Abstract
In its canonical role the reverse transcriptase telomerase recovers the telomeric repeats that are lost during DNA replication. Other locations and activities have been recently described for the telomerase protein subunit TERT in mammalian cells. In the present work, using biochemistry, molecular biology, and electron microscopy techniques, we found that in the human parasite Leishmania major, TERT (and telomerase activity) shared locations between the nuclear, mitochondrial, and cytoplasmic compartments. Also, some telomerase activity and TERT protein could be found in ∼100-nm nanovesicles. In the mitochondrial compartment, TERT appears to be mainly associated with the kinetoplast DNA. When Leishmania cells were exposed to H2O2, TERT changed its relative abundance and activity between the nuclear and mitochondrial compartments, with the majority of activity residing in the mitochondrion. Finally, overexpression of TERT in Leishmania transfected cells not only increased the parasitic cell growth rate but also increased their resistance to oxidative stress.
INTRODUCTION
Mammalian telomeres are nucleoprotein complexes with double and single DNA strand (telomeric overhang) runs of TTAGGG repeats, a chromatin organization containing methylated H3 and H4 histones, and a heavily methylated DNA, which are features of a transcriptionally repressed zone. At the ends of linear chromosomes, since DNA polymerases do not initiate DNA synthesis de novo, the new DNA synthesized over the lagging strand lacks the primer for the completion of its synthesis, leaving a gap; how to seal this gap has been named the end replication problem (1). Most eukaryotes solve the problem using mechanisms in which the enzyme telomerase plays an important role (2). Telomerase has been described for most eukaryotic cells, and in fact, the first report of telomerase was made for the protozoan Tetrahymena (2). The telomerase core has two components: a reverse transcriptase (TERT) protein and an RNA subunit (TER) that provides the template for copying the hexameric repeats to the telomeric overhang (2). In addition, mammalian telomeres contain a six-protein complex called shelterin that regulates the telomerase activity and protects the chromosomal ends from nucleolytic degradation and chromosomal rearrangements (3).
The TERT component has the typical motifs of reverse transcriptase enzymes (1, 2, A, B′, C, and E) and a telomerase-specific T-domain (4), whereas the TER subunit has been sequenced in a large number of organisms, and the length of the RNA varies from ∼200 to 1,300 nucleotides (nt) (5).
Growing evidence indicates that TERT plays multiple roles in addition to its telomeric function, including (i) acting as a transcriptional modulator of the β-catenin signaling pathway in mice; (ii) interacting with an RNA component of mitochondrial RNA processing endoribonuclease (RMRP), creating a new activity that synthetizes RNA in a RNA-dependent manner and generating a double-stranded RNA (dsRNA) that enters into the RNA interference (RNAi) cascade; (iii) regulating apoptotic processes; and (iv) conferring cell resistance to oxidative stress. For a more detailed review, see reference 6.
In the case of unicellular organisms such as the parasitic trypanosomatid Leishmania major, the etiological agent of cutaneous leishmaniasis in the Old World, the parasite switches between two morphological and physiological forms, i.e., promastigotes, which are mobile flagellated forms 7 μm long striving in the guts of a Phlebotomus fly, and ∼2-μm-diameter amastigotes with a reduced flagellum living inside the mammalian host cell. Since both forms undergo cell division, telomerase seems to be an essential activity for their survival.
Trypanosomatid telomerase activities were first reported by Cano and coworkers (7). There have been subsequent reports for Leishmania amazonensis (8), L. major (9), Trypanosoma cruzi (10, 11), and Trypanosoma brucei (7, 12). Trypanosomatid TERT DNA sequences show identities of 51% within the Trypanosoma genus, 87% in species included in the Leishmania genus, and 33% between these two genera. Knockout experiments with T. brucei TERT have shown that null mutants suffer telomeric shortening at the slow pace of 3 to 6 bp per duplication (12); thus, detection on telomeric shortening is difficult to assess.
Recently the TER subunit of T. brucei was sequenced; this RNA is around 900 nucleotides long and is produced from a larger transcript that is processed by transsplicing (13).
In a previous study, we characterized molecularly and biochemically the telomerase of L. major promastigotes (9). During those experiments, we noted that in nuclear preparations, despite showing apparently good integrity, half of the total telomerase activity remained in the supernatant. Although this extranuclear activity may have arisen from leaking nuclei, the reports on alternative roles of telomerase in mammalian cells (14) led us to reexamine the nature of this extranuclear activity in L. major. First, we did a subcellular fractionation of L. major promastigote forms and confirmed the presence of telomerase activity in the mitochondrial and cytoplasmic fractions. Further analysis revealed that part of the activity was associated with ∼100-nm nanovesicles (NV) excreted by the parasitic cells. Second, electron microscopic (EM) analysis combined with immunogold labeling showed the presence of telomerase in the parasite's mitochondria, mainly associated with the kinetoplast structure, and in what appear to be vesicles containing telomerase outside the cells. Further binding experiments using a recombinant L. major TERT confirmed the affinity of this protein for the mitochondrial kinetoplast DNA (kDNA).
As mentioned above, mammalian cell telomerase extratelomeric activity has been linked to oxidative stress response either protecting or damaging the mitochondrial functions (15–19). To evaluate the effect of oxidative stress in L. major, parasite cells were exposed to hydrogen peroxide (H2O2), with the finding that telomerase enzyme activity and protein localization in the mitochondrial fraction increased with a concomitant decrease in the nuclear fraction. Furthermore, we checked the effect of TERT overexpression in L. major promastigotes, observing that transformed cells had a 3-fold growth rate increase and a higher resistance to the oxidative stress than wild-type (WT) cells.
MATERIALS AND METHODS
Parasite culture.
Leishmania major MHOM/JL/80/Friedlin promastigotes were maintained at 25°C in 1× medium 199 with Hanks' salt (Sigma) supplemented with 10% (vol/vol) fetal bovine serum (FBS). For nanovesicle preparation, FBS was centrifuged at 120,000 × g for 1 h to deplete any serum nanovesicles. For oxidative stress induction experiments, cells were incubated for 1 h at 25°C in 1× medium 199 without FBS and supplemented with H2O2 (10 to 25 μM).
Protein preparation from subcellular fractions.
The parasite population was divided in two; half (8 × 107 cells) was used to obtain the mitochondrion-enriched fraction using a mitochondrion isolation kit for culture cells (Thermo Scientific) by following the manufacturer's instructions for achieving maximum purity and organelle integrity. The integrity of isolated mitochondria was evaluated by measuring the mitochondrial membrane potential using the fluorescent dye rhodamine 123 as described by Benaim et al. (20). Briefly, the isolated mitochondrial pellet was resuspended in 1× phosphate-buffered saline (PBS) and loaded with 20 μM rhodamine 123 for 45 min at 29°C under dark conditions. Subsequently, the loaded mitochondrial suspension was collected, washed twice and resuspended in the same buffer, and then transferred into a magnetically stirred cuvette. After adding the uncoupler of mitochondrial oxidative phosphorylation FCCP (carbonilcyanide p-trifluoromehoxyphenylhydrazone; 2 μM), the exit of fluorescent dye versus time (excitation wavelength, 488 nm; emission wavelength, 530 nm) was measured in a Hitachi 7000 spectrofluorimeter.
For preparation of mitochondrial protein extracts, the mitochondrion-enriched fraction was lysed with 2% (wt/vol) CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} buffer prepared in 1× PBS and then centrifuged at 16,000 × g for 10 min, and the supernatant was recovered. The purity of the mitochondrial fraction obtained was evaluated by assaying the succinate dehydrogenase enzyme activity according to the method of Johnson et al. (21). For this assay, we used 4.5 μg of proteins from the premitochondrial pellet, mitochondrion-enriched fraction, and postmitochondrial supernatant.
The second half of the parasite's population (8 × 107 cells) was used to prepare the nuclear and cytoplasmic fractions. Cells were processed with the NE-PER nuclear and cytoplasmic extraction reagent kit (Thermo Scientific) by following the manufacturer's instructions. The cytoplasmic fraction was recovered after spinning down most cell organelles at 16,000 × g for 10 min. Cross contamination with nuclear fractions was checked by Western blotting of each cell fraction (4.5 μg of proteins from the nuclear, mitochondrion-enriched, and cytoplasmic fractions) using antibodies produced against the human nuclear protein lamin B.
Telomerase activity assay.
For assaying total L. major telomerase activity, we used the telomere repeat amplification protocol (TRAP) (22), with some modifications (11). TRAP products were separated on 12% polyacrylamide gels and were run in 1× Tris-borate-EDTA (TBE) buffer at 250 V for 1.5 h. After staining with a SYBR green (Sigma) solution (diluted 1:10,000), gels were visualized on a Typhoon 9410 high-performance gel and blot imager (Amersham). Quantification of telomerase activity was done by densitometry analysis using the software ImageQuant TL (Amersham). The density of each band in the ladder of products was measured, and all the peaks' areas were integrated using the software to obtain the arbitrary units (AU).
Nanovesicle preparation.
Excreted parasite nanovesicles (NV) were obtained according to the method described by Thery et al. (23). Briefly, culture medium was cleared of larger debris and parasite cells by low-speed centrifugation (500 × g) for 30 min. The resulting supernatant was filtered through a 0.22-μm filter (Sartolab 20; Sartorius), and this filtered medium was centrifuged at 100,000 × g for 1 h. The resulting pellet was washed three times with 0.1 M Tris HCl (pH 7.2) using the same centrifugation conditions. For gel electrophoresis and Western blot analysis, an aliquot of the pellet was resuspended in 20 μl of lysis buffer (20 mM PBS, 0.25 mM sucrose, 1 mM EDTA, 1 mM dithiothreitol [DTT]) at pH 7.4 plus Complete Mini inhibitor protease cocktail (Roche Molecular Biochemicals). After treatment for 10 min, this suspension was sonicated, adjusted to the same protein concentration, and electrophoresed using 10% SDS-PAGE. To confirm the presence of NV in our preparations, an aliquot of the pellet was resuspended in 0.1 M Tris HCl (pH 7.2) and stained with 2% (vol/vol) uranyl acetate for direct observation by transmission electron microscopy (TEM) (24).
Western blotting.
After SDS-PAGE electrophoresis, separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Amersham) according to the method described by Towbin et al. (25). Electroblotting was done overnight at 4°C, and membranes were then exposed to the following primary antibodies for 1 h at room temperature (RT): (i) a polyclonal rabbit antibody produced against the L. major telomerase N-terminal domain including the GQ motif (anti-TEL-1) (see Fig. S1 in the supplemental material) at a 1:2,000 dilution (this antibody was purified by immunoabsorption using a recombinant L. major TERT protein fragment produced in E. coli cells as previously described [9]; anti-TEL-1 was able to immunoprecipitate a protein band with the expected molecular mass of L. major TERT [LmTERT] and with telomerase activity [see Fig. S2 in the supplemental material]), (ii) a monoclonal mouse antibody produced against human laminin B (Santa Cruz Biotechnology) tested at a 1:500 dilution (see Fig. S3A in the supplemental material), and (iii) a polyclonal mouse antibody produced against human α-tubulin (Santa Cruz Biotechnology) at a 1:750 dilution, used for gel loading control. Next, depending on the primary antibody's source, membranes were incubated for 1 h at RT with either horseradish peroxidase (HRP)-conjugated polyclonal goat anti-mouse immunoglobulin (Santa Cruz Biotechnology) or HRP-conjugated goat anti-rabbit immunoglobulin (Pierce), both at a 1:2,000 dilution. The HRP reaction was developed with InmunoCruz Western blot luminol reagent kit (Santa Cruz Biotechnology) by following the manufacturer's instructions.
DNA constructs and protein expression.
The LmTERT open reading frame (ORF; LmjF36.3930) was amplified from L. major genomic DNA (gDNA) by PCR using forward primer p1F (5′-TAAGGATCCGTATGTCCGCCTCGTTTCCAT-OH-3′) and reverse primer p1R (5′-ATAACCCGGGTCAAGTCTGCGAGAGTCG-OH-3′), and then the PCR product was inserted into the BamHI and XmaI sites of the expression vector pGEX5X2 (Amersham). Recombinant LmTERT expression was done using E. coli strain BL21 pLysS by following the protocol previously described (26). Briefly, cells transformed with recombinant plasmid (see Fig. S4 in the supplemental material) were grown in 2× YT medium (10 mg/ml of yeast extract and 16 mg/ml of tryptone) supplemented with ampicillin (100 μg/ml) and chloramphenicol (30 μg/ml) at 30°C with slight agitation until the culture reached an optical density at 595 nm (OD595) of 0.2. At this point, IPTG (isopropyl-β-d-1-thiogalactopyranoside; 1 mM) was added and the culture was kept at 30°C for 4 h. Later, cells were collected and resuspended in PBS supplemented with lysozyme (1 mg/ml) and 0.2% (vol/vol) Triton X-100. This mixture was kept on ice for 10 min and later centrifuged at 4,000 × g for 30 min at 4°C. The supernatant and insoluble fractions were recovered and analyzed by SDS-PAGE for the presence of recombinant LmTERT. The insoluble fraction was treated with a solubilization buffer (1.5% [wt/vol] N-lauryl sarcosine, 25 mM triethanolamine, 1 mM EDTA) and later exposed to 0.5% (wt/vol) glutathione agarose bead suspension for 30 min at 4°C. Recovery of recombinant protein attached to beads was done with elution buffer (10 mM reduced glutathione, 50 mM Tris-HCl [pH 8.0]). The identity of the recombinant protein was checked with immunopurified anti-TEL-1 polyclonal antibody, revealing a unique band with the expected molecular mass of ∼184 kDa (157 kDa of TERT and 27 kDa corresponding to the glutathione S-transferase [GST] tag) (see Fig. S4B in the supplemental material). The LmTERT ORF was also amplified by PCR using the same forward primer, p1F, and reverse primer p2R (5′-ATAATCTAGATCAAGTCTGCGAGAGTCG-OH-3′). The PCR product was first cloned into pGEM-T Easy vector (Promega), and then after digesting this plasmid with BamHI and NdeI endonucleases, the TERT fragment was inserted into the Leishmania expression vector pSP72NEO (27) digested with the same enzymes. Overexpression of the inserted LmTERT gene was driven by the intergenic region of the L. major α-tubulin gene (see Fig. S5 in the supplemental material). Ten to 20 μg of this construct was used to transfect L. major promastigotes by electroporation by following a protocol previously described (28). Transfectant cells were selected in 1× medium 199 with Hanks' salt (Sigma) supplemented with 10% (vol/vol) FBS, adjusted to 10 μg/ml of G418 (Geneticin; Gibco-BRL).
Testing oxidative stress resistance.
To evaluate the effect of oxidative stress in L. major cell lines (WT cells or TERT-overexpressing [+TERT] cell lines), we used a modified MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay as a cell viability test (29). MTT conversion from a yellow substrate to a blue crystal product (formazan) measures total mitochondrial dehydrogenase activity. A total of 2 × 106 parasites in medium 199 were incubated in a 96-well plate with concentrations of H2O2 ranging from 0.5 to 2.5 mM for 18 h at 25°C. Plates were centrifuged at 1,500 × g for 10 min, and the parasite pellet was resuspended with 100 μl of 0.5-mg/ml MTT solution and incubated for 4 h at 25°C. Later, plates were centrifuged again at 1,500 × g for 10 min. Solubilization of the pelleted formazan crystals was done with 100 μl of 100% (vol/vol) dimethyl sulfoxide (DMSO), and the absorbance of each sample was read at a wavelength of 595 nm using an iMARK microplate reader (Bio-Rad).
Isolation of kDNA and dot blot analysis.
Isolation of kDNA from L. major cells was done by a fast method described by Shapiro et al. (30). A total of 1 × 108 parasites were collected and resuspended in NET-100 buffer (100 mM NaCl, 100 mM EDTA, 10 mM Tris-HCl [pH 8.0]). Proteinase K and SDS were also added to final concentrations of 0.1 mg/ml and 1% (vol/vol), respectively. This mixture was kept at 37°C for 1 h with mild agitation. The lysate was sheared through a 27-gauge needle from a 5-ml syringe barrel using hand pressure, and then it was layered onto a cushion of 20% (wt/vol) sucrose prepared in NET-100 and centrifuged at 20,000 × g at 15°C for 1 h. The pelleted kDNA network was recovered and extracted twice with phenol-chloroform (24:1) and then precipitated overnight at −20°C with 2 volumes of 99% (vol/vol) 2-propanol and 1/10 volume of 3 M sodium acetate (pH 5.2). The precipitated kDNA was washed with 70% (vol/vol) ethanol and resuspended in Tris-EDTA (TE) buffer. For minicircle DNA (mcDNA) purification, kDNA was digested with EcoRI enzyme for 1 h at 37°C. Fragments were separated in preparative gels, and the minicircle bands were sliced from gel and recovered with a DNA gel extraction kit (Axygen). This procedure was repeated several times to collect enough DNA for our dot blot assays. For dot blot analysis, we fixed the DNA samples into a positively charged nylon membrane (Amersham) by UV cross-linking (1.2 × 106 μJ/cm2 for 1 min). Samples loaded were 5 to 10 μg of kDNA network; 10 μg of isolated mcDNA; purified total Leishmania genomic DNA (gDNA), intact and doubly digested with RsaI and HaeIII enzymes; and circular and linearized bacterial plasmid DNA. Membranes were equilibrated in 1× telomerase attaching buffer (100 mM Tris-HCl [pH 8.3], 7.5 mM MgCl2, 315 mM KCl, 0.25% [vol/vol] Tween 20, 5 mM EGTA [pH 8.0], BSA [0.5 mg/ml]) at 28°C for 15 min and later incubated with an LmTERT recombinant protein dilution (0.5 μg/ml) in the same buffer at 28°C for 2 h. After incubation, the membrane was blocked and incubated with the anti-TEL-1 antibody (1:2,000 dilution) and subsequently with anti-rabbit immunoglobulin-HRP (Santa Cruz Biotechnology) (1:2,000 dilution) for 1 h at room temperature. Signal was revealed using an InmunoCruz Western blot luminol reagent kit (Santa Cruz Biotechnology) by following the manufacturer's instructions. Binding capacity was estimated by densitometric analysis using the public-domain program ImageJ (Wayne Rasband, National Institutes of Health, USA [http://rsb.info.nih.gov/ij/]).
Transmission electron microscopy.
L. major cells were fixed with Karnosvsky fixative (31) (2.5% [vol/vol] glutaraldehyde and 2% [vol/vol] formaldehyde in 0.1 M cacodylate buffer; 50 mg CaCl2 in 100 ml) at 37°C for 30 min. Cells were then dehydrated and embedded in Spurr resin (Sigma). Ultrathin sections were stained with 8% (vol/vol) uranyl acetate followed by 0.2% (vol/vol) lead citrate and examined in a LIBRA 120 PLUS Carl Zeiss SMT.
Immunogold identification.
Grids containing slices of resin-embedded cells were immersed for 10 min at RT in a solution containing 1% (wt/vol) gelatin from cold-water fish skin (Sigma-Aldrich) prepared in 1× PBS, followed by a 15-min immersion in 0.02 M glycine in PBS and 45 min in 1% (wt/vol) ovalbumin prepared in 1× PBS. After this blocking step, slices were covered for 90 min by 1:20 dilutions of anti-TEL-1 antibody. After this incubation, grids were immersed five times in PBS for 10 min and then incubated with 1:20 dilutions of anti-rabbit secondary antibodies labeled with 25-nm gold particles (Electron Microscope Sciences). Incubations with antibodies were done in a humid chamber at 22°C, and after the secondary antibodies' incubation, grids were washed five times with 1× PBS and five times with distilled water and then contrasted with a 2% (vol/vol) uranyl acetate solution. Control grids were treated with preimmune serum as the primary antibody (see Fig. S6 in the supplemental material). EM observations were done with a Zeiss (Libra-2000) transmission microscope.
Nanovesicle negative staining and immunodetection.
For immune-labeling observations, aliquots of the NV pellet were resuspended in 30 μl of Tris-HCl (pH 7.3) and 5 μl of this suspension was adsorbed directly onto Formvar/carbon-coated grids. After 30 min of incubation at RT, grids were washed three times for 10 min with 1× PBS and then dried out at RT and fixed with 1% glutaraldehyde for 30 min. Finally, grids were washed three times with 1× PBS, blocked with a solution of 0.02 M glycine prepared in 0.1 M PBS (pH 7.3) for 30 min, and blocked for 30 min with blocking buffer (0.05% Tween in PBS [pH 7.4] plus 1% nonfat milk). Grid incubation with primary and secondary antibodies labeled with Au and contrasted with 2% (vol/vol) uranyl acetate was done as described above.
RESULTS
Telomerase is present in L. major mitochondrial and cytoplasmic subcellular fractions.
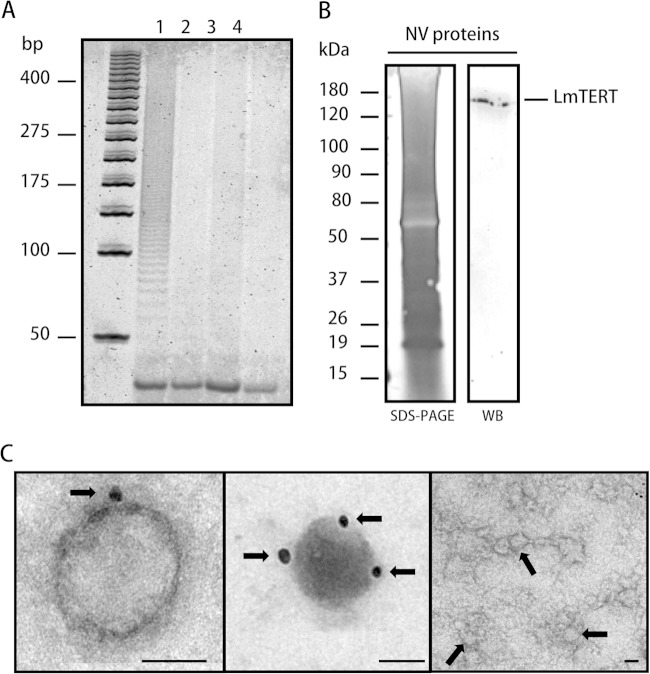
Nuclear and mitochondrial protein fractions from L. major promastigotes were prepared as indicated in Materials and Methods. The cytoplasmic proteins were also obtained during the preparation of the nuclear fraction. The purity and identity of these fractions were corroborated by Western blotting using an anti-human laminin B antibody (see Fig. S3A in the supplemental material) and by succinate dehydrogenase enzymatic assay for the mitochondrial fraction. Mitochondrial integrity was evaluated through a mitochondrial membrane potential assay (see Fig. S3C and D). A Western blot analysis of these three fractions using anti-TEL-1 immunopurified antibodies revealed the presence of a protein band matching the predicted molecular mass of LmTERT of ∼157 kDa (Fig. 1A). Two extra bands of lower molecular mass, likely products of LmTERT degradation, were observed in the cytoplasmic fraction. Although the band intensity for the mitochondrial fraction was bigger than the one for the nuclear fraction, the estimated total amount of LmTERT in the nuclear fraction almost doubles the amount present in the mitochondrial fraction (Table 1). The assays of telomerase activity confirmed the presence of this enzyme in the nuclear, mitochondrial, and cytoplasmic subcellular fractions of Leishmania (Fig. 1B). This fact led us to assume that the mitochondrial fraction is enriched in telomerase protein. Trying to understand whether the presence of LmTERT in cytoplasmic fraction is transient and possibly related to a secretion mechanism, we harvested parasites in culture and recovered the supernatant, and this was further treated to obtain NV. Protein preparation from NV showed telomerase activity (Fig. 2A, lane 1), whereas no enzyme activity was observed in the remaining supernatants (Fig. 2A, lanes 2 and 3). The presence of LmTERT in these NV was corroborated by Western blotting (Fig. 2B) and TEM immunochemistry analysis with anti-TEL-1 antibodies (Fig. 2C). A control sample of NV not treated with anti-TEL-1 antibodies is also shown in Fig. 2C.
FIG 1.
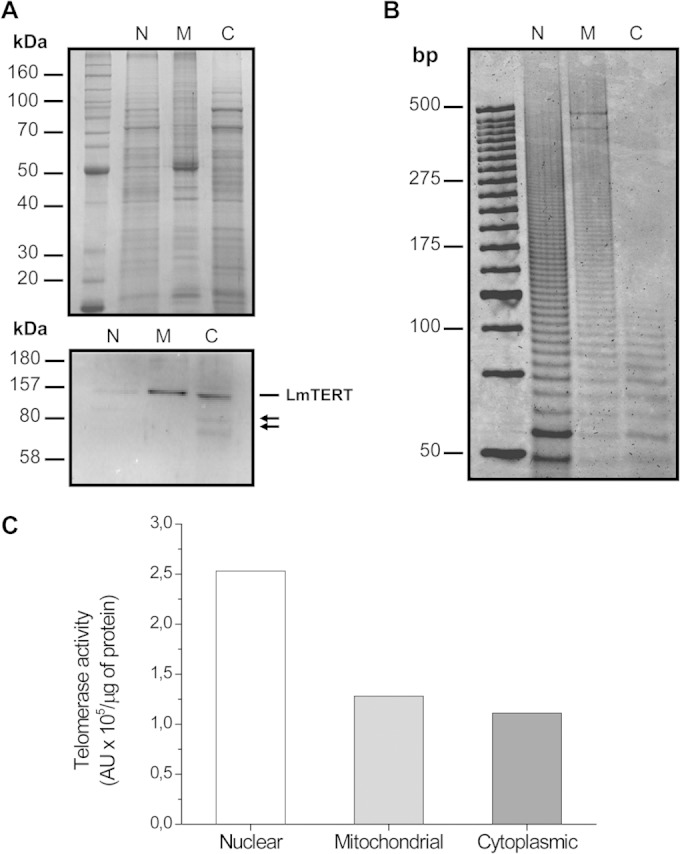
Subcellular distribution of L. major telomerase TERT component. (A) SDS-PAGE and Western blot analysis of nuclear (N), mitochondrion-enriched (M), and cytoplasmic (C) protein fractions. A polyacryalmide gel was loaded with 4.5 μg of protein from each cellular fraction and then incubated with immunopurified anti-TEL-1 antibody. Arrows point to lower-molecular-mass bands likely associated with LmTERT protein degradation. (B) Polyacrylamide gel electrophoresis of telomerase product N, M, and C fractions from L. major promastigotes. The total volume of the reaction mixture was loaded on the gel. (C) Telomerase specific activity in subcellular fractions is expressed in arbitrary units (AU) per μg of protein.
TABLE 1.
Estimation of LmTERT protein content in nuclear and mitochondrial fractions of L. major cells based on Western blot analysisa
| Cell fraction | Density (AU) | AU/μg of protein loaded on gel | Total estimated in fraction (AU) |
|---|---|---|---|
| Nuclear | 1,216.062 | 270.236 | 14,862.980 |
| Mitochondrial | 7,533.326 | 1,674.072 | 9,207.398 |
“Density” refers to areas of the protein bands. Total TERT protein content was calculated for each fraction after estimating the areas of the protein bands in arbitrary units (AU), dividing them by the quantity (μg) of proteins loaded on each lane of the gel, and then multiplying these values by the total protein content of the fractions.
FIG 2.
LmTERT and telomerase enzyme activities in L. major NV preparations. (A) Telomerase activity was detected in NV protein preparations (lane 1), whereas no activity was registered in the two supernatants obtained after pelleting NV (lanes 2 and 3). A blank control with no proteins was loaded (lane 4). (B) SDS-PAGE and silver staining with 5 μg of NV protein preparation and a corresponding Western blot (WB) revealed with Leishmania anti-TEL-1 immunopurified antibody. (C) Transmission electron microscopy and immunodetection of LmTERT in NV preparations. Arrows in the left and the middle photographs point to the 25-nm gold particles that indicate the presence of LmTERT. The photograph on the right shows a control of immunodetection in NV preparation without anti-TEL-1 antibodies; arrows point to nanovesicles.
H2O2 treatment causes TERT redistribution.
After treating the Leishmania cells with 10 or 25 μM H2O2, the cell fractionation experiments were repeated. As shown in Fig. 3, the telomerase enzyme activity increased in the mitochondrial and cytoplasmic fractions and decreased in the nuclear one at both H2O2 concentrations. Next, we examined H2O2-treated cells by TEM immunochemistry analysis. To confirm the specificity of the labeling with anti-TEL-1 antibodies, we first showed that the secondary anti-IgG rabbit alone did not produce any gold labeling in the cells (see Fig. S6 in the supplemental material). A second control experiment consisting of a sample of Leishmania cells with no H2O2 treatment but incubated with the anti-TEL-1 antibodies and with the gold-labeled anti-rabbit antibodies showed that some gold particles were located at the nuclear periphery, some of which were in a doublets or pairs (Fig. 4A). In the mitochondria, gold particles were mostly located at the central part of the kinetoplast, likely interacting with the very abundant minicircle DNA (31, 32).
FIG 3.
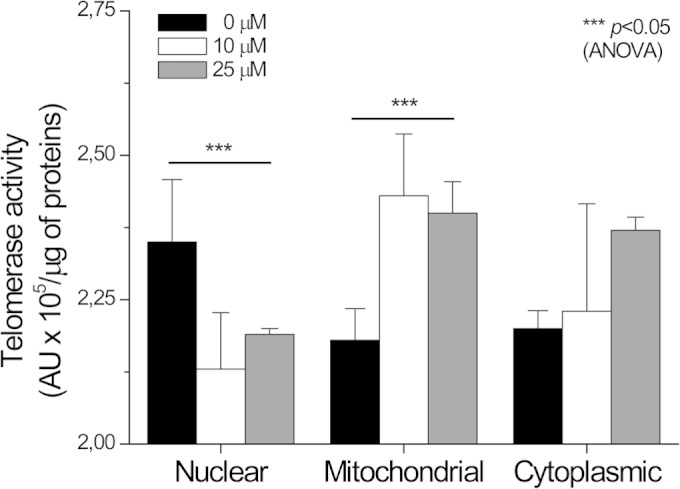
Redistribution of telomerase enzyme activity after H2O2 treatment. Telomerase enzyme activity was measured in L. major nuclear, mitochondrion-enriched, and cytoplasmic fractions after exposing cells for 1 h to 10 or 25 μM H2O2. Data are means ± SEMs of three independent measurements. Telomerase activity values for nuclear and mitochondrion-enriched fractions from treated cells show statistically significant difference from untreated cell fractions (analysis of variance [ANOVA]).
FIG 4.
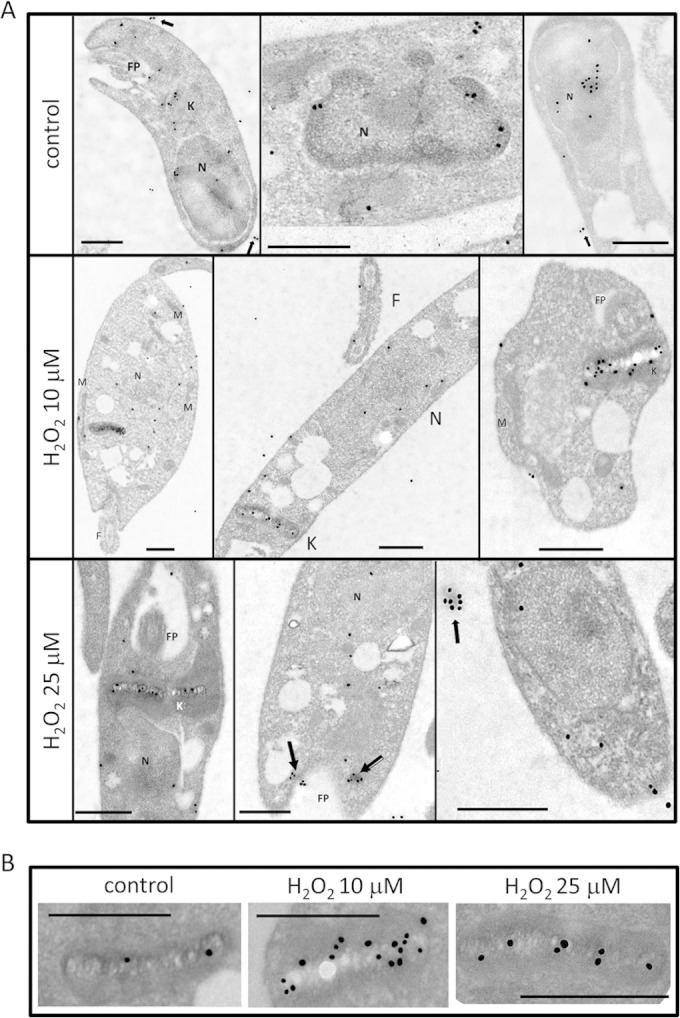
Immunodetection of TERT in L. major promastigotes by TEM. (A) EM images of L. major cells after incubation with anti-TEL-1 as the first antibody and anti-rabbit labeled with 25-nm gold particles as the secondary antibody. The top row shows control promastigotes, the middle row shows promastigotes treated with 10 μM H2O2, and the bottom row shows parasites treated with 25 μM H2O2. Hydrogen peroxide treatment was followed for 2 h before cells were processed for EM. N, nuclei; K, kinetoplast; M, mitochondria; FP, flagellar pocket. Arrows indicate LmTERT localized in nanovesicles. (B) Englarged view of kinetoplasts in control and H2O2-treated parasites. Bars in each image are 0.5 μm.
When Leishmania cells were treated with 10 μM H2O2, we observed a 50% reduction of gold particles within the nuclei, a slight increase associated with the kinetoplast, but a higher number in the whole mitochondrial compartment (Table 2 and Fig. 4B). At 25 μM H2O2, the number of gold particles within the nuclei decreased even further, and there was little variation in the kinetoplast and a decrease in the whole mitochondrial compartment. When we examined the number of gold particles in other cell compartments and in nanovesicles, we found an increase when the H2O2 concentration went from 0 to 25 μM (Table 2 and Fig. 4). Beyond 25 μM H2O2, nuclear telomerase activity decreased even further and there were very few gold particles associated either with the nuclei or the mitochondria (data not shown).
TABLE 2.
Gold particle distribution in Leishmania major promastigotes under control conditions and treated with H2O2a
| Location | No. of gold particles (mean ± SD) under indicated condition |
||
|---|---|---|---|
| Control | Treatment with 10 μM H2O2 | Treatment with 25 μM H2O2 | |
| Nucleus | 9.25 ± 1.90 | 4.25 ± 1.85 | 1.57 ± 0.20 |
| Kinetoplast | 3.66 ± 0.90 | 3.25 ± 1.04 | 3.00 ± 0.35 |
| Mitochondrion | 3.00 ± 0.50 | 4.77 ± 0.46 | 1.79 ± 0.27 |
| Flagellum | 3.05 ± 1.25 | 3.06 ± 1.02 | 2.19 ± 0.30 |
| Membrane | 2.33 ± 0.30 | 3.33 ± 0.55 | 4.05 ± 1.20 |
| V/FP/C | 1.25 ± 0.75 | 1.75 ± 0.47 | 3.21 ± 0.32 |
| Nanovesicles | 1.50 ± 0.60 | 4.50 ± 0.90 | 7.11 ± 0.70 |
Cell slices were first incubated with anti-TEL-1 antibodies and then with anti-rabbit antibodies labeled with 25-nm gold particles. Sixty-five cells were examined for each condition. V, vesicles; FP, flagellar pocket; C, cytoplasm.
Experiments of binding TERT to kDNA.
To answer the question of whether TERT is able to recognize naked kDNA, we isolated this DNA from promastigotes (Fig. 5A) and incubated it with LmTERT protein produced in E. coli cells (see Fig. S4 in the supplemental material). As shown in spots 1 and 5 in Fig. 5C, recombinant LmTERT protein strongly bound to purified kDNA in a dose-response way; it also showed binding to total gDNA in a dose-response way (spots 2 and 4) but at least three times less than the kDNA interaction, and it did not interact with 5 μg of purified bacterial plasmid pGEX5X2. The mcDNA fraction liberated from the kDNA network by EcoRI digestion (Fig. 5B) showed less capacity for binding to recombinant TERT than the intact kDNA. We also evaluated interaction of LmTERT with 5 μg of doubly digested gDNA (RsaI plus HaeIII) and 5 μg of intact bacterial plasmid pGEM3zF or linearized with BamHI endonuclease, and we found that TERT recombinant protein did not interact with either of these samples.
FIG 5.

DNA-protein binding assays with LmTERT expressed in bacterial cells. (A) Agarose gel electrophoresis showing kDNA and genomic DNA (gDNA) isolated from Leishmania cells after digestion with endonucleases. Lane 1, 1 μg of gDNA fully digested with PstI and HindIII; lane 2, 1 μg undigested kDNA network trapped in the gel's pocket. (B) Agarose gel electrophoresis showing 2 μg of kDNA digested with EcoRI endonuclease (lane 1) and 0.2 μg of EcoRI mcDNA purified from the gel (lane 2). The arrow points to the minicircle bands ranging from 0.8 to 1 kbp. (C) Recombinant LmTERT-DNA binding assay. DNA samples were eluted from agarose blocks (A and B), spotted, linked to nylon positively charged membranes, and incubated with recombinant LmTERT (1 μg/ml). Spots were revealed with anti-TEL-1 antibody. Spots are as follows: 1, 5 μg of total kDNA; 2, 5 μg of gDNA; 3, 10 μg of purified minicircle DNA; 4, 10 μg of gDNA; 5, 10 μg of kDNA; and 6, 5 μg of purified pGEX5X2 plasmid DNA (encircled).
TERT overexpression in Leishmania cells caused an increase in cell growth rate and resistance to H2O2.
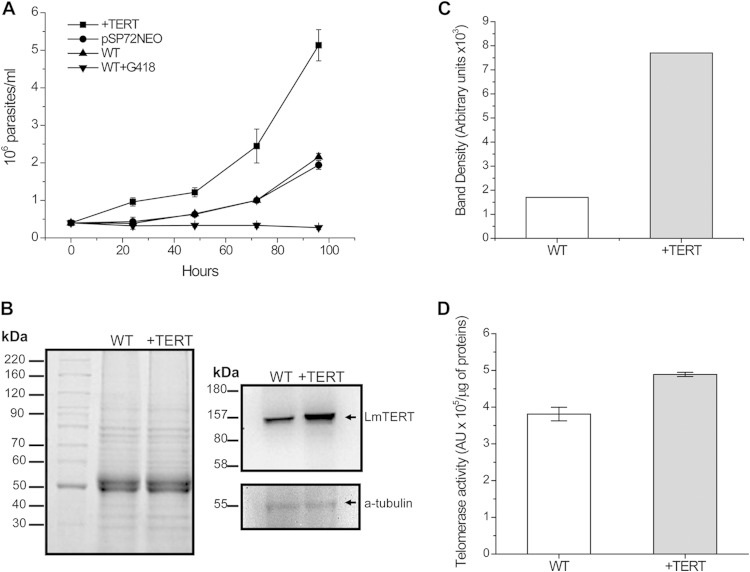
Next, to determine whether an increase in LmTERT protein quantity conferred any advantage on Leishmania cells, we transfected L. major promastigote forms with its homologous TERT gene inserted into Leishmania pSP72NEO expression vector (see Fig. S5 in the supplemental material). As shown in Fig. 6A, pSP72NEO+TERT-transfected cells grew 3-fold faster than the WT cells or cells transfected with pSP72NEO empty vector. Western blot analysis confirmed a 5-fold increase in LmTERT protein compared with that in WT cells (Fig. 6B and C). +TERT cells also showed a slight increase in telomerase enzyme activity compared with that of WT cells (Fig. 6D). When pSP72NEO+TER-transfected cells were exposed to 25 μM H2O2, we observed a decrease of telomerase activity and LmTERT protein quantity in the nuclear fraction and the opposite in the mitochondrial fraction, where we scored at least a 2-fold increase. Telomerase activity in cytoplasmic fraction did not show any significant variation (Fig. 7A and B). Finally, we calculated the 50% inhibitory concentration (IC50) for H2O2 in pSP72NEO+TERT-transfected and WT cells. Transfected cells displayed a higher IC50 (1.68 ± 0.03 mM) than did WT cells (1.33 ± 0.07 mM) (Fig. 7C).
FIG 6.
Effect of LmTERT overexpression in L. major cell growth. (A) Growth curves of L. major: wild-type cells (WT) with and without Geneticin (G418), Leishmania cells transfected with empty expression vector pSP72NEO, and L. major overexpressing TERT (+TERT). (B) SDS-PAGE of total protein extracts from L. major WT and L. major +TERT. A total of 4 × 106 cells were used in each case. Also shown is Western blot analysis of each extract with anti-TEL-1 antibody. As a loading control, the membrane was stripped and treated again with α-tubulin antibody. (C) Relative content of LmTERT protein estimated by densitometric analysis of the Western blot bands. (D) Telomerase specific activity assayed in L. major WT versus L. major +TERT. Data are the integrated areas of the telomerase products in AU per microgram of protein from three independent experiments.
FIG 7.
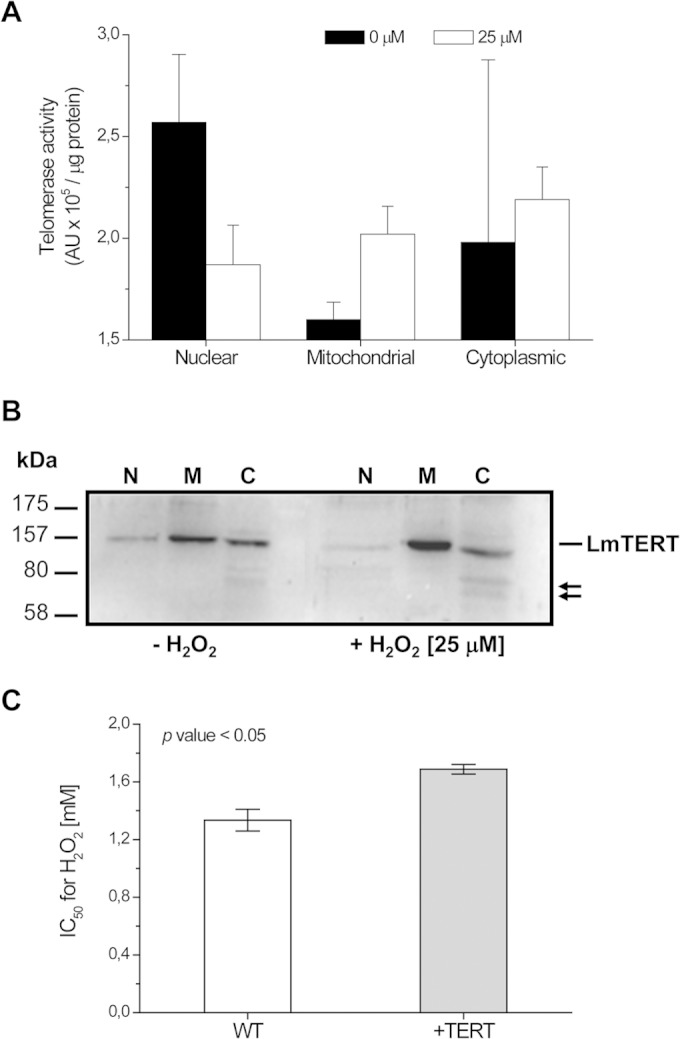
Redistribution of telomerase enzyme activity and TERT in L. major transfected cells after treatment with 25 μM H2O2, and viability test in the presence of H2O2. (A) Telomerase enzyme activity was assayed in nuclear, mitochondrion-enriched, and cytoplasmic protein fractions of L. major +TERT cells not treated or treated with 25 μM H2O2 for 1 h. (B) Telomerase Western blots of nuclear (N), mitochondrial (M), and cytoplasmic (C) fractions from control cells or cells treated with 25 μM H2O2. Arrows point to lower-molecular-mass bands likely associated with LmTERT degradation. (C) H2O2 IC50 in wild-type L. major promastigotes (WT) and TERT-overexpressing parasites (+TERT). L. major +TERT cells showed a higher tolerance than WT cells to increasing H2O2 concentrations. Data are the results of three independent experiments. Differences between IC50s for WT and +TERT cells are significant, with a P value of <0.05 (Student t test).
DISCUSSION
Characteristics of L. major telomerase.
L. major's telomerase activity was first characterized by Cano and coworkers (7). In their work, they noticed a low processivity for this enzyme compared with their Trypanosoma brucei and Leishmania tarentolae counterparts. In a more recent work, we showed that a high activity in L. major telomerase can be obtained when the assay conditions are adjusted, including specific extension primers and heating of the nuclear extracts prior to the assay reaction (9). The LmTERT subunit is encoded by a single gene located at chromosome 36 of this parasite (32); the size of its ORF corresponds to 1,451 amino acids (aa), which is larger than for the Trypanosoma brucei or Trypanosoma cruzi counterparts (approximately 1,198 aa) or the human protein (1,132 aa). Despite this size disparity, LmTERT protein has a high level of preservation in key amino acid (see Fig. S1 in the supplemental material) residues compared with human TERT (8). LmTERT, like TERT in other lower eukaryotes, lacks a canonical mitochondrial import signal (15). The TER subunit of L. major has not been sequenced, but the size of this RNA in another trypanosomatids, namely, T. brucei, has been estimated as 900 nucleotides, which is within the range of the budding yeast Kluyveromyces (930 nt) and Saccharomyces cerevisiae (1,300 nt) TER (13).
Telomeric and subtelomeric organization of Leishmania.
Leishmania has a unique telomeric and subtelomeric structure in which TTAGGG repeats can be found mixed with other sequences followed by long stretches of the TTAGGG repeats at the telomeric terminus (33, 34). This atypical telomeric structure led Fu and Barker (33) to propose a telomerase-free sequence slippage mechanism for the replenishment of telomeres. Nonetheless, the report of a telomerase activity in L. major by Cano et al. (7) suggests that if such a mechanism exists, it may not be the primary source for telomere recovery in Leishmania.
Subcellular localization of L. major telomerase.
The first report that telomerases can be found in the mitochondrial compartment was made by Hertzog-Santos et al. (15), who noted an increase of TERT relocated in this compartment when cells were subjected to oxidative stress. Haendeler et al. (18) reported that when TERT was overexpressed in mouse cells, it bound exclusively to two regions of mitochondrial DNA (mtDNA) containing the coding sequences for NADH ubiquinone oxidoreductase (complex I) subunits 1 and 2 (ND1 and ND2). However, more recently, Sharma et al. (35) found a less specific binding of TERT to mtDNA including the coding regions for 12S and 16S rRNAs, ND1, -2, -4, and -5, COX I and III, tRNAs, and subunits 6 and 8 of the ATP synthase. The multiple locations for a given protein have been called “eclipsed distribution” by Regev-Rudzki and Pines (36). In cancer cells the number of telomerase molecules has been estimated as 15 to 20 molecules per nucleus (37), and since it is assumed that the number in the mitochondrial compartment is smaller, the observation of TERT by microscopic methods is expected to be very difficult. Nonetheless, Sharma et al. (35) estimated that under normal conditions, around 10 to 20% of all TERT molecules are present in mammalian mitochondria.
When we searched for total telomerase activity in the Leishmania mitochondrial fraction, we registered a significant activity that was half of the nuclear fraction, and the mitochondrial location was further confirmed by TEM analysis using anti-Leishmania telomerase antibodies and immunogold techniques. In a rough estimation of LmTERT particles (Table 2), we observed twice the number of gold particles in the nuclei as in the mitochondria, suggesting that LmTERT is a normal resident of Leishmania mitochondria; this association mostly occurs with the kDNA.
Western blot experiments (Fig. 2A) revealed that when equal amounts of protein were loaded on the PAGE-SDS gels, the protein band corresponding to the mitochondrial fractions was six times bigger than the one of the nuclear fraction, although the total protein content and total telomerase activity were higher in the nuclear fraction (Table 1).
Binding assays using LmTERT protein expressed in E. coli cells (devoid of TER) confirmed the preference of this protein by kDNA. What TERT and the telomerase holoenzyme are doing at the mitochondria is not clear. It has been reported that in human cells TERT is able to interact with an RNA component of mitochondrial RNA processing endoribonuclease (RMRP), creating a new activity that synthetizes RNA in an RNA-dependent manner and generating a double-stranded RNA (dsRNA). This dsRNA is further processed by the RNA interference machinery (38). A different report by Sharma et al. (35) claims that TERT associates with mitochondrial tRNAs to generate a new reverse transcriptase activity. If the interaction of LmTERT with kDNA has a physiological significance, what is its role in Leishmania mitochondria? First, Leishmania major lacks RNAi machinery; therefore, it is unlikely that LmTERT has the dsRNA synthesizing capability previously mentioned. However, we cannot rule out that this LmTERT associates with mitochondrial tRNAs to generate new DNA polymerase activities. It is important to note that the kDNA complex consists of a large disc-shaped structure containing around 10,000 interlocked minicircles and around 20 maxicircles with the characteristics of mitochondrial DNA (39, 40). The DNA minicircles are transcribed into guide RNAs that participate in the important function of RNA editing (41). This process completes the information encrypted in the mitochondrial mRNAs, allowing the production of essential mitochondrial proteins. To our knowledge, though, LmTERT has not been described as a component of the editosome machinery (42, 43). Another possibility is that LmTERT may play a role in the kDNA replication, but again, it has not been found among the proteins that participate in this process (44). A third possibility is that the LmTERT role, similar to what has been found in mouse cells by Haendeler et al. (18), is to protect kDNA integrity against oxidative or UV stress: without the correct annealing and guiding process during mitochondrial RNA editing, the respiratory system of the parasite would fail. However, a great deal of experimental work is necessary to elucidate the role of LmTERT in Leishmania mitochondria.
Effect of oxidative stress.
When Leishmania cells were treated with low doses of H2O2, there was a clear reduction of gold particles associated with the nuclei and an increase in telomerase activity in the mitochondrial compartment. This is similar to the findings reported by Amhed et al. (17) and Haendeler et al. (18), and they may be related to protection of the mitochondrial DNA or antiapoptotic activity. Also, Sharma et al. (35) showed that a lack of TERT has a negative impact on mitochondrial function.
The presence of telomerase activity in the cytoplasm is partly due to a transient TERT protein being synthesized at the endoplasmic reticulum; however, we also detected it in 100-nm nanovesicles, some of which were found outside the Leishmania cell, and the question is whether this means that telomerase is also secreted as a stress response to protect cells from free reactive oxygen species (ROS) radicals or is just released during cell destruction caused by H2O2. In this regard, in mouse mast cells exposed to oxidative stress it has been observed, with a different kind of proteins, that NV produced in one cell can influence the response of other cells by providing recipient cells with resistance against oxidative stress (45).
Effect of overexpression of TERT in L. major promastigote forms.
So far, we have shown that oxidative stress produces an increase of LmTERT protein in the mitochondrial and cytoplasmic fractions of L. major and that it could be associated with a cell protection strategy. But does a higher level of LmTERT enhance fitness in the Leishmania cells? To answer this question, we transfected L. major cells with its homologous TERT; the transfected cells showed a 3-fold-higher growth rate and a 5-fold increase in LmTERT protein quantity. In addition, transfected cells exhibited more resistance to oxidative stress induced by H2O2 than WT cells. Therefore, this evidenced that an increment of LmTERT protein helps cells to be better adapted to an in vitro growth environment even under stress conditions. This behavior has also been described for mouse embryonic stem cells, in which overexpression of TERT protein confers stress resistance and improved antioxidant defense (46). Also, TERT overexpression promotes proliferation of resting stem cells in the skin epithelium in a noncanonical pathway (47). Perhaps LmTERT, independently of its nuclear functions, is part of the SOS response of Leishmania. Finally, the potentially new roles of TERT in the parasite cell open new opportunities for the development of more efficient drugs to treat leishmaniasis.
Supplementary Material
ACKNOWLEDGMENTS
This work was financed by grant POA IDEA 0013 to J.L.R., a Spanish Agency of Cooperation grant (A/032068/10), and Regional Government of Andalusia grant JJAA 2011 to A. Osuna. Publication costs for this work are sponsored by UNU-BIOLAC.
Thanks are due to Sharon Sumpter for revising the English of the manuscript and Gustavo Benaim for the mitochondrial integrity assay.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02269-14.
REFERENCES
- 1.Watson JD. 1972. Origin of concatemeric T7 DNA. Nat New Biol 239:197–201. doi: 10.1038/newbio239197a0, . [DOI] [PubMed] [Google Scholar]
- 2.Greider CW, Blackburn EH. 1989. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 3.Martínez P, Blasco MA. 2010. Role of shelterin in cancer and aging. Aging Cell 9:653–666. doi: 10.1111/j.1474-9726.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt HD, West SC, Beattie TL. 2010. InTERTpreting telomerase structure and function. Nucleic Acids Res 38:5609–5622. doi: 10.1093/nar/gkq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J-L, Greider CW. 2004. An emerging consensus for telomerase RNA structure. Proc Natl Acad Sci U S A 101:14683–14684. doi: 10.1073/pnas.0406204101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiodi L, Mondello Ch. 2012. Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria. Front Oncol 2:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano MI, Dungan JM, Blackburn EH. 1999. Telomerase in kinetoplastid parasitic protozoa. Proc Natl Acad Sci U S A 96:3616–3621. doi: 10.1073/pnas.96.7.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giardini MA, Lira CB, Conte FF, Camillo LR, Siquiera JL, Ramos CH, Cano MI. 2006. The putative telomerase reverse transcriptase component of Leishmania spp.: gene cloning and characterization. Parasitol Res 98:447–454. doi: 10.1007/s00436-005-0036-4. [DOI] [PubMed] [Google Scholar]
- 9.Galindo MM, Rodriguez E, Rojas MG, Figarella K, Campelo R, Ramírez JL. 2009. A heat-activated and thermoresistant telomerase activity in Leishmania major Friedlin. Acta Trop 111:86–89. doi: 10.1016/j.actatropica.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz DP, Collins K. 2004. Biochemical properties of Trypanosoma cruzi telomerase. Nucleic Acids Res 32:5214–5222. doi: 10.1093/nar/gkh864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campelo R, Galindo MM, Ramírez JL. 2011. Characterization of Trypanosoma cruzi telomerase. Acta Trop 120:173–178. doi: 10.1016/j.actatropica.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Dreesen O, Li B, Cross GAM. 2005. Telomere structure and shortening in telomerase-deficient Trypanosoma brucei. Nucleic Acids Res 33:4536–4543. doi: 10.1093/nar/gki769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandhu R, Sanford S, Basu S, Park M, Pandya UM, Li B, Chakrabarti K. 2013. A trans-spliced telomerase RNA dictates telomere synthesis in Trypanosoma brucei. Cell Res 23:537–551. doi: 10.1038/cr.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung HK, Cheong C, Song J, Lee H. 2005. Extratelomeric functions of telomerase. Curr Mol Med 5:233–241. doi: 10.2174/1566524053586635. [DOI] [PubMed] [Google Scholar]
- 15.Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B. 2004. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 3:399–411. doi: 10.1111/j.1474-9728.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 16.Santos JH, Meyer JN, VanHouten B. 2006. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum Mol Gen 15:1757–1768. doi: 10.1093/hmg/ddl098. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S, Passos JF, Birket M, Beckmann T, Brings S, Peters H, Birch-Machin MA, Von Zglinicki Saretzki TG. 2008. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci 121:1046–1053. doi: 10.1242/jcs.019372. [DOI] [PubMed] [Google Scholar]
- 18.Haendeler J, Drose S, Buchner N, Jakob S, Altschmied J, Goy C, Spyrodopoulos I, Zeiher AM, Brandt U, Dimmeler S. 2009. Mitochondrial telomerase reverse transcriptase binds and protects mitochondrial DNA and function from damage. Arterioscler Thromb Vasc Biol 29:929–935. doi: 10.1161/ATVBAHA.109.185546. [DOI] [PubMed] [Google Scholar]
- 19.Gordon DM, Hertzog-Santos J. 2010. The emerging role of telomerase reverse transcriptase in mitochondrial DNA metabolism. J Nucleic Acids 2010:390791. doi: 10.4061/2010/390791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benaim G, Casanova P, Hernandez-Rodriguez V, Mujica-Gonzalez S, Parra-Gimenez N, Plaza-Rojas L, Concepcion J, Liu Y, Oldfield E, Paniz-Mondolfi A, Suarez A. 2014. Dronedarone, an amiodarone analog with improved anti-Leishmania mexicana efficacy. Antimicrob Agents Chemother 58:2295–2303. doi: 10.1128/AAC.01240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AR, Craciunescu CN, Guo Z, Teng Y, Thresher RJ, Blusztajn JK, Zeisel SH. 2010. Deletion of murine choline dehydrogenase results in diminished sperm motility. FASEB J 24:2752–2761. doi: 10.1096/fj.09-153718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Piatyszek M, Prowse K, Harley C, West M, Ho P, Coviello G, Wright W, Weinrich S, Shay J. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 23.Thery C, Amigorena S, Raposo G, Clayton A. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 30:3.22:3.22.1–3.22.29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 24.Cruz-Bustos T, Gonzalez-Gonzalez G, Morales-Sanfrutos J, Megia-Fernadez A, Santoyo-Gonzales F, Osuna A. 2012. Functionalization of immunostimulating complexes (ISCOMs) with lipid vinyl sulfones and their application in immunological techniques and therapy. Int J Nanomedicine 7:5941–5956. doi: 10.2147/IJN.S35556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed, vol 1 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 27.Papadopoulou B, Roy G, Ouellette M. 1994. Autonomous replication bacterial DNA plasmid oligomers in Leishmania. Mol Biochem Parasitol 65:39–49. doi: 10.1016/0166-6851(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 28.Beverley SM, Clayton CE. 1993. Transfection of Leishmania and Trypanosoma brucei by electroporation. Methods Mol Biol 21:333–348. [DOI] [PubMed] [Google Scholar]
- 29.Denizot F, Lang R. 1986. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:271–277. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro TA, Klein VA, Englund PT. 1999. Isolation of kinetoplast DNA. Methods Mol Biol 94:61–67. [DOI] [PubMed] [Google Scholar]
- 31.Karnovsky MJ. 1965. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137–138. [Google Scholar]
- 32.Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, Anupama A, Apostolou Z, Attipoe P, Bason N, Bauser C, Beck A, Beverley SM, Bianchettin G, Borzym K, Bothe G, Bruschi CV, Collins M, Cadag E, Ciarloni L, Clayton C, Coulson RM, Cronin A, Cruz AK, Davies RM, De Gaudenzi J, Dobson DE, Duesterhoeft A, Fazelina G, Fosker N, Frasch AC, Fraser A, Fuchs M, Gabel C, Goble A, Goffeau A, Harris D, Hertz-Fowler C, Hilbert H, Horn D, Huang Y, Klages S, Knights A, Kube M, Larke N, Litvin L, Lord A, Louie T, Marra M, Masuy D, Matthews K, Michaeli S, Mottram JC, Müller-Auer S, Munden H, Nelson S, Norbertczak H, Oliver K, O'Neil S, Pentony M, Pohl TM, Price C, Purnelle B, Quail MA, Rabbinowitsch E, Reinhardt R, Rieger M, Rinta J, Robben J, Robertson L, Ruiz JC, Rutter S, Saunders D, Schäfer M, Schein J, Schwartz DC, Seeger K, Seyler A, Sharp S, Shin H, Sivam D, Squares R, Squares S, Tosato V, Vogt C, Volckaert G, Wambutt R, Warren T, Wedler H, Woodward J, Zhou S, Zimmermann W, Smith DF, Blackwell JM, Stuart KD, Barrell B, Myler PJ. 2005. The genome of kinetoplastid parasite Leishmania major. Science 309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu G, Barker DC. 1998. Characterization of Leishmania telomeres reveals unusual telomeric repeats and conserved telomere-associated sequence. Nucleic Acids Res 26:2161–2167. doi: 10.1093/nar/26.9.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiurillo MA, Beck AE, Devos T, Myler PJ, Stuart K, Ramirez JL. 2000. Cloning and characterization of Leishmania donovani telomeres. Exp Parasitol 94:248–258. doi: 10.1006/expr.2000.4499. [DOI] [PubMed] [Google Scholar]
- 35.Sharma NK, Reyes A, Green P, Caron MJ, Bonini MG, Gordon DM, Holt IJ, Hertzog Santos J. 2012. Human telomerase acts as an hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res 40:712–725. doi: 10.1093/nar/gkr758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regev-Rudzki N, Pines O. 2007. Eclipsed distribution: a phenomenon of dual targeting of protein and its significance. Bioessays 29:772–782. doi: 10.1002/bies.20609. [DOI] [PubMed] [Google Scholar]
- 37.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. 2007. Protein composition of catalytically active human telomerase from immortal cells. Science 315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 38.Maida Y, Yasukawa M, Furuuchi M, Lassmann T, Possemato R, Okamoto N, Kasim V, Hayashizaki Y, Hahn WC, Masutomi K. 2009. An RNA dependent RNA polymerase formed by hTERT and the RNase MRP RNA. Nature 461:230–235. doi: 10.1038/nature08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson L. 1986. Kinetoplast DNA in tripanosomatid flagellates. Int Rev Cytol 99:119–179. doi: 10.1016/S0074-7696(08)61426-6. [DOI] [PubMed] [Google Scholar]
- 40.Lee ST, Liu HY, Lee SP, Tarn C. 1994. Selection of arsenite resistance causes reversible changes in minicircle composition and kinetoplast organization in Leishmania mexicana. Mol Cell Biol 14:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feagin JE, Abraham J, Stuart K. 1988. Extensive editing of the cytochrome c oxidase III transcript in Trypanosoma brucei. Cell 53:413–422. [DOI] [PubMed] [Google Scholar]
- 42.Stuart K, Schnaufer A, Ernst NL, Panigrahi AK. 2005. Complex management: RNA editing in trypanosomes. Trends Biochem Sci 30:97–105. doi: 10.1016/j.tibs.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Lerch L, Carnes J, Acestor N, Guo X, Schnaufer A, Stuart K. 2012. Editosome accessory factors KREPB9 and KREPB10 in Trypanosoma brucei. Eukaryot Cell 11:832–843. doi: 10.1128/EC.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen RE, Englund PT. 2012. Network news: the replication of kinetoplast DNA. Annu Rev Microbiol 66:473–491. doi: 10.1146/annurev-micro-092611-150057. [DOI] [PubMed] [Google Scholar]
- 45.Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J. 2010. Exosomes communicate protective messages during oxidative stress: possible role of exosomal shuttle RNA. PLoS One 5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armstrong L, Saretzki G, Peters H, Wappler I, Evans J, Hole N, von Zglinicki T, Lako M. 2005. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic linage. Stem Cells 23:516–529. doi: 10.1634/stemcells.2004-0269. [DOI] [PubMed] [Google Scholar]
- 47.Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE. 2005. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.