Abstract
Neisseria meningitidis asymptomatically colonizes the human upper respiratory tract but is also the cause of meningitis and severe septicemia. Carriage or disease evokes an immune response against the infecting strain. Hitherto, we have known little about the breadth of immunity induced by natural carriage of a single strain or its implications for subsequent infectious challenge. In this study, we establish that transgenic mice expressing human CEACAM1 support nasal colonization by a variety of strains of different capsular types. Next, we nasally challenged these mice with either of the N. meningitidis strains H44/76 (serogroup B, ST-32) and 90/18311 (serogroup C, ST-11), while following the induction of strain-specific immunoglobulin. When these antisera were tested for reactivity with a diverse panel of N. meningitidis strains, very low levels of antibody were detected against all meningococcal strains, yet a mutually exclusive “fingerprint” of high-level cross-reactivity toward certain strains became apparent. To test the efficacy of these responses for protection against subsequent challenge, CEACAM1-humanized mice exposed to strain 90/18311 were then rechallenged with different N. meningitidis strains. As expected, the mice were immune to challenge with the same strain and with a closely related ST-11 strain, 38VI, while H44/76 (ST-32) could still colonize these animals. Notably, however, despite the paucity of detectable humoral response against strain 196/87 (ST-32), this strain was unable to colonize the 90/18311-exposed mice. Combined, our data suggest that current approaches may underestimate the actual breadth of mucosal protection gained through natural exposure to N. meningitidis strains.
INTRODUCTION
Neisseria meningitidis is the causative agent of meningococcal meningitis and septicemia. Both manifestations of invasive meningococcal disease (IMD) primarily affect infants and toddlers and are characterized by a rapid course of progression (1). The fatality rate is particularly high in meningococcal sepsis, and treatment options are limited, especially when patients present in hospitals with late-stage disease (2). Therefore, preemptive measures such as vaccination are pivotal in the defense against this human-specific pathogen.
Although mainly recognized because of its devastating invasive potential, N. meningitidis bacteria are normal inhabitants of the human upper respiratory tract, as about 10% of the general population carry these bacteria in their throats (3, 4). Overall, progression into disease is uncommon and is limited to certain hypervirulent lineages of this diverse species (5, 6). One key aspect in meningococcal virulence is the polysaccharide capsule, which shields the bacteria against the bactericidal activities of the host through phagocytosis and the complement system and which allows for their survival and multiplication in the blood. In order to overcome this protective function, immunoglobulin specific for surface antigens is required to facilitate Fc receptor-mediated phagocytosis and serum complement activation and deposition directly onto the bacterial surface. The serum bactericidal antibody (SBA) assay has therefore become the gold standard by which to measure the efficacy of protection conferred by meningococcal vaccines (7). However, an individual's SBA activity can also rise in response to natural carriage (8, 9). Meningococcal colonization of the nasopharynx is usually asymptomatic and can last for months before it is spontaneously cleared (10, 11). It is interesting that carriers often mount an SBA response but continue to be colonized (8, 9), indicating two separate, or sequential, mechanisms required to gain serum versus mucosal protection. While research efforts have mostly focused on serum protection against N. meningitidis, little is known about the mucosal immune response to infection or its efficacy at eliminating N. meningitidis from the nasopharyngeal niche.
Experimental approaches to investigate the generation of mucosal protection during meningococcal colonization have thus far been hampered by the human-specific tropism of these bacteria, which has precluded establishment of an accepted animal infection model. Therefore, our current knowledge stems from observations in humans during cross-sectional or longitudinal trials that monitored colonization and immune parameters (8, 12–15). The only experimental insight from human carriage was obtained in a challenge study in which human volunteers were inoculated with Neisseria lactamica, a close but nonvirulent relative of N. meningitidis (16). An inherent obstacle in interpretation of the published data on the human response during N. meningitidis carriage or disease is the potential heterogeneity in undescribed effectors of human susceptibility within a population and the uncontrolled nature of exposure, including variables such as natural infection by cross-reacting N. meningitidis strains and commensal Neisseria species, strain-specific differences in virulence, and the potential for viral coinfection. Furthermore, in the absence of a known correlate of protection against N. meningitidis mucosal colonization, it remains unclear what immune responses should be measured.
We have recently established a mouse model which allows effective N. meningitidis nasal colonization, using transgenic mice expressing the human carcinoembryonic antigen-like cell adhesion molecule 1 (CEACAM1). This model allows N. meningitidis to intimately associate with the nasal mucosa as its Opa protein adhesins bind to human CEACAM1, allowing us to dissect both innate and adaptive responses to infection (17). In this study, we take advantage of this mouse model to assess strain-specific cross-protection conferred upon N. meningitidis colonization of the respiratory mucosa. While the antibody response to different N. meningitidis strains showed a narrow range of cross-specificity by enzyme-linked immunosorbent assay (ELISA) and SBA, the nasal challenge model indicated a broader cross-protection against mucosal infection. Specifically, while serogroup- and/or serotype-specific immunity was acquired, protection was also apparent when strain-specific antibody was not detectable in serum. Together, these findings imply the presence of protective antigens not captured by standard serologic-based classification and indicate that serum antibody-based ELISAs underrepresent the breadth of colonization-induced cross-protection existing within the respiratory mucosa.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains used in this study are listed in Fig. 2A. N. meningitidis and Neisseria gonorrhoeae were grown overnight on GC agar supplemented with IsoVitaleX and VCNT inhibitor (Becton Dickinson, Sparks, MD, USA) at 37°C and 5% CO2 and with a humidified atmosphere. Likewise, N. lactamica, Neisseria cinerea, and Neisseria flavescens were grown on GC agar supplemented with IsoVitaleX (Becton Dickinson, Sparks, MD, USA). Nontypeable Haemophilus influenzae was grown on chocolate agar (Becton Dickinson, Sparks, MD, USA), and Moraxella catarrhalis was grown on brain heart infusion (BHI) agar (Becton Dickinson, Sparks, MD, USA) under the same conditions. Escherichia coli strains expressing Opa proteins of N. meningitidis strain MC58 were the same as in reference 17 and were grown overnight at 37°C on Luria agar, supplemented with IPTG (isopropyl-β-d-thiogalactopyranoside; Sigma-Aldrich, Oakville, Canada) to induce Opa expression.
FIG 2.
Strain-specific IgG response toward N. meningitidis in CEACAM1-humanized mice. (A) CEACAM1-humanized mice were intranasally infected with 105 CFU of N. meningitidis strain 90/18311 or H44/76 at 6 weeks of age and again at 9 weeks of age. Three weeks after the second intranasal inoculation, serum samples were taken and pooled by infection strain. Serum pools were incubated in 96-well plates coated individually with various N. meningitidis isolates as listed in the table, and cross-reactive IgG was monitored. Data in the graph to the right represent mean values and standard deviations from two individual serum pools consisting of each half of the total cohort size assayed in duplicate. Data are normalized to signal obtained with the homologous strain for intranasal inoculation (i.e., 90/18311 or H44/76, respectively), of which signal obtained with pools of preinfection sera of the same cohorts was subtracted. (B) Western blot analysis of the expression of PorB (using panspecific anti-PorB rabbit antiserum) and PorA (using monoclonal typing antibodies recognizing VR epitopes indicated to the left of each blot) in all strains typed with any of the following PorA epitopes: P1.7, P1.16, P1.5, and/or P1.2. Asterisks represent lanes in which bands reactive with the chosen typing antibody would be expected if the PorA was expressed.
Mouse intranasal infection.
All animal experiments were conducted in accordance with legal requirements under the Province of Ontario's Animal for Research Act and the Federal Council on Animal Care (CCAC). Experimental protocols were approved by the University of Toronto's Animal Ethics Review Committee (permit numbers 2008657 and 20009370). Generation of CEACAM1-humanized mice was described in detail in the work of Gu et al. (18). The infection procedure was carried out as previously described (17). Inocula were obtained by resuspending the lawn of growth from an overnight agar plate into 1 ml of phosphate-buffered saline (PBS) containing 1 mM MgCl2 (PBS/Mg), with adjustment to 107 CFU/ml, and 32 mg/ml of human holotransferrin was added (Sigma-Aldrich, Oakville, Canada). Six-week-old CEACAM1-humanized mice (FVB genetic background) were intranasally inoculated with a 10-μl inoculum (105 CFU) under light isoflurane anesthesia. Serial dilutions of the inoculum were routinely plated in order to ensure accuracy and viability of the suspension used for infection. For experiments in Fig. 1A, mice were sacrificed and sampled 3 days later, as described below. For reinfection experiments to monitor immune responses, mice received one dose of the indicated N. meningitidis strain at the age of 6 weeks and another dose at 9 weeks. Challenge infections were then carried out at 12 weeks of age with the indicated strain. During the time course of infection, blood samples were obtained from the facial vein. Three days after challenge infection, the mice were euthanized in a CO2 chamber and cardiac puncture was performed to exsanguinate the animals. Nasal wash was obtained by retrograde lavage through the trachea with 500 μl of PBS/Mg. The nasal cavity was then opened using scissors, and the nasal cavity was swabbed thoroughly with an ultrafine aluminum shaft applicator with a calcium alginate fiber tip (Puritan Medical Products, Guilford, ME, USA) to break up the mucosa, which was then resuspended in 500 μl of PBS/Mg. This procedure ensures harvesting of mucosal as well as submucosal tissues to probe for tightly associated N. meningitidis. Nasal washes were centrifuged at 13,000 × g, with the supernatant retained for further analysis and the pellet resuspended in 50 μl supernatant. Resuspended pellet and nasal swab samples were plated onto GC agar supplemented with IsoVitaleX and VCNT inhibitor (Becton Dickinson, Sparks, MD, USA) to enumerate recovered CFU after overnight incubation at 37°C and 5% CO2 as the sum of both nasal wash and nasal swab samples.
FIG 1.
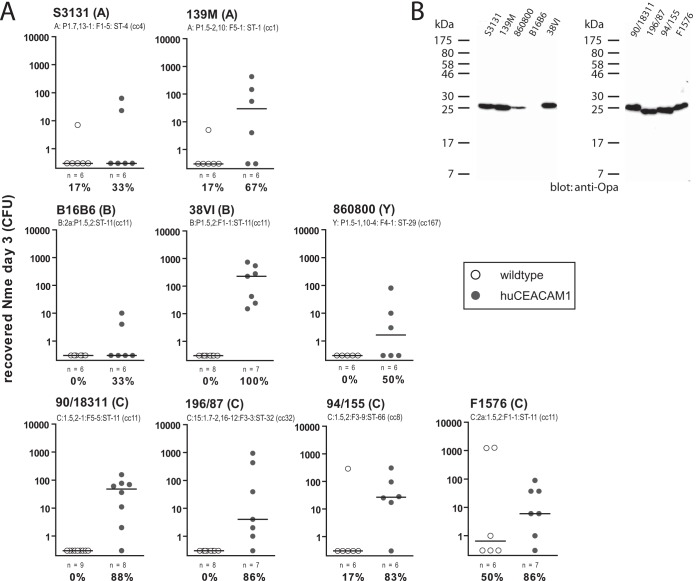
Diverse N. meningitidis strains can colonize CEACAM1-humanized mice. (A) Cohorts of CEACAM1-humanized mice (gray circles) or wild-type mice (white circles) were intranasally inoculated with 105 CFU of N. meningitidis. Strain designation and type are indicated above individual graphs. Each circle represents the number of viable N. meningitidis bacteria recovered from nasal samples enumerated 3 days postinfection. Group sizes and percentages of colonized mice within each cohort are indicated below each graph. Note that data obtained using strains 90/18311, 38VI, and 196/87 are identical to those in Fig. 4c (in the context of which the data were generated) but are shown here as well for qualitative comparison to the other strains. (B) Western blotting of lysates of N. meningitidis strains to monitor expression of Opa protein.
Whole-cell ELISA for detection of specific immunoglobulins.
MaxiSorp 96-well flat-bottom immunoplates (Nunc, Rochester, NY, USA) were coated with 50 μl per well of a suspension at an optical density at 600 nm (OD600) of 0.2 of heat-inactivated (56°C for 30 min) N. meningitidis strains in PBS and allowed to dry. Wells were washed four times with wash buffer (PBS containing 0.05% Tween 20) and blocked for 1 h with PBS containing 5% bovine serum albumin (BSA) before addition of 50 μl per well of diluted sample. After incubation for 2 h at room temperature, wells were washed three times before addition of 50 μl per well of a 1:10,000 dilution of alkaline phosphatase (AP)-goat anti-mouse IgG Fc(γ), AP-goat anti-mouse IgM (Jackson ImmunoResearch, West Grove, PA, USA), or AP-goat anti-mouse IgA (Abcam, Cambridge, MA, USA). After 1 h of incubation, wells were washed three times before 100 μl per well of Bluephos AP detection substrate (KPL, Gaithersburg, MD, USA) was added, and plates were then incubated at 37°C. This reaction was stopped by adding 100 μl/well of AP-Stop solution (KPL, Gaithersburg, MD, USA), and OD620 was measured.
Serum bactericidal antibody (SBA) assay.
About 10 colonies of an overnight growth of the N. meningitidis test strains were used to inoculate a fresh Columbia blood agar plate (bioMérieux, Nürtingen, Germany). After 4 h of incubation at 37°C and 5% CO2, the bacteria were harvested by swab and resuspended into assay buffer (Hanks balanced salt solution [HBSS]; Life Technologies, Darmstadt, Germany) containing 5 U/ml heparin (Biochrom, Berlin, Germany) and 0.5% BSA (Applichem, Darmstadt, Germany). The assay was performed by mixing approximately 500 CFU of bacteria in assay buffer containing serial 2-fold dilutions of the test sera and 25% baby rabbit complement (Cedarlane, Burlington, Canada), and then, the reaction mixtures were incubated at 37°C for 1 h. After plating, CFU were enumerated and a positive SBA value was assigned as the serum dilution below which less than 50% of the bacteria survived compared to the no-serum control. Where indicated, the bacteria were subjected to iron limitation by addition of 500 μM deferoxamine mesylate (Sigma-Aldrich, Seelze, Germany) when growing for 4 h prior to dilution in assay buffer and addition to the reaction.
Western blotting.
An overnight lawn of growth of the N. meningitidis strains was harvested and suspended into 1 ml of PBS to yield approximately 2 × 1010 CFU/ml. After addition of 2× Laemmli buffer, samples were boiled for 10 min and 5 μl hereof was subjected to analysis on a 12% SDS gel and subsequently transferred onto a nitrocellulose membrane. After blocking in PBS containing 0.05% Tween 20 and 5% skim milk, antigens were detected by incubation with the appropriate antibodies: Opa proteins with monoclonal antibody (MAb) 4B12/C11 (19), PorB with a panspecific polyclonal rabbit antiserum, P1.5 with MAb MN22A9.19 (National Institute for Biological Standards and Control [NIBSC] code 03/226), P1.7 with MAb MN14C11.6 (NIBSC code 01/514), P1.2 with MAb MN16C13F4 (NIBSC code 02/178), and P1.16 with MAb MN5C11G (NIBSC code 01/538). The typing antibodies were kindly provided by Heike Claus and Ulrich Vogel (Institute for Hygiene and Microbiology, University of Würzburg, Würzburg, Germany). Primary antibodies were detected by incubation with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (Dianova, Hamburg, Germany), followed by enhanced chemiluminescence (ECL) detection.
RESULTS
CEACAM1-humanized mice facilitate nasal carriage with a diverse spectrum of meningococcal isolates.
Previously, we observed carriage of serogroup B meningococci after nasal inoculation of transgenic mice expressing human CEACAM1 but not their wild-type (WT) littermates (17). In order to assess the applicability of this model for other meningococcal isolates, we intranasally infected CEACAM1-humanized mice versus WT littermates with inocula of serogroup A, B, C, and Y strains and then recovered viable bacteria in the nasal tissues at day 3 postinfection. In each case, the strains preferentially colonized CEACAM1-humanized mice regardless of serogroup or sequence type (Fig. 1A). Only one of the serogroup A strains (S3131) and one of the serogroup B strains (B16B6) showed a low success rate (two out of six mice) in colonization. In contrast, all other isolates tested showed persistence in at least half of the transgenic mice at 3 days postinfection. Of note, only strain F1576 (serogroup C) displayed marked success in colonization of WT mice (three out of six mice), although even this strain preferably colonized CEACAM1-humanized mice (six out of seven mice).
With the exception of strain B16B6, all isolates displayed expression of Opa proteins as determined by Western blotting (Fig. 1B). Hence, it appears that lack of Opa expression might account for the low success rate of B16B6 in colonization of CEACAM1-humanized mice.
In conclusion, the CEACAM1-humanized mouse is a susceptible host to colonization with most Opa-expressing meningococcal strains and, thus, is a suitable model for the analysis of strain-dependent immunity toward meningococcal colonization.
Nasal colonization with N. meningitidis induces a strain-specific serum response in CEACAM1-humanized mice.
We have previously established that nasal colonization with a prototypical N. meningitidis serogroup B isolate, H44/76, elicits an immune response that impedes subsequent carriage of the same strain (17). Here, we sought to determine the specificity of the immune response induced by nasal carriage with respect to cross-reactivity of serum antibodies to heterologous strains. Therefore, sera were obtained from CEACAM1-humanized mice that underwent two consecutive intranasal inoculations at 6 and 9 weeks of age with 105 CFU of either serogroup B isolate H44/76 or serogroup C isolate 90/18311. Serum pools of both cohorts were incubated in 96-well plates which had been coated with a collection of meningococcal isolates to measure cross-reactive antibodies by whole-cell ELISA (Fig. 2A). As expected, the serum IgG response was most pronounced against the homologous strain. In addition, there was significant cross-reactivity toward certain other meningococcal strains in each of the serum pools. Yet, the serum pools of H44/76-reinfected and 90/18311-reinfected mice displayed a mutually exclusive fingerprint, indicating the strain specificity of the elicited immune response to either isolate. It is pertinent that the values graphed in Fig. 2A represent normalized data from which the background (as measured by pooled preimmune serum of the same animals) has already been subtracted. Thus, it appears that a low-level response against all meningococcal strains in Fig. 2A is mounted by infection with either H44/76 or 90/18311.
The table with Fig. 2A lists the meningococcal strains used to test for cross-reactive serum IgG, along with a panel of typing antigens (serogroup, clonal complex, serotype, serosubtype, and FetA as well as the antigens of the Bexsero vaccine, fHbp [factor H binding protein], NHBA [neisserial heparin binding antigen], and NadA). Cross-reactivity of the serum samples tended to correlate with overall similarity of the test strains to the strains used for reinfection (H44/76 or 90/18311, respectively). Yet, no single antigen could be identified that would allow an unambiguous prediction of cross-reactivity: while strains typed B:15:P1.7,16:F3-3:ST-32(cc32) (fHbp peptide ID 1; NHBA peptide ID 3) clustered at high cross-reactivity with H44/76 and strains typed C:P1.5,2:F5-5:ST-11(cc11) (fHbp peptide ID 22; NHBA peptide ID 29; NadA allele 3) clustered at high cross-reactivity with 90/18311, individual antigens in these types are also found in strains which do not display marked immunologic cross-reactivity.
The outer membrane porins PorA and PorB are highly immunogenic proteins and have been reported to be the primary target of serum bactericidal antibodies generated during colonization (9, 15, 20), invasive disease (21–24), and vaccination with outer membrane vesicle (OMV) vaccines (25, 26). We analyzed their expression by all test strains, in which at least one of the two variable regions (VRs) of PorA matched one of the corresponding infection strains (i.e., expressing P1.7, P1.16, P1.5, or P1.2; shaded in black or gray in the table) by Western blotting (Fig. 2B). All analyzed strains expressed PorB. However, we found expression of PorA only in the majority of the strains clustering with H44/76, whereas no PorA expression was observed in the high-cross-reactivity cluster around 90/18311. Sequence analysis revealed that the lack of PorA expression in strain 90/18311 was due to phase variation, as the homopolymeric adenine tract following the start codon consisted of nine instead of seven adenine bases, thus leading to a frameshift (27). Even 90/18311, which was recovered from CEACAM1-humanized mice after 3 days, did not express detectable levels of PorA. Hence, PorA is an unlikely target for antigen presentation in the 90/18311-infected mice, and therefore, no significant levels of anti-PorA antibodies may have been generated that could lead to cross-protection. Furthermore, the failure to detect PorA in 90/18311 clones that were recovered after nasal carriage suggests that it plays a minor role for the successful colonization in this animal model.
Given that the prototypical strains used in this study have been isolated decades ago and have been distributed to numerous laboratories worldwide, the high reproducibility of results obtained with homologous strains (H44/76, MC58, and B16B6) obtained from different laboratories in this assay is interesting (Fig. 3).
FIG 3.
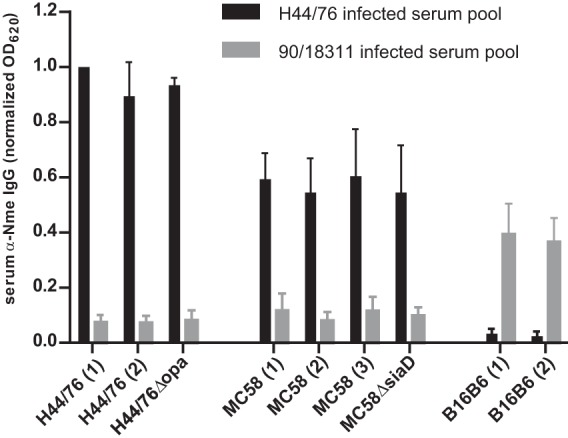
Effect of Opa protein and capsule expression on cross-reactivity. Cross-reactive serum IgG was measured as in Fig. 2A. Isogenic N. meningitidis strains from various laboratories (indicated by numbers in parentheses) displayed consistent cross-reactivity. Opa-deficient mutant or capsule-deficient mutants displayed signals comparable to those of parental strains.
Opa adhesins are crucial for interaction with CEACAM1 and facilitate nasopharyngeal colonization in the CEACAM1-humanized mice (17). While they are highly immunogenic proteins and have been proposed as vaccine antigens (28), they do not seem to be dominant epitopes for the immune response in CEACAM1-humanized mice, as (i) only modest amounts of Opa-specific antibodies are raised in H44/76-reinfected animals as evident by only a weak signal emanating from Opa-expressing E. coli (Fig. 2A) and (ii) an H44/76 isogenic mutant lacking all four opa genes does not display a significant reduction in signal strength compared to the parental strain (Fig. 3). Also, no effect was observed in an isogenic mutant lacking capsule (ΔsiaD), indicating (i) the lack of serogroup B-specific anticapsular antibodies, which is expected, given the nonimmunogenic nature of this particular antigen, and (ii) the lack of an observable inhibitory or steric effect of the capsule on antigen recognition by antibodies in the immune sera.
Cross-protection against nasal carriage in strain 90/18311-reinfected mice is greater than estimated by the serum antibody cross-reactivity pattern.
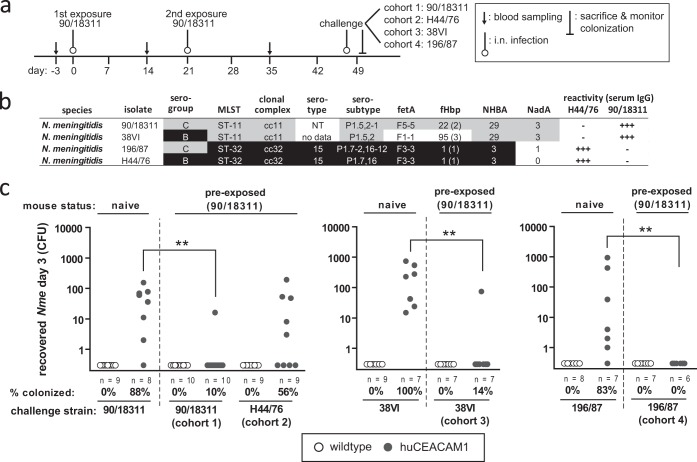
We sought to determine whether intranasal infection of CEACAM1-humanized mice impacted on subsequent nasal colonization with heterologous meningococcal isolates. Therefore, cohorts of CEACAM1-humanized mice and WT mice were intranasally infected at the ages of 6 weeks and 9 weeks with 105 CFU of serogroup C strain 90/18311 (infection schedule depicted in Fig. 4a). At 4 weeks after the second infection, the mice were intranasally challenged with 105 CFU of either the homologous strain (90/18311) or one of the three heterologous strains H44/76, 38VI, and 196/87. The antigenic composition of each of the four strains used in the challenge infection is outlined in Fig. 4b. The colonization status of the mice was assessed 3 days postchallenge (Fig. 4c). Significant reduction of the colonization was evident upon rechallenge with the isogenic serogroup C strain (90/18311) and the heterologous serogroup C strain (196/87), as well as the multilocus sequence typing (MLST)-matched serogroup B strain (38VI). In contrast, strain H44/76 still successfully colonized the preexposed mice.
FIG 4.
Induction of cross-protection against colonization with heterologous N. meningitidis strains in CEACAM1-humanized mice. (a) Schematic representation of experiment. Mice were intranasally infected at day 0 and day 21 with 105 CFU of strain 90/18311, inoculated at day 47 with either the homologous strain (90/18311) or heterologous strains (H44/76, 38VI, or 196/87, respectively), and then sacrificed 3 days later to monitor colonization. (b) Typing characteristics of the four strains used for terminal intranasal challenge. (c) Results of CFU enumeration of viable N. meningitidis in nasal samples of mouse cohorts 3 days after infection of naive mice (left in each panel) or mice that were previously infected with strain 90/18311 (right in each panel) as indicated above each graph. Each circle (wild-type mice, white circles; CEACAM1-humanized mice, gray circles) represents viable CFU recovered from one mouse. Group sizes, percentages of mice colonized in each cohort, and the N. meningitidis strain used for terminal intranasal mouse challenge are indicated below each graph. Statistical analysis to compare colonization frequencies in naive mice and previously with strain 90/18311-infected mice was performed using GraphPad Prism. ** indicates a P value of <0.005, applying Fisher's exact test.
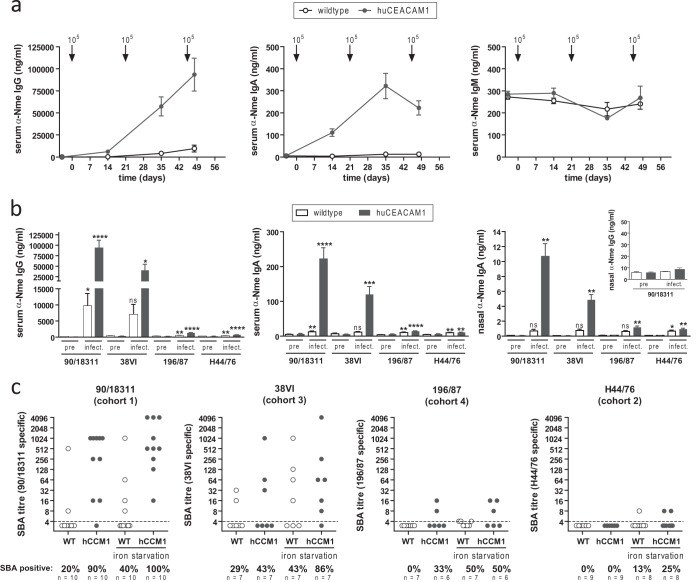
During the course of the reinfection experiment, CEACAM1-humanized mice mounted robust serum titers of meningococcus-specific (strain 90/18311) IgG and IgA but not IgM (Fig. 5a). As expected, WT mice showed only a weak response, due to their inability to carry N. meningitidis, which is pivotal to the development of adaptive immune responses in this system (17). Terminal serum samples as well as nasal wash samples of all animals were analyzed by ELISA for cross-reactive antibodies against the challenge strains (90/18311, 38VI, 196/87, and H44/76). In accordance with the data obtained from serum pools (Fig. 2A), the highest specific titers of serum IgG, serum IgA, and nasal IgA were found when testing with the isogenic serogroup C strain, 90/18311, followed by strain 38VI, which has high similarity with 90/18311 but expresses a serogroup B capsule. There were only low levels of cross-reactive IgG against strains 196/187 and H44/76 in these mice, although they were significantly higher than those in preimmune sera of the same animals (Fig. 5b). Of note, no meningococcus-specific IgG response was evident in nasal wash samples (inset in Fig. 5b), suggesting that serum IgG does not necessarily reflect mucosal protection against meningococcal colonization after nasal infection.
FIG 5.
Cross-reactive antibody responses in 90/18311-infected mice. (a) Time course of generation of specific anti-N. meningitidis serum IgG (left), IgA (middle), and IgM (right) in serum samples obtained from mouse cohorts identical to those in Fig. 4c, analyzed by whole-cell ELISAs using plates coated with strain 90/18311. Arrows indicate time points of intranasal infection (also shown in Fig. 4a). Data are expressed as means ± standard errors of the means of values obtained from individual serum samples. (b) Cross-reactive titers of serum IgG (left), serum IgA (middle), nasal IgA (right), and nasal IgG (inset), comparing terminal serum samples by whole-cell ELISAs using microtiter plates coated with N. meningitidis strains as indicated below graphs. Data are expressed as means ± standard errors of the means. Statistical analysis was performed using GraphPad Prism software. ns, not significant; *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001, using Student‘s t test comparing serum samples after infection with preimmune serum samples of the same cohorts. (c) Serum bactericidal antibody (SBA) titers in individual serum samples. For each serum sample, the N. meningitidis strain used for the SBA assay (indicated above graphs) was identical to that used for the terminal challenge of the corresponding cohorts. As indicated below the x axis, N. meningitidis test strains were exposed, or not, to iron starvation conditions by 500 μM deferoxamine mesylate to mimic in vivo stress conditions.
The serum bactericidal antibody (SBA) assay is the gold standard to determine serum protection induced by meningococcal vaccines. Bactericidal antibodies direct complement activation toward the N. meningitidis surface, which efficiently eliminates the bacteria. We investigated a possible involvement of bactericidal antibodies in nasal immunity, in order to explain the unexpected immunity of the 90/18311-infected mice against challenge with strain 196/87 in the absence of high titers of cross-reactive antibodies measured by ELISA. SBA activity against the four different N. meningitidis strains used for the terminal nasal challenge was tested in the individual cohorts (Fig. 5c). To mimic stress conditions that are faced by the bacteria in vivo in the nasopharyngeal mucosa, we also performed SBA titer measurements with N. meningitidis strains grown under iron-limiting conditions prior to the assay. This procedure is known to upregulate virulence factors (29) on the N. meningitidis outer membrane, which in turn might precipitate higher SBA titers of immune sera and reflect better the actual in vivo situation. High SBA titers were evident in preexposed CEACAM1-humanized mice after terminal challenge with the isogenic strain, 90/18311. About half of the CEACAM1-humanized mice in the cohort terminally challenged with strain 38VI displayed SBA activity against the challenge strain, yet this proportion was higher (6 out of 7 mice) when the N. meningitidis test strain was grown under iron limitation. Surprisingly, given the low levels of antibodies cross-reacting with 196/87, two out of six mice (and three out of six mice when the assay was conducted with iron-starved bacteria) in the corresponding cohorts also mounted notable SBA activity against 196/87. Consistent with the very low cross-reactive IgG against H44/76 and lack of mucosal protection, no SBA activity could be measured using H44/76 as the target strain. Considering the almost complete protection of the mice against nasal carriage with 38VI or 196/87, these findings indicate that the presence or absence of SBA activity does not correlate with protection.
DISCUSSION
Our finding that CEACAM1-humanized mice facilitate carriage of diverse meningococcal strains establishes the broad utility of this model for investigation of the meningococcal lifestyle in the upper respiratory tract mucosa, as well as the host response elicited by N. meningitidis colonization. This prompted us to consider whether the adaptive response elicited by mucosal colonization conferred protection specific for one or more major classes of antigens used either in vaccines or as a correlate of protection.
Classical epidemiological studies suggest that nasal carriage triggers the production of meningococcus-specific antibodies in human carriers, which often precipitate a protective serum bactericidal activity against the colonizing strain (8, 30). In agreement with this, the induction of strain-specific humoral immunity in mice requires extended colonization of the nasal tissues, since nasal and humoral immunoglobulin is not elicited in wild-type mice, or when Opa-deficient N. meningitidis bacterial are intranasally administered to the CEACAM1-humanized mice (17). Here, we observed that the cross-reactivity of the antibody response elicited by infection, not unexpectedly, tended to correlate with the overall similarity. This is highlighted by the fact that serum from H44/76-preexposed animals most strongly cross-reacted with isolates belonging to the clonal complex ST-32, while those most avidly recognized by 90/18311 immune serum were of ST-11. Conversely, all of the isolates without significant cross-reactivity belonged to clonal complexes other than ST-32 or ST-11. An exception to this is 204/92, which is ST-33 (i.e., it belongs to the same clonal complex as ST-32) but was not recognized by the H44/76 immune serum; however, this strain expressed a PorA variant (typed as P1.19,15) different from that of H44/76 (P1.7,16) and expressed immunologically distinct variants of all typing antigens listed in the table in Fig. 2A except for fHbp.
While PorA has been described as the immunodominant antigen in the N. meningitidis outer membrane (9, 15, 20), strain 8680 displays high cross-reactivity to H44/76 but does not express detectable PorA (Fig. 2B), indicating that cross-reactivity is not solely determined by this normally abundant antigen. Indeed, given the lack of its expression in 90/18311 or any of the highly cross-reacting ST-11 strains, this antigen is unlikely to contribute significantly to the cross-reactivity of the serum of 90/18311-infected mice. Notably, PorA expression in 90/18311 does not appear to be selected for during in vivo colonization (Fig. 2B). This is in contrast to our previous findings with respect to expression of Opa protein, which undergoes phase variation to become expressed when transparent (OpaOFF) colonies are inoculated into CEACAM1-humanized mice (17).
Besides PorA, other outer membrane protein and carbohydrate antigens would obviously be expected to contribute to the cross-reactivity pattern of the immune sera. The table in Fig. 2A lists the most commonly used typing antigens as well as the surface-exposed antigens of the protein-based vaccine against serogroup B meningococci, Bexsero (fHbp, NHBA, and NadA). As would be expected, strains clustering at high cross-reactivity with the respective immune sera usually expressed identical variants of these antigens. However, there were also some outliers where conservation in these antigens did not predict heightened reactivity with the respective immune serum. Examples for strains that harbor genes for antigen variants matching those of either H44/76 or 90/18311 but that did not detectably react with the corresponding immune sera include 860060 (same FetA variant as 90/18311), 204/92 (same fHbp variant as H44/76), S3131 (same NHBA variant as 90/18311), and NG144/82 (same NadA variant as 90/18311). On the other hand, not all strains which clustered at high serum cross-reactivity perfectly matched the antigenic variant profile of the colonizing strain. Given the multitude of surface-exposed antigens and their variable nature in both expression levels (phase variability) and cross-reactivity (antigenic variation), the antigens discussed here can only serve as a proxy for the actual complexity of the surface antigens recognized by colonization-induced immune serum rather than absolutely predict cross-reactivity patterns.
In addition to the typing antigens and Bexsero antigens, Opa proteins merit attention in the context of this study, as they mediate tissue adhesion in the CEACAM1-humanized mice (17). In good accordance with the typing antigens and vaccine antigens, there was a high level of congruence between the Opa alleles harbored by H44/76 (alleles 96, 288, 147, and 218) or 90/18311 (alleles 244, 132, and 347) and their respective cluster of strains with high cross-reactivity toward the corresponding immune sera (28, 31). Opa proteins identical to those from H44/76 (but cloned from MC58) expressed in E. coli displayed only minor cross-reactivity with the immune sera from H44/76-exposed mice (Fig. 2A). Furthermore, there was no reduction of cross-reactivity in an H44/76 mutant devoid of all four Opa genes compared to wild-type H44/76 (Fig. 3), perhaps related to the fact that CEACAM1-humanized mice display a reduced humoral response toward Opa proteins (32). Thus, Opa proteins do not appear to contribute substantially to the strong cross-reactivity patterns among N. meningitidis isolates, at least following natural infection.
As expected, given the high cross-reactivity of serum IgG (Fig. 2A and 5), we found that CEACAM1-humanized mice that were previously colonized by the ST-11 strain 90/18311 could thereafter not be successfully colonized by either the isogenic strain or the closely related ST-11 strain 38VI (Fig. 4c). Notably, 90/18311 exposure also conferred protection against intranasal challenge with the unrelated ST-32 strain 196/87; this was not an ST-related effect since H44/76 (also ST-32) could still colonize these animals. The only obvious difference between 196/87 and H44/76 is their capsule: 196/87 expresses serogroup C capsule, while H44/76 is serogroup B. While serogroup C capsule polysaccharide is immunogenic (8), we observed no differences in the cross-reactivities of the 90/18311 immune serum with these two strains. On the other hand, serogroup B polysaccharide is not immunogenic because it mimics mammalian embryonic tissue antigens. Consistent with this, several serogroup B strains were poorly recognized by the serum from H44/76. Cross-reactivity elicited by the serogroup B strains is, therefore, most likely protein based, which would explain the effective anti-196/87 response by H44/76-infected animals (Fig. 2A).
One of the most notable findings of this study relates to the lack of correlation between strain-specific cross-reactivity of SBAs and protection against colonization. Exemplifying this, there was very weak cross-reactivity of serum from 90/18311-infected mice with either 196/87 or H44/76 (Fig. 2A), yet 90/18311 infection protected against subsequent 196/87 challenge (Fig. 4c). The presence of detectable 196/87-specific SBA activity can explain this outcome in certain animals; however, other protected mice showed no detectable SBAs against this strain (Fig. 5c). Whether this results from very-low-abundance antibodies or T-cell-specific immunity remains to be tested in future studies.
The CEACAM1-humanized mice have proven to be an excellent tool to analyze mucosal as well as humoral immunity toward N. meningitidis. However, it is pertinent that this model cannot mimic all aspects of the human-specific host interaction of N. meningitidis; therefore, we will need to consider combining the CEACAM1-humanized mice with other transgenic models expressing human forms of factor H (33), transferrin binding protein (34), and other factors in the future. Since, despite all similarities, the immune systems of humans and mice may differ in some regards, novel insights gained using this or other infection models would obviously require verification in humans. However, the humanized mouse models provide an experimentally tractable system by which to explore the contribution of immune processes to infection of the nasal mucosa.
Since valid measures of existing protection are required for studies aimed at defining the impact of vaccines, and since the ideal vaccine is one that confers sterilizing immunity, understanding these protective effects will provide more prognostic value to guide the effective implementation of new and improved meningococcal vaccines.
ACKNOWLEDGMENTS
This work has been supported by the Alberta Heritage Foundation for Medical Research through the Interdisciplinary Team in Vaccine Design and Implementation and by the Canadian Institutes of Health Research operating grant MOP-130273.
We thank Ulrich Vogel and Heike Claus for the PorA typing antibodies and the PorB antiserum.
REFERENCES
- 1.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. 2001. Meningococcal disease. N Engl J Med 344:1378–1388. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 2.van Deuren M, Brandtzaeg P, van der Meer JW. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev 13:144–166. doi: 10.1128/CMR.13.1.144-166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartwright KA, Stuart JM, Jones DM, Noah ND. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect 99:591–601. doi: 10.1017/S0950268800066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claus H, Maiden MC, Maag R, Frosch M, Vogel U. 2002. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology 148:1813–1819. [DOI] [PubMed] [Google Scholar]
- 5.Caugant DA, Froholm LO, Bovre K, Holten E, Frasch CE, Mocca LF, Zollinger WD, Selander RK. 1986. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci U S A 83:4927–4931. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caugant DA, Mocca LF, Frasch CE, Froholm LO, Zollinger WD, Selander RK. 1987. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J Bacteriol 169:2781–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow R, Balmer P, Miller E. 2005. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 23:2222–2227. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 8.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med 129:1327–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordens JZ, Williams JN, Jones GR, Christodoulides M, Heckels JE. 2004. Development of immunity to serogroup B meningococci during carriage of Neisseria meningitidis in a cohort of university students. Infect Immun 72:6503–6510. doi: 10.1128/IAI.72.11.6503-6510.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Wals P, Bouckaert A. 1985. Methods for estimating the duration of bacterial carriage. Int J Epidemiol 14:628–634. doi: 10.1093/ije/14.4.628. [DOI] [PubMed] [Google Scholar]
- 11.Ala'Aldeen DA, Neal KR, Ait-Tahar K, Nguyen-Van-Tam JS, English A, Falla TJ, Hawkey PM, Slack RC. 2000. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J Clin Microbiol 38:2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reller LB, MacGregor RR, Beaty HN. 1973. Bactericidal antibody after colonization with Neisseria meningitidis. J Infect Dis 127:56–62. doi: 10.1093/infdis/127.1.56. [DOI] [PubMed] [Google Scholar]
- 13.Davenport V, Guthrie T, Findlow J, Borrow R, Williams NA, Heyderman RS. 2003. Evidence for naturally acquired T cell-mediated mucosal immunity to Neisseria meningitidis. J Immunol 171:4263–4270. doi: 10.4049/jimmunol.171.8.4263. [DOI] [PubMed] [Google Scholar]
- 14.Robinson K, Neal KR, Howard C, Stockton J, Atkinson K, Scarth E, Moran J, Robins A, Todd I, Kaczmarski E, Gray S, Muscat I, Slack R, Ala'Aldeen DA. 2002. Characterization of humoral and cellular immune responses elicited by meningococcal carriage. Infect Immun 70:1301–1309. doi: 10.1128/IAI.70.3.1301-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones GR, Christodoulides M, Brooks JL, Miller AR, Cartwright KA, Heckels JE. 1998. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonization. J Infect Dis 178:451–459. doi: 10.1086/515622. [DOI] [PubMed] [Google Scholar]
- 16.Evans CM, Pratt CB, Matheson M, Vaughan TE, Findlow J, Borrow R, Gorringe AR, Read RC. 2011. Nasopharyngeal colonization by Neisseria lactamica and induction of protective immunity against Neisseria meningitidis. Clin Infect Dis 52:70–77. doi: 10.1093/cid/ciq065. [DOI] [PubMed] [Google Scholar]
- 17.Johswich KO, McCaw SE, Islam E, Sintsova A, Gu A, Shively JE, Gray-Owen SD. 2013. In vivo adaptation and persistence of Neisseria meningitidis within the nasopharyngeal mucosa. PLoS Pathog 9:e1003509. doi: 10.1371/journal.ppat.1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu A, Zhang Z, Zhang N, Tsark W, Shively JE. 2010. Generation of human CEACAM1 transgenic mice and binding of Neisseria Opa protein to their neutrophils. PLoS One 5:e10067. doi: 10.1371/journal.pone.0010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achtman M, Neibert M, Crowe BA, Strittmatter W, Kusecek B, Weyse E, Walsh MJ, Slawig B, Morelli G, Moll A, Blake M. 1988. Purification and characterization of eight class 5 outer membrane protein variants from a clone of Neisseria meningitidis serogroup A. J Exp Med 168:507–525. doi: 10.1084/jem.168.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams J N, Jones GR, Christodoulides M, Heckels JE. 2003. Serological correlates of protection against meningococci in a cohort of university students, before and during an outbreak of serogroup C infection. J Infect Dis 187:1433–1441. doi: 10.1086/374648. [DOI] [PubMed] [Google Scholar]
- 21.Guttormsen HK, Wetzler LM, Solberg CO. 1994. Humoral immune response to class 1 outer membrane protein during the course of meningococcal disease. Infect Immun 62:1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandrell RE, Zollinger WD. 1989. Human immune response to meningococcal outer membrane protein epitopes after natural infection or vaccination. Infect Immun 57:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wedege E, Hoiby EA, Rosenqvist E, Bjune G. 1998. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect Immun 66:3223–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milagres LG, Gorla MC, Rebelo MC, Barroso DE. 2000. Bactericidal antibody response to Neisseria meningitidis serogroup B in patients with bacterial meningitis: effect of immunization with an outer membrane protein vaccine. FEMS Immunol Med Microbiol 28:319–327. doi: 10.1111/j.1574-695X.2000.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 25.Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Hoiby EA, Holst J, Nokleby H, Rosenqvist E, Sierra G, Campa C, Sotolongo F, Vega J, Garcia J, Herrera P, Poolman J T, Perkins BA. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520–1527. [DOI] [PubMed] [Google Scholar]
- 26.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27(Suppl 2):B3–B12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 27.van der Ende A, Hopman CT, Dankert J. 2000. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis. Infect Immun 68:6685–6690. doi: 10.1128/IAI.68.12.6685-6690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callaghan MJ, Lewis S, Sadarangani M, Bailey SE, Chan H, Ferguson DJ, Derrick JP, Feavers I, Maiden MC, Pollard AJ. 2011. Potential of recombinant opa proteins as vaccine candidates against hyperinvasive meningococci. Infect Immun 79:2810–2818. doi: 10.1128/IAI.01338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Ulsen P, Kuhn K, Prinz T, Legner H, Schmid P, Baumann C, Tommassen J. 2009. Identification of proteins of Neisseria meningitidis induced under iron-limiting conditions using the isobaric tandem mass tag (TMT) labeling approach. Proteomics 9:1771–1781. doi: 10.1002/pmic.200800642. [DOI] [PubMed] [Google Scholar]
- 30.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 129:1307–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callaghan MJ, Jolley KA, Maiden MC. 2006. Opacity-associated adhesin repertoire in hyperinvasive Neisseria meningitidis. Infect Immun 74:5085–5094. doi: 10.1128/IAI.00293-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zariri A, van Dijken H, Hamstra HJ, van der Flier M, Vidarsson G, van Putten JP, Boog CJ, van den Dobbelsteen G, van der Ley P. 2013. Expression of human CEACAM1 in transgenic mice limits the Opa-specific immune response against meningococcal outer membrane vesicles. Vaccine 31:5585–5593. doi: 10.1016/j.vaccine.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 33.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 186:3606–3614. doi: 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarantonelli ML, Szatanik M, Giorgini D, Hong E, Huerre M, Guillou F, Alonso JM, Taha MK. 2007. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect Immun 75:5609–5614. doi: 10.1128/IAI.00781-07. [DOI] [PMC free article] [PubMed] [Google Scholar]