Abstract
Decades of success with live adenovirus vaccines suggest that replication-competent recombinant adenoviruses (rAds) could serve as effective vectors for immunization against other pathogens. To explore the potential of a live rAd vaccine against malaria, we prepared a viable adenovirus 5 (Ad5) recombinant that displays a B-cell epitope from the circumsporozoite protein (CSP) of Plasmodium falciparum on the virion surface. The recombinant induced P. falciparum sporozoite-neutralizing antibodies in mice. Human adenoviruses do not replicate in mice. Therefore, to examine immunogenicity in a system in which, as in humans, the recombinant replicates, we constructed a similar recombinant in an adenovirus mutant that replicates in monkey cells and immunized four Aotus nancymaae monkeys. The recombinant replicated in the monkeys after intratracheal instillation, the first demonstration of replication of human adenoviruses in New World monkeys. Immunization elicited antibodies both to the Plasmodium epitope and the Ad5 vector. Antibodies from all four monkeys recognized CSP on intact parasites, and plasma from one monkey neutralized sporozoites in vitro and conferred partial protection against P. falciparum sporozoite infection after passive transfer to mice. Prior enteric inoculation of two animals with antigenically wild-type adenovirus primed a response to the subsequent intratracheal inoculation, suggesting a route to optimizing performance. A vaccine is not yet available against P. falciparum, which induces the deadliest form of malaria and kills approximately one million children each year. The live capsid display recombinant described here may constitute an early step in a critically needed novel approach to malaria immunization.
INTRODUCTION
Live oral adenovirus vaccines have been used successfully for decades to protect United States military recruits from serious respiratory disease due to adenovirus (Ad) infection (1–3). The military adenovirus vaccines contain live virus incorporated into enteric-coated tablets. Oral administration of the tablets leads to an asymptomatic enteric infection that, in trials involving over 42,000 soldiers, resulted in protective immunity against respiratory disease caused by the vaccine adenovirus types (types 4 and 7 [Ad4 and Ad7]) in 70 to 100% of vaccinees (1–3). The oral adenovirus vaccines possess properties that suit them for use in settings with limited public health resources and infrastructure, including ease of administration by minimally trained personnel, efficacy in a single dose, and freedom from the hazards associated with needles. Successful development of oral recombinant adenovirus vaccines against diseases of the developing world therefore would provide a valuable new tool in the global effort to control infectious disease. To assess the promise of a replicating recombinant adenovirus in immunization, we prepared and have begun characterization of viable adenovirus recombinants intended to elicit immunity to the malaria parasite Plasmodium falciparum.
Immunity to malaria infection can be induced by vaccination with radiation-attenuated sporozoites, the infectious form of the parasite injected by mosquitoes, administered either by hundreds of mosquito bites or multiple intravenous injections (4, 5). The principal target of irradiated sporozoite-induced immunity is the circumsporozoite protein (CSP), which coats the exterior of the sporozoite. The central region of CSP contains 25 to 50 repeats of the amino acid sequence Asn-Ala-Asn-Pro (NANP) interspersed with a few variants (6). This repeat is conserved in all P. falciparum isolates (7) and is the immunodominant B-cell epitope of CSP (8). Antibody to the NANP repeat is sufficient to confer protection in animal models (9–12), and protection by the most advanced malaria vaccine candidate, RTS,S, is correlated with antibody responses to the repeat region (13, 14). However, no candidate vaccine yet induces high-level, durable protection against sporozoite infection (15, 16), and an urgent need for novel malaria immunization strategies remains.
Adenoviruses that display exogenous epitopes on the surfaces of their capsids (capsid display recombinants) are potently immunogenic in mice (10–12, 17). Capsid display recombinants that expressed either the central repeat B-cell epitope (NANP)5 or a combined B- and T-cell epitope NANPNVDP(NANP)4 induced high-titer P. falciparum CSP antibodies, and serum from (NANP)5-immunized mice (the only ones examined) neutralized sporozoites in an in vitro assay (11). However, human adenoviruses do not replicate in mice, and immunological responses to capsid display recombinants in mice are not likely to accurately predict responses to the recombinants as they replicate in humans. To explore the properties of the capsid display recombinants under conditions that permit replication, we prepared a recombinant that displays the P. falciparum CSP central repeat epitope NANPNVDP(NANP)4 on hexon, the major adenovirus capsid protein. This recombinant, Ad5hr-NVDP, was constructed in an adenovirus type 5 (Ad5) background that includes a mutation that confers the ability to grow in monkey cells and macaques (18, 19). Here, we report that the recombinant induces potentially protective humoral immune responses to the CSP repeat in Aotus monkeys, which also provide a challenge model for P. falciparum sporozoite infection.
MATERIALS AND METHODS
Animals.
Four Aotus nancymaae monkeys (An6012, An6209, An6160, An6166), two males and two females, were used in this study. The Aotus monkeys were supplied by the Michael E. Keeling Center for Comparative Medicine and Research, University of Texas M.D. Anderson Cancer Center. C57BL/6 mice were obtained from the National Cancer Institute.
All experiments were conducted with the approval of the Johns Hopkins University Institutional Animal Care and Use Committee.
Viruses.
Ad5hr-NVDP is a viable recombinant that displays the P. falciparum CSP epitope NANPNVDP(NANP)4 in hypervariable region 1 (HVR1) of hexon (11). Ad5hr-FFIG is a viable Ad5 recombinant that expresses green fluorescent protein from the major late transcriptional unit derived from Ad5-FFIG (20). Both viruses carry the hr404 mutation in the adenovirus DNA binding protein gene that allows Ad5 to replicate in Old World monkey cells, macaques (18, 19), and cells derived from the New World monkey genus Aotus (C. Palma and G. Ketner, unpublished data). Because of preexisting immunity to Ad5 in the human population, Ad5 is not likely to be the platform ultimately chosen for viable recombinant adenovirus (rAd) immunization. However, because Ad5hr404 (and similar mutants of its close relative Ad2) are the only well-studied human adenoviruses with the capacity to replicate in monkeys, studies in monkeys can be performed only in the Ad5hr background. Capsid-modified derivatives of other, less ubiquitous Ad serotypes have been made, and data derived from the Ad5 experiments described here should be applicable to construction of antimalarial viable rAds in those serotypes.
Recombinants were purified by CsCl density gradient centrifugation (21), dialyzed into storage buffer (5% sucrose, 0.15 M NaCl, 0.5 M CaCl2, 0.9 M MgCl2, 20 mM HEPES, pH 7.4), and stored overnight at 4°C before use. Particle concentration was determined by A260 (22), and numbers of PFU were calculated assuming a particle/PFU ratio of 20:1.
Parasites.
Hybrid P. berghei/P. falciparum is a transgenic P. berghei parasite whose CSP bears the P. falciparum CSP central repeat region and that is sensitive to anti-NANP antibodies (23). Hybrid sporozoites were obtained by dissection of Anopheles stephensi mosquitoes fed 21 to 22 days previously on mice infected with the transgenic parasite provided by the Johns Hopkins Malaria Research Institute Mosquito and Parasite Core Facilities.
Immunizations.
Two Aotus monkeys (An6012 and An6209) were initially given a single dose of ∼104 PFU of Ad5hr-FFIG administered in enteric-coated capsules (24) by using a gastric tube. The remaining immunizations were by delivery of 200 μl of a virus suspension containing 1 × 107 PFU directly into the trachea of anesthetized animals by percutaneous puncture with a 25-gauge needle.
Sample preparation.
Peripheral blood (3 ml) was drawn from Aotus monkeys just prior to each immunization, every other week for 4 to 8 weeks, and periodically thereafter (Fig. 1). Blood was fractionated using Lympholyte cell separation medium (Cedarlane), and plasma was stored at −80°C.
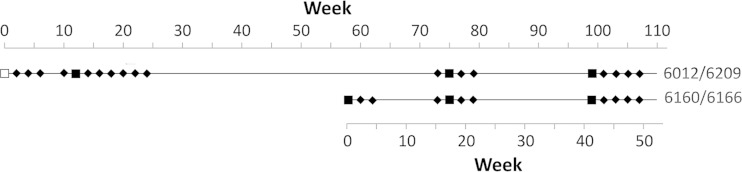
FIG 1.
Immunization schedule. Squares indicate times of immunization with Ad5hr-NVDP (filled squares, intratracheal) or Ad5hr-FFIG (empty square, oral). Times of pre- and postimmunization blood collection are indicated by diamonds.
Enzyme-linked immunosorbent assay (ELISA).
The wells of a 96-well plate (Immulon 2HB) were coated with either urea-disrupted antigenically wild-type Ad5 particles or recombinant CSP (rCSP; MRA272) at 1 μg protein per well. The wells were washed thrice with phosphate-buffered saline-0.1% Tween 20 (PBS-T) and thrice with PBS and were blocked in 5% nonfat dry milk (NFDM) in PBS. Threefold or 5-fold serial dilutions of the monkey plasma or the human or mouse positive-control serum were made in 5% NFDM, spanning the range of 1:100 to 1:312,500. Positive-control serum for Ad5 was obtained from a human source with previous exposure to Ad5. CSP monoclonal antibody (MAb) 2A10 (MRA-183) (25) was used as the positive control for rCSP. Preimmunization samples for each monkey were used as negative controls along with antigen-coated wells that received no serum. Incubation with serum or plasma was for 1 h at room temperature followed by washing as described above, the addition of horseradish peroxidase (HRP)-conjugated secondary antibodies against either mouse or human Ig diluted 1:1,000 in 5% NFDM, and incubation for an additional hour. After being washed, the wells were incubated with ABTS peroxidase substrate system (KPL) for 15 min at room temperature. The reaction was stopped with an equal volume of 1% SDS in PBS, and the A405 was determined by a plate reader. Wells were scored as positive if the optical density was at least double the average optical density from wells with no serum. Each experiment was done in triplicate. For the Ig isotype determination, bovine serum albumen (BSA) was used in place of NFDM, and the secondary antibodies used were anti-human IgG2 and IgM (1:3,000) (Invitrogen).
Adenovirus neutralization.
Plasma samples were serially diluted in PBS and 1% BSA. A total of 125 μl of diluted plasma was incubated with 125 μl of an Ad5-NVDPhr404 stock with a titer of approximately 2 × 103 PFU per ml. After 30 min at room temperature, 100 μl of the mixture was added to each of two 6-cm tissue culture dishes, and plaques were allowed to develop under standard conditions. Neutralizing titers are given as the reciprocal of the greatest dilution of plasma able to reduce plaque numbers by 90% compared to those of preimmune controls.
Immunoblots.
For the detection of anti-CSP antibodies, 10,000 hybrid sporozoites were boiled in loading buffer, separated on a 12% SDS-polyacrylamide gel, and electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane. For detection of anti-Ad5 100k antibodies, lysates of cells transfected with a 100k expression plasmid (gift from Antony Rosen, Johns Hopkins School of Medicine) were fractionated on a 7.5% SDS-polyacrylamide gel and transferred to a PVDF membrane. Membranes were probed with mouse anti-CSP MAb 2A10 tissue culture supernatant, rabbit anti-late Ad protein antiserum (1:1,000), rabbit anti-100k antibody (26) (1:1,000), or monkey plasma diluted as indicated in PBS containing 3% BSA. Appropriate HRP-conjugated secondary antibodies were diluted to 1:10,000 in 5% NFDM.
Immunofluorescence.
A total of 5,000 hybrid sporozoites were spotted in each well of an 18-well depression slide and air dried at room temperature overnight. Dry slides were stored at −20°C. Plasma from immunized monkeys (10 μl diluted in PBS containing 1% BSA), the 2A10 anti-CSP MAb (positive control; 1:100), or fetal bovine serum (FBS; negative control) was placed onto each well and incubated in a humidified chamber for 30 min at room temperature. Wells were washed twice, and 10 μl of the appropriate fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:500 in 1% BSA in PBS) was pipetted onto each well. Slides were incubated for 30 min in a wet chamber at room temperature. Slides were washed, mounted with Vectashield, dried, and examined under UV illumination using a Nikon Upright 90i fluorescence microscope and Volocity software (PerkinElmer). Immunofluorescence assay (IFA) titers are expressed as the highest plasma dilution that visibly stained sporozoites. Slides were examined independently by three investigators who unanimously agreed on titers.
Sporozoite neutralization assays.
The sporozoite neutralizing ability of plasma was determined by an assay that measures the ability of sporozoites to invade and traverse cells in culture (27, 28). A total of 50,000 hybrid sporozoites were incubated at room temperature for 30 min with heat-inactivated monkey plasma at a dilution of 1:4 or 2A10 MAb at 50 μg/ml. Treated sporozoites were added to 50,000 HC-04 (29) cells growing in a 48-well plate in Iscove's modified Dulbecco's medium (IMDM) containing 2.5% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Rhodamine-labeled dextran was added to the medium at 200 μg/ml just prior to infection. At 4 h postinfection, the cells were washed with IMDM and incubated with 1 mM Advasep-7 for 5 min. The cells were then washed with PBS, harvested by incubation with trypsin, washed again with PBS, and suspended in stain buffer (BD Pharmingen). Flow cytometry was performed on the samples using the FL2 channel (BD FACSCalibur). FloJo software was used to gate appropriately and enumerate cells that had taken up the labeled dextran. All test and control incubations were carried out in triplicate. Data were submitted to the Johns Hopkins Biostatistics Center for statistical analysis by analysis of variance (ANOVA) to determine the significance of differences in traversal inhibition between the postimmunization and preimmunization samples for each monkey.
Passive immunization.
Pools were prepared from the plasma samples with the highest anti-CSP titers for An6160 (weeks 43, 45, and 49) and An6166 (weeks 43, 45, 47, and 49). Female C57BL/6 mice, 6 to 8 weeks of age, were injected with 250 μl of pooled plasma followed immediately by 10,000 hybrid sporozoites. Injections were delivered into the tail veins of five mice per group with a 28.5-gauge needle. Forty to 42 h after sporozoite injection, the mice were sacrificed, whole livers were homogenized, and total liver RNA was prepared by the single-step method (30). Parasite liver burden was determined by reverse transcription-quantitative PCR (qRT-PCR) of P. berghei 18S rRNA (31). A modified Thompson τ test was used to identify outliers, and a Mann-Whitney test was used to determine significance of the differences among samples.
RESULTS
Immunizations.
The intended route of immunization with our viable adenovirus recombinants is oral. To test the feasibility of oral infection of Aotus monkeys, two monkeys (An6012 and An6209) were given enteric-coated capsules (24) containing approximately 104 PFU of antigenically wild-type green fluorescent protein (GFP)-expressing recombinant Ad5hr-FFIG (20). Attempts to demonstrate virus replication by detection either of GFP-expressing cells or of viral DNA in stool were unsuccessful. Further, only very low levels of adenovirus antibodies were detected in immunized animals by ELISA up to 12 weeks after enteric immunization (however, see below). Because of lack of evidence of robust replication, enteric immunization was not pursued, and subsequent immunization with Ad5hr-NVDP was by delivery of 5 × 107 PFU of virus to the respiratory epithelium by intratracheal (IT) instillation. An6012 and An6209 received their first Ad5hr-NVDP IT immunization 12 weeks after the attempted enteric immunization. They were immunized IT at week 75 and again at week 99 with the same dose. An6160 and An6166 were immunized IT with Ad5hr-NVDP at the same dose at weeks 0, 17, and 41.
Viral replication in Aotus.
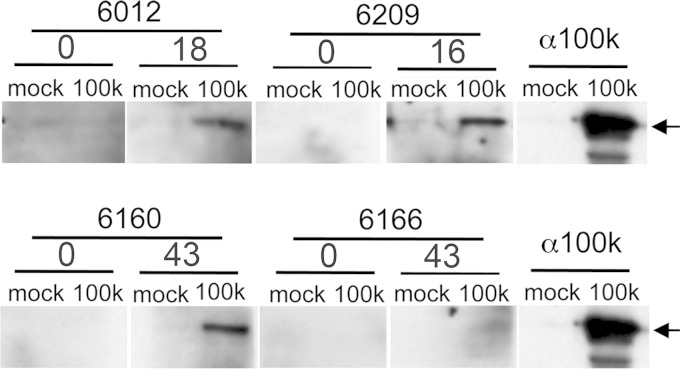
To determine whether the recombinants replicated in immunized monkeys, we examined plasma samples for antibodies to the viral protein 100k. Ad5 100k is not a component of the adenovirus virion and is produced late in infection only by viruses capable of conducting a full lytic cycle. Therefore, antibody responses to 100k will be seen only in monkeys in which Ad5hr-NVDP has replicated. Immunoblots of lysates of 293 cells transfected with an Ad5 100k expression plasmid were probed with pre- and postimmunization plasma samples with high anti-Ad5 antibody titers (below). All four of the Aotus monkeys produced anti-Ad5 100k antibody detectable for at least one time point (Fig. 2), and we conclude that Ad5hr-NVDP replicated in Aotus monkeys.
FIG 2.
100k antibody responses in Aotus monkeys. Aotus pre- and postimmunization plasma samples or anti-100k monoclonal antibodies were used in individual immunoblots to probe lysates of 293 cells mock transfected or transfected with a 100k-expressing plasmid. The numbers above pairs of lanes are the weeks on which plasma samples were taken. The anti-100k panel is duplicated in each row to indicate the position of the 100k band. 0 = preimmunization plasma.
Antibody responses to immunization.
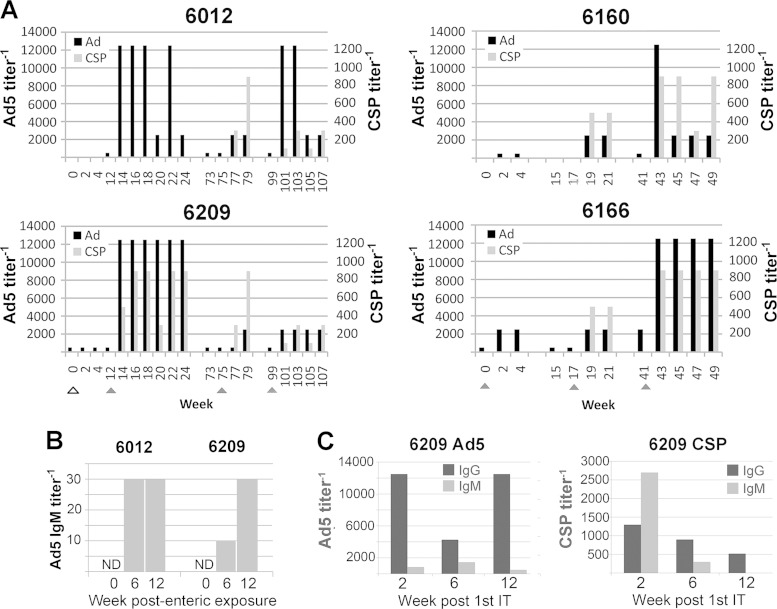
IT immunization elicited antibody responses to both Ad5 and CSP in all Aotus monkeys, but the dynamics of the responses differed strikingly between and within pairs of animals (Fig. 3A). The Aotus monkeys that received the enteric dose of Ad5hr-FFIG (An6012 and An6209) developed high levels of anti-Ad5 antibodies immediately after the first intratracheal immunization. The anti-Ad5 responses were evident through weeks 22 to 24 in these animals but waned over the year that elapsed prior to the second IT immunization (week 73). Boosts with Ad5hr-NVDP via the intratracheal route had a modest transient effect on anti-Ad5 antibody titers in both animals. Ad5 titers were again boosted to transiently high levels by the third immunization in An6012.
FIG 3.
Antibodies in Aotus plasma. (A) Anti-Ad5 titers (black bars; scale on the left axis) and anti-CSP titers (gray bars, scale on the right axis) were determined by ELISA using total immunoglobulin (IgG, IgM, and IgA) secondary antibody. Triangles indicate immunizations; the open triangle indicates enteric immunization of An6012 and An6209. Fivefold serial dilutions were performed beginning at 1:500 for Ad5, and 3-fold serial dilutions were performed beginning at 1:100 for CSP. (B) Anti-CSP and anti-Ad5 IgG and IgM levels were measured in An6209 plasma samples drawn at weeks 2, 6, and 12 after the first IT immunization with Ad5hr-NVDP. These times correspond to weeks 14, 18, and 24 in panel A. (C) Anti-Ad5 IgM titers in An6012 and An6209 following enteric immunization. The first IT immunization was at week 12, following the collection of the week 12 plasma sample. The time axis is the same as in panel A. ND, not detected at 1:10.
An6209 showed an immediate dramatic anti-CSP response to the first intratracheal immunization in addition to its Ad5 response, and both An6209 and An6012 developed high-level anti-CSP responses following the second intratracheal immunization. Neither sustained these anti-CSP levels, but both responded modestly to the third immunization (Fig. 3A).
The immediate Ad5 antibody response after IT immunization in An6012 and An6029 suggested that their previous enteric immunization had primed the Ad5 response. To address that possibility, we determined the isotypes of the Ad5 antibodies present prior to and early after the first IT immunization in An6012 and An6209. At both 6 and 12 weeks after administration of the enteric capsules, both animals displayed IgM responses to Ad5 (Fig. 3B), consistent with priming by the enteric dose. We then examined the antibodies induced by the first IT dose in An6209, which had responded immediately to both Ad and CSP (Fig. 3C). Two weeks after the first IT immunization, Ad5 IgG2 levels were already high in An6209, while very little Ad5-specific IgM was observed at any time point (Fig. 2C). In contrast, CSP-specific IgM was abundant at week 2 in An6209, decreasing thereafter. These observations are consistent with priming of the Ad5 response but not the CSP response by the initial enteric exposure to Ad5hr-FFIG.
An6160 and An6166, which had not been immunized orally, responded weakly to the first exposure to Ad5hr-NVDP (Fig. 3A). Both animals showed increased anti-CSP antibodies after the second exposure and high levels of anti-CSP after the third. The Ad5 responses were also boosted by the second (An6160) and third immunizations, although only transiently in An6160.
Ad5hr-NVDP neutralizing titers also were determined for critical time points in An6209 (enterically immunized) and An6166 (Table 1). Neutralizing titers roughly paralleled the ELISA titers shown in Fig. 3, although in An6209 neutralizing antibodies were boosted more rapidly than Ad5 ELISA antibodies by the second IT immunization and remained elevated in An6209 after ELISA titers had returned to low levels.
TABLE 1.
Neutralization titersa
| Monkey | Wk | Description | Neutralizing titer (90%) | Ad5 titer (ELISA) |
|---|---|---|---|---|
| An6209 | 0 | Preimmunization | <50 | 500 |
| 12 | 12 wks postenteric, pre-IT1 | <50 | 500 | |
| 16 | 4 wks post-IT1 | 4,050 | 12,500 | |
| 24 | 12 wks post-IT1 | 12,150 | 12,500 | |
| 75 | 63 wks post-IT1, pre-IT2 | 1,350 | 500 | |
| 79 | 4 wks post-IT2 | 4,050 | 2,500 | |
| 99 | 24 wks post-IT2, pre-IT3 | 1,350 | 500 | |
| 107 | 8 wks post-IT3 | 4,050 | 2,500 | |
| An6166 | 0 | Preimmunization | <50 | 500 |
| 17 | 17 wks post-IT1, pre-IT2 | 1,350 | 500 | |
| 41 | 24 wks post-IT2, pre-IT3 | 36,450 | 2,500 | |
| 47 | 6 wks post-IT3 | 36,450 | 12,500 |
Neutralization titers of monkey plasma samples were determined by plaque reduction using Ad5hr-NVDP. Titers are expressed as the highest serum dilution that reduced plaque-forming titer by 90% or more. ELISA titers are from Fig. 3. IT, intratracheal immunization.
Antibody recognition of sporozoite CSP.
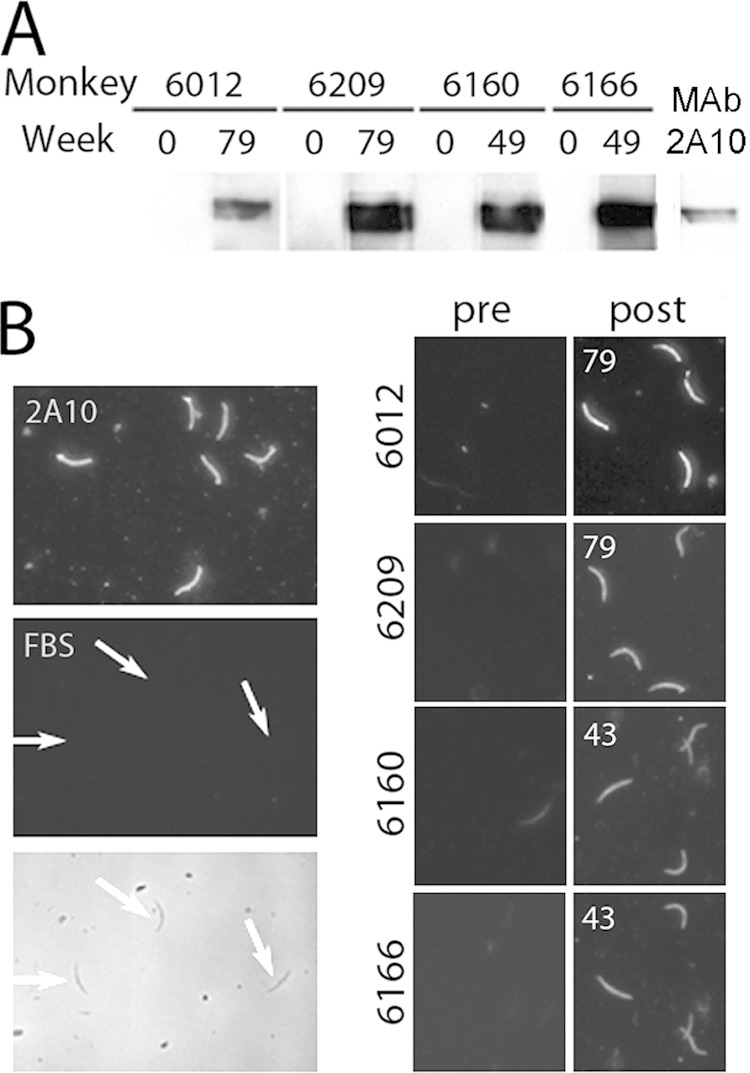
To confirm that the ELISA data reflect reactivity to CSP epitopes that are expressed by the parasites, immunoblot and immunofluorescence analyses were performed with hybrid sporozoites. For the immunoblots, whole sporozoite lysates were probed with one preimmunization plasma sample and one anti-CSP-positive postimmunization plasma sample from each Aotus monkey (Fig. 4A). The samples from all four animals robustly recognized the parasite-derived full-length CSP protein. For immunofluorescence, hybrid sporozoites were stained with preimmunization or selected ELISA-positive postimmunization plasma samples or with CSP MAb 2A10 (32). Preimmunization samples showed no or very weak (An6160) reactivity with sporozoites, while postimmunization samples from all four Aotus monkeys showed bright sporozoite staining at a dilution of 1:20 (Fig. 4B). Together, these experiments demonstrate that the antibodies raised in the Aotus monkeys recognize parasite-derived CSP and, in particular, full-length CSP as it is expressed on the sporozoite surface. Despite similar ELISA titers, plasma from An6160 and An6166 differed in potency in sporozoite neutralization and passive immunization assays (see below). Therefore, IFA titers also were determined by examining sporozoites stained by serially diluted plasma samples taken from those animals at the peak of CSP ELISA titers (week 43). Both samples stained sporozoites strongly at 1:20. Plasma from An6166 but not An6160 stained sporozoites weakly at 1:100. Therefore, by this assay, anti-CSP titers were somewhat higher in An6166 than in An6160.
FIG 4.
Recognition of hybrid sporozoites by Aotus antibodies. (A) Whole sporozoite lysates fractionated by SDS-PAGE were probed by immunoblotting using pre- and postimmunization plasma samples or anti-CSP 2A10 MAb. Plasma samples from weeks with positive anti-CSP ELISA results were used as indicated. (B) Purified sporozoites were stained using pre- and postimmunization plasma samples, anti-CSP 2A10 MAb, or FBS and FITC-conjugated secondary antibody. Postimmunization plasma samples were taken at the weeks indicated. The FBS panels are phase-contrast (bottom) and fluorescent (top) images of the same field; arrows show the location of sporozoites.
Sporozoite neutralization.
Using an in vitro assay, we determined if Aotus anti-CSP antibodies were capable of blocking sporozoite invasion and traversal of liver cells, a process that is required to establish infection in vivo. In this assay, uptake of otherwise cell-impermeable rhodamine-labeled dextran reflects successful invasion and traversal of cultured hepatocyte-derived cells (HC-04) by sporozoites (28). Sporozoite inactivation by antibody decreases the number of fluorescent cells generated by sporozoite exposure.
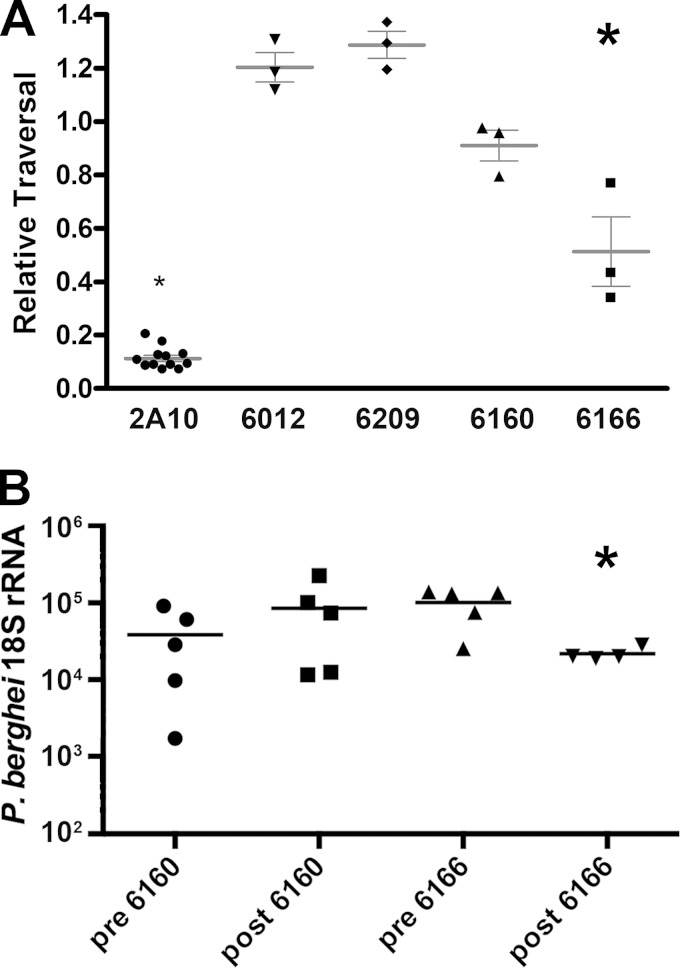
We tested preimmunization plasma and the postimmunization sample with the highest CSP ELISA titer for each Aotus monkey at a final dilution of 1:4. Samples were tested in three independent experiments, each experiment consisting of three independent neutralizations. Figure 5A presents the post-/preimmunization ratios of the fractions of dextran-positive cells from each monkey. The postimmunization sample from Aotus monkey An6166 was able to inhibit cell traversal by sporozoites by approximately 50% (P < 0.0001). The postimmunization plasma samples from An616012, An6209, and An6160 had no statistically significant impact on the ability of sporozoites to traverse the HC-04 cells.
FIG 5.
In vitro neutralization and passive immunization by Aotus plasma. (A) Sporozoite neutralization in vitro. Hepatocyte invasion by sporozoites was measured by uptake of fluorescent dextran during cell traversal in tissue culture. Relative traversal was calculated as the ratio of traversal by sporozoites treated with postimmunization plasma to that of sporozoites treated with preimmunization plasma for each Aotus monkey, or as the ratio of traversal by sporozoites treated with MAb 2A10 to untreated sporozoites. The relative traversal determined in each of three experiments is plotted for each monkey and for 2A10 with standard error bars. The significance of differences in traversal between pre- and postimmunization samples was determined by ANOVA. *, P < 0.0001. (B) Passive immunization with An6160 and An6166 plasma. C57BL/6 mice were injected with 250 μl of pooled pre- or postimmunization plasma from An6160 or An6166 and immediately challenged by 10,000 hybrid sporozoites. The resulting liver parasite burden was measured by qRT-PCR of P. berghei 18S rRNA. Quantities normalized to GAPDH are plotted, with the mean of the group indicated by the bar. A modified Thompson τ test was used to exclude an outlier from the postimmunization An6166 plasma sample (value of 8 × 105), and a Mann-Whitney test was used to determine P values for differences between pre- and postimmunization samples. *, P < 0.03.
Passive immunization.
Neutralizing activity in vitro may or may not accurately predict protective ability. Therefore, we assessed plasma from An6166 (neutralization-positive) and An6160 (neutralization-negative) in a passive immunization experiment. Five mice were injected in the tail vein with 250 μl of Aotus plasma followed immediately by 10,000 hybrid sporozoites. After 40 to 42 h, whole livers were homogenized and RNA was extracted for quantitation of P. berghei 18S rRNA by qRT-PCR. Successful sporozoite invasion of liver cells is reflected in the amount of P. berghei rRNA in the liver, and neutralization reduces this liver burden. Compared to preimmunization plasma, postimmunization plasma from monkey An6166 decreased parasite liver burden with P values of <0.03 (Fig. 5B) and <0.06 in two independent experiments. There was no significant difference in liver burden between An6160 pre- and postimmunization plasma. Thus, in vitro neutralization correlates with in vivo protection experiments for these animals, and we conclude that antibodies raised in An6166 are both neutralizing and significantly protective in mice.
DISCUSSION
There is a clear need for fresh approaches to immunization both against diseases refractory to current vaccination methods and against diseases whose vaccines are financially or logistically burdensome. Decades of success with live oral adenovirus vaccines developed by the U.S. military suggest that orally administered recombinant adenoviruses may provide a route to economical, logistically undemanding immunization against other pathogens. Malaria caused by P. falciparum imposes enormous health and economic burdens worldwide, but even the best conventional malaria vaccine candidates are not ideal. Thus, malaria offers an especially high value target for novel approaches to immunization. To explore the promise of live recombinant adenoviruses as vaccines against malaria, we constructed a series of viable adenovirus recombinants that display protective epitopes from P. falciparum CSP on the surface of their capsids.
Capsid display recombinants bearing the P. falciparum CSP central repeat B-cell epitope are highly immunogenic in mice; however, they do not replicate in this model system. To characterize our recombinants in a system where replication occurs, we immunized four Aotus nancymaae monkeys, a New World monkey species that provides a challenge model for P. falciparum sporozoite infection (33), with Ad5hr-NVDP, a recombinant bearing the CSP B-cell/T-cell epitope, NANPNVDP(NANP)4, and a host range mutation (hr404) that permits Ad5 growth in certain monkey cells. Each immunized Aotus monkey produced antibodies against the nonstructural late adenoviral protein 100k, indicating that a productive infection had occurred. Virus was not detectable in stool or saliva, however (not shown), suggesting that replication was modest. This is the first demonstration of the growth of human adenoviruses in an Aotus species, extends the range of species in which live adenovirus recombinants can be examined, and confirms the utility of Aotus in the evaluation of our malaria capsid display recombinants.
An initial effort to immunize two animals (An6012 and An6209) by oral administration of lyophilized virus in an enteric-coated capsule did not yield evidence of viral replication nor elicit an obvious antibody response (however, see below). This was unexpected in light of the vigorous replication of the Ad4 and Ad7 vaccines in humans and the ability of Ad5hr404 to form plaques in primary Aotus kidney fibroblasts (Palma and Ketner, unpublished). Potential explanations include the small virus dose that could be administered in the very small capsules that must be used in Aotus, failure of the hand-coated capsules to survive passage through the stomach or open in the gut, or difference in the susceptibility to Ad5 infection of cells in the intestines of Aotus and humans. If enteric delivery is to be used in Aotus, then additional work will be required to develop the technique. However, the goal of this study was to examine the immunogenicity of replicating rAds in Aotus monkeys, and we therefore chose to conduct subsequent immunizations by direct delivery of virus to the trachea. Following IT immunization, each animal mounted an ELISA antibody response to the Ad5 vector, confirming the immunogenicity of capsid-display recombinants in a replication-permissive animal. However, the kinetics of the responses dramatically differed between pairs of animals: after their first IT immunization, the enterically exposed An6012/An6209 pair responded immediately and strongly with an anti-Ad5 response to Ad5hr-NVDP immunization, while the An6160/An6166 pair responded modestly to the first IT immunization and ultimately required three inoculations to achieve Ad5 titers comparable to the initial responses of An6012 and An6209. It seemed plausible that the prior attempted enteric immunization of An6012 and An6209 with the reporter virus Ad5hr-FFIG had primed the immune system to respond to Ad5 antigens. To explore this possibility, we examined Ad5 antibodies present prior to and early after the first IT immunization in An6012 and An6209. Following enteric immunization, both animals had developed low IgM responses to Ad5 that had not been detected in our earliest assays. The presence of IgM is consistent with priming by the enteric exposure. In the enterically exposed An6209, the Ad5 antibody present 2 weeks post-IT was predominantly IgG2, with little IgM detectable. Thus, in An6209, the Ad5 response had undergone class switching within 2 weeks of IT immunization. Since antibody class switching from IgM to other immunoglobulin isotypes occurs over the course of a few weeks, these data support the idea that the Ad5 response was primed by the previous enteric immunization 12 weeks earlier. If this is the case, enteric infection must have primed the immune response very efficiently, since in the An6160/An6166 Aotus pair, comparable responses were seen only after the third IT immunizations. This observation provides a persuasive argument for reinvestigation of enteric immunization in this system.
Strikingly, An6209 (but not An6012) also developed an immediate, strong anti-CSP antibody response. In contrast to the anti-Ad5 IgG present 2 weeks after the first IT immunization, the predominant CSP response 2 weeks post-IT immunization was IgM. This suggests that the CSP response was new, induced de novo by the first IT immunization. This is consistent with the absence of the CSP epitope from the FFIGhr404 used for the enteric dose. The origin of the dramatic response of An6209 to the CSP epitope remains uncertain. A vigorous T-cell response to hexon, primed by enteric Ad5 immunization and recalled by the Ad5hr-NVDP immunization, might have enhanced a bystander B-cell response to the hexon-embedded malaria epitope in An6209.
Responses to the subsequent IT immunizations in An6012 and An6209 varied. Both animals showed an increased CSP response after the second IT dose but not after the third. Responses to Ad5 were slightly increased after the second IT immunization in both animals and substantially but transiently after the third in An6012.
While Aotus nancymaae monkeys are susceptible to P. falciparum sporozoite infection and provide a challenge model (34) that we plan to use for vaccine efficacy measurements, sporozoite challenge in Aotus is inefficient, and to provide the power to detect even high protective efficacy, a study would require substantially larger numbers of animals than were available here. Therefore, no Aotus challenges were attempted. Instead, potential protective ability was assessed by in vitro neutralization and passive transfer of Aotus plasma to mice. A cell traversal assay was used to measure sporozoite neutralization in vitro by plasma from immunized animals. Despite comparable ELISA titers in all animals, only An6166 plasma significantly inhibited sporozoite invasion of hepatocytes. Similarly, of the two animals tested in mice by passive transfer (An6166 and An6160), plasma only from An6166, which was also active in the in vitro sporozoite neutralization assay, provided protection in mice. An6166 had an IFA titer slightly higher than that of An6160, and it is possible that a slightly higher antibody titer not detected in the ELISA is responsible for this difference. Alternatively, genetic diversity among outbred Aotus monkeys may have led to diversity in the specific antibodies induced by the malaria epitope and to functional differences that account for the difference in neutralizing activity.
The consistent induction of anti-CSP antibodies in Aotus monkeys by a replicating capsid display recombinant is an encouraging early step in the development of live recombinant adenovirus vaccines against malaria and other targets. However, protection from sporozoite infection by antibody alone is likely to require high antibody levels. Approaches to improving the performance of capsid display recombinants therefore will be required. Several are available. First, the immunogenicity of a particular epitope in a capsid display recombinant depends upon its configuration, including which capsid protein or which hexon HVR is modified, whether the epitope is an insertion or a substitution, and the length and sequence of the specific antigenic peptide inserted (35). Incorporation of identical or distinct epitopes in multiple hexon HVRs or multiple capsid proteins is possible and may enhance the magnitude or breadth of an antibody response to capsid display recombinants. We and others have demonstrated induction of robust T-cell responses against antigens delivered by live adenovirus recombinants that express whole proteins as transgenes (17, 18, 36). Recombinants that both contain a transgene and display a relevant epitope on the capsid therefore might effectively induce both humoral and cellular immunity. Recently, a nonreplicating rAd expressing CSP from early region 1 and the repeat epitope on its hexon protein has been characterized with promising results in mice (17). The ability of oral immunization to potently prime a response to subsequent IT immunization in two Aotus monkeys further suggests that optimization of the route of administration might substantially enhance immunogenicity of capsid display recombinants.
ACKNOWLEDGMENTS
This work was supported by a pilot grant from the Johns Hopkins Malaria Research Institute. K.A.K., D.A.E., and J.X. were supported by NIH grant T32 AI007417 from the National Institutes of Health. C.D. is a recipient of a Bloomberg School of Public Health Sommer Scholarship. F.Z. was supported by NIH grant R01AI044375.
We gratefully acknowledge the indispensable assistance of Christopher Kizito and Godfree Mlambo of the JHMRI Mosquito and Parasite Cores and instruction in traversal assays from Stefanie Trop and Peter Dumoulin.
REFERENCES
- 1.Top FH Jr, Buescher EL, Bancroft WH, Russell PK. 1971. Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. J Infect Dis 124:155–160. [DOI] [PubMed] [Google Scholar]
- 2.Top FH Jr, Grossman RA, Bartelloni PJ, Segal HE, Dudding BA, Russell PK, Buescher EL. 1971. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J Infect Dis 124:148–154. doi: 10.1093/infdis/124.2.148. [DOI] [PubMed] [Google Scholar]
- 3.Gaydos CA, Gray GC. 2008. Adenovirus vaccine, p 1103–1122. In Plotkin SA, Orenstein MD (ed), Vaccines. Saunders, Philadelphia, PA. [Google Scholar]
- 4.Clyde DF, Most H, McCarthy VC, Vanderberg JP. 1973. Immunization of man against sporozoite-induced falciparum malaria. Am J Med Sci 266:169–177. [DOI] [PubMed] [Google Scholar]
- 5.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL. 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341:1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 6.Sinnis P, Nardin E. 2002. Sporozoite antigens: biology and immunology of the circumsporozoite protein and thrombospondin-related anonymous protein. Chem Immunol. 80:70–96. doi: 10.1159/000058840. [DOI] [PubMed] [Google Scholar]
- 7.Zavala F, Masuda A, Graves PM, Nussenzweig V, Nussenzweig RS. 1985. Ubiquity of the repetitive epitope of the CS protein in different isolates of human malaria parasites. J Immunol 135:2790–2793. [PubMed] [Google Scholar]
- 8.Zavala F, Cochrane AH, Nardin EH, Nussenzweig RS, Nussenzweig V. 1983. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med 157:1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zavala F, Tam JP, Hollingdale MR, Cochrane AH, Quakyi I, Nussenzweig RS, Nussenzweig V. 1985. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 228:1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- 10.Crompton J, Toogood CI, Wallis N, Hay RT. 1994. Expression of a foreign epitope on the surface of the adenovirus hexon. J Gen Virol 75(Part 1):133–139. doi: 10.1099/0022-1317-75-1-133. [DOI] [PubMed] [Google Scholar]
- 11.Palma C, Overstreet MG, Guedon JM, Hoiczyk E, Ward C, Karen KA, Zavala F, Ketner G. 2011. Adenovirus particles that display the Plasmodium falciparum circumsporozoite protein NANP repeat induce sporozoite-neutralizing antibodies in mice. Vaccine 29:1683–1689. doi: 10.1016/j.vaccine.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Worgall S, Krause A, Rivara M, Hee KK, Vintayen EV, Hackett NR, Roelvink PW, Bruder JT, Wickham TJ, Kovesdi I, Crystal RG. 2005. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J Clin Invest 115:1218–1219. doi: 10.1172/JCI200523135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olotu A, Fegan G, Wambua J, Nyangweso G, Awuondo KO, Leach A, Lievens M, Leboulleux D, Njuguna P, Peshu N, Marsh K, Bejon P. 2013. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N Engl J Med 368:1111–1120. doi: 10.1056/NEJMoa1207564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, Ockenhouse CF, Ghani AC. 2013. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS One 8:e61395. doi: 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, et al. 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Nhamuave A, Quelhas D, Bassat Q, Mandjate S, Macete E, Alonso P, Abdulla S, Salim N, Juma O, Shomari M, Shubis K, Machera F, Hamad AS, Minja R, Mtoro A, Sykes A, Ahmed S, Urassa AM, Ali AM, Mwangoka G, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Tahita MC, Kabore W, Ouedraogo S, Sandrine Y, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Odero C, et al. 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 17.Shiratsuchi T, Rai U, Krause A, Worgall S, Tsuji M. 2010. Replacing adenoviral vector HVR1 with a malaria B cell epitope improves immunogenicity and circumvents preexisting immunity to adenovirus in mice. J Clin Invest 120:3688–3701. doi: 10.1172/JCI39812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buge SL, Richardson E, Alipanah S, Markham P, Cheng S, Kalyan N, Miller CJ, Lubeck M, Udem S, Eldridge J, Robert-Guroff M. 1997. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol 71:8531–8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klessig DF, Grodzicker T. 1979. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell 17:957–966. [DOI] [PubMed] [Google Scholar]
- 20.Hoti N, Li Y, Chen CL, Chowdhury WH, Johns DC, Xia Q, Kabul A, Hsieh JT, Berg M, Ketner G, Lupold SE, Rodriguez R. 2007. Androgen receptor attenuation of Ad5 replication: implications for the development of conditionally replication competent adenoviruses. Mol Ther 15:1495–1503. doi: 10.1038/sj.mt.6300223. [DOI] [PubMed] [Google Scholar]
- 21.Ketner G, Boyer J. 1998. Isolation, growth, and purification of defective adenovirus deletion mutants, p 19–28. In Wold WSM. (ed), Adenovirus methods and protocols. The Humana Press Inc., Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 22.Mittereder N, March KL, Trapnell BC. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 70:7498–7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson C, Oliveira AG, Sultan AA, Bhanot P, Nussenzweig V, Nardin E. 2002. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol 169:6681–6685. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- 24.Mercier GT, Nehete PN, Passeri MF, Nehete BN, Weaver EA, Templeton NS, Schluns K, Buchl SS, Sastry KJ, Barry MA. 2007. Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine 25:8687–8701. doi: 10.1016/j.vaccine.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nardin EH, Nussenzweig V, Nussenzweig RS, Collins WE, Harinasuta KT, Tapchaisri P, Chomcharn Y. 1982. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med 156:20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade F, Bull HG, Thornberry NA, Ketner GW, Casciola-Rosen LA, Rosen A. 2001. Adenovirus L4-100K assembly protein is a granzyme B substrate that potently inhibits granzyme B-mediated cell death. Immunity 14:751–761. doi: 10.1016/S1074-7613(01)00149-2. [DOI] [PubMed] [Google Scholar]
- 27.Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, Nussenzweig V, Rodriguez A. 2001. Migration of Plasmodium sporozoites through cells before infection. Science 291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 28.Mishra S, Nussenzweig RS, Nussenzweig V. 2012. Antibodies to Plasmodium circumsporozoite protein (CSP) inhibit sporozoite's cell traversal activity. J Immunol Methods 377:47–52. doi: 10.1016/j.jim.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim PL, Tan W, Latchoumycandane C, Mok WC, Khoo YM, Lee HS, Sattabongkot J, Beerheide W, Lim SG, Tan TM, Boelsterli UA. 2007. Molecular and functional characterization of drug-metabolizing enzymes and transporter expression in the novel spontaneously immortalized human hepatocyte line HC-04. Toxicol In Vitro 21:1390–1401. doi: 10.1016/j.tiv.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 31.Bruna-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol 31:1499–1502. doi: 10.1016/S0020-7519(01)00265-X. [DOI] [PubMed] [Google Scholar]
- 32.Hollingdale MR, Nardin EH, Tharavanij S, Schwartz AL, Nussenzweig RS. 1984. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol 132:909–913. [PubMed] [Google Scholar]
- 33.Collins WE. 1992. South American monkeys in the development and testing of malarial vaccines—a review. Mem Inst Oswaldo Cruz 87(Suppl 3):401–406. doi: 10.1590/S0074-02761992000700068. [DOI] [PubMed] [Google Scholar]
- 34.Collins WE, Sullivan JS, Williams A, Nace D, Williams T, Galland GG, Barnwell JW. 2006. Aotus nancymaae as a potential model for the testing of anti-sporozoite and liver stage vaccines against Plasmodium falciparum. Am J Trop Med Hyg 74:422–424. [PubMed] [Google Scholar]
- 35.Krause A, Joh JH, Hackett NR, Roelvink PW, Bruder JT, Wickham TJ, Kovesdi I, Crystal RG, Worgall S. 2006. Epitopes expressed in different adenovirus capsid proteins induce different levels of epitope-specific immunity. J Virol 80:5523–5530. doi: 10.1128/JVI.02667-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berg M, Adams RJ, Gambhira R, Siracusa MC, Scott AL, Roden RB, Ketner G. 2014. Immune responses in macaques to a prototype recombinant adenovirus live oral HPV16 vaccine. Clin Vaccine Immunol 21:1224–1231. doi: 10.1128/CVI.00197-14. [DOI] [PMC free article] [PubMed] [Google Scholar]