Abstract
Targeting antigens (Ag) to Fcγ receptors (FcγR) intranasally (i.n.) enhances immunogenicity and protection against intracellular and extracellular pathogens. Specifically, we have demonstrated that targeting fixed (inactivated) Francisella tularensis (iFT) organisms to FcR in mice i.n., with MAb-iFT immune complexes, enhances F. tularensis-specific immune responses and protection against F. tularensis challenge. Furthermore, traditional adjuvant is not required. In addition, we have demonstrated that the increased immunogenicity following the targeting of iFT to FcR is due, in part, to enhanced dendritic cell (DC) maturation, enhanced internalization, and processing and presentation of iFT by DCs, as well as neonatal FcR (FcRn)-enhanced trafficking of iFT from the nasal passage to the nasal mucosa-associated lymphoid tissue (NALT). Using this immunization and challenge model, we expanded on these studies to identify specific in vivo immune responses impacted and enhanced by FcR targeting of iFT i.n. Specifically, the results of this study demonstrate for the first time that targeting iFT to FcR increases the frequency of activated DCs within the lungs of MAb-iFT-immunized mice subsequent to F. tularensis LVS challenge. In addition, the frequency and number of gamma interferon (IFN-γ)-secreting effector memory (EM) CD4+ T cells elicited by F. tularensis infection (postimmunization) is increased in an interleukin 12 (IL-12)-dependent manner. In summary, these studies build significantly upon previously published work utilizing this vaccine platform. We have identified a number of additional mechanisms by which this novel, adjuvant-independent, FcR-targeted mucosal vaccine approach enhances immunity and protection against infection, while further validating its potential as a universal vaccine platform against mucosal pathogens.
INTRODUCTION
The limited success of immunization against mucosal pathogens and the lack of effective and safe adjuvants highlight the urgent need for mucosal vaccines. As a result, researchers have utilized novel strategies to target antigens (Ags) to receptors expressed on the surfaces of antigen-presenting cells (APCs), such as dendritic cells (DCs) and macrophages, in an effort to more effectively activate the mucosal immune system and elicit robust and protective immune responses (1). Importantly, in this regard, we have previously shown that targeting fixed (inactivated) Francisella tularensis (iFT) to FcR intranasally (i.n.), via the formation of monoclonal antibody (MAb)-iFT immune complexes (ICs), generated enhanced protection against lethal respiratory challenge with F. tularensis live vaccine strain (LVS) and the category A F. tularensis agent SchuS4 (2). This protection was dependent upon the expression of FcγR and the neonatal Fc receptor (FcRn), as immunization of FcγR- or FcRn-deficient mice, or i.n. administration of F(ab′)2 MAb-iFT ICs, abrogated protection (2). Furthermore, protection was not mediated by the administration of the antilipopolysaccharide (anti-LPS) IgG2a antibody alone (2). In a different study using the same vaccine platform, targeting the pneumococcal protective Ag, PspA, to human FcγRI in a human FcγRI transgenic mouse model also elicited enhanced protection against Streptococcus pneumoniae challenge (3).
In regard to the mechanisms involved in FcR-enhanced immune protection following i.n. immunization with MAb-iFT ICs, we have recently demonstrated that iFT presentation to F. tularensis-specific T cells is significantly enhanced. This is, in part, due to the increased binding and internalization of iFT by APCs (4). Furthermore, targeting iFT to Fc receptors enhances DC activation and maturation in vitro and also extends the period over which antigen-loaded APCs stimulate T cells (4, 5). Lastly, we have also shown that targeting iFT to FcR i.n. also enhances trafficking of iFT Ag from the nasal passage to the nasal mucosa-associated lymphoid tissue (NALT) (4). Nevertheless, questions remain regarding the in vivo impact of MAb-iFT immunization and whether DC activation and T cell priming also occur in vivo when utilizing this FcR-targeted vaccine strategy.
In this study, we have expanded on our previous work utilizing this vaccine platform by examining the effect of FcR-targeted mucosal vaccination on DC activation and memory CD4+ T cell formation in vivo during lethal challenge with F. tularensis LVS. For the first time, we show that FcR targeting increases the frequency and activation status of DCs in the lungs of immunized mice and mediates the generation of F. tularensis-specific, gamma interferon (IFN-γ)-secreting, effector memory (EM) CD4+ T cells during infection, thus further elucidating the immunological mechanisms involved in enhanced immune protection utilizing this novel mucosal vaccine platform.
MATERIALS AND METHODS
Mice and bacteria.
C57BL/6 mice were purchased from Taconic Laboratories (Hudson, NY), and B6.129S1-Il12atm1Jm/J mice (interleukin 12p35 [IL-12p35]-deficient mice) were purchased from Jackson laboratories (Bar Harbor, ME). All mice were housed at the Animal Resources Facility at Albany Medical College. The mice were used at 6 to 10 weeks of age. All protocols were reviewed and approved by the Albany Medical College Ethics Committee, utilizing NIH standards.
F. tularensis LVS (ATCC 29684; American Type Culture Collection) was provided by K. Elkins (U.S. Food and Drug Administration, Bethesda, MD).
Antibodies.
Mouse IgG2a anti-F. tularensis LPS MAb used to generate MAb-iFT immune complexes was purchased from Fitzgerald (catalog number 10-F02, clone number M0232621; Acton, MA). The following flow cytometry antibodies were purchased from BD Biosciences (San Jose, CA): anti-CD3 (fluorescein isothiocyanate [FITC]), anti-CD4 (allophycocyanin [APC]), anti-CD44 (phycoerythrin [PE]), anti-CD44 (FITC), anti-CD62L (peridinin chlorophyll protein [PerCP] Cy5.5) anti-CCR7 (PE-Cy7), anti-CD11c (APC), anti-DEC-205 (PerCP Cy5.5), anti-B7.1 (PE), anti-B7.2 (PE), anti-major histocompatibility complex (anti-MHC) class II (FITC), and anti-IFN-γ (PE). For neutralizing IL-12p35 in vivo, rat IgG2a anti-mouse IL-12 (anti-mIL-12; clone C18.2) and its isotype control were purchased from eBioscience (San Diego, CA).
Inactivation and labeling of F. tularensis.
iFT LVS was generated by growing F. tularensis LVS in Mueller-Hinton broth (MHB) medium (BD Biosciences) up to a density of 1 × 109 CFU/ml. The culture was then spun down at 22,000 × g for 20 min at 40°C, washed 3 times with phosphate-buffered saline (PBS), resuspended in 2% paraformaldehyde (Sigma), and incubated 2 h at room temperature on a rocker. Bacteria were then washed 3 more times with PBS, and 1 × 109 organisms were plated on a chocolate agar plate (BD Biosciences) and incubated for 7 days at 37°C to confirm inactivation. The final concentration of iFT organisms was determined by optical density (OD) at 610 nm.
MAb-iFT and F(ab′)2-iFT IC generation.
To generate ICs, 1 × 109 iFT organisms were incubated at 34°C overnight on a rocker with 0 or 1 μg/ml of anti-F. tularensis MAb or anti-F. tularensis F(ab′)2 in PBS. Following the incubation, iFT, MAb-iFT, or F(ab′)2-iFT preparations were administered to mice i.n. Generation of immune complexes (ICs) has been previously confirmed by enzyme-linked immunosorbent assay (ELISA) and SDS-PAGE (4).
Immunization and challenge studies.
C57BL/6 and IL-12p35-deficient mice 8 to 12 weeks of age were divided into three groups consisting of 5 or 6 mice/group. Each mouse was immunized on days 0 and 21 with 2 × 107 iFT organisms alone or in the form of ICs [MAb-iFT or F(ab′)2-iFT]. On day 35, the mice were challenged with 20,000 CFU of live F. tularensis LVS. Following challenge, survival was monitored for 21 to 25 days. Exact CFU administered were also verified by culturing and counting the inoculum subsequent to challenge on chocolate agar plates.
Lung WBC isolation.
Lungs of immunized mice were harvested, perfused with cold 1× PBS, shredded into small pieces, and placed in digestion buffer containing RPMI medium (Life Technologies), 0.2 mg/ml of DNase I (Sigma), 0.4 mg/ml of collagenase D (Sigma), and 1 M MgCl2. After a 30-min incubation at 37°C, the digested tissue samples were forced through a cell strainer and the cell suspension obtained was washed and resuspended in RPMI medium containing 2% fetal bovine serum (FBS). The cell suspension was then carefully layered on 5 ml of Lympholyte M (Cedarlane Laboratories, Burlington, NC) and spun down at 15,000 × g for 30 min at room temperature. Following centrifugation, the interface containing the majority of immune cells was obtained and added in RPMI medium with 2% FBS prior to enumeration. In the case of DC isolation, lung leukocytes (WBCs) were cultured in petri dishes containing complete medium at 2 × 106 cells/ml for 1 h at 37°C. Identification and enumeration of dendritic cells were based on the expression of CD11c and DEC-205. These markers are primarily expressed on mouse dendritic cells (6, 7). Nevertheless, a small percentage of alveolar macrophages (3 to 4%) are also positive for these markers (8). Therefore, in order to further increase dendritic cell purity, lung WBCs were cultured for 30 min at 37°C in petri dishes to remove (via adherence) the lung macrophages.
Neutralization of IL-12 in vivo.
Two doses of rat anti-murine anti-IL-12p35 MAb (C18.2) was administered intraperitoneally (i.p.) (500 μg in 250 μl of PBS) on days −1, 0, and 1 after F. tularensis LVS infection. This method has been proven to neutralize IL-12 in vivo for a minimum of 5 days after administration of the IL-12p35 MAb (9).
Flow cytometry.
Splenocytes (SPCs) or lung parenchymal cells were obtained from immunized mice at different time points after F. tularensis LVS infection as described above. For cell surface marker staining, cells were washed with PBS-bovine serum albumin (BSA)-azide, resuspended in blocking buffer (PBS-BSA-azide plus 30 μg/ml of normal mouse IgG [Sigma]), and incubated on ice for 30 min. Cells were then washed with PBS-BSA-azide, and fluorescently labeled antibodies to CD11c, DEC-205, MHC class II, B7.1, B7.2, CD3, CD4, CD44, CD62L, CCR7, or their corresponding isotype controls were added. The cells were then incubated on ice for 30 min, washed, and fixed with 2% paraformaldehyde. Cells were then analyzed by flow cytometry on an LSRII flow cytometer (BD Biosciences).
Intracellular cytokine staining.
Splenocytes from immunized mice were obtained at 2 days after F. tularensis LVS infection and cultured for 5 h at 37°C in the absence or presence of F. tularensis LVS (1 × 103 CFU per 2 × 105 SPCs). For a positive control, SPCs were cultured in complete medium (RPMI medium, 2% FBS, l-glutamine), with 50 ng/ml of phorbol myristate acetate (PMA; Sigma) and 500 ng/ml of ionomycin (Sigma). Cell media also contained protein transport inhibitor with 10 μg/ml of brefeldin A (GolgiPlug; BD Biosciences). Following incubation, the cells were washed with PBS-BSA-azide (containing the protein transport inhibitor), resuspended in blocking buffer, stained with the cell surface marker antibodies for CD3, CD4, and CD44, and fixed with 2% paraformaldehyde as described above.
After fixation, cells were washed, resuspended, and incubated in permeabilization buffer (BD Biosciences) for 15 min at 4°C to facilitate pore formation. Anti-mIFN-γ was diluted in permeabilization buffer and added to each cell sample. Cells were further incubated on ice for 30 min. Following incubation, cells were washed, resuspended in PBS-BSA-azide, and analyzed by flow cytometry on an LSRII flow cytometer (BD Biosciences).
For intracellular cytokine staining of lung DCs, these cells were obtained from immunized mice, as previously described, at 2 days after LVS challenge. DCs were stained for the cell surface markers DEC-205 and CD11, and intracellularly for IL-12, as described above. The results were analyzed by flow cytometry on an LSRII flow cytometer (BD Biosciences).
Cytokine measurements.
C57BL/6 mice were immunized intranasally (i.n.) with PBS, iFT (1 × 109 CFU), or MAb-iFT, boosted on day 21, and challenged on day 35 with 20,000 CFU of iFT LVS. Two days postinfection, lung DCs were obtained from all groups and cultured for 24 h or 48 h with F. tularensis LVS (1 × 103 CFU/well). Supernatants were collected at designated time points, and the levels of IL-12p70 were measured using a BD Biosciences cytometric bead array (CBA) according to the vendor's instructions.
Statistical analysis.
The log rank (Mantel-Cox) test was used for survival curves. One-way analysis of variance (ANOVA) or the unpaired, one-tailed Student t test was used for the remaining figures. GraphPad Prism 4 provided the software for the statistical analysis (San Diego, CA).
RESULTS
Enhanced maturation and activation of DCs in the lungs of mice immunized intranasally with MAb-iFT ICs during F. tularensis LVS infection.
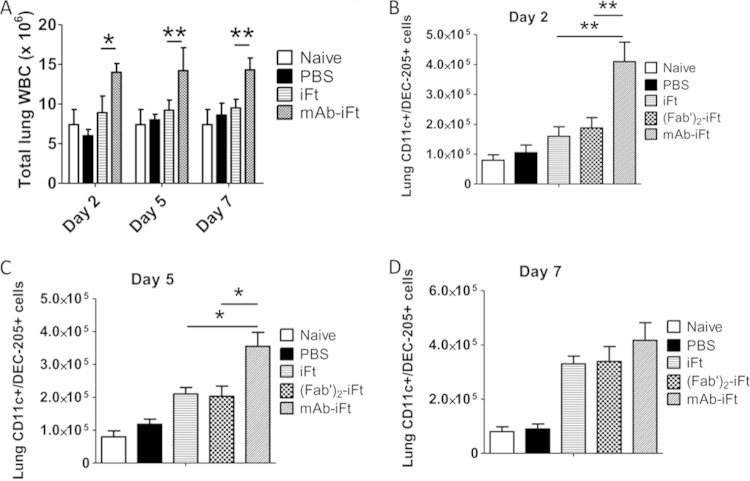
We have previously demonstrated that targeting iFT to FcγRs via the intranasal administration of preformed immune complexes (MAb-iFT ICs) elicits protective responses against lethal challenges with F. tularensis LVS and the category A F. tularensis agent SchuS4 (1). The protection observed was based on survival studies, bacterial burdens, and immunohistochemical analysis of mouse tissues. The protective effect was due to targeting of bacterial antigens to FcR through opsonization with an anti-LPS IgG2a MAb. The antibody alone had no protective effect on mice against F. tularensis challenge (2). Furthermore, we have shown that targeting iFT to FcγRs on APCs mediates internalization, processing, and presentation of the immunogen to an F. tularensis-specific T cell hybridoma in vitro (2, 4). Since DCs play a key role in processing and presenting antigens to T lymphocytes, effectively bridging the innate and adaptive immune systems, we wanted to focus on the effect immunization with MAb-iFT ICs has on the number of activated DCs in the lungs of immunized mice during F. tularensis LVS challenge. For this purpose, C57BL/6 mice were immunized with PBS, iFT, F(ab′)2-iFT, or MAb-iFT and boosted on day 21. On day 35 postimmunization, lungs were harvested and digested, and the DCs were purified using Lympholyte, followed by a 1-h incubation at 37°C on petri dishes to negatively select for the adherent cells, such as macrophages. Following selection, we used the expression of CD11c and DEC-205 markers to define lung DCs (6, 7, 10). All the selected cells that were CD11c+ were also positive for the dectin marker (DEC-205), indicating a DC purity of >98% (data not shown). The activation status of lung DCs was based upon the expression of MHC class II, B7.1, and B7.2, and the analysis was performed by flow cytometry. Interestingly, no significant differences were noted among the three groups of immunized mice (data not shown). In contrast, when immunized mice were infected with a lethal dose of F. tularensis LVS on day 35 postimmunization and their DC profile was analyzed at different time points postinfection, there was found to be a significant difference in the frequency and activation status of lung DCs among the iFT, F(ab′)2-iFT, and MAb-iFT groups. More specifically, on days 2 and 5 postinfection, there was a significantly higher number of CD11c+ DEC-205+ cells in the lungs of MAb-iFT-immunized mice than in those of the mice immunized with iFT alone or F(ab′)2iFT (Fig. 1B and C), potentially indicating increased recruitment of DCs in the lungs during infection, an effect that was dependent upon FcγR targeting. These differences were not significant on day 7 (Fig. 1D). It is also interesting that the overall leukocyte (WBC) cellularity in the lungs of MAb-iFT-immunized mice was significantly increased following F. tularensis LVS challenge (Fig. 1A). On a similar note, the expression (Fig. 2) and absolute numbers (Fig. 3) of CD11c+ cells expressing MHC class II, B7.1, and B7.2 were significantly higher in the MAb-iFT-immunized group than in the group immunized with iFT alone or F(ab′)2-iFT, indicating for the first time that mucosal targeting of an immunogen to FcγR not only enhances the frequency of DCs in the lungs upon subsequent infection but also mediates in vivo maturation and activation of DCs, enabling them to become better antigen-presenting cells.
FIG 1.
Immunization of mice with MAb-iFT immune complexes enhances the number of CD11c+ cells in the lungs of immunized mice following F. tularensis LVS challenge. C57BL/6 mice were immunized i.n. with PBS, iFT (1 × 109 CFU), F(ab′)2-iFT, or MAb-iFT, boosted on day 21, and challenged on day 35 with 20,000 CFU of F. tularensis LVS. On days 2, 5, and 7 postinfection, the lungs of immunized mice were harvested and the white blood cells were obtained and counted (A). Lungs were also harvested from unimmunized and uninfected mice (naive) as a baseline control. The frequency of CD11c+ DEC-205+ cells was measured by flow cytometry and translated into absolute numbers for days 2 (B), 5 (C), and 7 (D) postinfection. Naive mice were neither immunized nor challenged. The P value for iFT versus MAb-iFT in panel A (day 2) was 0.008. The P value for iFT versus MAb-iFT in panel B (day 4) was 0.021. There was no significant difference between the two groups on day 7 after F. tularensis LVS infection (*, P < 0.05; **, P < 0.01).
FIG 2.
Increased frequency of activated CD11c+ dendritic cells in the lungs of mice immunized with MAb-iFT immune complexes and challenged with F. tularensis LVS. C57BL/6 mice were immunized i.n. with PBS, iFT (1 × 109 CFU), F(ab′)2-iFT, or MAb-iFT, boosted on day 21, and challenged on day 35 with 20,000 CFU of F. tularensis LVS. On day 2 after F. tularensis LVS infection, the lungs of immunized mice were harvested and the white blood cells were stained and analyzed by flow cytometry to obtain the expression levels of MHC class II (A), B7.1 (B), and B7.2 (C) on lung dendritic cells (CD11c+ DEC-205+). Three mice were used for each group, and the results are representative of those from three experiments.
FIG 3.
Immunization of mice with MAb-iFT immune complexes increases the number of activated CD11c+ lung dendritic cells following F. tularensis LVS challenge. C57BL/6 mice were immunized and challenged as for Fig. 2. On days 2 and 5 after F. tularensis LVS infection, the lungs of immunized mice were harvested and the white blood cells were obtained and counted. The frequencies of CD11c+ DEC-205+ MHC class II+ (A), CD11c+ DEC-205+ B7.1+ (B), and CD11c+ DEC-205+ B7.2+ (C) cells were translated into absolute cell numbers. Six mice were used for each group, and the results are representative of those from three experiments. *, P < 0.05; **, P < 0.01.
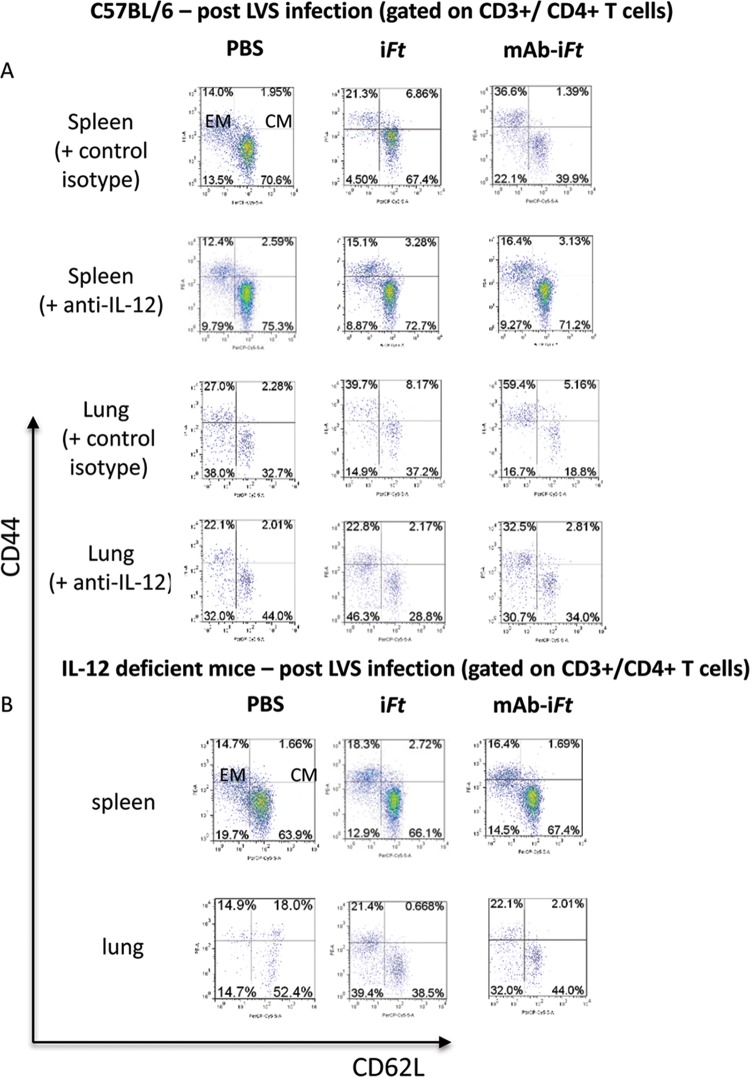
Immunization with MAb-iFT ICs enhances the generation of effector memory CD4+ T cells during F. tularensis LVS challenge.
Memory T cells responses are often required to resolve intracellular bacterial infections; hence, effective vaccines should have the ability to generate long-lasting memory T cell populations. Memory cells are loosely divided into central memory (CM) and effector memory (EM) cells based on their expression of cell surface receptors. More specifically, although both types of memory cells are positive for the adhesion molecule CD44, CM T cells are also positive for CD62L and the chemokine receptor CCR7, while EM T cells lack both (11–13).
We have previously shown that targeting iFT to FcγR via intranasal immunization with ICs generates protection against lethal F. tularensis challenge in an IFN-γ-dependent fashion, indicative of a T cell recall response (2). Having established that intranasal immunization with MAb-iFT ICs increases the number of activated DCs in the lungs during F. tularensis LVS infection, and antigen presentation by DCs can effectively program T lymphocytes to differentiate into memory cells (12), we sought to determine the effect of FcγR targeting on the generation of memory CD4+ T cells. For this purpose, C57BL/6 mice were immunized with PBS, iFT, F(ab′)2-iFT, or MAb-iFT and boosted on day 21. On day 35 postimmunization, the lungs and spleens of immunized mice were harvested and the lymphocytes were stained with the relevant MAb followed by flow cytometric analysis as described in Materials and Methods. In accordance with previous studies, EM CD4+ T cells were characterized as CD3+ CD4+ CD44high CD62Llow CCR7−, while the phenotype of CM memory CD4+ T cells was CD3+ CD4+ CD44high CD62Lhigh CCR7+ (11–13). Interestingly, regardless of the type of immunization, there was no generation of EM CD4+ T cells in the spleens or lungs of immunized mice (Fig. 4A). In fact, similar populations of CM T cells were present in all three groups, regardless of the type of immunogen used (Fig. 4A). Most importantly, though, F. tularensis LVS infection of immunized mice generated a population of EM CD4+ T cells in both the lungs and spleens of mice within 2 days postinfection (Fig. 4B). The levels of EM CD4+ T cells elicited during bacterial challenge were significantly higher in the MAb-iFT-immunized group than in mice immunized with iFT or F(ab′)2-iFT (Fig. 4B). The cells characterized as CM CD4+ T cells were also CCR7+, while the EM CD4 cells had very low levels of CCR7 expression (data not shown).
FIG 4.
Enhanced generation of effector memory CD4+ T cells in the spleens and lungs of mice immunized with MAb-iFT ICs, following F. tularensis LVS challenge. C57BL/6 mice were immunized with PBS, iFT, MAb-iFT, or F(ab′)2-iFT and challenged as described for Fig. 1. On day 35 postimmunization or on day 2 after F. tularensis LVS challenge, the lungs and spleens of immunized mice were harvested and the white blood cells were obtained and counted. Expression levels of CD3+ CD4+ CD44+ CD62L+ CCR7+ T cells postimmunization (A) and postinfection (B), as well as their absolute numbers (C), were analyzed by flow cytometry. The effector memory cells are presented as CD44+ CD62L−, while the central memory cells are presented as CD44+ CD62L+. Three mice were used per group per experiment. The data presented are the averages of nine mice per group. Statistical analyses were carried out using the one-way ANOVA. The P value for iFT versus MAb-iFT in the spleen is 0.006, and that for the lungs is 0.008. The P value for F(ab′)2-iFT versus MAb-iFT in both the spleen and lungs is 0.007 (*, P < 0.05; **, P < 0.01).
The enhancement of the effector T cell population in the MAb-iFT IC-immunized group indicated that targeting antigens to FcγR can drive the generation of effector memory immune responses both locally (lung) and systemically (spleen) during bacterial infection. Mice immunized with F(ab′)2-iFT ICs and then challenged with F. tularensis LVS generated levels of EM CD4+ T cells similar to those generated by the iFT-immunized group, confirming that Fc receptor targeting of the immunogen is essential for this observation (Fig. 4B and C). Importantly, the EM CD4+ T cells generated following Fc receptor targeting of iFT were persistent for at least 25 days after F. tularensis LVS infection (Fig. 4B).
Interestingly, the population of central memory CD4+ T cells was negligible within 2 days following F. tularensis LVS infection in all three groups of mice (Fig. 4B). The qualitative (Fig. 4B) and quantitative (Fig. 4C) enhanced generation of effector versus central memory T cells observed in the MAb-iFT-immunized group suggests that EM CD4+ T cells have a more pivotal role in mediating protection against lethal F. tularensis LVS challenge than their CM counterparts.
We are currently investigating the generation of effector versus central memory CD8+ T lymphocytes in our F. tularensis model since this subpopulation of T cells has also been shown to play an important role in protection against this mucosal bacterial pathogen (14, 15).
IL-12 is necessary for protection against lethal F. tularensis LVS challenge following immunization with MAb-iFT ICs.
The importance of T cells and IFN-γ in protection against F. tularensis LVS infection following immunization with MAb-iFT ICs provides evidence for the generation of a TH1-like protective immune response. It is widely established that IL-12, secreted mainly by innate immune cells, such as DCs and macrophages, drives the differentiation of TH1 effector T cells (16). Thus, we hypothesized that the protective immune responses elicited following targeting of iFT to FcγR are dependent upon IL-12. In order to confirm our hypothesis, IL-12p35-deficient mice, and their control counterparts (C57BL/6), were immunized with either PBS, iFT, or MAb-iFT, boosted on day 21, and infected with a lethal dose of F. tularensis LVS on day 35 postimmunization. We selected mice deficient for the p35 IL-12 subunit (as opposed to p40-deficient mice) due to the role of the p35 gene in the development of TH1 responses (17). In addition, the p40 subunit is not unique to IL-12, as it is also shared by IL-23.
As anticipated, based on our previous studies, 100% of the C57BL/6 mice that were immunized with MAb-iFT ICs survived the F. tularensis LVS challenge, while immunization with iFT alone provided only 50% protection (Fig. 5A). IL-12p35-deficient mice, though, succumbed to infection within 13 days, regardless of the type of immunization, indicating the importance of IL-12 in protection generated by FcR targeting (Fig. 5B). The role of IL-12 in protection against lethal F. tularensis challenge is evident in both the iFT- and MAb-iFT-immunized IL-12p35-deficient mice, as both groups completely succumbed to the infection. This indicates that targeting of our fixed bacterial immunogen to FcR on APCs drives the production of higher levels of IL-12 during F. tularensis LVS challenge than obtained with iFT alone, pointing toward a quantitative versus a qualitative difference between the two immunization approaches. In addition, there were significantly more lung DCs producing IL-12 from MAb-iFT IC-immunized mice following F. tularensis LVS challenge than from challenged mice immunized with iFT alone. The numbers of lung DCs positive for IL-12 from challenged mice immunized with the F(ab′)2-iFT ICs were also comparable to those in the iFT group, indicating that the increase in IL-12 production from lung DCs is FcR-targeting dependent (Fig. 5C). Furthermore, culture of peritoneal cells (PECs) from mice immunized with MAb-iFT and challenged with live F. tularensis LVS (1 × 103 CFU/well) led to significantly higher levels of IL-12p70 secretion than found with PECs from challenged mice immunized with iFT alone (Fig. 5D). These data, collectively, show for the first time the importance of IL-12 in generating protective responses against F. tularensis LVS challenge when utilizing our FcR targeting vaccine model.
FIG 5.
Targeting of iFT to FcR provides protection against F. tularensis LVS challenge in an IL-12-dependent fashion. C57BL/6 (A) or IL-12-deficient (B) mice were immunized i.n. with PBS, iFT (1 × 109 CFU), or MAb-iFT, boosted on day 21, and challenged on day 35 with 20,000 CFU of F. tularensis LVS. Survival was monitored for 25 days postinfection. The P value for iFT versus MAb-iFT in panel A (C57BL/6) was 0.041. There was no significant difference between iFT and MAb-iFT-immunized IL-12-deficient mice (B). (C and D) C57BL/6 mice were immunized i.n. with PBS, iFT (1 × 109 CFU), MAb-iFT, or F(ab′)2-iFT, boosted on day 21, and challenged on day 35 with 20,000 CFU of F. tularensis LVS iFT. Two days postinfection, lung DCs were obtained as previously described and stained intracellularly for IL-12 (C). Peritoneal exudate cells (PECs) were also obtained from all groups and cultured for 24 h or 48 h with F. tularensis LVS (1 × 103 CFU/well). Supernatants were collected at designated time points, and the levels of IL-12p70 were measured by CBA (D). Six mice were used per group, and the statistical analyses were carried out using one-way ANOVA. *, P < 0.05; **, P < 0.01.
IL-12 is required for the generation of effector memory CD4+ T cells upon intranasal immunization with MAb-iFT ICs and subsequent F. tularensis LVS challenge.
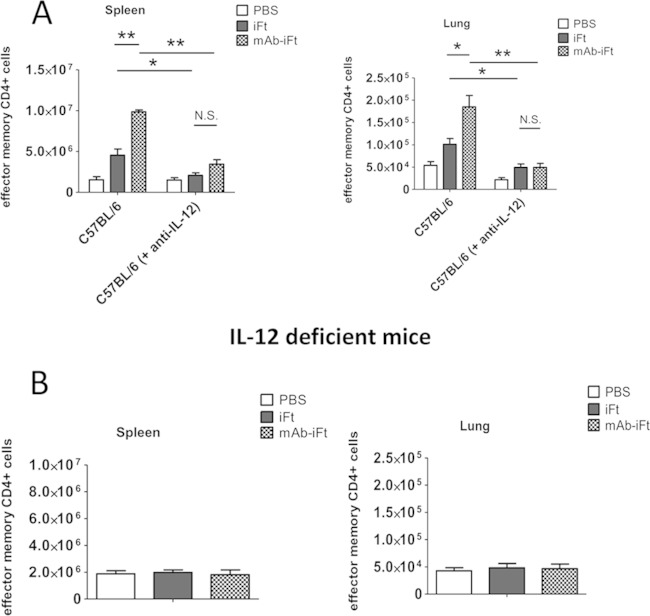
Having established that protection against F. tularensis LVS challenge via intranasal immunization with MAb-iFT ICs is IL-12 dependent, we hypothesized that the generation of effector memory CD4+ T cells following FcγR targeting also required IL-12. In order to test our hypothesis, C57BL/6 mice were immunized i.n. with PBS, iFT, or MAb-iFT, boosted on day 21, and challenged on day 35 with a lethal dose of F. tularensis LVS. Mice were also injected i.p. with rat IgG2a anti-IL-12p35, or the control isotype, on days 0 and 1 after F. tularensis LVS infection. On day 2 after F. tularensis LVS challenge, the levels of effector memory CD4+ T cells in the lungs and spleens of immunized mice were analyzed by flow cytometry. Our data indicated that neutralization of IL-12 in vivo via the administration of anti-IL-12 MAb significantly reduced the frequency and number of effector memory CD4+ T cells in both the lungs and spleens of immunized mice during F. tularensis LVS challenge (Fig. 6A and 7A). In accordance with these results, immunization of IL-12-deficient mice with iFT or MAb-iFT ICs, followed by lethal F. tularensis LVS infection, failed to enhance the generation of effector memory CD4+ T cells in either the lungs or spleens of immunized mice (Fig. 6B and 7B). Collectively, our data suggest that the necessity of IL-12 for protection against lethal LVS challenge is, in part, due to the ability of IL-12 to generate EM CD4+ T cells following targeting of bacterial immunogens to FcR.
FIG 6.
Generation of effector memory CD4+ T cells following targeting of iFT to FcR, during F. tularensis LVS challenge, is IL-12 dependent. C57BL/6 (A) or IL-12-deficient (B) mice were immunized and challenged as described for Fig. 5. C57BL/6 mice were also injected i.p. with rat IgG2a anti-IL-12 (500 mg/dose), or the control isotype, on days −1, 0, and 1 after F. tularensis LVS infection. On day 2 after F. tularensis LVS challenge, the lungs and spleens of immunized C57BL/6 mice (A) and IL-12-deficient mice (B) were harvested, and the white blood cells were obtained and counted. Expression levels of CD3+ CD4+ CD44+ CD62L+ CCR7+ T cells in the lungs and spleens of immunized C57BL/6 mice (A) and IL-12-deficient mice (B) were obtained by flow cytometry. The effector memory cells are presented as CD44+ CD62L−, while the central memory cells are presented as CD44+ CD62L+. Four mice were used per group, and data are representative of those from three experiments.
FIG 7.
Immunization of mice with MAb-iFT immune complexes increases the absolute numbers of effector memory CD4+ T cells during F. tularensis LVS infection, in an IL-12-dependent fashion. Immunization and challenge studies were conducted in C57BL/6 mice (A) or IL-12-deficient mice (B) as for Fig. 5. On day 2 after F. tularensis LVS challenge, the lungs and spleens of immunized mice were harvested and the lymphocytes were obtained and counted. C57BL/6 mice were also injected i.p. with rat IgG2a anti-IL-12 (500 mg/dose), or the control isotype, on days −1, 0, and 1 after F. tularensis LVS infection. Expression levels of CD3+ CD4+ CD44+ CD62L+ CCR7+ T cells in the lungs and spleens of immunized C57BL/6 mice (A) and IL-12-deficient mice (B) were obtained by flow cytometry, and their absolute numbers were calculated. Four mice were used per group per experiment. The data present the pooled averages of those from three experiments (12 mice per group). Statistical analyses were carried out using one-way ANOVA. *, P < 0.05; **, P < 0.01.
Enhanced generation of IFN-γ-secreting CD4+ effector memory T cells during F. tularensis LVS challenge in mice immunized i.n. with MAb-iFT ICs.
The importance of IFN-γ in protection against F. tularensis LVS challenge, and the enhanced generation of effector CD4+ memory T cells following MAb-iFT immunization, prompted us to hypothesize that the latter cell population is involved in the production of IFN-γ during F. tularensis LVS infection. To test this, C57BL/6 mice were immunized i.n. with PBS, iFT, or MAb-iFT, boosted on day 21, and challenged on day 35 with a lethal dose of F. tularensis LVS. Two days postchallenge, splenocytes from immunized mice were obtained and cultured for 5 h in the presence or absence of F. tularensis LVS (1 × 103 CFU/well), while PMA and ionomycin were used a positive control. Following incubation, cells were collected and pooled from each group and stained extracellularly for the markers CD4, CD44, CD62L, and CCR7 and intracellularly for IFN-γ. As depicted in Fig. 8, there were significantly more F. tularensis-specific, CD4+ splenocytes from MAb-iFT-immunized mice that secreted IFN-γ upon in vitro restimulation. The CD4 and IFN-γ double-positive cells also possessed the EM phenotype (CD44+ CD62Llow CCR7−). These observations lead us to believe that the source of IFN-γ, essential for protection against F. tularensis LVS challenge, is at least in part the effector memory CD4+ T cells generated following i.n. immunization with MAb-iFT ICs. Surprisingly, the CD4+ IFN-γ+ population observed was not CD8+ T lymphocytes (data not shown).
FIG 8.
Increased generation of IFN-γ-producing effector CD4+ T cells in mice immunized with MAb-iFT ICs compared to that in mice immunized with iFT alone. C57BL/6 were immunized and challenged as described for Fig. 1. On day 2 after F. tularensis LVS challenge, the spleens were harvested and splenocytes were cultured for 5 h with F. tularensis LVS (1 × 103 CFU per 2 × 105 SPCs/well). Following incubation, cells from each group were pooled, counted, and stained extracellularly for CD4, CD44, CD62L, and CCR7 and intracellularly for IFN-γ. Analysis (A) and enumeration (B) of IFN-γ+ effector CD4+ T cells were done by flow cytometry. Four mice were used per group, and the data are representative of those from two experiments. The P value for the iFT versus the MAb-iFT group in panel B was 0.03 (**, P < 0.05).
DISCUSSION
We have previously shown that i.n. targeting of fixed (inactivated) F. tularensis to FcγR in mice generates protection against lethal doses of F. tularensis. Due to the lack of an identified F. tularensis protective antigen, we focus on an inactivated vaccine (iFT), which we have shown can protect against the highly virulent SchuS4 organism in the highly stringent C57BL/6 mouse model (2). In this study, we have advanced our in vitro work by dissecting the in vivo cellular mechanisms involved in this protection. For the first time, we show that our mucosal vaccination strategy increases the frequency and activation of DCs in the lungs of immunized mice during F. tularensis LVS infection, while it enhances the generation of effector memory CD4+ T cells, both locally (lung) and systemically (spleen), in an IL-12-dependent fashion. We believe that this effector memory cell population plays an important role in the observed protection against F. tularensis LVS challenge, partly via its increased ability to produce IFN-γ. Despite the ability of antibodies to form bacterial aggregates, our findings depend upon specifically targeting antigens to FcγRs. Specifically, studies were conducted and published in which this issue was addressed using F(ab′)2 MAb-iFT (2). Importantly, the latter mimics MAb-iFT aggregation, except that the Fc domain is absent, thus preventing FcγR interaction. In the cases where F(ab′)2 MAb-iFT ICs were utilized, enhanced immune responses and protection were not observed (2). We have also published on the nature of the MAb-iFT complexes, in that the amount of MAb added to iFT can impact the MAb/iFT ratio within the immune complex, the level of MAb-iFT binding to APCs, and the level of protection provided, dependent in part on the challenge organism utilized (4). In addition, in these studies, immunization with F(ab′)2-iFT ICs failed to enhance recruitment and activation of DCs in the lungs of immunized mice (Fig. 1 to 3), as well as to generate effector memory CD4+ T cells during infection (Fig. 4).
Modulating DCs, one of the most important types of professional APCs of the immune system, present an attractive target for vaccine design. Indeed, DCs migrating from the periphery to secondary and tertiary lymphoid organs, loaded with exogenous antigens and activated by “danger signals,” identify naive T cells and induce their proliferation and differentiation to effector and memory phenotypes. Indeed, the superior capacity of DCs to activate naive T cells following nasal and oral immunizations has been determined in several studies (18–22). In our study, we observed that targeting iFT to FcγR via intranasal immunization of mice with MAb-iFT ICs increased the overall WBC cellularity of immunized mice during F. tularensis LVS infection compared to that in mice immunized with iFT alone. Most importantly, the frequency and activation status of lung DCs were also significantly increased postchallenge. Mice immunized with F(ab′)2-iFT ICs failed to recruit and increase the activation of DCs in their lungs, indicating that this effect is dependent upon targeting of the immunogen to FcγR (Fig. 1 to 3). However, it is currently unclear whether expression of FcγR specifically on DCs is required for their recruitment and activation. It would be of interest to assess whether expression of FcγR on other cell populations in the airway mucosa plays an important role in our observations. Nevertheless, the enhanced generation of activated DCs in the lungs of mice immunized with ICs provides evidence of enhanced mucosal immunity.
Although we have previously established that FcR targeting of a fixed bacterial immunogen (iFT) in vitro leads to DC activation (4), the exact mechanism that leads to their in vivo maturation and potential recruitment in the lungs remains unclear. At different developmental stages and at various anatomical sites, DCs may express various chemokine receptors, including CCR1, CCR2, CCR4, CCR5, CCR6, CCR7, CXCR1, and CXCR4 (11, 23–26). These findings suggest that chemokines and chemokine receptor expression levels may regulate DC trafficking. Indeed, in various infection models, including Mycobacterium bovis, Schistosoma mansoni, and Cryptococcus neoformans, expression of CCR2 in mice was necessary for DC recruitment and activation (27, 28). Similarly, in two studies using F. tularensis LVS, CCR7+ DCs were found to be recruited in areas of high CCL-19 and CCL-21 chemokine concentrations (29, 30). Whether the levels of these chemokines are increased significantly in the lungs of IC-immunized mice over mice administered with iFT alone, essentially recruiting mature DCs from peripheral sites, remains to be addressed.
Chemokine expression also plays a role in the recruitment of CD4+ T cells at the sites of infection, where they are exposed to concentrated amounts of polarizing cytokines, such as IL-12, secreted by the activated DCs (28). In our study, lung DCs from MAb-iFT IC-immunized mice had an increased ability to secrete IL-12 upon restimulation in vitro compared to those from mice immunized with the untargeted iFT. The activated DCs would efficiently present F. tularensis antigens to CD4+ T cells, polarizing them toward a TH1 phenotype via the secretion of IL-12 and driving their differentiation toward a protective effector memory phenotype. Furthermore, the observation that PECs showed increased secretion of IL-12 upon restimulation in vitro is of great interest, as it demonstrates the ability of this vaccine strategy to elicit both mucosal and systemic protective immune responses.
Indeed, in our work, immunizing mice with MAb-iFT ICs increased the expression of MHC class II and costimulatory molecules (B7.1 and B7.2) on the surfaces of lung DCs in vivo, hence facilitating a more robust antigen presentation to T lymphocytes and subsequent generation of memory. We believe that the activation of lung DCs is a direct effect of FcγR targeting and is not aided by the LPS present in the bacterial cell wall. This is evident due to the significant differences in DC maturation between the iFT- and MAb-iFT-immunized mice. In addition, F. tularensis LPS is structurally different from the typical enteric LPS and has a reduced stimulatory effect on APCs (31, 32).
Although it is clear that memory T cells can be distinguished into central and effector subtypes, their relevance during recall responses has been controversial. CM T cells usually reside in the secondary lymphoid tissues due to their high expression of CD62L and CCR7 (33, 34). EM T cells, on the other hand, have a greater capacity to migrate to inflamed tissues, partly due to increased expression of chemokine receptors, such as CCR2 and CCR5 (13). Generation of memory lymphocytes is often used to assess vaccination strategies, especially against intracellular pathogens. In our F. tularensis model, we have previously shown that immunization with MAb-iFT immune complexes provides protection 30 days postimmunization, indicating that targeting of bacterial antigens to FcγR results in the generation of protective memory lymphocytes (2). The lack of an increased CM T cell population though following FcγR targeting was surprising (Fig. 4A). This led us to believe that the significant enhancement of EM T cells during F. tularensis LVS infection is of vital importance for the protection observed in our model (Fig. 4B). Indeed, it has been shown that at early times after bacterial infection, effector cells dominate the memory pool and provide potent protective immunity (35).
The origin of the effector memory CD4+ T cells in our study remains unclear. One possibility is that these cells were generated in the secondary lymphoid organs, such as the spleen and lymph nodes, and homed to the lungs early after F. tularensis LVS challenge, possibly in a chemokine-dependent manner. Alternatively, the effector memory T cells may have been generated locally from the central memory pool. Indeed, in an elegant study from Blander et al., use of a transgenic T cell line showed that a significant percentage of central memory T lymphocytes can acquire effector functions upon restimulation (36). Although the levels of CM T cells in the lungs and spleens of IC-immunized mice were not different from the levels observed in mice immunized with iFT alone, we speculate that the differences at the postimmunization stage are qualitative. It is likely that the enhanced activation status of DCs enables them to generate central memory T cells that are more effectively programmed to switch to an effector memory phenotype during infection, both in the mucosa and systemically. Therefore, it is clear that the ability of vaccines to induce EM T cells is a requirement for protection. In addition, generation of EM cells in the secondary lymphoid organs requires prolonged and systemic exposure of the immunogen, which often poses a challenge, especially for nonreplicative vaccine vectors. We believe that by targeting iFT to FcγR, we aid the widespread distribution of antigen, increasing the number of antigen encounters by naive T cells and thus circumventing this problem.
Currently, we are not certain whether the effector memory CD4+ T cells are independently capable of providing protection against pulmonary infection with F. tularensis. Previous studies have implicated both CD4+ and CD8+ T cell subtypes in protection against this mucosal pathogen, as neutralization of either CD4+ or CD8+ T cells in vivo was not detrimental to the host (14, 15). We believe that protection mediated by CD4+ T cells is mainly IFN-γ dependent, while the cytotoxic effects of CD8+ T lymphocytes are important in clearing the infection. The independent protective role of CD4+ T cells in our model is under investigation via adoptive-transfer experiments, while the effect of MAb-iFT IC immunization on the generation of CD8+ memory T lymphocytes is also currently being studied.
Another aspect to be considered in vaccine design is the maintenance of effector memory cells. Apart from persistence of the immunogen, the cytokine milieu may mediate the long-term survival of these memory cells. More specifically, Esser et al. suggested that IL-12 may be a candidate factor to maintain CD4+ memory cells (12). In addition, IL-12 has been shown to enhance the potency and increase the durability of a leishmanial protein vaccine in mice (37). In our study, IL-12 was necessary in protection against lethal F. tularensis LVS challenge as well as for generating effector memory CD4+ T cells (Fig. 5 and 6). This is in accordance with previous studies showing that both subunits of IL-12 (p35 and p40) are essential in resolving pulmonary F. tularensis infection, while the administration of recombinant IL-12 i.n. facilitates bacterial clearance from the lungs of infected mice (38–40). Therefore, it is likely that targeting iFT to FcγR on DCs (and potentially macrophages as well) increases the levels of IL-12 in vivo, which drives EM T cell differentiation that is long-lived and possesses TH1 phenotype characteristics. In our model, effector memory CD4+ T cells from IC-immunized mice had a higher frequency as well as an increased ability to secrete IFN-γ compared to cells obtained from mice immunized with iFT alone, upon in vitro restimulation with F. tularensis LVS (Fig. 8). We believe that the CD4+ EM T lymphocytes generated in this vaccine platform are also long-lived, as we could still detect a significant population of these cells 25 days after F. tularensis LVS challenge (Fig. 4). The presence of IFN-γ in the memory CD4+ T lymphocytes following in vitro restimulation with F. tularensis LVS indicates specificity against F. tularensis. Enhanced production of IFN-γ in vivo can activate macrophages, thus facilitating bacterial clearance while driving the switching toward TH1-like antibody isotypes, such as IgG2a or IgG2c. Indeed, a type 1-like antibody response against F. tularensis has been previously shown following targeting of bacterial antigens to FcR (2). Hence, these observations lead us to believe that FcγR targeting mediates TH1 polarization of effector memory lymphocytes through IL-12 secretion, while it generates a greater frequency of antigen-specific memory T cells. The lack of protection against lethal F. tularensis LVS challenge following immunization of IFN-γ-deficient mice with MAb-iFT ICs (2) led us to conclude that the ability of effector memory T cells to produce IFN-γ may be indispensable for protection in our immunization model.
The specific F. tularensis responses observed in this study also revealed a CD4-negative cell population that expressed IFN-γ during restimulation with F. tularensis LVS in vitro. This population had a higher frequency in the MAb-iFT-immunized mice, suggestive of a memory recall response, but surprisingly, these cells were not CD8+ T lymphocytes either. Despite this observation, we believe that CD8+ cytotoxic T cells do play a role in protection against lethal F. tularensis LVS challenge; thus, potential CD8-mediated mechanisms are under investigation.
We have previously shown that targeting of the fixed immunogen, iFT, to FcγR provided 20 to 50% protection in C57BL/6 mice against the more lethal F. tularensis strain SchuS4 (2). It would be of interest to investigate whether SchuS4 infection generates similar cellular immune responses, although we suspect that the observed partial protection against this strain would account for more subtle differences among the immunized groups. For this reason, we focused primarily on our F. tularensis LVS model, although the analysis of cell-mediated mechanisms involved in SchuS4 protection is in our scope of future studies.
In summary, in our present work we advanced our understanding on the in vivo, protective mechanisms involved following FcR targeting of a fixed bacterial immunogen. Intranasal immunization of mice with MAb-iFT ICs increased the frequency of activated DCs as well as the generation of F. tularensis-specific, IFN-γ-secreting, EM CD4+ T cells. Generation and maintenance of the EM T cell population were dependent upon IL-12, which is partially produced by activated lung DCs but also systemically produced by other professional APCs. We believe that the enhanced ability of the EM T cells to secrete IFN-γ is necessary for protection against F. tularensis challenge.
This is the first study that implicates FcγR in modulating DC and T cell responses in an infectious model in vivo, essentially bridging the innate and adaptive branches of the immune system. The enhanced cellular immune responses in the lungs and spleens of immunized mice observed in this model provide evidence for the generation of pathogen-specific mucosal and systemic immunity. We believe that our targeting approach is highly applicable and promising, as it fills a significant gap left by the paucity of FDA-approved adjuvants and the lack of effective vaccines against mucosal pathogens.
ACKNOWLEDGMENTS
We thank the Immunology Core at Albany Medical College as well as the Animal Care Facility at Albany Medical College for significant contributions to this work.
This study was funded by the National Institutes of Health (R01 AI076408, R01 AI100138, and P01 AI056320).
REFERENCES
- 1.Czerkinsky C, Holmgren J. 2012. Mucosal delivery routes for optimal immunization: targeting immunity to the right tissues. Curr Top Microbiol Immunol 354:1–18. doi: 10.1007/82_2010_112. [DOI] [PubMed] [Google Scholar]
- 2.Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, Metzger DW, Gosselin EJ. 2008. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol 180:5548–5557. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitsaktsis C, Iglesias BV, Li Y, Colino J, Snapper CM, Hollingshead SK, Pham G, Gosselin DR, Gosselin EJ. 2012. Mucosal immunization with an unadjuvanted vaccine that targets Streptococcus pneumoniae PspA to human Fcgamma receptor type I protects against pneumococcal infection through complement- and lactoferrin-mediated bactericidal activity. Infect Immun 80:1166–1180. doi: 10.1128/IAI.05511-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iglesias BV, Bitsaktsis C, Pham G, Drake J R, Hazlett KR, Porter K, Gosselin EJ. 2013. Multiple mechanisms mediate enhanced immunity generated by mAb-inactivated F. tularensis immunogen. Immunol Cell Biol 91:139–148. doi: 10.1038/icb.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch J V, Young JW. 2005. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest 115:2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kronin V, Wu L, Gong S, Nussenzweig MC, Shortman K. 2000. DEC-205 as a marker of dendritic cells with regulatory effects on CD8 T cell responses. Int Immunol 12:731–735. doi: 10.1093/intimm/12.5.731. [DOI] [PubMed] [Google Scholar]
- 7.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. 2004. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins DM, Sanchez-Campillo J, Rosas-Taraco AG, Higgins JR, Lee EJ, Orme IM, Gonzalez-Juarrero M. 2008. Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with Mycobacterium tuberculosis. J Immunol 180:4892–4900. doi: 10.4049/jimmunol.180.7.4892. [DOI] [PubMed] [Google Scholar]
- 9.Meyts I, Hellings PW, Hens G, Vanaudenaerde BM, Verbinnen B, Heremans H, Matthys P, Bullens DM, Overbergh L, Mathieu C, De Boeck K, Ceuppens JL. 2006. IL-12 contributes to allergen-induced airway inflammation in experimental asthma. J Immunol 177:6460–6470. doi: 10.4049/jimmunol.177.9.6460. [DOI] [PubMed] [Google Scholar]
- 10.Witmer-Pack MD, Swiggard WJ, Mirza A, Inaba K, Steinman RM. 1995. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. II. Expression in situ in lymphoid and nonlymphoid tissues. Cell Immunol 163:157–162. [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 12.Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N, Washabaugh MW. 2003. Memory T cells and vaccines. Vaccine 21:419–430. doi: 10.1016/S0264-410X(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 13.Kaech SM, Wherry EJ, Ahmed R. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol 2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 14.Conlan J W, Sjostedt A, North RJ. 1994. CD4+ and CD8+ T-cell-dependent and -independent host defense mechanisms can operate to control and resolve primary and secondary Francisella tularensis LVS infection in mice. Infect Immun 62:5603–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yee D, Rhinehart-Jones TR, Elkins KL. 1996. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol 157:5042–5048. [PubMed] [Google Scholar]
- 16.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Cao S, Herman LM, Ma X. 2003. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J Exp Med 198:1265–1276. doi: 10.1084/jem.20030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. 2005. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol 35:1831–1840. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 19.Anjuère F, Luci C, Lebens M, Rousseau D, Hervouet C, Milon G, Holmgren J, Ardavin C, Czerkinsky C. 2004. In vivo adjuvant-induced mobilization and maturation of gut dendritic cells after oral administration of cholera toxin. J Immunol 173:5103–5111. doi: 10.4049/jimmunol.173.8.5103. [DOI] [PubMed] [Google Scholar]
- 20.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. 2008. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med 205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wikstrom ME, Batanero E, Smith M, Thomas J A, von Garnier C, Holt PG, Stumbles PA. 2006. Influence of mucosal adjuvants on antigen passage and CD4+ T cell activation during the primary response to airborne allergen. J Immunol 177:913–924. doi: 10.4049/jimmunol.177.2.913. [DOI] [PubMed] [Google Scholar]
- 22.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. 2001. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med 193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cyster JG. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lanzavecchia A. 1999. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med 189:611–614. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A. 1999. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol 29:1617–1625. doi:. [DOI] [PubMed] [Google Scholar]
- 26.Dieu MC, Vanbervliet B, Vicari A, Bridon J M, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu BC, Freeman CM, Stolberg VR, Hu J S, Zeibecoglou K, Lu B, Gerard C, Charo IF, Lira SA, Chensue SW. 2004. Impaired lung dendritic cell activation in CCR2 knockout mice. Am J Pathol 165:1199–1209. doi: 10.1016/S0002-9440(10)63380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. 2008. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol 181:610–620. doi: 10.4049/jimmunol.181.1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar-Haim E, Gat O, Markel G, Cohen H, Shafferman A, Velan B. 2008. Interrelationship between dendritic cell trafficking and Francisella tularensis dissemination following airway infection. PLoS Pathog 4:e1000211. doi: 10.1371/journal.ppat.1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiavolini D, Rangel-Moreno J, Berg G, Christian K, Oliveira-Nascimento L, Weir S, Alroy J, Randall TD, Wetzler LM. 2010. Bronchus-associated lymphoid tissue (BALT) and survival in a vaccine mouse model of tularemia. PLoS One 5:e11156. doi: 10.1371/journal.pone.0011156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ancuta P, Pedron T, Girard R, Sandstrom G, Chaby R. 1996. Inability of the Francisella tularensis lipopolysaccharide to mimic or to antagonize the induction of cell activation by endotoxins. Infect Immun 64:2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dueñas AI, Aceves M, Orduna A, Diaz R, Sanchez Crespo M, Garcia-Rodriguez C. 2006. Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than E. coli LPS. Int Immunol 18:785–795. [DOI] [PubMed] [Google Scholar]
- 33.Campbell JJ, Butcher EC. 2000. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol 12:336–341. doi: 10.1016/S0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 34.Gunn MD, Tangemann K, Tam C, Cyster J G, Rosen SD, Williams LT. 1998. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A 95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts AD, Ely KH, Woodland DL. 2005. Differential contributions of central and effector memory T cells to recall responses. J Exp Med 202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blander JM, Sant'Angelo DB, Metz D, Kim SW, Flavell RA, Bottomly K, Janeway CA Jr. 2003. A pool of central memory-like CD4 T cells contains effector memory precursors. J Immunol 170:2940–2948. doi: 10.4049/jimmunol.170.6.2940. [DOI] [PubMed] [Google Scholar]
- 37.Gurunathan S, Prussin C, Sacks DL, Seder RA. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat Med 4:1409–1415. doi: 10.1038/4000. [DOI] [PubMed] [Google Scholar]
- 38.Elkins KL, Cooper A, Colombini SM, Cowley SC, Kieffer TL. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect Immun 70:1936–1948. doi: 10.1128/IAI.70.4.1936-1948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pammit MA, Budhavarapu VN, Raulie EK, Klose KE, Teale J M, Arulanandam BP. 2004. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob Agents Chemother 48:4513–4519. doi: 10.1128/AAC.48.12.4513-4519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duckett NS, Olmos S, Durrant DM, Metzger DW. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun 73:2306–2311. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]