Abstract
Mutations that alter virulence and antibiotic susceptibility arise and persist during Staphylococcus aureus bacteremia. However, an experimental system demonstrating transmission following bacteremia has been lacking, and thus implications of within-host adaptation for between-host transmission are unknown. We report that S. aureus disseminates to the gastrointestinal tract of mice following intravenous injection and readily transmits to cohoused naive mice. Both intestinal dissemination and transmission were linked to the production of virulence factors based on gene deletion studies of the sae and agr two-component systems. Furthermore, antimicrobial selection for antibiotic-resistant S. aureus displaced susceptible S. aureus from the intestine of infected hosts, which led to the preferential transmission and dominance of antibiotic-resistant bacteria among cohoused untreated mice. These findings establish an animal model to investigate gastrointestinal dissemination and transmission of S. aureus and suggest that adaptation during the course of systemic infection has implications beyond the level of a single host.
INTRODUCTION
Staphylococcus aureus is a leading cause of bacteremia and the most common cause of infectious endocarditis worldwide (1, 2). Despite advances in patient care, S. aureus cardiovascular infections remain associated with considerable morbidity and mortality as a result of complications arising from hematogenous dissemination. In addition, treatment of methicillin-resistant S. aureus (MRSA) strains is complicated by the emergence of intermediate and fully vancomycin-resistant strains. Thus, how antibiotic-resistant strains spread in the community and hospital environment has become an area of intense investigation. Development of an animal model of S. aureus transmission would facilitate this effort and complement ongoing epidemiological studies that track the movement of specific strains and the acquisition of mutations.
Adaptation of S. aureus to different environments involves a regulatory network including but not limited to two global regulatory determinants, agr and sae. agr and sae are two-component systems that sense a quorum-sensing peptide and host signals, respectively, to tailor the production of S. aureus virulence factors in an environment-dependent manner (3, 4). Both regulators have been shown to be important for virulence in several animal models of infection (5–9). For example, the expression of important virulence determinants like hemolysins, Panton-Valentine leukocidin, enterotoxins, and protein A is controlled by agr and sae, partly in a cooperative fashion (10, 11). Whether agr and sae also contribute to host-to-host transmission is less clear (12).
Our work on the pathogenesis of cardiovascular infections serendipitously revealed that S. aureus bacteremia results in strikingly high levels of fecal shedding of the bacteria. Although the gastrointestinal (GI) tract is not the relevant organ for understanding staphylococcal pathogenesis, it is a relevant compartment for S. aureus colonization and transmission in the health care environment (13–15). Since we are unaware of previous studies demonstrating that bacteremic infection results in dissemination to the GI tract, we sought to extend this observation by determining whether bacteria in the blood can be transmitted to new hosts. We found that S. aureus can be readily detected in naive mice cohoused with mice infected by the intravenous (i.v.) route. Optimal transmission to the naive host was dependent on agr and sae, and an in vivo competition assay revealed the preferential transmission of antibiotic-resistant bacteria. All together, these results suggest that bacteria can lead to GI dissemination and transmission and establish a new animal model to investigate mechanisms that contribute to S. aureus spread.
MATERIALS AND METHODS
Mice.
Eight-week-old female C57BL/6 mice (Jackson Laboratories) were injected via the tail vein with the indicated amount of bacteria resuspended in 100 μl of phosphate-buffered saline (PBS). Preparation of hematoxylin and eosin (H&E)-stained intestinal tissue sections was performed as described previously (16). Briefly, 2 cm of ileal or colonic tissue was removed, flushed with PBS, and pinned on black wax while fixed in 10% formalin, before tissue was embedded in agar. We confirmed that S. aureus was not present in mice prior to infection or in mice that were not infected by plating stool homogenized in 1 ml PBS. No colonies were detected when 100 μl of the suspension was plated on mannitol salt agar (MSA) (BD Biosciences) overnight at 37°C, in contrast to when samples from mice infected intravenously with S. aureus were plated under the same conditions. Animal studies were performed according to approved protocols by the NYU School of Medicine Institutional Animal Care and Use Committee (IACUC).
Bacterial growth conditions and quantification of bacterial burden.
MRSA strains USA300-LAC, MW2, and USA500-BK2395 were used in this study (17). The USA300 Δsae, USA300 Δagr, and USA300 Δsae Δagr mutant strains were previously described (18). S. aureus strains were cultured overnight from a single colony in 5 ml tryptic soy broth (TSB) with shaking at 37°C. Cultures were diluted 1:100 in 5 ml of TSB and grown with shaking at 37°C for an additional 3 h until the optical density at 600 nm (OD600) was 1.5 (109 CFU/ml). Bacterial burden was quantified by plating serial dilutions of homogenized stool and organs on MSA plates. Colon and small intestine were washed in PBS to remove nonadherent bacteria to distinguish lumenal and mucosa-associated bacteria. The gallbladder was carefully removed, and the tissue and lumen were separated and diluted in PBS for plating on MSA plates. For analysis of the in vitro growth curve, overnight cultures of indicated strains were diluted 1:100 in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetal calf serum (FCS), and the OD600 was measured regularly. For the competition growth curve, cells of the USA300-LAC wild-type (WT) strain and USA300 Δsae Δagr double mutant were mixed at a 1:1 ratio and diluted 1:100 in DMEM plus 10% FCS in triplicate, and grown at 37°C with shaking. At 0, 2, 4, and 8 h, 100 μl was plated on plates containing MSA or MSA with tetracycline (5 μg/ml) to distinguish tetracycline-resistant (USA300 Δsae Δagr) and -sensitive (USA300-LAC WT) bacteria. The amount of USA300-LAC WT bacteria was calculated by subtracting the number of colonies formed on the tetracycline-containing plates from the number of colonies formed on the antibiotic-free plates.
Competition experiments in mice.
The mecA+ reference strain USA300-JE2 was grown separately from the mecA-negative competitor (mecA::bursa NE1868 obtained from the Nebraska Transposon Mutant Library) (19) overnight at 37°C. Cultures were diluted 1:100, grown to the exponential phase, and mixed at a 1:1 ratio of 5 × 106 CFU each, which was confirmed by colony determination following plating on agar and incubation. Mice were injected via the tail vein with a combined inoculum of 107 CFU, and 400 mg/kg oxacillin was administered by intraperitoneal (i.p.) injection 20 h later. Relative numbers of CFU in feces were determined by plating on MSA (nonselective) and Chromagar (BD Biosciences) plates to select resistant strains. The number of susceptible bacteria was calculated by subtracting CFU obtained by plating on selective media from the CFU from plating on nonselective media.
Statistics.
GraphPad Prism v6 was used for statistical analysis. Bacterial burdens were compared after log transformation. Differences between means were evaluated by two-tailed unpaired t test or one-way analysis of variance (ANOVA) and the Holm-Sidak test for experiments involving multiple comparisons.
RESULTS
S. aureus disseminates to the intestine following intravenous infection.
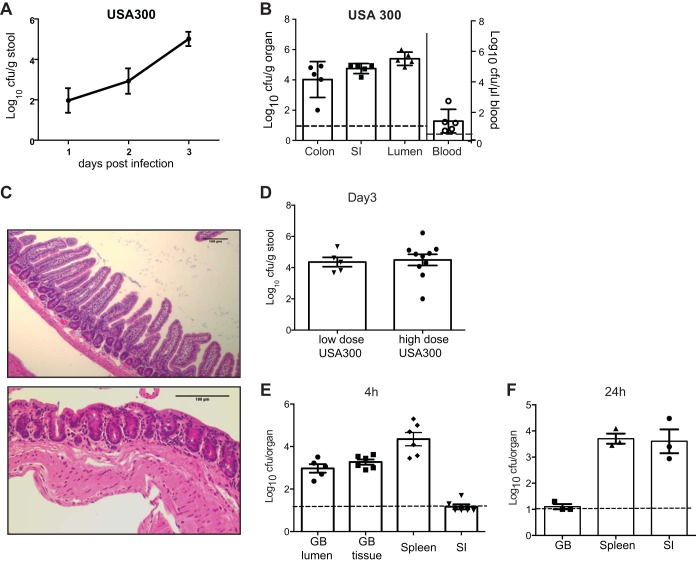
To investigate dissemination during bacteremia, mice were infected with 1 × 107 CFU of the community-acquired (CA) MRSA strain USA300-LAC by tail vein injection. Remarkably, the presence of S. aureus in stool was detected as early as 1 day postinfection and continued to rise during the course of acute infection to 3.9 × 105 CFU/g stool at day 3 (Fig. 1A). Examination of the colon, small intestine, and intestinal lumen on day 3 postinfection revealed bacteria from each of these sites with no obvious regional preference (Fig. 1B). It is unlikely that the presence of S. aureus in the stool or intestine was due to contamination from the blood, because we detected no intestinal bleeding. Also, based on the amount of S. aureus remaining in the blood on day 3 postinfection (∼20 CFU/μl) (Fig. 1B), a large volume of contaminating blood would have been required to account for such high bacterial levels in tissue or feces. We conclude that intravenous (i.v.) injection of S. aureus leads to dissemination from the blood to the lower GI tract followed by shedding in the feces.
FIG 1.
Intravenous S. aureus infection leads to gastrointestinal dissemination and shedding. (A) Quantification of bacteria in stool following i.v. injection with 1 × 107 CFU of MRSA strain USA300 (n = 10 mice). (B) Quantification of bacteria per gram of colon, small intestine (SI), and lumenal contents of small intestine or per microliter of whole blood on day 3 after i.v. injection with 1 × 107 CFU of MRSA USA300 (n = 5 mice). (C) H&E-stained section of small intestine (ileum [top panel]) and colon (bottom panel) on day 3 after i.v. injection with 1 × 107 CFU MRSA USA300. Images are representative of 5 mice. Scale bar, 100 μm. (D) Quantification of bacteria in stool on day 3 after i.v. injection with either a low dose of 1 × 105 CFU (n = 5 mice) or high dose of 1 × 107 CFU (n = 10 mice) of MRSA USA300. (E) Quantification of bacteria in the gallbladder (GB) lumen, GB tissue, spleen, or 2 cm of small intestinal tissue 4 h after i.v. injection with 1 × 107 CFU MRSA USA300. (F) Quantification of bacteria in organs 24 h after injection as in panel E. The data represent the mean ± standard error of the mean (SEM).
Intestinal dissemination did not appear to be a consequence of hematogenous seeding of the GI tract because MRSA was detected in stool on day 1 postinfection, a time at which we observed no symptoms of illness, such as lethargy, ruffled fur, or hunched posture. Also, mice had no diarrhea, and macroscopic and microscopic examination of intestinal tissue showed no obvious sign of infectious abscesses or inflammation (Fig. 1C). Additionally, the same fecal burden (5.8 × 104 CFU/g stool) was obtained following i.v. injection with the lower dose of 1 × 105 CFU of USA300, indicating that the infecting concentration of bacteria was saturating even at a nonlethal dose (Fig. 1D). Another route by which S. aureus could reach the intestine is through biliary excretion following infection of the gallbladder during the initial high-grade bacteremia. Consistent with this possibility, the presence of S. aureus in the gallbladder preceded its presence in the intestine. S. aureus was retrieved from the gallbladder (1.3 × 104 CFU in the lumen and 2.2 × 104 CFU in the tissue) as early as 4 h after i.v. injection, a time at which bacterial numbers were at or below the limit of detection in intestinal tissue (Fig. 1E). By comparison, bacterial burden in the spleen was apparent from the onset of infection (Fig. 1E). Bacteria were not recovered from the gallbladder at 24 h postinfection. Thus, any seeding of S. aureus into the GI tract from the gallbladder would have been transient. Although these results do not exclude other routes of spread, our findings suggest that biliary excretion contributes to early GI dissemination.
Intestinal dissemination of S. aureus is followed by transmission.
Cohousing of infected mice with naive mice is often used as a model to investigate fecal-oral transmission of a variety of pathogens (20–23). To determine whether S. aureus from a systemic infection can be transmitted to another host, mice injected i.v. as described above with 1 × 107 CFU of USA300 were cohoused with naive mice (Fig. 2A). Strikingly, analysis of bacteria in the stool indicated that S. aureus was transmitted to naive animals. Cohoused mice displayed a similar amount of S. aureus in stool (1.3 × 105 CFU/g on day 4) to mice infected by i.v. injection (Fig. 2B). Thus, S. aureus can transmit to uninfected cage mates via the fecal-oral route following bacterial dissemination from the blood to the intestine.
FIG 2.
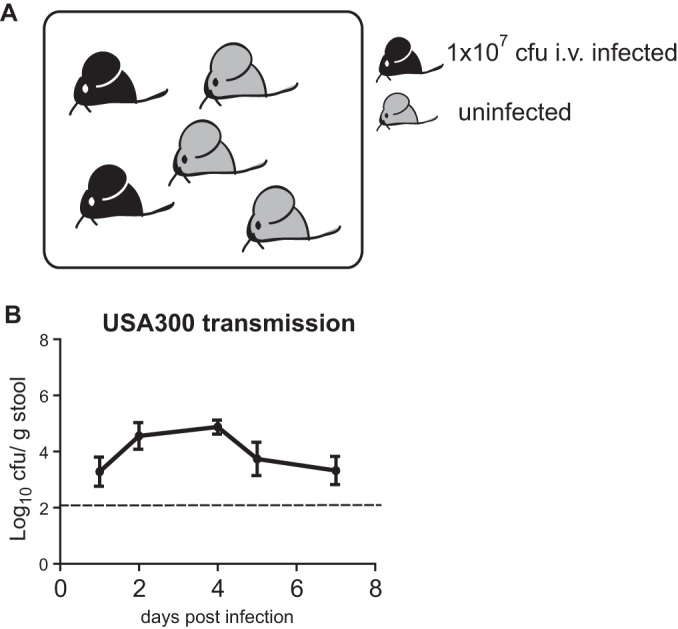
S. aureus transmission following intravenous infection. (A) Schematic of the transmission assay. Two mice injected i.v. with 1 × 107 CFU of S. aureus were cohoused with three uninfected mice per cage. (B) Quantification of bacteria in stool over time in untreated mice that were cohoused with mice injected with MRSA USA300 as depicted in panel A (n = 6 untreated mice per group). Data represent the mean ± SEM.
To determine if the intestinal dissemination and transmission reflect a specific property of USA300, we injected mice i.v. with 1 × 107 CFU of CA-MRSA strains USA400 (MW2) and USA500. S. aureus in the stool was readily detected following bacteremic infection by these strains as well (Fig. 3A). Moreover, using the same cohousing scheme described above, we found a similar degree of transmission with USA400 and USA500 to that observed with USA300 (Fig. 3B and C). Thus, fecal shedding and transmission are not strain specific.
FIG 3.
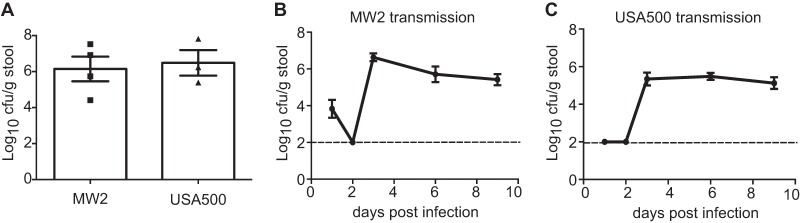
Gastrointestinal dissemination and transmission of S. aureus are not strain specific. (A) Quantification of bacteria in stool on day 3 after i.v. injection with 1 × 107 CFU of MRSA strains MW2 and USA500 (n = 3 to 4 mice each). (B) Quantification of bacteria in stool over time in untreated mice that were cohoused with mice injected with MRSA MW2 as depicted in Fig. 2A (n = 6 untreated mice per group). (C) Quantification of bacteria in stool over time in untreated mice that were cohoused with mice injected with MRSA USA500 as depicted in Fig. 2A (n = 6 untreated mice per group). Data represent the mean ± SEM.
sae and agr cooperatively facilitate intestinal dissemination and transmission.
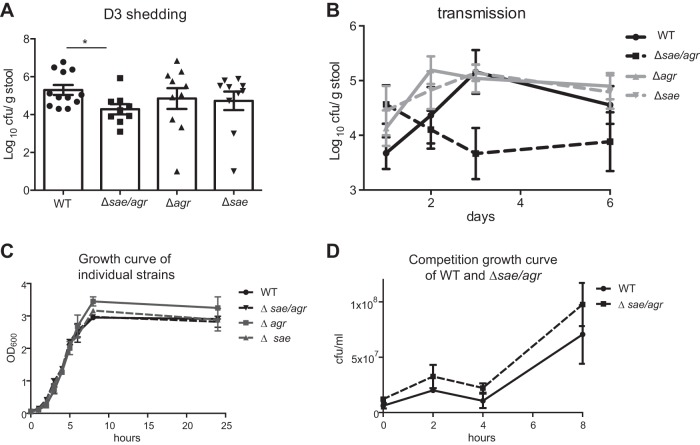
The observed linkage between intestinal dissemination and transmission raises the intriguing possibility that virulence can promote transmission. Although no single virulence determinant has been demonstrated to account for the ability of S. aureus to disseminate to peripheral organs following blood infection (24), many of the characterized virulence factors are controlled by the sae and agr regulatory systems (25). We therefore infected mice with either USA300 isogenic sae and agr single mutants (Δsae and Δagr), a double mutant (Δsae Δagr), or the wild-type (WT) strain (18). Whereas loss of either sae or agr alone did not significantly affect shedding of the bacteria on day 3 after i.v. infection, the double mutant strain USA300 Δsae Δagr displayed a 10-fold decrease in fecal shedding compared to USA300 WT (Fig. 4A). The effect of the double mutation on transmission was even more pronounced. We found a 103-fold reduction in the amount of bacteria recovered from the stool of mice cohoused with mice injected with USA300 Δsae Δagr (280 CFU/g stool USA300 Δsae Δagr compared with 1.55 × 106 CFU/g stool USA300 WT on day 3). In contrast, the USA300 Δsae and USA300 Δagr single mutants did not exhibit a transmission disadvantage (Fig. 4B). When USA300 WT and the Δsae Δagr mutant were grown side by side or together in an in vitro competition assay, USA300 Δsae Δagr did not display a growth defect (Fig. 4C and D). These results suggest that the fitness cost for USA300 Δsae Δagr in vivo was not attributable to an intrinsic growth defect: if anything, it showed a growth advantage (Fig. 4D). Reduced transmission may reflect either a requirement for a threshold level of bacteria in the feces of donor hosts or the role of agr- or sae-mediated virulence once the recipient is exposed. Regardless, our observations indicate that S. aureus is transmitted from a bacteremic host to uninfected mice in the same cage through a process that is facilitated by sae and agr.
FIG 4.
sae and agr facilitate fecal shedding and transmission of S. aureus. (A) Quantification of bacteria in stool on day 3 after i.v. injection with 1 × 107 CFU of the WT strain or USA300 Δsae, USA300 Δagr, or USA300 Δsae Δagr mutant (n = 9 to 12 mice each). (B) Quantification of bacteria in stool over time in untreated mice cohoused with mice injected with the WT strain or USA300 Δsae, USA300 Δagr, or USA300 Δsae Δagr mutant from panel D (n = 6 untreated mice per group). (C) Growth curve of the individually grown WT strain or USA300 Δsae, USA300 Δagr, or USA300 Δsae Δagr mutant in DMEM plus 10% FCS. (D) Quantification of the WT and USA300 Δsae Δagr mutant grown together in DMEM plus 10% FCS. Data represent the mean ± SEM.
Antibiotic selection in the bacteremic host has consequences for transmission.
The above findings suggest that an event occurring in a bacteremic host can determine which strains successfully transmit to new hosts. Since the selection and spread of antibiotic-resistant strains of S. aureus have become a major medical threat, we examined the consequence of antibiotic treatment on the transmission of resistant bacteria in our mouse model of dissemination and transmission. In an in vivo competition assay, mice were challenged i.v. with equal numbers of mecA-negative and isogenic WT (mecA+ oxacillin-resistant) USA300 bacteria (Fig. 5A). Antibiotic (oxacillin) was administered 20 h after bacteremic infection, a time after hepatobiliary dissemination. Four hours after antibiotic administration (i.e., 24 h after i.v. injection), two infected “donor” mice were cohoused with three uninfected “recipient” mice in a fresh cage to ensure that recipient mice would not be exposed to S. aureus cells that were shed from donor mice prior to antibiotic treatment. Recipient mice did not receive antibiotic. Thus, by measuring the ability of mecA+ and mecA-negative strains to reach the stool of untreated recipient mice, we can ascertain the consequence of selection within the bacteremic donor mice independently of subsequent events that occur within the recipient mice. As expected, the proportion of mecA+ bacteria in the stool of antibiotic-treated donor mice increased compared with the initial starting frequency of approximately 50% (Fig. 5B). Furthermore, selection for antimicrobial resistance in the GI tract of treated mice also resulted in preferential transmission of resistant bacteria to uninfected cage mates. Two days after cohousing, the majority of bacteria recovered from the stool of recipient mice were antibiotic resistant (Fig. 5C). Thus, a disproportionate amount of resistant bacteria entering the stool of treated donor mice allowed resistant bacteria to preferentially transmit and become the dominant strain in untreated recipient mice.
FIG 5.
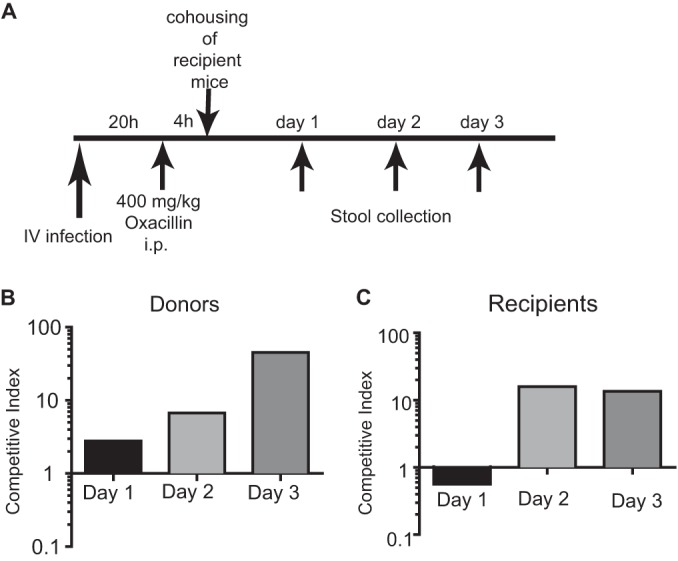
Antibiotic administration to bacteremic mice leads to preferential transmission of resistant S. aureus. (A) Schematic of competition assay for effect of antibiotic treatment on transmission. Donor mice injected i.v. with a mixture of 5 × 106 CFU each of mecA+ and mecA-negative USA300 strain cells were subsequently injected i.p. with oxacillin and then cohoused with untreated recipient mice at the indicated time points. (B) Competitive index representing the ratio of antibiotic-resistant versus -susceptible bacteria in the stool from donor mice. (C) Competitive index representing the ratio of antibiotic-resistant versus -susceptible bacteria in the stool from recipient mice (n = 6 mice). The bar graph represents the geometric mean.
DISCUSSION
Our observations establish a new animal model to investigate host-to-host transmission following within-host dissemination. A key finding in the present work is that large numbers of S. aureus cells can transmit to new hosts via the GI tract as a result of bloodstream infection, indicating a novel route connecting compartments relevant to pathogenesis and transmission. S. aureus may have seeded the intestinal lumen from the gallbladder after the initial high-grade bacteremia in a process resembling biliary excretion of Salmonella and Listeria into the GI tract after i.v. infection of mice (26, 27). The bacterial load in the gallbladder was transient, suggesting that (i) the gallbladder is not a permissive site for S. aureus replication, and (ii) high bacterial loads after i.v. infection of mice are transient. However, unlike the temporal staphylococcal discharge into the gallbladder of bacteremic mice, persistently bacteremic human patients may shed bacteria for long periods of time. Although further investigation is required to determine the exact way in which S. aureus reaches the intestine, transmission by the fecal route is considered important in the hospital environment (15, 28), where exposure of hospitalized patients to antibiotics could disrupt GI microbial flora and promotes colonization of antibiotic-resistant bacteria, including MRSA. Thus, one implication of our findings is that bacteremic patients in a hospital can be a source of further MRSA infections and contribute to a nosocomial outbreak. The S. aureus GI dissemination and transmission model described herein is an important step forward toward an understanding of the S. aureus life cycle in the health care environment.
Our initial results suggest that the ability of S. aureus to disseminate and transmit following blood infection is not strain specific, although there were subtle differences in the kinetics of transmission when comparing the three strains we analyzed (compare Fig. 2B with 3B and C). As an initial step toward elucidating the mechanisms behind S. aureus dissemination and transmission, we examined the role of agr and sae in this model. Transmission to naive hosts subsequent to intestinal dissemination depended on the regulatory circuits cooperatively controlled by agr and sae, consistent with the importance of these factors in other models of invasive staphylococcal infection (25). Interestingly, loss of only one of the regulators of these important two-component systems did not affect the ability of S. aureus to transmit to new hosts in this assay. This finding may reflect redundancies in virulence factors that mediate within-host dissemination (24). Alternatively, it is possible that reducing the initial bacterial load or coinfecting mice with WT and mutant strains will reveal a role for the individual factors. Such competition assays may be particularly useful in determining whether some strains have an increased capacity to disseminate and transmit.
S. aureus and MRSA strains can acquire mutations that alter antibiotic resistance and virulence during disease progression (29–31). Although a disease-promoting mutation that occurs during the course of bacteremia could confer a transient advantage to the bacterium, such an adaption would be a dead end for the bacterium in the absence of transmission. Thus, another implication of the present work is that selective processes taking place over the course of blood infection can go beyond a single host. Further experimental support for this hypothesis was provided by in vivo competition assays demonstrating preferential transmission of resistant strains over susceptible ones following antibiotic treatment. The displacement time in naive cohoused mice that did not receive the antibiotic mirrored the period of selection observed in the antibiotic-treated bacteremic mice, thus bridging within- and between-host dynamics.
Two additional comments are relevant to the work described above. First, the barrier to infection in humans is higher than that in mice, where coprophagia permits fecal-oral spread of S. aureus. However, even if S. aureus is not able to sustain transmission by the fecal-oral route in humans, the introduction of large doses of bacteria into the hospital environment may provide opportunities for the bacterium to leave one host and enter a new one at more conventional sites from which it is transmitted onwards. Second, evolution of S. aureus within the bacteremic host is not instantaneous, which may affect the within-host dissemination and spread of adapted mutants to new hosts. Additional studies are needed to demonstrate clinical significance and test specific host adaptive mutations for functional trade-offs in transmission efficiency.
ACKNOWLEDGMENTS
We thank Karl Drlica for suggestions on the manuscript.
This work was supported by National Institutes of Health (NIH) grants R01 DK093668 (K.C.), R01 AI103268 (B.S. and V.J.T.), T32 GM007308 (K.M.), T32 AI100853 (K.M.), and F30 DK098925 (K.M.), American Heart Association grant 12GRNT12030041 (K.C.), the Vilcek Fellowship (E.K.), and the Erwin Schrodinger Fellowship of the FWF (Austrian Science Fund) (E.K.). V.J.T. is a Burroughs Wellcome Fellow in the Pathogenesis of Infectious Diseases.
The authors declare they have no conflicts of interest.
REFERENCES
- 1.Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Novick RP, Geisinger E. 2008. Quorum sensing in staphylococci. Annu Rev Genet 42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 4.Novick RP, Jiang D. 2003. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 149:2709–2717. doi: 10.1099/mic.0.26575-0. [DOI] [PubMed] [Google Scholar]
- 5.Fowler VG Jr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS. 2004. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 190:1140–1149. doi: 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- 6.Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP. 2008. agr function in clinical Staphylococcus aureus isolates. Microbiology 154:2265–2274. doi: 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, McGregor JC, Thom KA, Perencevich EN. 2011. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother 55:1082–1087. doi: 10.1128/AAC.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montgomery CP, Boyle-Vavra S, Daum RS. 2010. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One 5:e15177. doi: 10.1371/journal.pone.0015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voyich JM, Vuong C, DeWald M, Nygaard TK, Kocianova S, Griffith S, Jones J, Iverson C, Sturdevant DE, Braughton KR, Whitney AR, Otto M, DeLeo FR. 2009. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis 199:1698–1706. doi: 10.1086/598967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogasch K, Ruhmling V, Pane-Farre J, Hoper D, Weinberg C, Fuchs S, Schmudde M, Broker BM, Wolz C, Hecker M, Engelmann S. 2006. Influence of the two-component system SaeRS on global gene expression in two different Staphylococcus aureus strains. J Bacteriol 188:7742–7758. doi: 10.1128/JB.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arya R, Princy SA. 2013. An insight into pleiotropic regulators Agr and Sar: molecular probes paving the new way for antivirulent therapy. Future Microbiol 8:1339–1353. doi: 10.2217/fmb.13.92. [DOI] [PubMed] [Google Scholar]
- 12.Massey RC, Horsburgh MJ, Lina G, Hook M, Recker M. 2006. The evolution and maintenance of virulence in Staphylococcus aureus: a role for host-to-host transmission? Nat Rev Microbiol 4:953–958. doi: 10.1038/nrmicro1551. [DOI] [PubMed] [Google Scholar]
- 13.Boyce JM, Havill NL, Maria B. 2005. Frequency and possible infection control implications of gastrointestinal colonization with methicillin-resistant Staphylococcus aureus. J Clin Microbiol 43:5992–5995. doi: 10.1128/JCM.43.12.5992-5995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce JM, Havill NL, Otter JA, Adams NM. 2007. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 28:1142–1147. doi: 10.1086/520737. [DOI] [PubMed] [Google Scholar]
- 15.Donskey CJ. 2004. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis 39:219–226. doi: 10.1086/422002. [DOI] [PubMed] [Google Scholar]
- 16.Marchiando AM, Ramanan D, Ding Y, Gomez LE, Hubbard-Lucey VM, Maurer K, Wang C, Ziel JW, van Rooijen N, Nunez G, Finlay BB, Mysorekar IU, Cadwell K. 2013. A deficiency in the autophagy gene Atg16L1 enhances resistance to enteric bacterial infection. Cell Host Microbe 14:216–224. doi: 10.1016/j.chom.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonzo F III, Benson MA, Chen J, Novick RP, Shopsin B, Torres VJ. 2012. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol Microbiol 83:423–435. doi: 10.1111/j.1365-2958.2011.07942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson MA, Lilo S, Nygaard T, Voyich JM, Torres VJ. 2012. Rot and SaeRS cooperate to activate expression of the staphylococcal superantigen-like exoproteins. J Bacteriol 194:4355–4365. doi: 10.1128/JB.00706-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4(1):e00537–12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, Monack DM. 2008. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect Immun 76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. 2011. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron T. 2002. Mouse models of prion disease transmission. Trends Mol Med 8:495–500. doi: 10.1016/S1471-4914(02)02416-4. [DOI] [PubMed] [Google Scholar]
- 23.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. 2005. Citrobacter rodentium of mice and man. Cell Microbiol 7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 24.Edwards AM, Massey RC. 2011. How does Staphylococcus aureus escape the bloodstream? Trends Microbiol 19:184–190. doi: 10.1016/j.tim.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 26.Hardy J, Francis KP, DeBoer M, Chu P, Gibbs K, Contag CH. 2004. Extracellular replication of Listeria monocytogenes in the murine gall bladder. Science 303:851–853. doi: 10.1126/science.1092712. [DOI] [PubMed] [Google Scholar]
- 27.Menendez A, Arena ET, Guttman JA, Thorson L, Vallance BA, Vogl W, Finlay BB. 2009. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J Infect Dis 200:1703–1713. doi: 10.1086/646608. [DOI] [PubMed] [Google Scholar]
- 28.Salyers AA, Gupta A, Wang Y. 2004. Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol 12:412–416. doi: 10.1016/j.tim.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Young BC, Golubchik T, Batty EM, Fung R, Larner-Svensson H, Votintseva AA, Miller RR, Godwin H, Knox K, Everitt RG, Iqbal Z, Rimmer AJ, Cule M, Ip CL, Didelot X, Harding RM, Donnelly P, Peto TE, Crook DW, Bowden R, Wilson DJ. 2012. Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc Natl Acad Sci U S A 109:4550–4555. doi: 10.1073/pnas.1113219109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cameron DR, Ward DV, Kostoulias X, Howden BP, Moellering RC Jr, Eliopoulos GM, Peleg AY. 2012. Serine/threonine phosphatase Stp1 contributes to reduced susceptibility to vancomycin and virulence in Staphylococcus aureus. J Infect Dis 205:1677–1687. doi: 10.1093/infdis/jis252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, Bruce D, Rubin E, Myers E, Siggia ED, Tomasz A. 2007. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A 104:9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]