet-Martin S. Lack of IL-10 and IL-13 production by human polymorphonuclear neutrophils. Cytokine. 1998;10:192–198. doi: 10.1006/cyto.1997.0272. [DOI] [PubMed] [Google Scholar]
- 29.Vicioso MA, Garaud JJ, Reglier-Poupet H, Lebeaut A, Gougerot-Pocidalo MA, Chollet-Martin S. Moderate inhibitory effect of interleukin-10 on human neutrophil and monocyte chemotaxis in vitro. Eur Cytokine Netw. 1998;9:247–253. [PubMed] [Google Scholar]
- 30.Bussolati B, Mariano F, Montrucchio G, Piccoli G, Camussi G. Modulatory effect of interleukin-10 on the production of platelet-activating factor and superoxide anions by human leucocytes. Immunology. 1997;90:440–447. doi: 10.1111/j.1365-2567.1997.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reglier-Poupet H, Hakim J, Gougerot-Pocidalo MA, Elbim C. Absence of regulation of human polymorphonuclear oxidative burst by interleukin-10, interleukin-4, interleukin-13 and transforming growth factor-beta in whole blood. Eur Cytokine Netw. 1998;9:633–638. [PubMed] [Google Scholar]
- 32.Ballabh P, Simm M, Kumari J, Califano C, Aghai Z, Laborada G, Sison C, Cunningham-Rundles S. Respiratory burst activity in bronchopulmonary dysplasia and changes with dexamethasone. Pediatr Pulmonol. 2003;35:392–399. doi: 10.1002/ppul.10279. [DOI] [PubMed] [Google Scholar]
- 33.Kotecha S, Mildner RJ, Prince LR, Vyas JR, Currie AE, Lawson RA, Whyte MK. The role of neutrophil apoptosis in the resolution of acute lung injury in newborn infants. Thorax. 2003;58:961–967. doi: 10.1136/thorax.58.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grigg JM, Savill JS, Sarraf C, Haslett C, Silverman M. Neutrophil apoptosis and clearance from neonatal lungs. Lancet. 1991;338:720–722. doi: 10.1016/0140-6736(91)91443-x. [DOI] [PubMed] [Google Scholar]
- 35.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154:4719–4725. [PubMed] [Google Scholar]
- 36.Ward C, Murray J, Clugston A, Dransfield I, Haslett C, Rossi AG. Interleukin-10 inhibits lipopolysaccharide-induced survival and extracellular signal-regulated kinase activation in human neutrophils. Eur J Immunol. 2005;35:2728–2737. doi: 10.1002/eji.200425561. [DOI] [PubMed] [Google Scholar]
- 37.Coldren CD, Nick JA, Poch KR, Woolum MD, Fouty BW, O’Brien JM, Gruber MP, Zamora MR, Svetkauskaite D, Richter DA, He Q, Park JS, Overdier KH, Abraham E, Geraci MW. Functional and genomic changes induced by alveolar transmigration in human neutrophils. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1267–L1276. doi: 10.1152/ajplung.00097.2006. [DOI] [PubMed] [Google Scholar]
- 38.Ballabh P, Simm M, Kumari J, Krauss AN, Jain A, Califano C, Lesser ML, Cunningham-Rundles S. Neutrophil and monocyte adhesion molecules in bronchopulmonary dysplasia, and effects of corticosteroids. Arch Dis Child Fetal Neonatal Ed. 2004;89:F76–F83. doi: 10.1136/fn.89.1.F76. [DOI] [PMC free article] [PubMed] [Google Scholar]