Abstract
Hemolytic-uremic syndrome (HUS), caused by Shiga toxin (Stx)-producing Escherichia coli (STEC), remains untreatable. Production of human monoclonal antibodies against Stx, which are highly effective in preventing Stx sequelae in animal models, is languishing due to cost and logistics. We reported previously that the production and evaluation of a camelid heavy-chain-only VH domain (VHH)-based neutralizing agent (VNA) targeting Stx1 and Stx2 (VNA-Stx) protected mice from Stx1 and Stx2 intoxication. Here we report that a single intramuscular (i.m.) injection of a nonreplicating adenovirus (Ad) vector carrying a secretory transgene of VNA-Stx (Ad/VNA-Stx) protected mice challenged with Stx2 and protected gnotobiotic piglets infected with STEC from fatal systemic intoxication. One i.m. dose of Ad/VNA-Stx prevented fatal central nervous system (CNS) symptoms in 9 of 10 animals when it was given to piglets 24 h after bacterial challenge and in 5 of 9 animals when it was given 48 h after bacterial challenge, just prior to the onset of CNS symptoms. All 6 placebo animals died or were euthanized with severe CNS symptoms. Ad/VNA-Stx treatment had no impact on diarrhea. In conclusion, Ad/VNA-Stx treatment is effective in protecting piglets from fatal Stx2-mediated CNS complications following STEC challenge. With a low production cost and further development, this could presumably be an effective treatment for patients with HUS and/or individuals at high risk of developing HUS due to exposure to STEC.
INTRODUCTION
Infection with Shiga toxin (Stx)-producing Escherichia coli (STEC) is the most significant cause of hemolytic-uremic syndrome (HUS), the leading cause of acute renal failure in children (1–4) and in some adults. Of the two antigenically distinct toxins, Stx1 and Stx2, Stx2 is more firmly linked with the development of HUS, since STEC strains producing this toxin are more frequently associated with HUS than strains that produce both Stx1 and Stx2, while Stx1 alone has rarely been associated with HUS (5–7). Stx1 and Stx2 are similar in basic structure (8), binding specificity (8), and mode of action (9, 10). Both toxins consist of an A-subunit monomer and a B-subunit pentamer (8, 11, 12). The pentameric B subunit binds to its cell surface receptor, CD77, also called globotriaosyl ceramide (Gb3; Galα1-4 Galβ1-4 glucosyl ceramide) (13, 14). This binding triggers endocytosis of the holotoxin, mainly through clathrin-coated pits (15). Internalization of the catalytically active A subunit, delivered to the cytosol via retrograde transport, causes the shutdown of protein synthesis and leads to cell death (9, 10). In addition to blocking protein synthesis, a long-term effect of the toxin in several types of cells is the induction of apoptosis (16).
We previously reported the production of human monoclonal antibodies (HuMAbs) against Stx1 and Stx2 and their evaluation in animal models for efficacy against systemic toxin challenge (17–19) or oral STEC infection (17, 19–21). Clinical evaluation of these monoclonal antibodies has been slow and is still pending, due largely to the logistics and cost. We also reported the use of an alternative antitoxin strategy that employs “VHH-based neutralizing agents” (VNAs) consisting of linked 14-kDa camelid heavy-chain-only VH domains (VHHs), produced as heteromultimers, that bind and neutralize toxin targets (22, 23). Linking VHHs to form VNAs results in agents with much greater therapeutic efficacy in preventing intoxication in animals due to exposure to Stx1 and Stx2 (23), botulinum neurotoxin (22), ricin (24), or Clostridium difficile toxins TcdA and TcdB (25) than equivalent pools of the VHH components. VNAs also contain several copies of an epitopic tag recognized by an anti-tag MAb. Coadministration of the anti-tag MAb, called the effector antibody (efAb), can enhance the therapeutic efficacy of VNA in some intoxication models (22–25), probably by promoting toxin clearance through the liver (26). Inclusion of an albumin-binding peptide (ABP) substantially prolonged the functional half-life of VNA in serum, from 1 to 2 h to more than a day (27).
VNA antitoxins offer the potential for genetic delivery using vehicles that lead to the expression of antitoxin protein by patients. A wide variety of genetic delivery vehicles have already been developed, including direct administration of DNA and RNA, recombinant adenovirus (Ad) (28–30), and adeno-associated virus (AAV) (31, 32). Furthermore, gene delivery vehicles can effectively promote in vivo expression of a range of antibody species for passive immunotherapy (28, 29, 33, 34). We have shown that gene therapy with an Ad expressing a VNA that neutralizes botulinum neurotoxin serotype A (VNA-BoNT/A) resulted in sustained high levels of VNA-BoNTA in serum that protected mice from BoNT/A challenge for several months (27).
In this study, we report the use of a recombinant, replication-incompetent human Ad serotype 5 (Ad5) vector that promotes de novo secretion of antitoxin VNAs into the circulation. The Ad/VNA-Stx vector produces a potent anti-Stx VNA, a VHH heterotrimer (A9/A5/G1 [23]) that recognizes both Stx1 and Stx2. Here we demonstrate that a single administration of Ad/VNA-Stx protects mice against Stx2 intoxication following parenteral toxin challenge and protects piglets against fatal systemic intoxication when given 24 h after oral STEC infection.
MATERIALS AND METHODS
Ethics statement.
The mouse and piglet studies described in this report were carried out in strict accordance with the National Institutes of Health guidelines for the ethical treatment of animals and were approved by the Institutional Animal Care and Use Committee of Tufts University.
Bacteria and toxin.
Enterohemorrhagic E. coli (EHEC) O157:H7 strain 86-24, which produces Stx2, was isolated in 1986 from a patient with hemorrhagic colitis in Seattle, WA (35). Stx2, purified as described previously (36), was obtained from the Phoenix Laboratory (Tufts University–NEMC Microbial Products and Services Facility). Before use, the toxin was passed through a Polyacrylamide 6000 Desalting column (Thermo Scientific, IL) in order to suspend it in phosphate-buffered saline (PBS) (pH 7.2). The toxin was quantified by UV spectrophotometry (ND-1000 spectrophotometer; NanoDrop), aliquoted, and stored at −20°C.
Adenovirus vector construction and preparation.
The recombinant replication-incompetent Ad5-based vector was generated essentially as described elsewhere (37). Briefly, the genome of the Ad/VNA-Stx vector was generated using the pShCMV-JHQ9 shuttle plasmid, which was constructed by subcloning DNA encoding VNA-Stx, a VHH heterotrimer (A9/A5/G1 [23]) recognizing both Stx1 and Stx2, with a carboxyl-terminal ABP under the control of a cytomegalovirus (CMV) promoter within plasmid pAdTrack (38) while replacing the CMV-driven green fluorescent protein (GFP) gene. The pShCMV-JHQ9 plasmid was linearized with BstZ17I and was employed for homologous recombination with the pAdEasy-1 plasmid (38) using E. coli BJ5183-AD-1 cells as recommended by the manufacturer (Agilent Technologies, Inc., Santa Clara, CA) to generate the pAd/VNA-Stx rescue plasmid containing the viral genome. The resultant pAd/VNA-Stx plasmid, which was validated by PCR, restriction analysis, and partial sequencing, was first digested with PacI to release the inverted terminal repeats of the viral genomic DNA and then transfected into 293 cells (39) to rescue the replication-incompetent Ad/VNA-Stx vector. The newly rescued Ad vector was propagated on 911 cells (40), purified by centrifugation on CsCl gradients according to the standard protocol, and dialyzed against PBS (8 mM Na2HPO4, 2 mM KH2PO4 [pH 7.4], 137 mM NaCl, 2.7 mM KCl) containing 10% glycerol. The titers of physical virus particles (vp) were determined by the methods of Maizel et al. (41).
Mouse toxicity model.
The mouse toxicity model (19, 20) was used to determine the efficacy of Ad/VNA-Stx in protecting mice against a parenteral challenge with a lethal Stx2 dose. Two groups of 10 ∼4-week-old female Swiss Webster mice (Charles River Laboratories, Inc.) were injected intraperitoneally (i.p.) with 60 ng (∼2.5 100% lethal doses [LD100]) of Stx2. One of the two groups was injected i.p. with 1 × 1010 Ad/VNA-Stx vp in 100 μl PBS/mouse 4 h prior to administration of the toxin, and another group was injected with PBS at this time. Mice were monitored for illness and death 4 or more times daily for 1 week. Mice that were severely ill were humanely euthanized. Similarly, surviving animals were humanely euthanized 1 week after Stx2 challenge. Blood was collected for the measurement of serum VNA-Stx concentrations before euthanasia.
Gnotobiotic piglet model of STEC infection.
The Ad/VNA-Stx treatment was further evaluated in the gnotobiotic piglet infection model of STEC using E. coli strain 86-24 (17, 20). A total of 35 piglets were used to determine the protective efficacy of the Ad/VNA-Stx treatment, given intramuscularly (i.m.), either 18 h before (5 piglets treated, 5 given a placebo) or 24 h (10 treated, 6 given a placebo) or 48 h (9 treated) after bacterial challenge with ∼1010 CFU of 86-24. This high inoculum usually induces neurological signs and lesions associated with Stx2 activity in 95% of control piglets within 48 to 96 h postinfection (20, 42). The placebo group received either PBS or an Ad5-based vector expressing VNA against botulinum neurotoxin serotype A (Ad/VNA-BoNTA) (27) or C. difficile toxins TcdA and TcdB (Ad/VNA-Tcd) (unpublished data). Piglets were monitored ≥4 times daily for diarrhea, dehydration, and central nervous system (CNS) symptoms, which included ataxia, paresis, head pressing, paddling, convulsions, and opisthotonos. Animals with severe CNS symptoms, as well as surviving animals 7 to 12 days after the bacterial challenge, were humanely euthanized. Brain tissue (cerebral cortex and cerebellum) and gut sections were fixed in formalin and were processed for histology, and blood was collected for the measurement of serum antibody concentrations.
Another group of 4 control piglets was intramuscularly administered 1 × 1011 Ad/VNA-Stx vp/kg of body weight 48 h after birth but was not challenged with STEC. These piglets were bled every day for 7 days in order to determine the kinetics of serum VNA-Stx.
Capture ELISAs to measure serum VNA levels.
MaxiSorp C96 immunoplates (Nunc) or 96-well tissue culture plates (Corning) were coated overnight with 100 μl/well of 1 μg/ml Stx2 (Phoenix Laboratory, Tufts University, Boston, MA). Affinity-purified recombinant VNA-Stx was used as the standard in every plate to estimate VNA-Stx levels in piglet/mouse sera. This standard was “spiked” into normal mouse/piglet serum at 80 nM (4 μg/ml). Enzyme-linked immunosorbent assays (ELISAs) were performed in which spiked serum or test sera were applied to a well at a 1:10 dilution, followed by seven 1:5 serial dilutions. VNA bound to the coated Stx2 was detected with a horseradish peroxidase (HRP)-conjugated anti-E-tag MAb (GE Healthcare). By use of the linear portion of the VNA-Stx standard-curve plot, the VNA-Stx content of each piglet/mouse test serum sample was determined and was expressed as micrograms of VNA-Stx per milliliter. Typically, signals 2-fold above background were achieved in dilutions of the VNA-Stx standard down to about 30 pM (1.5 ng/ml). Thus, all assays measuring levels below 30 pM were considered to have results “below the detection limit.” VNA-BoNTA and VNA-Tcd levels in serum were determined in the same manner, by employing the target of the control VNA in place of Stx2 in the ELISA.
Statistical analysis.
The grouped survival data were analyzed by applying a Kaplan-Meier log rank test using SigmaPlot (version 13.0; Systat Software, Inc.). Resulting P values of <0.05 were considered significant.
RESULTS
Efficacy of Ad/VNA-Stx treatment in the mouse toxicity model.
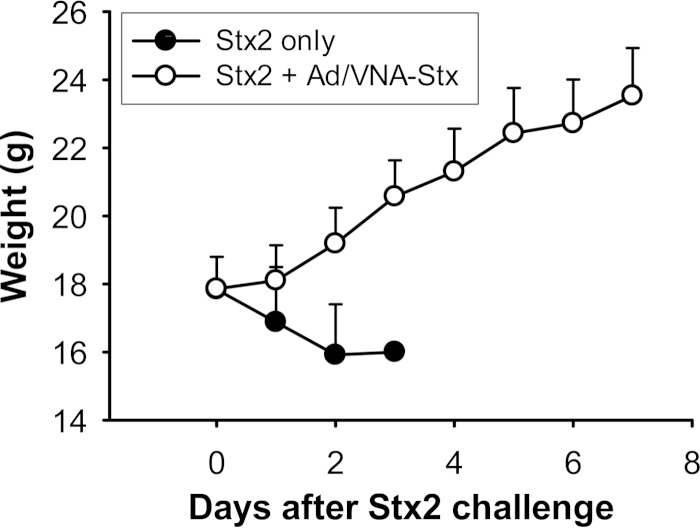
A replication-defective Ad5 vector was engineered to promote mammalian-cell secretion of the recombinant heterotrimeric VHH-based neutralizing agent (VNA) that potently neutralizes both Shiga toxins, Stx1 and Stx2 (23) (VNA-Stx). The resulting vector, called Ad/VNA-Stx, was administered i.p. to groups of mice (1010 vp/mouse) 4 h prior to intoxication with Stx2 (60 ng/mouse; ∼2.5 LD100). All mice given Ad/VNA-Stx survived, while all placebo (PBS)-treated mice succumbed to Stx2 toxicity within 2 to 3 days (Table 1). The difference in survival time between the 2 groups was statistically significant (P < 0.001). All the mice in the Ad/VNA-Stx-treated group gained weight following the toxin challenge (mean weight gain, 1.33 g on day 2 [Fig. 1]). In contrast, all mice in the placebo group lost weight (mean weight loss, 1.92 g on day 2 [Fig. 1]). Serum VNA-Stx concentrations in mice, measured at the end of the experimental period (1 week after Ad/VNA-Stx treatment), ranged from 0.5 to 6.0 μg/ml, with a mean of 2.55 μg/ml (Table 1).
TABLE 1.
Survival of mice given an Stx2-neutralizing VNA treatment or a placebo intraperitoneally 4 h before challenge with Stx2
| Treatmenta | Dose (vp/mouse) | No. of surviving mice (% survival)b | Mean time (days) to death/euthanasia (± SD)c | Mean serum VNA concn (μg/ml) (range)d |
|---|---|---|---|---|
| Ad/VNA-Stx | 1 × 1010 | 10 (100) | 7.0 (±0.0) A | 2.55 (0.5–6.0) |
| Placebo | PBS | 0 (0) | 2.4 (±0.5) B |
There were 10 mice in each group. Mice in the Ad/VNA-Stx group were given an Stx2-neutralizing VNA treatment intraperitoneally 4 h before Stx2 challenge. Mice in the placebo group received PBS only.
Mice in the placebo group either died overnight or were euthanized due to severe illness. All mice in the Ad/VNA-Stx group survived and were euthanized after 7 days.
Different capital letters following survival times indicate statistically significant differences (P < 0.001) between those treatment groups.
Estimated concentration of VNA in mouse serum at the time of euthanasia.
FIG 1.
Change in body weight. The group that received Stx2 only consistently lost weight and succumbed to Stx2 toxicity within 2 to 3 days of toxin administration. In contrast, the group that received Ad/VNA-Stx treatment 4 h prior to toxin administration (Stx2 + Ad/VNA-Stx) consistently gained weight, and all mice in this group survived.
Efficacy of Ad/VNA-Stx treatment in the piglet diarrhea model of oral STEC infection.
Piglets received Ad/VNA-Stx treatment either 18 h before (Table 2) or 24 h or 48 h after (Table 3) bacterial infection. Almost all piglets in all groups, irrespective of the time of Ad/VNA-Stx treatment, developed diarrhea within 20 to 55 h (the majority within 42 to 48 h) after the challenge. The Ad/VNA-Stx treatments were intramuscular (i.m.) and were administered before the onset of systemic complications/CNS symptoms, which tended to develop ∼48 h after the oral STEC challenge.
TABLE 2.
Survival of piglets given either a placebo or an Stx2-neutralizing VNA treatment intramuscularly 18 h before oral challenge with 1 × 1010 CFU of STEC 86-24
| Treatmenta | Dose (vp/kg) | No. of surviving piglets (% survival)b | Mean time (days) to death/euthanasia (± SD)c | Mean serum VNA concn (μg/ml) (range)d |
|---|---|---|---|---|
| Ad/VNA-Stx | 1 × 1012 | 5 (100) | 12 (±0.0) A | 0.05 (0.025–0.1) |
| Placebo | PBS | 0 (0) | 5.0 (±2.0) B |
There were 5 piglets per group.
Piglets in the placebo group either died overnight or were euthanized due to severe illness.
All piglets in the Ad/VNA-Stx group were euthanized after 12 days. Different capital letters following survival times indicate statistically significant differences (P < 0.001) between those treatment groups.
Estimated concentration of VNA in piglet serum at the time of euthanasia.
TABLE 3.
Survival of piglets orally challenged with 1010 CFU of STEC 86-24 followed by intramuscular administration of either a placebo or an Stx2-neutralizing VNA treatment 24 h or 48 h later
| Treatment (h post-STEC challenge) | Dose (vp/kg) | No. of piglets | No. of surviving piglets (% survival)a | Mean time (days) to death/euthanasia (± SD)b | Mean serum VNA concn (μg/ml) (range)c |
|---|---|---|---|---|---|
| Placebod (24) | 1 × 1011 or 1 × 1012 | 6 | 0 (0) | 3.50 (±0.54) A | Not relevante |
| Ad/VNA-Stx (24) | 1 × 1012 | 4 | 3 (75) | 5.0 BC | 0.70 (0.2–2.0) |
| 1 × 1011 | 6 | 6 (100) | 9.0 (±0.0) BC | 0.08 (0.03–1.0) | |
| Ad/VNA-Stx (48) | 1 × 1012 | 7 | 4 (57) | 3.33 (±0.57) AC | 0.23 (0.05–0.4) |
| 1 × 1011 | 2 | 1 (50) | 3.0 AC | 0.15 |
Piglets that did not survive either died overnight or were euthanized due to severe illness.
Surviving piglets in the Ad/VNA-Stx groups were euthanized after 9 days. The survival times of piglets that either were euthanized or died in Ad/VNA-Stx groups are shown. Survival times followed by the same capital letter are not statistically significantly different. Different capital letters following survival times indicate statistically significant differences (P < 0.05).
Estimated concentration of VNA in piglet serum at the time of euthanasia.
Four piglets in the placebo group received 1012 vp of Ad/VNA-BoNTA/kg, and two received 1011 vp of Ad/VNA-Tcd/kg.
The estimated mean VNA concentration in the sera of pigs receiving 1012 vp of Ad/VNA-BoNTA/kg was 3.75 μg/ml, and that for pigs receiving 1011 vp of Ad/VNA-Tcd/kg was 0.02 μg/ml.
Treatment with 1 × 1012 vp of Ad/VNA-Stx per kg of body weight administered 18 h before oral STEC challenge significantly protected all piglets from fatal systemic intoxication, whereas all piglets in the placebo (PBS) group succumbed to Stx2-associated CNS symptoms after the oral STEC challenge (P < 0.001) (Table 2). Serum VNA-Stx concentrations ranged from 0.025 to 0.1 μg/ml, with a mean of 0.05 μg/ml (Table 2).
Table 3 summarizes piglet survival following treatment with either of two doses of Ad/VNA-Stx (1 × 1011 or 1 × 1012 vp/kg of body weight) administered 24 h or 48 h after oral STEC infection. All six pigs in the group receiving the 1 × 1011-vp/kg dose 24 h after infection were fully and significantly protected (P = 0.009) against systemic intoxication. Similarly, the 1 × 1012-vp/kg dose given 24 h postinfection significantly protected piglets (3 of 4 piglets protected; P = 0.04). Mean serum VNA-Stx concentrations were 0.7 μg/ml for pigs given the 1012-vp/kg dose and 0.08 μg/ml for pigs given 1011 vp/kg. The one Ad/VNA-Stx-treated pig that succumbed survived for 5 days after infection. In contrast, in pigs treated 48 h after infection, either dose of Ad/VNA-Stx protected only about 50% (5/9) of piglets from STEC sequelae and death; the survival of piglets in these groups was not significantly different from that of placebo-treated piglets. Serum VNA-Stx concentrations averaged 0.23 μg/ml in the group receiving the 1012-vp/kg dose and 0.15 μg/ml in the group receiving 1011 vp/kg. All piglets in the placebo (Ad/VNA-BoNTA or Ad/VNA-Tcd) group succumbed after challenge, with a mean survival time of 3.5 days. The mean concentrations of VNA-BoNTA (in the group receiving 1012 vp of Ad/VNA-BoNTA/kg) and VNA-Tcd (in the group receiving 1011 vp of Ad/VNA-Tcd/kg) in serum were 3.75 μg/ml and 0.02 μg/ml, respectively.
We also studied the kinetics of VNA-Stx in serum after the administration of Ad/VNA-Stx to 4 piglets that were not challenged with STEC in order to determine VNA-Stx levels in the absence of the toxin over 7 days. The mean serum VNA-Stx concentrations for these pigs on days 1, 2, 3, 4, 5, 6, and 7 were 11.3, 16.7, 41.3, 65.0, 10.0, 10.0, and 10.0 ng/ml, respectively. VNA-Stx was clearly detectable within a day after Ad/VNA-Stx administration, peaked on day 4, and remained detectable for at least several more days.
Clinical observations.
Untreated piglets challenged orally with STEC typically develop profound gastrointestinal (GI) tract symptoms within 2 days, which include diarrhea, anorexia, depression, dehydration, and moderate but rapid loss of body weight. Dehydration often requires fluid therapy, but the piglets in this study were mildly dehydrated and did not need fluid therapy. The appearance of GI tract symptoms was highly variable. Half of the piglets listed in Table 2, which received Ad/VNA-Stx or a placebo at 18 h before bacterial challenge, developed GI tract symptoms within 20 to 23 h, and another half developed these symptoms within 44 h of infection. Five of the 6 placebo-treated piglets listed in Table 3 developed diarrhea within 44 to 50 h of infection, and 1 developed diarrhea within 76 h after infection. Fourteen of the 19 Ad/VNA-Stx-treated piglets survived (Table 3); the majority of them (12 piglets) developed diarrhea within 44 to 48 h of infection, and 2 developed diarrhea within 65 h after infection. The 5 Ad/VNA-Stx-treated piglets that did not survive (Table 3) developed diarrhea within 44 to 48 h after infection. As expected, while parenteral administration of Ad/VNA-Stx had a major impact on animal survival, it had no effect on the GI tract symptoms. Typically, all piglets challenged with STEC develop diarrhea and other GI tract symptoms due to an intimate attachment of bacteria to the mucosal surfaces of the terminal ileum and the entire large bowel, including the colon and cecum. The nature, distribution, and extent of the mucosal lesions in gnotobiotic piglets induced by STEC strain 86-24 were consistent with those described in the past by us (20, 43–45) and others (46).
DISCUSSION
This project builds on earlier work that describes in detail the VNA technology developed in this lab (22, 23), specifically, a single VNA, now called VNA-Stx, that targets both Stx1 and Stx2 for potent neutralization (23). This VNA, consisting of three different Stx-binding VHHs linked into a heterotrimer, fully protected mice from lethal doses of Stx1 or Stx2 when coadministered with an effector antibody to promote toxin clearance. In this study, we show that de novo expression of a VNA-Stx transgene following treatment with a recombinant Ad vector can lead to protection from Stx2 exposure in mice and from the fatal systemic intoxication caused by STEC infection in piglets.
The gnotobiotic piglet has been used extensively over the last 2 decades by several investigators, including us (17, 20, 21), to study the sequence of events leading to diarrhea and systemic intoxication due to Stx uptake from severely damaged colonic mucosae. STEC infections can cause severe kidney injury, at least temporarily, in subsets of infected children and adults. Although neurological complications are less common in human patients, systemic complications manifest largely in the form of fatal CNS vascular injury in piglets. Therefore, we use fatal CNS manifestations as the endpoint. In children, the prodromal period is 3 to 7 days between the onset of diarrhea due to STEC and HUS, while in piglets, this period is typically 2 to 4 days (17, 20, 21).
While acute STEC diarrhea tends to be transient, with rapid recovery, in humans, a few infected individuals (especially young children and the elderly) go on to develop serious, even fatal consequences, such as hemolytic anemia, thrombocytopenia, acute kidney failure, and sometimes neurological symptoms (7). In some survivors of HUS, long-term kidney damage may require dialysis. Therefore, there is a critical need for an effective treatment that can prevent the sequelae when given to those at risk of developing HUS after the onset of bloody diarrhea. Targeting the two toxins for neutralization seems an obvious approach, and their interception is more likely to be effective in the circulation than in the lumen of the large bowel, where they are liberated by the bacteria. With this in mind, we developed HuMAbs to neutralize Stx (17–19), which were shown to be highly effective in protecting STEC-infected piglets (17, 20, 21). Other investigators have also developed Stx-neutralizing chimeric (47) or humanized (48, 49) MAbs. HuMAb 5C12, specific for Stx2, was found to be highly effective in protecting piglets 48 h after STEC infection; a dose of 0.4 mg/kg of body weight protected 80% of piglets (20). This product is slowly making its way through to a phase I clinical trial with human volunteers (unpublished data). However, HuMAb production and delivery to individuals at risk are expected to be very costly, requiring intravenous administration; thus, they may be offered only to patients presenting with HUS, at which point it may be too late for a complete recovery. Hence, our group continued the search for a treatment that is easy to manufacture at a low cost, simple to administer by i.m. injection, and safe and that can be given to all individuals at risk who either present with diarrhea, have been exposed to a known source of infection, or show symptoms of HUS. We believe that treatment with a product such as the Ad/VNA-Stx described in this paper could provide these benefits.
The outcome of the experiments in the piglet model is compelling evidence of the efficacy of this therapeutic approach to a very serious disease against which there is currently no specific treatment or prevention. These experiments show that a single i.m. injection of an Ad vector-delivered VNA-Stx gene, given before challenge with STEC, fully protected piglets against fatal systemic intoxication, in contrast with a placebo Ad vector. More significant, however, is the demonstration that a single i.m. injection of Ad/VNA-Stx, given 24 h after bacterial challenge, protected 9 of 10 animals. Treatment at 48 h after bacterial challenge, shortly before the onset of systemic intoxication, resulted in the survival of more than half of the VNA-treated animals. This corresponds to the treatment of children just before the onset of HUS. Given the hyperacute nature of HUS, protection is critical over a relatively short period of 4 to 10 days after the onset of diarrhea or exposure to STEC, which makes the half-life of the VNA in the circulation less critical. VNA is also a one-time treatment, and therefore, there should be no concern associated with possible VNA immunogenicity from multiple dosing.
For this work, we employed a standard Ad5 vector at doses of 1011 to 1012 vp/kg, based on prior literature and experience. We observed no significant difference in efficacy between the two doses, so additional studies are required to establish the minimal effective dose, as it relates to the time of delivery after bacterial challenge. It is more than likely that the earlier the time of delivery to an infected individual, the smaller the dose required for full protection. For ultimate clinical use, it will likely be necessary to develop novel nonreplicating Ad vectors specifically designed for therapeutic expression of VNAs in patient serum. Such vectors might be targeted to the vascular endothelium for optimal secretion. Additionally, it may be advantageous to engineer the regulated expression of the VNA such that expression can be induced or repressed as needed for optimal efficacy, and to further reduce the risk of developing neutralizing Abs to the VNA, which is already expected to be poorly immunogenic (50, 51). Finally, it may be beneficial for the Ad vector to coexpress an anti-tag effector antibody, since this would increase the potency of the epitopically tagged VNA and promote more rapid clearance of the Shiga toxins from the system (22, 23, 26).
As expected, the systemic production of VNA-Stx, which targets the toxins in the circulation, had no effect on the extent and duration of diarrhea in piglets due to STEC infection. We made the same observation with HuMAb treatment of STEC-infected piglets (17, 20, 21). However, this treatment is intended to protect against or treat HUS, and not the diarrhea due to STEC, which in itself is less life-threating.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Patricia Boucher and Rachel Nieminen in performing the piglet experiments. We thank Yimin Zhang for statistical analysis.
REFERENCES
- 1.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol 37:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin PM, Tauxe RV. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev 13:60–98. [DOI] [PubMed] [Google Scholar]
- 3.Milford DV, Taylor CM, Guttridge B, Hall SM, Rowe B, Kleanthous H. 1990. Haemolytic uraemic syndromes in the British Isles 1985–8: association with verocytotoxin producing Escherichia coli. Part 1. Clinical and epidemiological aspects. Arch Dis Child 65:716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostroff SM, Tarr PI, Neill MA, Lewis JH, Hargrett-Bean N, Kobayashi JM. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J Infect Dis 160:994–998. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich AW, Bielaszewska M, Zhang WL, Pulz M, Kuczius T, Ammon A, Karch H. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis 185:74–84. doi: 10.1086/338115. [DOI] [PubMed] [Google Scholar]
- 6.Rüssmann H, Schmidt H, Heesemann J, Caprioli A, Karch H. 1994. Variants of Shiga-like toxin II constitute a major toxin component in Escherichia coli O157 strains from patients with haemolytic uraemic syndrome. J Med Microbiol 40:338–343. doi: 10.1099/00222615-40-5-338. [DOI] [PubMed] [Google Scholar]
- 7.Tzipori S, Sheoran A, Akiyoshi D, Donohue-Rolfe A, Trachtman H. 2004. Antibody therapy in the management of Shiga toxin-induced hemolytic uremic syndrome. Clin Microbiol Rev 17:926–941. doi: 10.1128/CMR.17.4.926-941.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser ME, Fujinaga M, Cherney MM, Melton-Celsa AR, Twiddy EM, O'Brien AD, James MN. 2004. Structure of Shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J Biol Chem 279:27511–27517. doi: 10.1074/jbc.M401939200. [DOI] [PubMed] [Google Scholar]
- 9.Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. 1988. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem 171:45–50. [DOI] [PubMed] [Google Scholar]
- 10.Saxena SK, O'Brien AD, Ackerman EJ. 1989. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28 S RNA when microinjected into Xenopus oocytes. J Biol Chem 264:596–601. [PubMed] [Google Scholar]
- 11.Ling H, Boodhoo A, Hazes B, Cummings MD, Armstrong GD, Brunton JL, Read RJ. 1998. Structure of the Shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry 37:1777–1788. doi: 10.1021/bi971806n. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu H, Field RA, Homans SW, Donohue-Rolfe A. 1998. Solution structure of the complex between the B-subunit homopentamer of verotoxin VT-1 from Escherichia coli and the trisaccharide moiety of globotriaosylceramide. Biochemistry 37:11078–11082. doi: 10.1021/bi980946+. [DOI] [PubMed] [Google Scholar]
- 13.Lingwood CA, Law H, Richardson S, Petric M, Brunton JL, De Grandis S, Karmali M. 1987. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem 262:8834–8839. [PubMed] [Google Scholar]
- 14.Waddell T, Head S, Petric M, Cohen A, Lingwood C. 1988. Globotriosyl ceramide is specifically recognized by the Escherichia coli verocytotoxin 2. Biochem Biophys Res Commun 152:674–679. doi: 10.1016/S0006-291X(88)80091-3. [DOI] [PubMed] [Google Scholar]
- 15.Lingwood CA. 1996. Role of verotoxin receptors in pathogenesis. Trends Microbiol 4:147–153. doi: 10.1016/0966-842X(96)10017-2. [DOI] [PubMed] [Google Scholar]
- 16.Tesh VL. 2010. Induction of apoptosis by Shiga toxins. Future Microbiol 5:431–453. doi: 10.2217/fmb.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee J, Chios K, Fishwild D, Hudson D, O'Donnell S, Rich SM, Donohue-Rolfe A, Tzipori S. 2002. Human Stx2-specific monoclonal antibodies prevent systemic complications of Escherichia coli O157:H7 infection. Infect Immun 70:612–619. doi: 10.1128/IAI.70.2.612-619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee J, Chios K, Fishwild D, Hudson D, O'Donnell S, Rich SM, Donohue-Rolfe A, Tzipori S. 2002. Production and characterization of protective human antibodies against Shiga toxin 1. Infect Immun 70:5896–5899. doi: 10.1128/IAI.70.10.5896-5899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheoran AS, Chapman S, Singh P, Donohue-Rolfe A, Tzipori S. 2003. Stx2-specific human monoclonal antibodies protect mice against lethal infection with Escherichia coli expressing Stx2 variants. Infect Immun 71:3125–3130. doi: 10.1128/IAI.71.6.3125-3130.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheoran AS, Chapman-Bonofiglio S, Harvey BR, Mukherjee J, Georgiou G, Donohue-Rolfe A, Tzipori S. 2005. Human antibody against Shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infect Immun 73:4607–4613. doi: 10.1128/IAI.73.8.4607-4613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong KI, Tzipori S, Sheoran AS. 2010. Shiga toxin 2-specific but not Shiga toxin 1-specific human monoclonal antibody protects piglets challenged with enterohemorrhagic Escherichia coli producing Shiga toxin 1 and Shiga toxin 2. J Infect Dis 201:1081–1083. doi: 10.1086/651198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee J, Tremblay JM, Leysath CE, Ofori K, Baldwin K, Feng X, Bedenice D, Webb RP, Wright PM, Smith LA, Tzipori S, Shoemaker CB. 2012. A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PLoS One 7:e29941. doi: 10.1371/journal.pone.0029941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tremblay JM, Mukherjee J, Leysath CE, Debatis M, Ofori K, Baldwin K, Boucher C, Peters R, Beamer G, Sheoran A, Bedenice D, Tzipori S, Shoemaker CB. 2013. A single VHH-based toxin neutralizing agent and an effector antibody protects mice against challenge with Shiga toxins 1 and 2. Infect Immun 81:4592–4603. doi: 10.1128/IAI.01033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vance DJ, Tremblay JM, Mantis NJ, Shoemaker CB. 2013. Stepwise engineering of heterodimeric single domain camelid VHH antibodies that passively protect mice from ricin toxin. J Biol Chem 288:36538–36547. doi: 10.1074/jbc.M113.519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Schmidt D, Liu W, Li S, Shi L, Sheng J, Chen K, Yu H, Tremblay JM, Chen X, Piepenbrink KH, Sundberg EJ, Kelly CP, Bai G, Shoemaker CB, Feng H. 2014. A novel multivalent, single-domain antibody targeting TcdA and TcdB prevents fulminant Clostridium difficile infection in mice. J Infect Dis 210:964–972. doi: 10.1093/infdis/jiu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sepulveda J, Mukherjee J, Tzipori S, Simpson LL, Shoemaker CB. 2010. Efficient serum clearance of botulinum neurotoxin achieved using a pool of small antitoxin binding agents. Infect Immun 78:756–763. doi: 10.1128/IAI.01084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee J, Dmitriev I, Debatis M, Tremblay JM, Beamer G, Kashentseva EA, Curiel DT, Shoemaker CB. 2014. Prolonged prophylactic protection from botulism with a single adenovirus treatment promoting serum expression of a VHH-based antitoxin protein. PLoS One 9:e106422. doi: 10.1371/journal.pone.0106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sofer-Podesta C, Ang J, Hackett NR, Senina S, Perlin D, Crystal RG, Boyer JL. 2009. Adenovirus-mediated delivery of an anti-V antigen monoclonal antibody protects mice against a lethal Yersinia pestis challenge. Infect Immun 77:1561–1568. doi: 10.1128/IAI.00856-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasuya K, Boyer JL, Tan Y, Alipui DO, Hackett NR, Crystal RG. 2005. Passive immunotherapy for anthrax toxin mediated by an adenovirus expressing an anti-protective antigen single-chain antibody. Mol Ther 11:237–244. doi: 10.1016/j.ymthe.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Skaricic D, Traube C, De B, Joh J, Boyer J, Crystal RG, Worgall S. 2008. Genetic delivery of an anti-RSV antibody to protect against pulmonary infection with RSV. Virology 378:79–85. doi: 10.1016/j.virol.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Fang J, Yi S, Simmons A, Tu GH, Nguyen M, Harding TC, VanRoey M, Jooss K. 2007. An antibody delivery system for regulated expression of therapeutic levels of monoclonal antibodies in vivo. Mol Ther 15:1153–1159. doi: 10.1038/sj.mt.6300142. [DOI] [PubMed] [Google Scholar]
- 32.Zuber C, Mitteregger G, Schuhmann N, Rey C, Knackmuss S, Rupprecht W, Reusch U, Pace C, Little M, Kretzschmar HA, Hallek M, Buning H, Weiss S. 2008. Delivery of single-chain antibodies (scFvs) directed against the 37/67 kDa laminin receptor into mice via recombinant adeno-associated viral vectors for prion disease gene therapy. J Gen Virol 89:2055–2061. doi: 10.1099/vir.0.83670-0. [DOI] [PubMed] [Google Scholar]
- 33.De BP, Hackett NR, Crystal RG, Boyer JL. 2008. Rapid/sustained anti-anthrax passive immunity mediated by co-administration of Ad/AAV. Mol Ther 16:203–209. doi: 10.1038/sj.mt.6300344. [DOI] [PubMed] [Google Scholar]
- 34.Tutykhina IL, Sedova ES, Gribova IY, Ivanova TI, Vasilev LA, Rutovskaya MV, Lysenko AA, Shmarov MM, Logunov DY, Naroditsky BS, Tillib SV, Gintsburg AL. 2013. Passive immunization with a recombinant adenovirus expressing an HA (H5)-specific single-domain antibody protects mice from lethal influenza infection. Antiviral Res 97:318–328. doi: 10.1016/j.antiviral.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Tarr PI, Neill MA, Clausen CR, Newland JW, Neill RJ, Moseley SL. 1989. Genotypic variation in pathogenic Escherichia coli O157:H7 isolated from patients in Washington, 1984–1987. J Infect Dis 159:344–347. doi: 10.1093/infdis/159.2.344. [DOI] [PubMed] [Google Scholar]
- 36.Donohue-Rolfe A, Acheson DW, Kane AV, Keusch GT. 1989. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect Immun 57:3888–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He TC. 2007. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 38.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. 1998. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham FL, Smiley J, Russell WC, Nairn R. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 40.Fallaux FJ, Kranenburg O, Cramer SJ, Houweling A, Van Ormondt H, Hoeben RC, Van Der Eb AJ. 1996. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther 7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 41.Maizel JV Jr, White DO, Scharff MD. 1968. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 36:115–125. [DOI] [PubMed] [Google Scholar]
- 42.Donohue-Rolfe A, Kondova I, Oswald S, Hutto D, Tzipori S. 2000. Escherichia coli O157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for gnotobiotic piglets than are isotypes producing only Stx1 or both Stx1 and Stx2. J Infect Dis 181:1825–1829. doi: 10.1086/315421. [DOI] [PubMed] [Google Scholar]
- 43.Tzipori S, Gunzer F, Donnenberg MS, de Montigny L, Kaper JB, Donohue-Rolfe A. 1995. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun 63:3621–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzipori S, Chow CW, Powell HR. 1988. Cerebral infection with Escherichia coli O157:H7 in humans and gnotobiotic piglets. J Clin Pathol 41:1099–1103. doi: 10.1136/jcp.41.10.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donohue-Rolfe A, Kondova I, Mukherjee J, Chios K, Hutto D, Tzipori S. 1999. Antibody-based protection of gnotobiotic piglets infected with Escherichia coli O157:H7 against systemic complications associated with Shiga toxin 2. Infect Immun 67:3645–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francis DH, Collins JE, Duimstra JR. 1986. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun 51:953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dowling TC, Chavaillaz PA, Young DG, Melton-Celsa A, O'Brien A, Thuning-Roberson C, Edelman R, Tacket CO. 2005. Phase 1 safety and pharmacokinetic study of chimeric murine-human monoclonal antibody cαStx2 administered intravenously to healthy adult volunteers. Antimicrob Agents Chemother 49:1808–1812. doi: 10.1128/AAC.49.5.1808-1812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura T, Co MS, Vasquez M, Wei S, Xu H, Tani S, Sakai Y, Kawamura T, Matsumoto Y, Nakao H, Takeda T. 2002. Development of humanized monoclonal antibody TMA-15 which neutralizes Shiga toxin 2. Hybrid Hybridomics 21:161–168. doi: 10.1089/153685902760173872. [DOI] [PubMed] [Google Scholar]
- 49.López EL, Contrini MM, Glatstein E, Gonzalez Ayala S, Santoro R, Allende D, Ezcurra G, Teplitz E, Koyama T, Matsumoto Y, Sato H, Sakai K, Hoshide S, Komoriya K, Morita T, Harning R, Brookman S. 2010. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in pediatric patients infected with Shiga-like toxin-producing Escherichia coli. Antimicrob Agents Chemother 54:239–243. doi: 10.1128/AAC.00343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muyldermans S. 2013. Nanobodies: natural single-domain antibodies. Annu Rev Biochem 82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 51.Hassanzadeh-Ghassabeh G, Devoogdt N, De Pauw P, Vincke C, Muyldermans S. 2013. Nanobodies and their potential applications. Nanomedicine (Lond) 8:1013–1026. doi: 10.2217/nnm.13.86. [DOI] [PubMed] [Google Scholar]