Abstract
Staphylococcus aureus is a common pathogen found in the community and in hospitals. Most notably, methicillin-resistant S. aureus is resistant to many antibiotics, which is a growing public health concern. The emergence of drug-resistant strains has prompted the search for alternative treatments, such as immunotherapeutic approaches. To date, most clinical trials of vaccines or of passive immunization against S. aureus have ended in failure. In this study, we investigated two ESAT-6-like proteins secreted by S. aureus, S. aureus EsxA (SaEsxA) and SaEsxB, as possible targets for a vaccine. Mice vaccinated with these purified proteins elicited high titers of anti-SaEsxA and anti-SaEsxB antibodies, but these antibodies could not prevent S. aureus infection. On the other hand, recombinant SaEsxA (rSaEsxA) and rSaEsxB could induce Th1- and Th17-biased immune responses in mice. Mice immunized with rSaEsxA and rSaEsxB had significantly improved survival rates when challenged with S. aureus compared with the controls. These findings indicate that SaEsxA and SaEsxB are two promising Th1 and Th17 candidate antigens which could be developed into multivalent and serotype-independent vaccines against S. aureus infection.
INTRODUCTION
Staphylococcus aureus is a common pathogen in the community and frequently found in hospitals (1). It is a facultative anaerobic Gram-positive bacterium commonly found as part of the normal flora on the skin and nasal passages of humans (2). Previously, S. aureus infections could be effectively treated with antibiotics. However, in the past 2 decades, an increasing number of strains of S. aureus have become resistant to a variety of antibiotics. Methicillin-resistant S. aureus (MRSA) is one of the more dangerous antibiotic-resistant S. aureus strains. MRSA strains are prevalent in hospitals and are fast becoming a common community-acquired infection (3, 4). For this reason, research into the development of immunotherapeutic approaches, either active or passive, has seen a resurgence in recent years (5).
Several studies have investigated the many surface proteins and virulence factors of S. aureus, many of which have been evaluated as potential vaccine targets (6–15). Current and past S. aureus vaccines or therapeutic antibody strategies have focused mainly on capsular polysaccharide (CPS), virulence factors, surface proteins, and iron-regulated proteins. The putative protective capsular polysaccharide antigen has been developed into potential anti-S. aureus vaccines. The leading candidate of this type of vaccine is StaphVAX, a bivalent polysaccharide and protein-conjugated vaccine (16, 17). Other strategies for developing S. aureus vaccines have targeted virulence factors and surface proteins, including alpha-toxin (a nontoxic derivative of H35L) (7, 18), clumping factor A (ClfA) (19), fibronectin binding protein A or B (FnBPA or FnBPB) (12), Panton-Valentine leukocidin (PVL) (20), and protein A (11). Iron-regulated proteins, such as Merck V710, which is based on iron-regulated surface determinant B (IsdB) (6, 21), have also been investigated as other possible targets for vaccines against S. aureus. Most strategies for passive immunization aim to eliminate major S. aureus virulence determinants, such as monoclonal alpha-toxin antibodies, polyclonal PVL antibodies, and anti-ClfA monoclonal antibodies (Aurexis).
To date, most of the clinical trials for vaccines or passive immunization against S. aureus, such as clinical trials of the Inhibitex vaccine, ClfA, SdrG (Veronate) (22, 23), types 5 and 8 CPS conjugated to pseudomonal exoprotein A (17), and polyclonal antibodies to types 5 and 8 CPS (Altastaph) (24, 25), have ended in failure. A recent review analyzed the reasons why many clinical trials of S. aureus vaccines have failed (26). The authors concluded the most important reason for the failure of these trials was that these vaccines are based on the production of antibodies against S. aureus infection. Furthermore, the above-named vaccines are based on either a single antigen or certain proteins from a protein family. An effective S. aureus vaccine might require several antigenic components (6), such as a sequence targeting multiple S. aureus virulence factors. A recent study indicated that a T-helper 17 (Th17)-interleukin 17 (IL-17) axis might provide avenues for the development of an effective broad vaccine against S. aureus infections (26). Therefore, targets for vaccines could be expanded to include any antigen that induces an immune response against S. aureus infection, for example, a Th1- and/or Th17-mediated immune response.
S. aureus is known to secrete many virulence factors through two mainly secretion systems, Tat and Sec (27, 28). Two virulence factors of S. aureus produced by the 6-kDa early-secretion antigen (ESAT-6) secretion system, S. aureus EsxA and -B (SaEsxA and SaEsxB) (29, 30), play important roles in establishing S. aureus infections in the host (29). Furthermore, a new study found that SaEsxA modulated host cell apoptosis and that, when combined with SaEsxB, it could mediate the release of staphylococci from the host cell (31). SaEsxA and SaEsxB proteins are highly conserved in the genomes of different clinical S. aureus strains (31). ESAT-6-like proteins are also found in many other Gram-positive bacteria, including Clostridium acetobutylicum, Bacillus anthracis, and Listeria monocytogenes (32). The ESAT-6 secretion system in S. aureus is similar to the Esx-1 protein secretion system in Mycobacterium tuberculosis, which also secretes 2 proteins, ESAT-6 and a 10-kDa culture filtrate antigen (CFP-10). ESAT-6 (EsxA) and CFP-10 (EsxB) virulence factors have been identified as antigenic proteins with potent T-cell stimulatory effects that trigger immune responses during tuberculosis (33).
In this study, we investigated SaEsxA and SaEsxB proteins as potential targets for the development of a vaccine against S. aureus. We expressed SaEsxA and SaEsxB in Escherichia coli and purified recombinant SaEsxA (rSaEsxA) and rSaEsxB. We investigated whether these two recombinant ESAT-6-like proteins had immunogenic activities to induce a host immune response against staphylococcal infection. We tested the immunoprotective effects of rSaEsxA and rSaEsxB, alone or combined (rSaEsxA+B), against invasive S. aureus in a murine model.
MATERIALS AND METHODS
Bacteria, plasmids, antibodies, and animals.
The S. aureus ATCC 25923, ATCC 29213, Newman, and USA 300 strains were stored at −80°C until use. E. coli strain BL21(DE3) was used for protein expression. The recombinant expression vector pETH was obtained from K. Y. Yuen. Specific-pathogen-free BALB/c mice were supplied by the Laboratory Animal Unit of the University of Hong Kong. All animal experiments were approved by the Committee on the Use of Live Animals in Teaching & Research of the University of Hong Kong (approval no. CULATR 2596-11).
Animal immunization.
Six-week-old female BALB/c mice (n = 6 per group) were immunized with a range of doses (Table 1) of rSaEsxA or rSaEsxB proteins with Freund's adjuvant (FA) by intraperitoneal (i.p.) injection or with aluminum hydroxide gel (Alhydrogel [AHG]) by intramuscular (i.m.) injection. The two recombinant proteins were emulsified at a ratio of 1:1 with complete FA for priming and with incomplete FA for boosting. The two recombinant proteins were formulated at a ratio of 9:1 with AHG (100 μl of 2% AHG per 900 μl of antigen). These treatments were administered to mice on days 0, 14, and 28. Blood samples were drawn from the tail vein on days 0, 21, and 35.
TABLE 1.
Immunization with different doses and forms of rSaEsxA and rSaEsxB against S. aureus infection
| Treatment | Dose (μg) | Adjuvanta | Titer ofb: |
% survivalc | Significanced | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-rSaEsxA IgG | Anti-rSaEsxA IgG1 | Anti-rSaEsxA IgG2a | Anti-rSaEsxB IgG | Anti-rSaEsxB IgG1 | Anti-rSaEsxB IgG2a | |||||
| rSaEsxA | 50 | N | 2.0 × 104 ± 5.0 × 103 | 1.5 × 104 ± 3.9 × 103 | 8.0 × 103 ± 1.8 × 103 | <20 | ND | ND | 66.70 | P < 0.05 |
| 50 | AHG | 8.5 × 104 ± 1.1 × 104 | 5.9 × 104 ± 1.2 × 104 | 9.7 × 104 ± 2.5 × 103 | <20 | ND | ND | 88.90 | P < 0.0001 | |
| 50 | FA | 9.6 × 104 ± 1.1 × 104 | 6.4 × 104 ± 1.1 × 104 | 1.1 × 104 ± 2.3 × 103 | <20 | ND | ND | 83.30 | P < 0.0001 | |
| 10 | FA | 7.2 × 104 ± 2.5 × 104 | ND | ND | <20 | ND | ND | 75.00 | P < 0.005 | |
| 3 | FA | 5.4 × 104 ± 3.0 × 104 | ND | ND | <20 | ND | ND | 75.00 | P < 0.005 | |
| rSaEsxB | 50 | N | <50 | ND | ND | 1.2 × 105 ± 3.1 × 104 | 9.6 × 105 ± 1.4 × 105 | 4.3 × 105 ± 6.7 × 104 | 50.00 | P < 0.05 |
| 50 | AHG | <50 | ND | ND | 1.2 × 106 ± 1.8 × 105 | 1.1 × 106 ± 1.7 × 105 | 3.3 × 105 ± 4.6 × 104 | 77.78 | P < 0.0001 | |
| 50 | FA | <50 | ND | ND | 2.6 × 106 ± 1.9 × 105 | 1.8 × 106 ± 1.4 × 105 | 3.8 × 105 ± 4.4 × 104 | 83.30 | P < 0.0001 | |
| 10 | FA | <10 | ND | ND | 2.6 × 106 ± 8.0 × 104 | ND | ND | 62.50 | P < 0.005 | |
| 3 | FA | <10 | ND | ND | 1.9 × 105 ± 6.4 × 104 | ND | ND | 50.00 | P < 0.05 | |
| rSaEsxA+B | 50 | AHG | 8.0 × 104 ± 1.1 × 104 | 6.9 × 104 ± 1.0 × 104 | 1.2 × 104 ± 2.1 × 103 | 1.3 × 106 ±1.9 × 105 | 1.0 × 106 ± 1.7 × 105 | 6.0 × 104 ± 1.2 × 104 | 83.30 | P < 0.0001 |
| PBS (mock) | 0 | FA | <10 | <10 | <10 | <10 | <10 | <10 | 22.20 | P = 0.7861 |
| 0 | AHG | <10 | <10 | <10 | <10 | <10 | <10 | 16.70 | P = 0.7250 | |
| 0 | N | <10 | <10 | <10 | <10 | <10 | <10 | 11.10 | ||
FA, Freund's adjuvant; AHG, aluminum hydroxide gel; N, no adjuvant.
Antibody titers (titers ± SEM) were detected by ELISA with purified rSaEsxA or rSaEsxB (1 μg/ml); the antibody endpoint titer was defined as the serum dilution that produced an OD450 of 0.5 absorbance unit in the ELISA. ND, not determined.
Vaccinated BALB/c mice were challenged with S. aureus ATCC 2593 (5 × 107 CFU) by intravenous injection, and survival was monitored.
A log rank (Mantel-Cox) test was used to compare the PBS mock treatment (no adjuvant) and treatment groups.
Antibody detection.
The rSaEsxA and rSaEsxB antibody titers were detected by enzyme-linked immunosorbent assay (ELISA). Briefly, rSaEsxA or rSaEsxB protein (1 μg/ml in 0.05 M carbonate-bicarbonate buffer, pH 9.6) were coated (200 μl/well) on ELISA plates (Nunc, Roskilde, Denmark) and incubated overnight at 4°C. The plates were blocked with phosphate-buffered saline (PBS) containing 5% (wt/vol) nonfat milk for 3 h at 37°C and washed four times with PBS containing 0.05% Tween 20. Twofold serially diluted mice sera were added into the wells and incubated for 1 h at 37°C. The plates were washed six times with PBS containing 0.05% Tween 20 and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG/IgG1/IgG2a for 1 h at 37°C. The color was developed using tetramethylbenzidine (TMB) solution (Sigma), and absorbance was measured using an ELISA reader at 450 nm. The antibody endpoint titer was defined as the serum dilution that produced an optical density at 450 nm (OD450) of 0.5 absorbance unit in the ELISA.
ELISPOT assay.
Mice were sacrificed 5 days after the third immunization. Gamma interferon (IFN-γ)- or interleukin 17A (IL-17A)-producing splenocytes from vaccinated or naive unvaccinated mice were analyzed using a cytokine-specific enzyme-linked immunospot (ELISPOT) assay (BD Pharmingen, United States). Briefly, plates were coated with capture antibodies (anti-IFN-γ or IL-17A monoclonal antibodies [MAb]) overnight at 4°C and then blocked with a blocking solution (RPMI 1640 containing 10% fetal bovine serum and 1% l-glutamine–streptomycin–penicillin) for 1 h at 37°C. Splenocytes isolated from immunized mice were plated at a concentration of 1 × 105 cells/well and stimulated with rSaEsxA (0.2 μg/well) or rSaEsxB (0.2 μg/well) at a final concentration of 10 μg/ml in triplicate and incubated for 20 h at 37°C. Ionomycin (1 μg/ml) (Sigma, USA) and phorbol myfismte acetate (PMA, 50 ng/ml) (Sigma) were used as positive controls. Splenocytes from naive mice stimulated with rSaEsxA or rSaEsxB, splenocytes from unstimulated mice (immunized mice), and RPMI 1640-treated splenocytes were used as negative controls. After the cells were washed, biotinylated anti-IFN-γ or IL-17A MAb was added for 1 h at 37°C, followed by streptavidin-HRP conjugate for 1 h at 37°C. The color was developed with TMB solution, and the spots were counted using an immunospot analyzer.
Renal abscess.
S. aureus strain ATCC 25923 was plated onto a TSA plate with 5% horse blood and cultured for 24 h at 37°C. The bacteria were harvested using endotoxin-free PBS, washed twice, and suspended in PBS at a concentration of 5 × 107 CFU/ml. On day 42 after the first vaccination, mice immunized with rSaEsxA (50 μg) or rSaEsxB (50 μg) were injected with 200 μl of the inoculums i.p. at a total bacterial suspension concentration of 1 × 107 CFU. Four days after bacterial challenge, infected mice were euthanized by compressed CO2 inhalation. The kidneys were removed and homogenized in 1% Triton X-100. Aliquots were diluted and plated on blood agar for CFU counting. Kidney tissue samples for histological analysis were incubated in 10% formalin for 24 h at room temperature. Tissues were embedded in paraffin, and thin sections were obtained using a microtome. Sections were stained with hematoxylin-eosin and examined under a microscope.
Lethal challenge.
On day 42 after the first vaccination, immunized mice were injected intravenously in the tail vein with 5 × 107 CFU of S. aureus ATCC 25923, Newman (methicillin-susceptible S. aureus [MSSA]) or USA 300 (community-associated MRSA [CA-MRSA]) strains. Mice were monitored for mortality and clinical signs.
Passive immunization.
Mouse polyclonal SaEsxA- or SaEsxB-specific antisera were generated and collected from the mice immunized with purified rSaEsxA or rSaEsxB. Female BALB/c mice (∼8 weeks old) were administered 100 μl of normal mouse sera or polyclonal SaEsxA- or SaEsxB-specific antisera (∼1:200,000 antibody titers) by i.p. injection 4 h before S. aureus challenge and then 2 days after S. aureus challenge. Mice were monitored for mortality and clinical signs.
Statistical analysis.
Student's t test was used to analyze the statistical significance of ELISPOT assay results and staphylococcal-load measurements. Log rank (Mantel-Cox) analysis was used to analyze the statistical significance of the data from the lethal-challenge experiments. Analyses were performed using GraphPad Prism 5 (GraphPad Software, USA), and a P value of <0.05 was considered statistically significant.
RESULTS
Immunogenicity of rSaEsxA and rSaEsxB in mice.
Mouse groups were vaccinated intraperitoneally with three doses of rSaEsxA or rSaEsxB protein in various adjuvants (Table 1). ELISA of serum samples obtained 7 days following the immunization showed rSaEsxA- and rSaEsxB-specific IgG, IgG1, and IgG2a antibody titers. The data from different mouse immunization groups are summarized in Table 1. The data showed that immunization with rSaEsxA or rSaEsxB resulted in the generation of specific antibodies. The rSaEsxA and rSaEsxB antibody responses were similar, and levels of specific IgG antibody titers increased with increasing doses of each protein. Immunization with rSaEsxA or rSaEsxA+B induced high levels of anti-rSaEsxA IgG (>1:104), reaching the highest titer after the second boost (day 35). Similarly, the majority of the mice immunized with rSaEsxB or rSaEsxA+B showed high levels of induced anti-rSaEsxB IgG (>1:105). Coadministration of AHG with rSaEsxA or rSaEsxB (50 μg) resulted in significant increases in anti-rSaEsxA IgG (5-fold) or anti-rSaEsxB IgG (10-fold). In addition, immunization with rSaEsxA or rSaEsxB in the absence or presence of AHG elicited both Th1- and Th2-associated rSaEsxA (or rSaEsxB)-specific IgG2a and IgG1 antibody responses. IgG, IgG1, and IgG2a specific for rSaEsxA or rSaEsxB were not detected in the sera of mice mock immunized with PBS (control), AHG, or FA.
SaEsxA- and SaEsxB-specific IFN-γ+ and IL-17A+ T cell responses.
IFN-γ and IL-17A play essential roles in the protective immunity against S. aureus infection. The release of IFN-γ and IL-17A are indicative of Th1- and Th17-biased immune responses (34). ELISPOT analysis of mice sacrificed 5 days after the third immunization showed that splenocytes from mice immunized with rSaEsxA or rSaEsxB had more IFN-γ (Fig. 1A)- and IL-17A (Fig. 1B)-producing cells than the control group. The number of IFN-γ-producing splenocytes in these immunized mice was also significantly greater than in naive mice stimulated with rSaEsxA and NMSB (naive mice stimulated with rSaEsxB) groups. Furthermore, immunization with rSaEsxA or rSaEsxB induced robust specific Th17 responses. Taken together, these data suggest that SaEsxA or SaEsxB promoted the induction of Th1- and Th17-biased immune responses.
FIG 1.
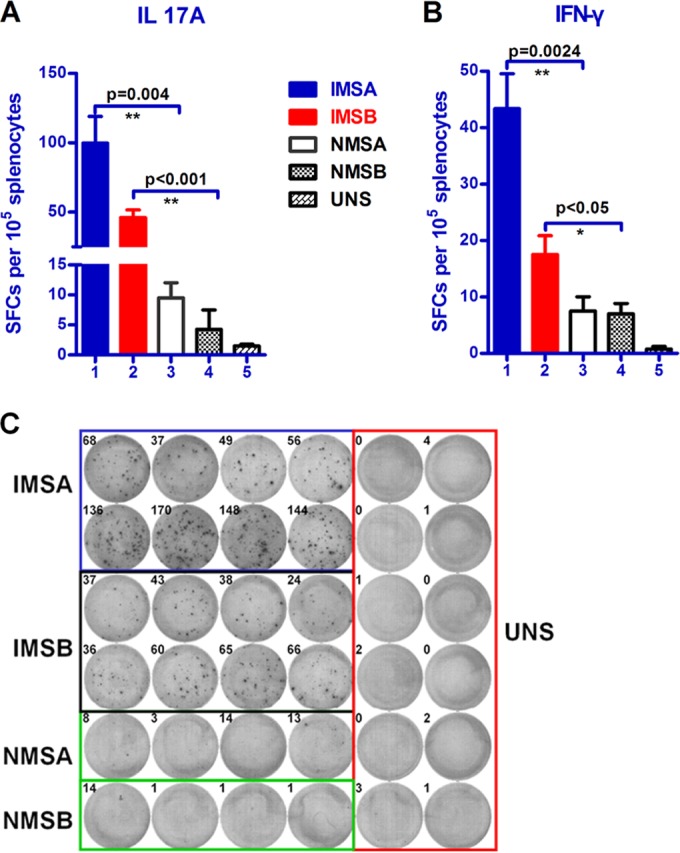
Antigen-specific IL-17A and IFN-γ responses elicited by rSaEsxA or rSaEsxB immunization. Immunized mice (rSaEsxA or rSaEsxB plus AHG) (n = 8) were sacrificed 5 days after the third immunization, and splenocytes were prepared and stimulated with rSaEsxA or rSaEsxB protein for 20 h. IL-17A (A)- and IFN-γ (B)-producing cells were detected by ELISPOT assay. (C) Representative images of splenic ELISPOT responses. Results of one of two representative experiments are shown. Student's t test was used for the statistical analysis. Data are expressed as means ± standard errors of the means (SEM). SFU, spot-forming units; IMSA, immunized mice stimulated with SaEsxA; IMSB, immunized mice stimulated with SaEsxB; NMSA, naive mice stimulated with SaEsxA; NMSB, naive mice stimulated with SaEsxB; UNS, unstimulated immunized mice.
Renal abscess.
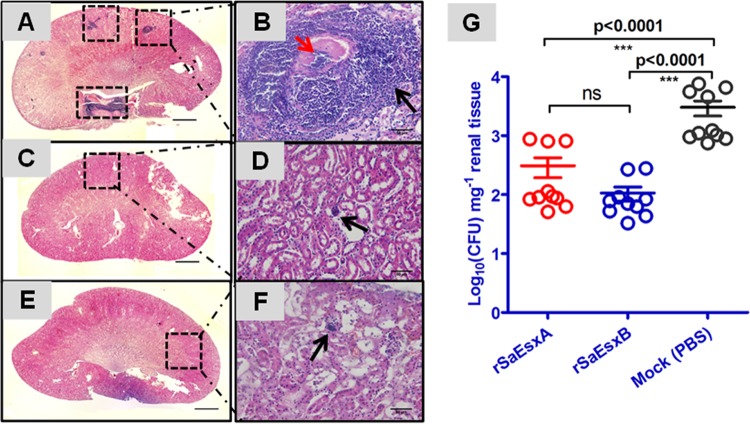
We evaluated the potential protective effect of the rSaEsxA and rSaEsxB in a mouse renal abscess model. Mice were infected with 1 × 107 CFU of S. aureus strain ATCC 25923. Four days after challenge, mice were sacrificed and their kidneys were collected. Renal tissue of animals treated with PBS displayed a staphylococcal load of 3.48 ± 0.95 log10 CFU/mg of kidney tissues. In contrast, significant decreases in bacterial number were observed in animals treated with rSaEsxA (2.38 ± 0.34 log10 CFU mg−1; P < 0.0001) and rSaEsxB (2.02 ± 0.09 log10 CFU mg−1; P = 0.0079) (Fig. 2G). Histological analysis of kidney tissues from mice immunized with rSaEsxA or rSaEsxB did not show any staphylococcal abscesses (Fig. 2C to F). In contrast, kidneys collected from control mice contained abscesses with staphylococci concentrated in the center, surrounded by large numbers of necrotic immune cells (Fig. 2A and B).
FIG 2.
Immunization with rSaEsxA and rSaEsxB generated protective immunity against S. aureus abscess formation in BALB/c mice. Mice treated with PBS plus AHG (A, B) or immunized with rSaEsxA plus AHG (C, D) and rSaEsxB plus AHG (E, F) were challenged with S. aureus ATCC 25923 by intraperitoneal injection. They were sacrificed after 4 days, and their kidneys were collected for histopathology (A to F) or bacterial-load measurements (G). Hematoxylin and eosin images of whole kidneys (A, C, and E) and magnified areas (B, D, and F) revealed abscess formation only in PBS control mice. Consistent results were obtained for 6 kidney tissues from each group. (B) Staphylococcal abscess (black arrow) with a central concentration of staphylococci (red arrow). (D, F) Small infiltrates of polymorphonuclear leukocytes (black arrow). Statistical significance was calculated by the Student t test. Bars represent means ± SEM. Scale bars: 1,000 μm (A, C, E) and 50 μm (B, D, F). ns, not significant (P > 0.05). Results of one of two representative experiments are shown.
Lethal challenge.
The protective effect of the recombinant SaEsxA and SaEsxB proteins against lethal infections was investigated in mice immunized with purified rSaEsxA or rSaEsxB antigens or a mixture of both. The immunized mice were challenged with 5 × 107 CFU of S. aureus ATCC 25923 by intravenous injection through the tail vein. Animals were monitored for more than 14 days. Survival rates between groups were compared using the pairwise, log rank (Mantel-Cox) test. The different survival rates of mice immunized with different treatments (protein with and without AHG or FA) and doses of rSaEsxA and rSaEsxB (3, 10, and 50 μg) against S. aureus ATCC 25923 are shown in Table 1. Survival rates increased with increasing doses of rSaEsxA and rSaEsxB, with AHA or FA possibly playing nonspecific roles against S. aureus infection. However, there were no significant differences between the adjuvant-only groups (FA group, 22.20% survival [P = 0.7861]; AHG group, 16.70% survival [P = 0.7250]) and the PBS control group (11.10% survival). The results in Fig. 3 show that the vaccinated-mouse groups (rSaEsxA, rSaEsxB, rSaEsxA+B) had significantly improved survival rates (P < 0.0001). Specifically, mice vaccinated with rSaEsxA had the highest survival rate (16/18), followed by those vaccinated with rSaEsxB (14/18) and rSaEsxA+B (14/18). In contrast, the majority of mice (16/18) in the control group died within 8 days after bacterial challenge. The survival rates between groups administered combined and individual (rSaEsxA+B, rSaEsxA, rSaEsxB) antigens were not significantly different (P > 0.05).
FIG 3.
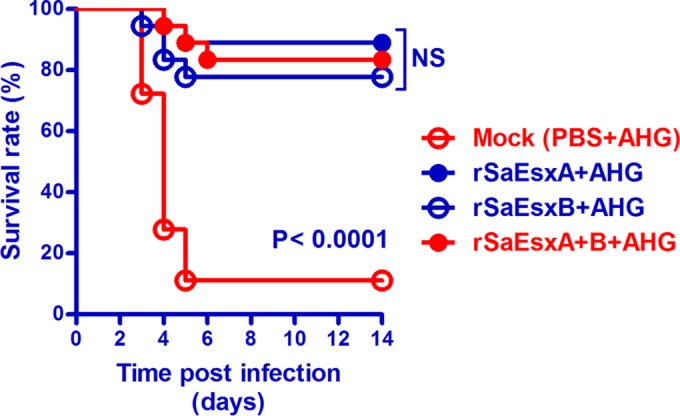
Survival curves of vaccinated BALB/c mice after S. aureus challenge. Mice (n = 18) were challenged with S. aureus ATCC 2593 (5 × 107 CFU) by intravenous injection. Pairwise log rank analysis between groups and log rank (Mantel-Cox) tests were performed. NS, not significant (P > 0.05). Data from three independent experiments are shown.
To test whether rSaEsxA and rSaEsxB could protect against a wide range of S. aureus clinical strains, two typical S. aureus strains, Newman and USA 300, were also tested. Newman is a methicillin-sensitive S. aureus strain, and USA 300 is a CA-MRSA strain. Mice were treated with these strains in a manner similar to that of the above-described experiments and were monitored over 14 days. Compared with the control mice treated with PBS plus AHG, mice vaccinated with rSaEsxA+B had significant protective immunity to the Newman (60% survival; P = 0.0085, log rank Mantel-Cox test) and USA 300 (50% survival; P = 0.0013, log rank Mantel-Cox test) S. aureus strains (Fig. 4).
FIG 4.
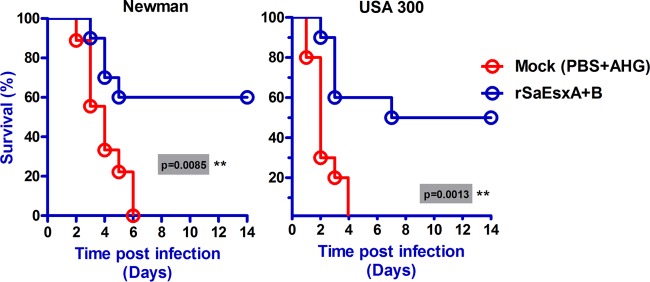
Immunization with rSaEsxA+B generated protective immunity against lethal challenge with two different clinical S. aureus strains. Mice (n = 10) immunized with rSaEsxA+B or treated with PBS plus AHG as controls were challenged with S. aureus Newman or USA 300 strains by intravenous injection. The survival of mice was monitored for 14 days. A log rank (Mantel-Cox) test was used to compare the protective immunities of control mice and the rSaEsxA+B-immunized mice. Data from two replicate experiments are shown.
Passive immunization.
Our results showed that mice treated with SaEsxA- or SaEsxB-specific antisera did not exhibit any significant protective effects against S. aureus challenge (P > 0.05, log rank Mantel-Cox test) (Fig. 5). Treatment with SaEsxA- or SaEsxB-specific antisera alone could not provide effective immunity.
FIG 5.
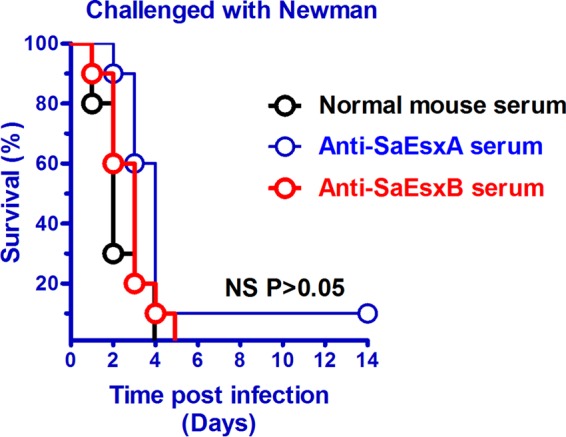
Survival rates of BALB/c mice passively treated with SaEsxA- or SaEsxB-specific mouse antisera and challenged with the Newman strain via the tail vein. BALB/c mice (n = 10) received 100 μl of normal mouse serum or specific mouse antiserum (anti-SaEsxA or anti-SaEsxB) 4 h before intravenous injection of S. aureus Newman strain (5 × 107 CFU) and on day 2 after infection. A log rank (Mantel-Cox) test was used to compare groups. NS, no significant differences (P > 0.05). Data from two replicate experiments are shown.
DISCUSSION
Multidrug-resistant (MDR) pathogens are a global problem. Their ability to adapt enables emerging strains to develop resistance to new antibiotics. Vaccinations may be a better strategy to control MDR-pathogen infections. Vaccination has been demonstrated to be effective in preventing many infectious diseases, including influenza, small pox, and hepatitis B virus (HBV). However, for many MDR pathogens, a serotype-independent immune response may be required. Despite eliciting high levels of anti-SaEsxA IgG and anti-SaEsxB IgG after vaccination with the purified SaEsxA and SaEsxB proteins, these antibodies could not prevent S. aureus infection in our murine model. Studies showed that healthy individuals naturally have high titers of antibody to S. aureus, but those with defects in B cell immunity are not particularly prone to S. aureus infections (35, 36). The lack of humoral immunity protection against S. aureus must be compensated for by other immune mechanisms.
At least 13 secreted proteins and 24 surface adhesion proteins from S. aureus have been implicated in bacterial immune evasion (37). The secretion of SaEsxA and SaEsxB represents an important bacterial virulence strategy, which leads to bacterial replication and abscess formation (29). To study whether mice immunized with rSaEsxA or rSaEsxB could prevent abscess formation, a murine model of staphylococcal load and abscess formation was chosen. Our results indicated that SaEsxA or SaEsxA proteins could induce protective immunity against S. aureus renal-abscess formation in our murine model. Furthermore, these results suggest that mice immunized with rSaEsxA+B, rSaEsxA, or rSaEsxB had significantly increased protection against lethal challenge by S. aureus ATCC 25923. Instead of a vaccine targeting a single antigen, multivalent antigens may have a better chance of inducing both B and T cell immune responses to achieve protection against S. aureus.
Similar ESAT-6-like proteins, EsxA (Rv3875) and EsxB (Rv3874), secreted by Mycobacterium tuberculosis are known to play a vital role in its pathogenesis. These two proteins can trigger cell-mediated immune responses and IFN-γ production during tuberculosis (33, 38). One study suggested that the ESAT-6 expressed by the virulent M. tuberculosis H37Rv strain, but not by Mycobacterium bovis BCG, promoted immunity by enhancing Th17 cell responses (39). Activation of naive T cells by pathogen antigens presented by antigen-presenting cells in the presence of various cytokines leads to the generation of T helper cell subsets, such as Th1, Th2, and Th17. Universally, Th1 cells regulate IFN-γ-dependent immunity against most intracellular pathogens. Th1 could be inhibited by IL-4, subsequently inducing another T cell subset, Th2, which produced IL-4, IL-5, and IL-13 against helminth infection (40). Indeed, our results showed that the rSaEsxA and rSaEsxB proteins specifically triggered not only high levels of IL-17A but also high levels of IFN-γ. These data indicate that SaEsxA and SaEsxB may promote the induction of Th1- and Th17-biased immune responses.
A new study by Misstear et al. showed that mouse nasal vaccination with targeted nanoparticles loaded with S. aureus protein could protect against systemic S. aureus infection in the absence of any antigen-specific antibodies (41). This study suggests that only a cellular response could protect against S. aureus infection. Moreover, many reports show the importance of Th17/IL-17 in the protection against S. aureus infections, which may shed light on this immune response (42, 43). Many mucosal vaccination approaches can induce robust Th17 responses, suggesting that Th17 cells may be useful targets for vaccines that induce immunity (44). Recently, several studies using mouse vaccine models showed that T helper cells, including Th17, are important for a CD4+ T-cell-dependent immune response (44). Th17 cells have a role in antimicrobial immunity at the epithelial/mucosal barrier (44). They produce cytokines that stimulate epithelial cells to produce antimicrobial proteins to clear out certain types of opportunistic microbes.
Th17-mediated protective responses involve the release of antimicrobial peptides, recruitment of neutrophils, and IL-17-driven Th1 immunity. These signaling mechanisms may offer immunity against a range of MDR pathogens through the production and induction of inflammatory cytokines and other proteins. For staphylococcal vaccines to be effective, protection must be achieved against a wide variety of different clinical strains. We showed that mice immunized with rSaEsxA+B were also protected against two typical S. aureus clinical strains, Newman (MSSA) and USA 300 (CA-MRSA). Although these two immunogens could prevent infection from different clinical S. aureus strains, it is not clear whether Th1- and/or Th17-biased immune responses conferred this protection.
In conclusion, we provide evidence for SaEsxA and SaEsxB proteins as vaccine targets against S. aureus infection. Experimental data from both immunization and lethal-challenge studies indicated that rSaEsxA and rSaEsxB could elicit immune responses leading to increased protective immunity in a murine model. Both SaEsxA and SaEsxB are promising Th1 and T17 cell candidates for developing into a multivalent, serotype-independent, effective vaccine against S. aureus infection. Future work in this area promises to be fertile and to lead to effective vaccines against S. aureus infection.
ACKNOWLEDGMENT
This work was supported by funding from the Research Fund for the Control of Infectious Diseases Commissioned Study, Food and Health Bureau of Hong Kong Government (grants RFCID HK-09-01-20 and HK-09-01-21).
REFERENCES
- 1.Blot S, Vandewoude K, Colardyn F. 1998. Staphylococcus aureus infections. N Engl J Med 339:2025–2026. doi: 10.1056/NEJM199812313392716. [DOI] [PubMed] [Google Scholar]
- 2.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. 2002. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 346:1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 3.Gould IM. 2005. The clinical significance of methicillin-resistant Staphylococcus aureus. J Hosp Infect 61:277–282. doi: 10.1016/j.jhin.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Abrahamian FM, Moran GJ. 2007. Methicillin-resistant Staphylococcus aureus infections. N Engl J Med 357:2090. doi: 10.1056/NEJMc072407. [DOI] [PubMed] [Google Scholar]
- 5.Schaffer AC, Lee JC. 2008. Vaccination and passive immunisation against Staphylococcus aureus. Int J Antimicrob Agents 32(Suppl 1):S71–S78. doi: 10.1016/j.ijantimicag.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Stranger-Jones YK, Bae T, Schneewind O. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A 103:16942–16947. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bubeck Wardenburg J, Schneewind O. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin RL, Li C, Yang ZT, Zhang YJ, Bai WL, Li X, Yin RH, Liu H, Liu S, Yang Q, Cao YG, Zhang NS. 2009. Construction and immunogenicity of a DNA vaccine containing clumping factor A of Staphylococcus aureus and bovine IL18. Vet Immunol Immunopathol 132:270–274. doi: 10.1016/j.vetimm.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson IM, Mazmanian SK, Schneewind O, Verdrengh M, Bremell T, Tarkowski A. 2002. On the role of Staphylococcus aureus sortase and sortase-catalyzed surface protein anchoring in murine septic arthritis. J Infect Dis 185:1417–1424. doi: 10.1086/340503. [DOI] [PubMed] [Google Scholar]
- 10.Inskeep TK, Stahl C, Odle J, Oakes J, Hudson L, Bost KL, Piller KJ. 2010. Oral vaccine formulations stimulate mucosal and systemic antibody responses against staphylococcal enterotoxin B in a piglet model. Clin Vaccine Immunol 17:1163–1169. doi: 10.1128/CVI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. 2010. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med 207:1863–1870. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrecubieta C, Matsunaga I, Asai T, Naka Y, Deng MC, Lowy FD. 2008. Vaccination with clumping factor A and fibronectin binding protein A to prevent Staphylococcus aureus infection of an aortic patch in mice. J Infect Dis 198:571–575. doi: 10.1086/590210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HK, DeDent A, Cheng AG, McAdow M, Bagnoli F, Missiakas DM, Schneewind O. 2010. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 28:6382–6392. doi: 10.1016/j.vaccine.2010.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth DM, Senna JP, Machado DC. 2006. Evaluation of the humoral immune response in BALB/c mice immunized with a naked DNA vaccine anti-methicillin-resistant Staphylococcus aureus. Genet Mol Res 5:503–512. [PubMed] [Google Scholar]
- 15.Gaudreau MC, Lacasse P, Talbot BG. 2007. Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus. Vaccine 25:814–824. doi: 10.1016/j.vaccine.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Fattom A, Fuller S, Propst M, Winston S, Muenz L, He D, Naso R, Horwith G. 2004. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 23:656–663. doi: 10.1016/j.vaccine.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, Muenz L, Fuller S, Johnson J, Fireman B, Alcorn H, Naso R. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 18.Menzies BE, Kernodle DS. 1994. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect Immun 62:1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spellberg B, Ibrahim AS, Yeaman MR, Lin L, Fu Y, Avanesian V, Bayer AS, Filler SG, Lipke P, Otoo H, Edwards JE Jr. 2008. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun 76:4574–4580. doi: 10.1128/IAI.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch F, Bowden MG. 2009. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect 15:156–164. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, Noble L, Brown MJ, Zorman JK, Wang XM, Pancari G, Fan H, Isett K, Burgess B, Bryan J, Brownlow M, George H, Meinz M, Liddell ME, Kelly R, Schultz L, Montgomery D, Onishi J, Losada M, Martin M, Ebert T, Tan CY, Schofield TL, Nagy E, Meineke A, Joyce JG, Kurtz MB, Caulfield MJ, Jansen KU, McClements W, Anderson AS. 2006. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun 74:2215–2223. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, Jung E, Millard D, Schelonka R, Eyal F, Morris A, Kapik B, Roberson D, Kesler K, Patti J, Hetherington S. 2007. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr 151:260–265. doi: 10.1016/j.jpeds.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 23.Patti JM. 2004. A humanized monoclonal antibody targeting Staphylococcus aureus. Vaccine 22(Suppl 1):S39–S43. doi: 10.1016/j.vaccine.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin DK, Schelonka R, White R, Holley HP, Bifano E, Cummings J, Adcock K, Kaufman D, Puppala B, Riedel P, Hall B, White J, Cotton CM. 2006. A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. J Perinatol 26:290–295. doi: 10.1038/sj.jp.7211496. [DOI] [PubMed] [Google Scholar]
- 25.Rupp ME, Holley HP Jr, Lutz J, Dicpinigaitis PV, Woods CW, Levine DP, Veney N, Fowler VG Jr. 2007. Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 51:4249–4254. doi: 10.1128/AAC.00570-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proctor RA. 2012. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis 54:1179–1186. doi: 10.1093/cid/cis033. [DOI] [PubMed] [Google Scholar]
- 27.Biswas L, Biswas R, Nerz C, Ohlsen K, Schlag M, Schafer T, Lamkemeyer T, Ziebandt AK, Hantke K, Rosenstein R, Gotz F. 2009. Role of the twin-arginine translocation pathway in Staphylococcus. J Bacteriol 191:5921–5929. doi: 10.1128/JB.00642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneewind O, Missiakas DM. 2012. Protein secretion and surface display in Gram-positive bacteria. Philos Trans R Soc Lond B Biol Sci 367:1123–1139. doi: 10.1098/rstb.2011.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burts ML, Williams WA, DeBord K, Missiakas DM. 2005. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci U S A 102:1169–1174. doi: 10.1073/pnas.0405620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galan JE, Cossart P. 2005. Host-pathogen interactions: a diversity of themes, a variety of molecular machines. Curr Opin Microbiol 8:1–3. doi: 10.1016/j.mib.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Korea CG, Balsamo G, Pezzicoli A, Merakou C, Tavarini S, Bagnoli F, Serruto D, Unnikrishnan M. 2014. Staphylococcal Esx proteins modulate apoptosis and release of intracellular Staphylococcus aureus during infection in epithelial cells. Infect Immun 82:4144–4153. doi: 10.1128/IAI.01576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pallen MJ. 2002. The ESAT-6/WXG100 superfamily—and a new Gram-positive secretion system? Trends Microbiol 10:209–212. doi: 10.1016/S0966-842X(02)02345-4. [DOI] [PubMed] [Google Scholar]
- 33.Ulrichs T, Munk ME, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro ML, Kaufmann SH. 1998. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur J Immunol 28:3949–3958. doi:. [DOI] [PubMed] [Google Scholar]
- 34.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr, Spellberg B. 2009. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holland SM. 2010. Chronic granulomatous disease. Clin Rev Allergy Immunol 38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 36.Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, Ariga T, Avcin T, de Boer M, Bustamante J, Condino-Neto A, Di Matteo G, He J, Hill HR, Holland SM, Kannengiesser C, Koker MY, Kondratenko I, van Leeuwen K, Malech HL, Marodi L, Nunoi H, Stasia MJ, Ventura AM, Witwer CT, Wolach B, Gallin JI. 2010. Hematologically important mutations: X-linked chronic granulomatous disease (third update). Blood Cells Mol Dis 45:246–265. doi: 10.1016/j.bcmd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy AJ, Lindsay JA. 2010. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol 10:173. doi: 10.1186/1471-2180-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun 63:1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatterjee S, Dwivedi VP, Singh Y, Siddiqui I, Sharma P, Van Kaer L, Chattopadhyay D, Das G. 2011. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog 7:e1002378. doi: 10.1371/journal.ppat.1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. 1991. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med 173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misstear K, McNeela EA, Murphy AG, Geoghegan JA, O'Keeffe KM, Fox J, Chan K, Heuking S, Collin N, Foster TJ, McLoughlin RM, Lavelle EC. 2014. Targeted nasal vaccination provides antibody-independent protection against Staphylococcus aureus. J Infect Dis 209:1479–1484. doi: 10.1093/infdis/jit636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller LS, Cho JS. 2011. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minegishi Y, Karasuyama H. 2009. Defects in Jak-STAT-mediated cytokine signals cause hyper-IgE syndrome: lessons from a primary immunodeficiency. Int Immunol 21:105–112. doi: 10.1093/intimm/dxn134. [DOI] [PubMed] [Google Scholar]
- 44.Kumar P, Chen K, Kolls JK. 2013. Th17 cell based vaccines in mucosal immunity. Curr Opin Immunol 25:373–380. doi: 10.1016/j.coi.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]